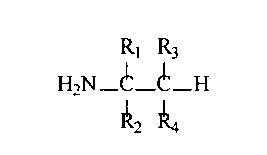Note: Descriptions are shown in the official language in which they were submitted.
CA 02307821 2000-OS-09
-1-
DIESEL FUEL COMPOSITIONS CONTAINING
TERTIARY ALKYL PRIMARY AMINES
BACKGROUND OF THE INVENTION
The present invention relates to diesel fuel additives and diesel fuel
compositions captaining such additives. In particular, the present invention
relates
to a diesel fuel additive and diesel fuel compositions containing such
additives
which are useful as thermal stabilizers and in reducing deposits on injection
nozzles
of compression ignition diesel engines.
Diesel fuel is principally a blend of petroleum-derived fractions called
middle
distillates (heavier than gasoline, but lighter than Tube oil) and may
optionally
contain additional additives useful for a variety of purposes. For example,
oxidation
inhibitors are added to reduce the formation of gums and insoluble residues
that can
clog fuel filters, and detergent-dispersant additives help keep fuel-insoluble
materials in suspension and are therefore helpful in maintaining a clean
engine and
fuel delivery system. Other additives include rust preventatives, anti-icing
additives
and cold-flow improvers. Ignition quality (cetane number) improvers are
another
type of common additive, and are added to increase the cetane number when the
base fuel cetane does not meet requirements.
During operation of a diesel engine, diesel fuel is injected into the
compressed, high-temperature air in the combustion chamber, where it ignites
spontaneously. The most desirable fate of the carbon present in diesel fuels
is to be
emitted as carbon dioxide in the exhaust gas. If this is the case then the
combustion
is at its most efficient, which means that the maximum calorific value of the
fuel is
being exploited. When combustion is incomplete, then some of the carbon can be
emitted as carbon monoxide (CO), hydrocarbon (HC), or as particulates.
Particulates
are of great concern as some studies are suggesting relationships between
concentrations of fine particulates (less than 10 ~ in size) in urban air and
human
health problems, including asthma and heart disease. Concerns over the
environment and health impact of the emissions from the exhaust of automotive
diesel engines have lead to several initiatives to reduce the levels of
harmful
CA 02307821 2000-OS-09
pollutants. These initiatives have embraced both advancements in engine design
and improvements in fuel quality. The improvements in fuel quality can be
divided
into tvvo main categories: changes in certain key physical and chemical
properties of
the fuel; and the addition of mufti-functional detergent additives packages.
Diesel fuel contains hydrocarbons having higher boiling point range than that
of gasoline. Diesel fuel is designed to ignite spontaneously, quickly (within
1-2
milliseconds), and without a spark. The time lag between the initiation of
injection
and the initiation of combustion is known as ignition delay. In high speed
diesel
engines, a long ignition delay produces rough operation and knocking. To
minimize
ignition delay, it is necessary to maintain the fuel injector's ability to
atomize a
precise amount of fuel and mix it with available air. This in turn depends on
the
operation of the fuel injector. The performance of the fuel injector can be
impaired
by the build-up of deposits derived from the fuel. Thermal degradation of both
fuel
and crankcase lubricant components leads to the formation of deposits within
fuel
injectors. Deposit formation is worsened by hot combustion gases entering the
nozzle. Deposits alter the close manufacturing tolerances of injectors and
change
fuel spray characteristics, leading to the observed degradation in engine
performance.
These deposits restrict the flow of fuel through the injector and can cause
needle sticking. Indirect ignition (IDI) engines with pintle-type nozzles are
the more
sensitive to such deposits. Engine symptoms resulting from IDI nozzle fouling
are
analogous to those caused by operation on fuel of inadequate cetane number.
Increased noise, black smoke and exhaust emissions are all associated with
severe
IDI nozzle fouling, together with reduction in fuel economy.
Pintle-type nozzles are designed to release a restricted initial fuel, known
as
the pilot injection. This pilot injection, which occurs bet~n.~een needle
lifts of 0 - 0.4
mm, initiates combustion ahead of the main fuel injection which occurs above
0.5
mm needle lift. This smoother, progressive combustion is both more efficient
and
quieter than previous type diesel engines. Deposits, which tend to form around
the
pintle tip, can strangulate the pilot injection by blocking the fuel flow.
This results in
CA 02307821 2000-OS-09
-3-
a serious deviation from the designed combustion characteristics for the
engine
causing increases in noise and unburnt fuel exhaust emissions. There is
published
data which correlates fouling reduction with reduction of HC, CO and
particulate
emissions. See, for example: R. F. Haycock and R. G. F. Thatcher, "Fuel
Additives
and the Environment," ATC document, CEFIC, N° 52 (1994); M. W. Vincent
et al.,
"Diesel Fuel Detergent Additive Performance And Assessment," J. Soc. Automot..
Eng., SP (1994), SP-1056 (Diesel Fuel),11-24; and K. Reading et al., "The
Effect of a
Fuel Detergents on Nozzle Fouling and Emissions in IDI Diesel Engines."
To counter the adverse effects of injector nozzle fouling, detergency
additives
are employed. The use of mufti-functional detergent additive packages has
become
more widespread in recent years. By reducing deposit formation in the injector
nozzle the detergent brings about a reduction in HC, CO and particulate
emissions.
There are currently different types of chemistries which are well known to
bring detergency to the diesel fuel. All are based on molecules having: a
polar
portion, bringing the dispersancy effect; and a lipophilic (often a polymeric)
portion,
allowing the entire molecule to be soluble in fuel. Most important chemistries
are
polybutenylsuccinic amides or imides (especially those known as PIBSA
derivatives).
Surfactant molecules, frequently based on polymeric succinimide chemistry,
help to control deposits within fuel injectors with significant benefits to
engine
performance. Detergent additives are effective in preserving an acceptable
pilot
flow by preventing deposit build up and removing performed deposits. Measuring
the performance of detergent additive formulations is an important aspect of
developing a high performance diesel fuel. Different test engines and test
procedures are employed in Europe and the USA; however, the common aim is to
show benefits from the use of detergent additives compared with untreated base
fuels, and thereby to allow the cost effective development of improved fuels.
Another approach to the problem of injector nozzle fouling has been to blend
together a number of additives to produce synergistic combinations that work
to
control fouling. Such additive combinations are disclosed in US 4,482,353; US
CA 02307821 2000-OS-09
4,482,355; US 4,482,356; and US 4,482,357 (all Hanlon). However, there is
still a need
for simple, multifunctional diesel fuel additives, such as those capable of
imparting
thermal stability and preventing or reducing deposit formation in injector
nozzles.
SUMMARY OF THE INVENTION
The present invention is directed to a fuel additive composition effective to
provide injector deposit inhibiting properties and thermal stability to diesel
fuel,
such composition consisting essentially of at least one tertiary alkyl primary
amine.
The present invention is also directed to diesel fuel compositions conprising
a
major amount of diesel fuel and a minor amount of the fuel additive
composition as
discussed above.
BRIEF DESCRIPTION OF THE DRAWING
Figure 1 is a cut-away side view of a typical fuel injector used in diesel
engines.
Figures 2 and 3 are close-ups of the fuel injector nozzle 20 of Figure 1.
Figure
2 shows the nozzle in open position, and Figure 3 shows the nozzle in closed
position. Bold lines in Figures 2 and 3 indicate typical deposit formation
areas.
DETAILED DESCRIPTION OF THE INVENTION
As used in this specification, the following terms have the following
definitions, unless the context clearly indicates otherwise. The terminology
"(C~-
Cn)" means a straight chain, branched chain or cyclic groups having 1 to 21
carbon
atoms per group. The term "major amount" is understood to mean greater than 50
percent by weight, and the term "minor amount" is understood to mean less than
50
percent by weight. The term "TAPA" means tertiary alkyl primary amine(s). The
following abbreviations are used throughout this specification: mL =
milliliters; L =
liters; ~ - microns; mm = millimeters; mg = milligrams; g = grams; rpm =
revolutions
per minute. Unless otherwise specified, ranges specified are to be read as
inclusive,
references to percentages are by weight and all temperatures are in degrees
centigrade (°C)
CA 02307821 2000-OS-09
-5-
The tertiary alkyl primary amines useful in the present invention are tertiary
alkyl primary amines according to the formula:
R~ R3
H2N-C- C-H
I I
R2 Ra
wherein:
Ri and Rz are each independently selected from: Ci-Cz1 alkyl or substituted Co-
Czv alkyl, Ci-Cn alkenyl or substituted Ci-Czl alkenyl; and
R~ and R~ are each independently selected from: hydrogen or Ci-Czi alkyl,
substituted Ci-Czi alkyl, Ci-Czl alkenyl or substituted Cl-Czn alkenyl.
Suitable examples of Ci-Cn alkyl include, but are not limited to: methyl,
ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, cyclobutyl, n-
pentyl,
isopentyl, neopentyl, cyclopentyl, n-hexyl, cyclohexyl, 2-ethylhexyl, octyl
cyclooctyl,
nonyl, cyclononyl, decyl, isodecyl, cyclodecyl, undecyl, dodecyl (also known
as
lauryl), tridecyl, tetradecyl (also known as myristyl), pentadecyl, hexadecyl,
heptadecyl, octadecyl, nonadecyl, cosyl, eicosyl and heneicosyl.
Suitable examples of Cl-Cn alkenyl include, but are not limited to: ethenyl, n-
propenyl, isopropenyl,1-butenyl, cis-2-butenyl, isobutylene, traps-2-butenyl,
2-3,
dimethyl-2-butenyl, 3-methyl-1-butenyl, 2-methyl-2-butene, 1-pentenyl, cis-2-
pentenyl, traps-2-pentenyl,1-hexenyl,1-heptenyl, 1-octenyl,1-nonenyl, and 1-
decenvl.
Suitable examples of Ci-Cn substituted alkyl and alkenyl include, but are not
limited to: the above recited alkyl and alkenyl groups substituted with
hydroxy,
halide such as fluorine, chlorine or bromine; cyano; alkoxy; haloalkyl;
carbalkoxy;
carboxy; amino; alkylamino derivatives and the like; or nitro groups.
Tertiary alkyl amines useful in the present invention include but are not
limited to: 1,1,3,3-tetramethylbutylamine; an isomeric mixture of C1~ to Czz
tertiary
alkyl primary amines; an isomeric mixture of Cio to Cl.~ tertiary alkyl
primary
CA 02307821 2000-OS-09
-6-
amines; an isomeric mixture of Cs to Cio tertiary alkyl primary amines; or
mixtures
thereof. In a preferred embodiment, tertiary alkyl amine is an isomeric
mixture of Cs
to Coo and C1o to C14 tertiary alkyl primary amines. In a most preferred
embodiment,
the tertiary alkyl primary amine is an isomeric mixture of Clo to Cla tertiary
alkyl
primary amines. Such tertiary alkyl primary amines are available from Rohm and
Haas Company (Philadelphia, PA) under the PRIMENE~ trademarks.
In general, the tertiary alkyl primary amines of the present invention are
present in diesel fuel compositions at a concentration of 1 to 2000 mg/ L,
preferably
to 800 mg/ L, more preferably 20 to 600 mg/ L and most preferably 40 to 500
10 mg/ L.
The tertiary alkyl primary amines used in the diesel fuel compositions of the
present invention are prepared using substrate compounds known as substrates
for
the Bitter reaction and include, for example, alcohols, alkenes, aldehydes,
ketones,
ethers. See, generally, L. I. Krimen and D. J. Cota, "The Bitter Reaction,"
Organic
Reactions,17(1969), pp. 213-325. The process for preparing the amines is known
in
the art and is described, for instance, in US 5,527,949 and in co-pending
provisional
application 60/ 051,867.
The fuel additives of the present invention are also useful in maintaining or
increasing the thermal stability of diesel fuels. Most users are knowledgeable
about
the fuel's primary role as an energy source; however, few are aware that
diesel fuel
performs multiple functions in a diesel engine and the associated fuel system.
Diesel
fuel is increasingly used as a circulating coolant for high pressure fuel
injections
systems. In addition to its primary role as an energy source, the fuel also
serves as
the sole lubricant of critical moving parts and as a heat-transfer fluid.
Adequate
thermal stability is a necessary requirement for the effective functioning of
diesel
fuel as a heat-transfer fluid. In modern heavy-duty diesel engines, only a
portion of
the fuel that is circulated to the fuel injectors is actually delivered to the
combustion
cylinders. The remainder is circulated back to the fuel tank carrying heat
with it,
consequently raising the bulk fuel temperature. Because of the recirculation
of fuel
through the newer engines, fuel can be exposed momentarily to temperatures as
CA 02307821 2000-OS-09
high as 350°C. This process, in some engines and fuel combinations,
causes a
problem in that as engine heat passes from injectors into the fuel, it can
trigger a
degradation process that leads to particle formation, clogging filters and
injectors.
Fuels resistant to such thermal degradation must get a minimum 80%
reflectance in the updated 150°C Accelerated Fuel Oil Stability Test
(F21-61) at 180
minutes. Good thermal stability may become even more important in the future.
Diesel engine manufacturers have indicated that engines under development to
meet
future exhaust emission standards will expose the fuel to more severe
operating .
environments (stress), e.g., higher pressures and longer contact with high-
temperature engine parts. the new premium diesel fuel specifications in USA
will
require a thermal stability and detergency pass test.
In the following Examples, performance testing of the diesel fuel detergency
additives of the present invention was based on the widely used Peugeot 1.9
litre IDI
engine XUD9A, in an industry standard test method developed by the Co-
ordinating
European Council (CEC: Working Group PF26 draft procedure). Details of the
test
methods used to evaluate nozzle fouling employed in the PF26 Peugeot XUD9A
engine test can be found in: Vincent, M. W. et al., "Diesel Fuel Detergent
Additive
Performance And Assessment," j. Soc. Automot. End, SP (1994), SP-1056 (Diesel
Fuel),11-24; and CEC document reference CEC F-23-X-95.
The test engine is the most widely used light duty diesel engine in Europe,
powering a substantial proportion of all vehicles in this class. The Peugeot
engine,
in common with many other IDI engines, employs a pintle type injector actuated
by
fuel pressure. Some of the main parameters of this test engine are provided
below.
Bore, mm 83.0
Stroke, mm 88.0
No of cylinders 4
Cubic capacity, 1.9
liters
Compression ratio 23.5:1
Aspiration Natural
Max Power G~ rpm, 4600, 48.5
kW
Max Torque rpm, 4000,120
Nm
Fuel injection pumpBosch Rotary
Fuel injector type Pintle
CA 02307821 2000-OS-09
_g_
Referring now to the Figures, a metered quantity of fuel under pressure,
delivered by the pump, lifts the injector needle 30 from its seat. The pintle
type
injector is normally closed by pressure from spring 20, thus preventing the
flow of
fuel. When the injector needle nozzle 40 is lifted from its seat by fuel
pressure, a
flow path is opened and a spray of small fuel droplets enters the combustion
chamber. Fuel pressure under injection is typically of the order of 100 bar.
The following Examples are provided as an illustration of the present
invention. Fuel samples A, B and C were, fresh test fuels without any
additives and
were obtained from commercial sources. The fuel samples were analyzed to
ensure
conformance with specifications and stored under ambient temperature, in dark,
and
under nitrogen atmosphere. All tests were started within a month of obtaining
the
fresh samples. All commercial additives used were as received without further
purification. The Cy, Ci2, and Clx tertiary alkyl primary amines samples were
commercial products sold by Rohm and Haas Company under the PRIMENE~
trademark. The results are shown below in Table 1.
Table 2: Detailed Anah s/ is of Test Ficel Samples
Fuel # % Sulfur % Aromatics Cetane Number
A 0.033 24.5 53.2
B 0.06 39 45
C 0.051 32 47
In the Peugeot engine tests, selected pintle nozzles were flow checked and
matched prior to the start of the test. The bed engine was prepared and stared
from
cold on "slave" injectors for 20 minutes, comprising two five minute no load
idle
periods, separated by operation for 10 minutes at 2000 rpm. After flushing the
fuel
pump with fresh test fuel to ensure thorough removal of all prior fuel,
matched and
flow-checked injectors were fitted for the test itself. The engine was
operated for 6
hours at constant speed and load (3000 rpm, and about 50% maximum load)
followed by a 5 minute idle at no load prior to shut down. Injectors were
removed
promptly from the engine and flows rechecked. Nozzle flows were checked both
before and after the test using air. The results were expressed in terms of
injector
CA 02307821 2000-OS-09
-9-
air-flow reduction measured at needle lift values of 0.05, 0.10, 0.20, 0.30,
0.=10, and
0.50 mm.
In the mode tested, the engine typically undergoes severe nozzle coking on
untreated base fuels. Flow loss at the onset of pilot fuel flow (0.1 mm needle
lift) is
typically 88-90% compared to the initial clean condition, after 6 hours test
bed
operation on an untreated base fuel.
Ex. ample 1: Injector Fouling
The efficiency of the tertiary alkyl primary amines was assessed with the
Peugeot XUD-9A engine test in comparison with current commercial dispersants
and detergent fuel additives (Control Reference). Table 2 contains the results
of
several Peugeot XUD9A engine tests at a needle lift of 0.10 mm. All the engine
tests
were run in a laboratory complying with ISO 14001 standard.
Table 2: Percent Flow Reduction at 0.1 mm Needle Lift znith Diesel Fuel A
Additive (mg/L) % Flow Reduction
Control Reference 80.4*
PRIMENE BC-9 ( 400) 76.7
PRIMENE 81-R (400) 75.1**
C9 - Cls TAPA (250)*** 68.5
C9 - Cls TAPA (300)*** 70.5
C9 - Cls TAPA (350)*** 77.1
C9 - Cis TAPA (400)*** 69.2
C9 - Cls TAPA (420)*** 76.3
C9 - Cls TAPA (460)*** 68.1
PRIMENE JM-T (200) 80.6
* Average of 6 independent runs
** Average of 4 independent runs
*** Additive was diluted with 10 - 20 % hydrocarbon
solvent
One can see that the results demonstrate that tertiary alkyl primary amines
provide significant reduction in injector nozzle fouling, and are comparable
to or
better than the combination of current commercial dispersants and fuel
additives.
CA 02307821 2000-OS-09
-10-
ExamQle 2: Thermal Stability Tests
Samples of Fuels B and C, having a sulfur concentration of 0.06%, and 0.051 %,
respectively, were also evaluated by 150 °C Accelerated Fuel Oil
Stability Test
("Octet F-21-61") for thermal stability improvement for 180 minutes.
The test procedure was as follows. A 50 mL sample of fuel oil in a test tube
was placed in a 150 °C bath for 180 minutes. After removal from the
bath the fuel
was allowed to cool in air to 21 - 26 °C over a period of 90 minutes to
4 hours. The
aged fuel was then filtered through 4.25 cm Whatman No 1 filter paper using
vacuum filtration assembly. The paper was then washed with three portions,
about
15 mL each, of iso-octane. The filter paper was dried under vacuum for one or
two
minutes. The filter paper was rated by measuring percent reflectance using a
photovolt meter (model 5TH using Search Unit Y with green filter. The filter
paper
was also rated for color by choosing a reference blotter which gave the best
visual
match. Generally, 80% or higher reflectance was considered a pass from thermal
stability standpoint. Color rating of the filter paper was measured on a scale
of 1 to
20, and the rating of up to 7 was considered a pass. Color rating higher than
7 is
reported as poor thermal stability for the test fuel.
The data presented in the Tables 3 and 4 below show that the addition of the
tertiary alkyl primary amines of the present invention to the fuel samples
improved
the thermal stability as seen by filter pad rating and reflection meter
reading.
Table 3: 150°C Accelerated Flsel Oil Stabilitu Test Results of Diesel
Fuel B
Filter pad % Reflection
Additive Dosage (mg/L) rating meter reading
None --- 8 71
Primene 81-R 5 4 86
Commercial #1 5 7 72
Commercial #2 5 5 76
Commercial #3 5 4 84
Commercial #4 5 9 70
Commercial #5 5 8 72
Primene 81-R 10 4 89
CA 02307821 2000-OS-09
-11-
Primene 81-R 20
2 95
Primene BC-9 20 2 97
Primene BC-9 + 81-R (1:1)20 2 96
C9 - C15 TAPA* 20 2 85
Commercial #3 20 2 94
Commercial #5 20 3 87
Commercial #6 - - - - 20 - - _ - 4 - - 89 - - -
Primene 81-R 40 2 96
Primene BC-9 40 2 97
Primene BC-9 + 81-R (1:1)40 1 98
Commercial #3 40 3 92
Commercial #5 40 4 84
Commercial #1 40 3 88
Commercial #6 40 3 91
* Additive was diluted with 10 - 20 °i° hydrocarbon solvent
Table 4 150°C Accelerated Fuel Oil Stabilihl Test Res~clts of Diesel
Fuel #C
Dosage Filter % Reflection
pad
Additive (mg/L) rating meter reading
None ---- 13 75
Primene 81-R 10 4 84
Primene BC-9 10 3 85
Commercial #3 10 4 82
Primene 81-R 15 7 89
Primene BC-9 15 7 88
Commercial #1 15 10 72
Commercial #3 15 9 80
Commercial #4 15 8 76
Commercial #5 15 3 85
Commercial #6 15 4 87
Commercial #7 15 5 85
Commercial #8 15 5 82
Commercial #9 15 7 74
CA 02307821 2000-OS-09
-12-
As can be clearly seen, addition of tertiary alkyl primary amines greatly
improves the thermal stability of the diesel.