Note: Descriptions are shown in the official language in which they were submitted.
CA 02307853 2000-04-27
WO 99123087 PCT/CA98/00998
TITLE F THE INVENTION
DIARYL-5-ALKYL-5-METHYL-2(SH)-FURANONES AS
SELECTIVE CYCLOOXYGENASE-2 INHIBITORS
_BACKGROUND OF THE INVENTIQN
This invention relates to methods of treating
cyclooxygenase mediated diseases and certain pharmaceutical
compositions therefor.
Non-steroidal, antiinflammatory drugs exert most of their
antiinflammatory, analgesic and antipyretic activity and inhibit
hormone-induced uterine contractions and certain types of cancer
growth through inhibition of prostaglandin G/H synthase, also known as
cyclooxygenase. Initially, only one form of cyclooxygenase was known,
this corresponding to cyclooxygenase-1 (COX-1) or the constitutive
enzyme, as originally identified in bovine seminal vesicles. More
recently the gene for a second inducible form of cyclooxygenase,
cyclooxygenase-2 (COX-2) has been cloned, sequenced and
characterized initially from chicken, murine and human sources. This
enzyme is distinct from the COX-1 which has been cloned, sequenced
and characterized from various sources including the sheep, the mouse
and man. The second form of cyclooxygenase, COX-2, is rapidly and
readily inducible by a number of agents including mitogens, endotoxin,
hormones, cytokines and growth factors. As prostaglandins have both
physiological and pathological roles, we have concluded that the
constitutive enzyme, COX-1, is responsible, in large part, for
endogenous basal release of prostaglandins and hence is important in
their physiological functions such as the maintenance of gastrointestinal
integrity and renal blood flow. In contrast, we have concluded that the
inducible form, COX-2, is mainly responsible for the pathological
effects of prostaglandins where rapid induction of the enzyme would
occur in response to such agents as inflammatory agents, hormones,
growth factors, and cytokines. Thus, a selective inhibitor of COX-2
will have similar antiinflammatory, antipyretic and analgesic properties
to a conventional non-steroidal antiinflammatory drug, and in addition
-1-
20064 . CA 02307853 2000-04-27
~ 4 09. 1999
s8
would inhibit hormone-induced uterine contractions and have potential
anti-cancer effects, but will have a diminished ability to induce some of the
mechanism-based side effects. In particular, such a compound should have
a reduced potential for gastrointestinal toxicity, a reduced potential for
renal side effects, a reduced effect on bleeding times and possit~ly a
lessened ab ~lity to induce asthma attacks in aspirin-sensitive astamatic
subjects.
Furthermore, such a compound will also inhibit prostanoid-
induced smooth muscle contraction by preventing the synthesis of
contractile prostanoids and hence may be of use in the treatment of
dysmenorrhea, premature labour, asthma and eosinophil related disorders.
It will also be of use in the treatment of Alzheimer's disease., for
decreasing bone loss particularly in postmenopausal women (i.e. treatment
of osteoporosis) and for the treatment of glaucoma.
The potential utilities of selective cyclooxygenase-2
inhibitors are discussed in John Vane, "Towards a better aspirin" in Nature,
Vol. 3 67, pp. 215-216, 1994; Bruno Battistini, Regina Bolting and Y.S.
Bakhle, " COX-I and COX-2: Toward the Development of More Selective
NSAIDs" in Drug News and Perspectives, Vol. 7, pp. 501-5129 1994; and
David B. Reitz and Karen Seibert, "Selective Cyclooxygenase Inhibitors"
in Annual Reports in Medicinal Chemistry, James A. Bristol, Editor, Vol.
30, pp. 179-188, 1995.
W095/00501 published on 5 January 1995 relates to phenyl-
substituted heterocycles and cyclopentyl compounds. The present
application is different in that R2 and R3 which represent substituents at
position 5 of the furanone ring cannot be the same, such that they are
attached to an asymmetric carbon atom.
W097/14691 published on 24 April 1997 relates to phenyl
substituted heterocycles in which position 3 is substituted with X-R2
instead of an aryl group as in the present application.
SUMMARY OF THE INVENTION
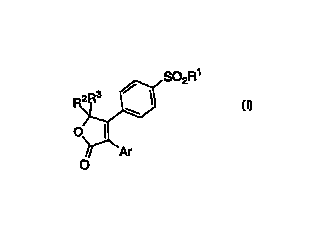
The invention encompasses compounds of Formula I as well
as a method of treating COX-2 mediated diseases comprising
administration to a patient in need of such treatment of a non-toxic
therapeutically effective amount of a compound of Formula 1:
-2-
AMENDED SHEET
IPEA/EP ~ -
26064 CA 02307853 2000-04-27
S02R~
O
The invention also encompasses certain pharmaceut,cal
compositions for treatment of COX-2 mediated diseases comprising
compounds of Formula I.
DETAILED DESCRIPTION OF THE IIWENTION
The invention encompasses compounds of Formula I as well
as a method of treating COX-2 mediated diseases comprising
administering to a patient in need of such treatment a non-toxic
therapeutically effective amount of a compound of Formula I
S02R~
O
or a pharmaceutically acceptable salt thereof, wherein:
Ar is an unsubstituted, mono or di substituted phenyl or pyridyl,
wherein the substituents are selected from halogen;
Rl is selected from the group consisting of NH2 and CH3;
RZ is selected from the group consisting of
C 1 _6 alkyl unsubstituted or substituted with C3_6 cycloalkyl,
and
C3_6 cycloalkyl;
R3 is selected from the group consisting of
C 1 _6 alkyl unsubstituted or substituted with one, two or three
fluoro atoms; and C3_6 cycloalkyl;
-3-
A~cNDED Si~EET
IPEAIEP ~
X0064 CA 02307853 2000-04-27
with the proviso that R2 and R3 are not the same.
In a class of compounds and pharmaceutically acceptable
salts of the invention, Ar is unsubstituted phenyl, phenyl substituted with
fluoro, or unsubstituted pyrindyl or pyridyl substituted with fluoro.
In a subclass of this class of compounds and
pharmaceutically acceptable salts of the invention, R2 is C2-3 alkyl,
CH2-cyclopropyl, or cyclopropyl.
In a group of this subclass of compounds and
pharmaceutically acceptable salts of the invention, R3 is CH3, CH2CH3,
CH2F, CHF2, or CF3.
In a subgroup of this group of compounds and
pharmaceutically acceptable salts of the invention, R3 is CH3.
The following abbreviations have the indicated meanings:
AA arachidonic acid
Ac acetyl
AIBN 2.2-azobisisobutyronitrile
Bn benzyl
DBU 1,8-diazobicyclo[5.4.0]undec-7-ene
DMAP 4-(dimethylamino)pyridine
DMF N,N-dimethylformamide
DMSO dimethyl sulfoxide
Et3N triethylamine
HBSSHanks balanced salt solution
HEPES N-(2-Hydroxyethyl]piperazine-Nl-[2
ethanesulfonic acid]
HWB human whole blood
KHMDS potassium hexamethyldisilazane
LDA lithium diisopropylamide
LPS lipopolysaccharide
MMPP magnesium monoperoxyphthalate
Ms methanesulfonyl = mesyl
Ms0 methanesulfonate = mesylate
NSAID non-steroidal anti-inflammatory drug
-4-
_.' .
- P :-,=.v" , ::: i i
.~_.
?0064 CA 02307853 2000-04-27
OXONE~ 2KHS05 - KHS04 - K2S04
PCC pyridinium chlorochromate
PDC pyridinium dichromate
Ph phenyl
r.t. room temperature
rac. racemic
'r' f trifluoromethanesulfonyl =
triflyl
T~ trifluoromethanesulfonate =
triflate
THF tetrahydrofuran
TLC thin layer chromatography
Ts p-toluenesulfonyl = tosyl
Ts0 p-toluenesulfonate = tosylate
S02Me methyl sulfone
S02NH2 sulfonamide
Alkyl group abbreviations
Me methyl
Et ethyl
n-Pr normal propyl
i-Pr isopropyl
n-Bu normal butyl
i-Bu isobutyl
s-Bu secondary butyl
t-Bu tertiary butyl
c-Pr cyclopropyl
c-Bu cyclobutyl
c-Pen cyclopentyl
c-Hex cyclohexyl
Dose Abbreviations
bid bis in die (twice daily)
qid quater in die (four times
a day)
tid ter in die (three times
a day)
-5-
AMENDED SHEET
IPEA/EP _
CA 02307853 2000-04-27
_ WO 99123087 PCT/CA98100998 _
Alkyl, alkenyl, and alkynyl mean linear and branched
structures and combinations thereof.
The term "alkyl" means linear and branched structures and
combinations thereof, containing the indicated number of carbon atoms.
Examples of alkyl groups include methyl, ethyl, propyl, isopropyl,
butyl, s- and t-butyl, pentyl, hexyl, heptyl, octyl, nonyl, undecyl,
dodecyl, tridecyl, tetradecyl, pentadecyl, eicosyl,
3,7-diethyl-2,2-dimethyl- 4-propylnonyl, and the like.
"Cycloalkyl" means a hydrocarbon having the indicated
number of carbon atoms, containing one or more rings. Examples of
cycloallcyl groups are cyclopropyl, cyclopropylmethyl, 2-
cyclohexylethyl, cyclopentyl, cycloheptyl, adamantyl,
cyclododecylmethyl, 2-ethyl-1- bicyclo[4.4.0]decyl and the like.
"Fluoroalkyl" means linear and branched alkyl groups and
combinations thereof, of the indicated number of carbon atoms, in
which one or more hydrogen is replaced by fluorine. Examples are -
CF3, -CH2CH2F, and -CH2CF3, and the like.
"Fluorocycloalkyl" means a hydrocarbon having the
indicated number of carbon atoms, containing one or more rings. in
which one or more hydrogen is replaced by fluorine. Examples are c-
Pr-F5, c-Pr-FSCH2~ c-Hex-F11 and the like.
Halogen includes F, Cl, Br, and I.
Illustrations of the invention are:
(a) 5-ethyl-5-methyl-4-[4-(methylsulfonyl)phenyl]-3-phenyl-2,5-
dihydro-2-furanone,
(b) 4-[4-(aminosulfonyl)phenyl]-5-ethyl-5-methyl-3-phenyl-2,5-
dihydro-2-furanone,
(c) 5-ethyl-3-(4-fluorophenyl)-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
(d) (5S)-5-ethyl-3-(4-fluorophenyl)-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
(e) (5R)-5-ethyl-3-(4-fluorophenyl)-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
-6-
CA 02307853 2000-04-27
WO 99/23087 PCTICA98/00998 _
(f) 5-ethyl-3-(3-fluorophenyl)-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
(g) 3-(3,4-difluorophenyl)-5-ethyl-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
(h) (5R)-3-(3,4-difluorophenyl)-5-ethyl-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
(i) (5S)-3-(3,4-difluorophenyl)-5-ethyl-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
(j) 4-[4-(aminosulfonyl)phenyl]-3-(3,4-difluorophenyl)-5-ethyl-5-
methyl-2,5-dihydro-2-furanone,
(k) 3-(4-chlorophenyl)-5-ethyl-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
(1) 3-(4-bromophenyl)-5-ethyl-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
(m) 5-methyl-4-[4-(methylsulfonyl)phenyl]-3-phenyl-5-propyl-2,5-
dihydro-2-furanone,
(n) 3-(4-fluorophenyl)-5-methyl-4-[4-(methylsulfonyl)phenyl]-5-
propyl-2,5-dihydro-2-furanone,
(o) 3-(3-fluorophenyl)-5-methyl-4-[4-(methylsulfonyl)phenyl]-5-
2(1 propyl-2,5-dihydro-2-furanone,
(p) 3-(3,4-difluorophenyl)-5-methyl-4-[4-
(methylsulfonyl)phenyl]-5-propyl-2,5-dihydro-2-furanone,
(q) 4-[4-(aminosulfonyl)phenyl]-3-(4-fluorophenyl)-5-methyl-5-
propyl-2, 5-dihydro-2-furanone,
{r) 4-[4-(aminosulfonyl)phenyl]-3-(3,4-difluorophenyl)-5-methyl-
5-propyl-2, 5-dihydro-2-furanone,
(s) 3-(4-fluorophenyl)-5-isopropyl-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
(x) 3-(3,4-difluorophenyl)-5-ethyl-4-[4-(methylsulfonyl)phenyl]-5-
(trifluoromethyl)-2,5-dihydro-2-furanone,
(y) 3-(3,4-difluorophenyl)-5-ethyl-5-(fluoromethyl)-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
(z) 5-(difluoromethyl)-3-(3,4-difluorophenyl)-5-ethyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
-7-
CA 02307853 2000-04-27
_ WO 99/23087 PCT/CA98/00998 _ -
(aa) 5-ethyl-5-methyl-4-[4-(methylsulfonyl)phenyl)-3-(2-pyridyl)-
2,5-dihydro-2-furanone,
(bb) 5-ethyl-5-methyl-4-[4-(methylsulfonyl)phenyl]-3-(3-pyridyl)-
2, 5-dihydro-2-furanone,
(cc) 5-ethyl-5-methyl-4-[4-(methylsulfonyl)phenyl]-3-(4-pyridyl)-
2, 5-dihydro-2-furanone,
{dd) 5-cyclopropyl-3-(3,4-difluorophenyl)-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone, and
(ee) 5-(cyclopropylmethyl)-3-(4-fluorophenyl)-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone.
A group of these compounds includes:
(c) 5-ethyl-3-(4-fluorophenyl)-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
(d) (5S)-5-ethyl-3-(4-fluorophenyl)-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
(e) (5R)-5-ethyl-3-(4-fluorophenyl)-5-methyl-4-[4-
{methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
(f) 5-ethyl-3-(3-fluorophenyl)-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
(g) 3-(3,4-difluorophenyl)-5-ethyl-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
(h) (5R)-3-(3,4-difluorophenyl)-5-ethyl-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
(i) (5S)-3-(3,4-difluorophenyl)-5-ethyl-5-methyl-4-[4-
(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone,
The following compounds are later referred to for
comparison purposes, but are not compounds of the invention.
(t) 5,5-diethyl-4-[4-(methylsulfonyl)phenyl]-3-phenyl-2,5-
dihydro-2-furanone,
(u) 5,5-diethyl-3-(4-fluorophenyl)-4-[4-(methylsulfonyl)phenyl]-
2,5-dihydro-2-furanone,
_g-
CA 02307853 2000-04-27
_ WO 99123087 PCT/CA98/00998 _ -
(v) 4-[4-(aminosulfonyl)phenyl]-5,5-diethyl-3-phenyl-2,5-
dihydro-2-furanone,
(w) 4-[4-(aminosulfonyl)phenyl]-5,5-diethyl-3-(4-fluorophenyl)-
2,5-dihydro-2-furanone,
In another embodiment, the invention encompasses
pharmaceutical compositions for inhibiting COX-2 and for treating
COX-2 mediated diseases as disclosed herein comprising a
pharmaceutically acceptable carrier and non-toxic therapeutically
effective amount of a compound of formula I as described above.
In yet another embodiment, the invention encompasses a
method of inhibiting cyclooxygenase and treating cyclooxygenase
mediated diseases, advantageously treated by an active agent that
selectively inhibits COX-2 in preference to COX-1 as disclosed herein
comprising administering to a patient in need of such treatment of a
non-toxic therapeutically effective amount of a compound of Formula I
as disclosed herein.
Some of the compounds described herein contain one or
more asymmetric centres and may thus give rise to diastereomers and
optical isomers. The present invention is meant to comprehend such
possible diastereomers as well as their racemic and resolved,
enantiomerically pure forms and pharmaceutically acceptable salts
thereof.
Some of the compounds described herein contain olefinic
double bonds, and unless specified otherwise, are meant to include both
E and Z geometric isomers.
The pharmaceutical compositions of the present invention
comprise a compound of Formula I as an active ingredient or a
pharmaceutically acceptable salt, thereof, and may also contain a
pharmaceutically acceptable carrier and optionally other therapeutic
ingredients. The term "pharmaceutically acceptable salts" refers to salts
prepared from pharmaceutically acceptable non-toxic bases including
inorganic bases and organic bases. Salts derived from inorganic bases
include aluminum, ammonium, calcium, copper, ferric, ferrous,
lithium, magnesium, manganic salts, manganous, potassium, sodium,
-9-
CA 02307853 2000-04-27
_ WO 99/23087 PCTICA98/00998 _
zinc, and the like. Particularly preferred are the ammonium, calcium,
magnesium, potassium, and sodium salts. Salts derived from
pharmaceutically acceptable organic non-toxic bases include salts of
primary, secondary, and tertiary amines, substituted amines including
naturally occurring substituted amines, cyclic amines, and basic ion
exchange resins, such as arginine, betaine, caffeine, choline,
N,N--dibenzylethylenediamine, diethylamine, 2-diethylaminoethanol,
2-dimethylaminoethanol, ethanolamine, ethylenediamine,
N-ethylmorpholine, N-ethylpiperidine, N-methylglucamine, glucamine,
glucosarnine, histidine, hydrabamine, N-(2-hydroxyethyl)piperidine, N-
(2-hydroxyethyl)pyrrolidine, isopropylamine, lysine, methylglucamine,
morpholine, piperazine, piperidine, polyamine resins, procaine, purines,
theobromine, triethylamine, trimethylamine, tripropylamine,
tromethamine, and the like.
When the compound of the present invention is basic, salts
may be prepared from pharmaceutically acceptable non-toxic acids,
including inorganic and organic acids. Such acids include acetic, adipic,
aspartic, 1,5-naphthalenedisulfonic, benzenesulfonic, benzoic,
camphorsulfonic, citric, 1,2-ethanedisulfonic, ethanesulfonic,
ethylenediaminetetraacetic, fumaric, glucoheptonic, gluconic, glutamic,
hydriodic, hydrobromic, hydrochloric, isethionic, lactic, malefic, malic,
mandelic, methanesulfonic, mucic, 2-naphthalenesulfonic, nitric, oxalic,
pamoic, pantothenic, phosphoric, pivalic, propionic, salicylic, stearic,
succinic, sulfuric, tartaric, p-toluenesulfonic acid, undecanoic, 10-
undecenoic, and the like. Particularly preferred are citric,
hydrobromic, hydrochloric, malefic, methanesulfonic, phosphoric,
sulfuric and tartaric acids. It will be understood that in the discussion of
methods of treatment which follows, references to the compounds of
Formula I are meant to also include the pharmaceutically acceptable
salts.
Compounds of Formula I are useful for the relief of pain,
fever and inflammation of a variety of conditions including rheumatic
fever, symptoms associated with influenza or other viral infections,
common cold, low back and neck pain, dysmenorrhea, headache,
-10-
CA 02307853 2000-04-27
WO 99123087 PCTlCA98/00998 _ .
toothache, sprains and strains, myositis, neuralgia, synovitis, arthritis,
including rheumatoid arthritis, degenerative joint diseases
(osteoarthritis), gout and ankylosing spondylitis, bursitis, burns,
injuries, following surgical and dental procedures. In addition, these
compounds may inhibit cellular neoplastic transformations and metastic
tumour growth and hence can be used in the treatment of cancer. The
compounds may also be of use in the treatment and/or prevention of
cyclooxygenase-mediated proliferative disorders such as may occur in
diabetic retinopathy and tumor angiogenesis.
Compounds of the invention will also inhibit prostanoid-
induced smooth muscle contraction by preventing the synthesis of
contractile prostanoids and hence may be of use in the treatment of
dysmenorrhea, premature labour, asthma and eosinophil related
disorders. It will also be of use in the treatment of Alzheimer's disease,
for decreasing bone loss particularly in postmenopausal women (i.e.
treatment of osteoporosis) and for treatment of glaucoma.
By virtue of its high inhibitory activity against COX-2
and/or its specificity for COX-2 over COX-1, compounds of the
invention will prove useful as an alternative to conventional NSAID'S,
particularly where such non-steroidal antiinflammatory drugs may be
contra-indicated such as in patients with peptic ulcers, gastritis, regional
enteritis, ulcerative colitis, diverticulitis or with a recurrent history of
gastrointestinal lesions; GI bleeding, coagulation disorders including
anaemia such as hypoprothrombinemia, haemophilia or other bleeding
problems; kidney disease; those prior to surgery or taking
anticoagulants.
For the treatment of any of these cyclooxygenase mediated
diseases, compounds of the invention may be administered orally,
topically, parenterally, by inhalation spray or rectally in dosage unit
formulations containing conventional non-toxic pharmaceutically
acceptable carriers, adjuvants and vehicles. The term parenteral as used
herein includes subcutaneous injections, intravenous, intramuscular,
intrasternal injection or infusion techniques. In addition to the
treatment of warm-blooded animals such as mice, rats, horses, cattle
-11-
CA 02307853 2000-04-27
_ WO 99/23087 PCT/CA98/00998 _
sheep, dogs, cats, etc., the compound of the invention is effective in the
treatment of humans.
As indicated above, pharmaceutical compositions for
treating COX-2 mediated diseases as defined may optionally include one
or more ingredients as listed above.
The pharmaceutical compositions containing the active
ingredient may be in a form suitable for oral use, for example, as
tablets, troches, lozenges, aqueous or oily suspensions, dispersible
powders or granules, emulsions, hard or soft capsules, or syrups or
elixirs. Compositions intended for oral use may be prepared according
to any method known to the art for the manufacture of pharmaceutical
compositions and such compositions may contain one or more agents
selected from the group consisting of sweetening agents, flavouring
agents, colouring agents and preserving agents in order to provide
pharmaceutically elegant and palatable preparations. Tablets contain the
active ingredient in admixture with non-toxic pharmaceutically
acceptable excipients which are suitable for the manufacture of tablets.
These excipients may be for example, inert diluents, such as calcium
carbonate, sodium carbonate, lactose, calcium phosphate or sodium
phosphate; granulating and disintegrating agents, for example, corn
starch, or alginic acid; binding agents, for example starch, gelatin or
acacia, and lubricating agents, for example, magnesium stearate, stearic
acid or talc. The tablets may be uncoated or they may be coated by
known techniques to delay disintegration and absorption in the
gastrointestinal tract and thereby provide a sustained action over a
longer period. For example, a time delay material such as glyceryl
monostearate or glyceryl distearate may be employed. They may also
be coated by the technique described in the U.S. Patent 4,256,108;
4,166,452; and 4,265,874 to form osmotic therapeutic tablets for
control release.
Formulations for oral use may also be presented as hard
gelatin capsules wherein the active ingredient is mixed with an inert
solid diluent, for example, calcium carbonate, calcium phosphate or
kaolin, or as soft gelatin capsules wherein the active ingredients is mixed
-12-
CA 02307853 2000-04-27
WO 99/Z3087 PCT/CA98/00998 _ -
with water or miscible solvents such as propylene glycol, PEGs and
ethanol, or an oil medium, for example peanut oil, liquid paraffin, or
olive oil.
Aqueous suspensions contain the active material in
admixture with excipients suitable for the manufacture of aqueous
suspensions. Such excipients are suspending agents, for example sodium
carboxymethylcellulose, methylcellulose, hydroxy-
propylmethycellulose, sodium alginate, polyvinyl-pyrrolidone, gum
tragacanth and gum acacia; dispersing or wetting agents may be a
naturally-occurring phosphatide, for example lecithin, or condensation
products of an alkylene oxide with fatty acids, for example
polyoxyethylene stearate, or condensation products of ethylene oxide
with long chain aliphatic alcohols, for example
heptadecaethyleneoxycetanol, or condensation products of ethylene
i5 oxide with partial esters derived from fatty acids and a hexitol such as
polyoxyethylene sorbitol monooleate, or condensation products of
ethylene oxide with partial esters derived from fatty acids and hexitol
anhydrides, for example polyethylene sorbitan monooleate. The
aqueous suspensions may also contain one or more preservatives, for
example ethyl, or n-propyl, p-hydroxybenzoate, one or more colouring
agents, one or more flavouring agents, and one or more sweetening
agents, such as sucrose, saccharin or aspartame.
Oily suspensions may be formulated by suspending the
active ingredient in a vegetable oil, for example arachis oil, olive oil,
sesame oiI or coconut oil, or in mineral oil such as liquid paraffin. The
oily suspensions may contain a thickening agent, for example beeswax,
hard paraffin or cetyl alcohol. Sweetening agents such as those set forth
above, and flavouring agents may be added to provide a palatable oral
preparation. These compositions may be preserved by the addition of
an anti-oxidant such as ascorbic acid.
Dispersible powders and granules suitable for preparation
of an aqueous suspension by the addition of water provide the active
ingredient in admixture with a dispersing or wetting agent, suspending
agent and one or more preservatives. Suitable dispersing or wetting
-13-
CA 02307853 2000-04-27
_ WO 99/23087 PCT/CA98/00998 _
agents and suspending agents are exemplified by those already
mentioned above. Additional excipients, for example sweetening,
flavouring and colouring agents; may also be present.
- The pharmaceutical compositions of the invention may also
be in the form of an oil-in-water emulsions. The oily phase may be a
vegetable oil, for example olive oil or arachis oil, or a mineral oil, for
example liquid paraffin or mixtures of these. Suitable emulsifying
agents may be naturally-occurring phosphatides, for example soy bean,
lecithin, and esters or partial esters derived from fatty acids and hexitol
anhydrides, for example sorbitan monooleate, and condensation
products of the said partial esters with ethylene oxide, for example
polyoxyethylene sorbitan monooleate. The emulsions may also contain
sweetening and flavouring agents.
Syrups and elixirs may be formulated with sweetening
agents, for example glycerol, propylene glycol, sorbitol or sucrose.
Such formulations may also contain a demulcent, a preservative and
flavouring and colouring agents. The pharmaceutical compositions may
be in the form of a sterile injectable aqueous or oleagenous suspension.
This suspension may be formulated according to the known art using
those suitable dispersing or wetting agents and suspending agents which
have been mentioned above. The sterile injectable preparation may also
be a sterile injectable solution or suspension in a non-toxic parenterally-
acceptable diluent or solvent, for example as a solution in 1,3-butane
diol. Among the acceptable vehicles and solvents that may be employed
are water, Ringer's solution and isotonic sodium chloride solution.
Cosolvents such as ethanol, propylene glycol or polyethylene glycols
may also be used. In addition, sterile, fixed oils are conventionally
employed as a solvent or suspending medium. For this purpose any
bland fixed oil may be employed including synthetic mono- or
diglycerides. In addition, fatty acids such as oleic acid find use in the
preparation of injectables.
Compounds of the invention may also be administered in
the form of a suppositories for rectal administration of the drug. These
compositions can be prepared by mixing the drug with a suitable non-
-14-
CA 02307853 2000-04-27
_ WO 99/23087 PCT/CA98/00998 _
irritating excipient which is solid at ordinary temperatures but liquid at
the rectal temperature and will therefore melt in the rectum to release
the drug. Such materials are cocoa butter and polyethylene glycols.
- For topical use, creams, ointments, gels, solutions or
suspensions, etc., containing the compound of Formula I are employed.
(For purposes of this application, topical application shall include mouth
washes and gargles.) Topical formulations may generally be comprised
of a pharmaceutical carrier, cosolvent, emulsifier, penetration enhancer,
preservative system, and emollient.
Dosage levels of the order of from about 0.01 mg to about
140 mg/kg of body weight per day are useful in the treatment of the
above-indicated conditions, or alternatively about 0.5 mg to about 7 g
per patient per day. For example, inflammation may be effectively
treated by the administration of from about 0.01 to 50 mg of the
compound per kilogram of body weight per day, or alternatively about
0.5 mg to about 3.5 g per patient per day.
The amount of active ingredient that may be combined with
the carrier materials to produce a single dosage form will vary
depending upon the host treated and the particular mode of
administration. For example, a formulation intended for the oral
administration of humans may contain from 0.5 mg to 5 g of active
agent compounded with an appropriate and convenient amount of
carrier material which may vary from about 5 to about 95 percent of
the total composition. Dosage unit forms will generally contain between
from about 1 mg to about 500 mg of an active ingredient, typically 25
mg, 50 mg, 100 mg, 200 mg, 300 mg, 400 mg, 500 mg, 600 mg, 800
mg, or 1000 mg.
It will be understood, however, that the specific dose level
for any particular patient will depend upon a variety of factors
including the age, body weight, general health, sex, diet, time of
administration, route of administration, rate of excretion, drug
combination and the severity of the particular disease undergoing
therapy.
-15-
CA 02307853 2000-04-27
WO 99/230$7 PCT/CA98/00998 _
Similarly, compounds of the invention will be useful as
partial or complete substitutes for conventional NSAID'S in
preparations wherein they are presently co-administered with other
agents or ingredients. Thus in further aspects, the invention
encompasses pharmaceutical compositions for treating C(3X-2 mediated
diseases as defined above comprising a non-toxic therapeutically
effective amount of compounds of Formula I as defined above and one
or more ingredients such as another pain reliever including
acetaminophen or phenacetin; a potentiator including caffeine; an H2-
antagonist, aluminum or magnesium hydroxide, simethicone, a
decongestant including phenylephrine, phenylpropanolamine,
pseudophedrine, oxymetazoline, ephinephrine, naphazoline,
xylometazoline, propylhexedrine, or levo-desoxyephedrine; an
antiitussive including codeine, hydrocodone, caramiphen,
carbetapentane, or dextramethorphan; a prostaglandin including
misoprostol, enprostil, rioprostil, ornoprostol or rosaprostol: a diuretic;
a sedating or non-sedating antihistamine. In addition the invention
encompasses a method of treating cyclooxygenase mediated diseases
comprising: administration to a patient in need of such treatment a non-
toxic therapeutically effect amount of a compound of Formula I,
optionally co-administered with one or more of such ingredients as
listed immediately above.
The compounds of the present invention can be prepared
according to the following methods.
-16-
CA 02307853 2000-04-27
_ WO 99/23087 PCTICA98100998 _
Method
A Friedel-Crafts reaction between an acid chloride 2 and
thioanisole 1 yields the substituted acetophenone 3. This acetophenone
is hydroxylated to the hydroxyketone 4 using CC14 and NaOH in toluene
with an appropriate phase transfer catalyst (U.S. Patent 4,740,624).
Sulfide 4 is oxidized to the sulfone 5 using MMPP or OXONEO. The
hydroxyketone 5 is acylated with a phenylacetic acid 6 using a
carbodiimide coupling reagent to afford ester 7 which is then cyclised,
using a suitable base such as DBU, to the lactone Ia.
Method 2
L-Lactic acid 8 is converted to the dioxolanone 9 with
valeraldehyde followed by alkylation using a suitable base such as LDA
and an alkylating to give dioxolanone 10 (D. Seebach, N. Reto, G.
Calderari, Tetrahedron 40, 1313 (1984)). Addition of aryl lithium 11 to
dioxolanone 10 afforded dioxolanol 12. Acid catalyzed hydrolysis of the
acetal followed by OXONEU oxidation of the methyl sulfide give the
hydroxyketone 13(R). The hydroxyketone 13(S) can be prepared
according to the method previously described in World Patent
Application W09714691 ( 1997.04.24), Example 144 steps 1 to 8.
-1'7 -
CA 02307853 2000-04-27
_ WO 99/23087 PCT/CA98/00998 _
- R2 COCI
2Y O
\ R2 CC14
-----~ \
MeS ~ AICI3 ~ ~ ~ Toluene
MeS NaOH
1 ~ Aliquat
O RR3 MMPP O ~3
\ ~ or OXONE ~ ~. p
MeS ~ H Me02S
4 5
S02Me
Ar~C02H ~ R3
g ~ DBU
CMC O
DMAP Ar
O
S02Me
O
la
-18-
CA 02307853 2000-04-27
_ Wo 99123087 PCT/CA98100998 _ -
the a 2
- Me H t-Bu-CHO Me LDA
--~. O
HO~C02H O
O R3_X
t-Bu~
L-Lactic Acid X = CI, Br, I, OTf
9
R3 Me
.,
O O ~ Li
t-Bu~ O
MeS 11
~Me
O '~ OH R3 Me ~ S02Me
1 ) p-TsOH
--.--
t-Bu~O ~ 2 ) OXONE HO .
0
12 SMe 13(R)
S02Me
R3Me
HO
.O
13{S)
5 Table 1 illustrates compounds of formula I, which are
representative of the present invention (except for those marked with
"*" which are shown for comparative purposes).
_ ~g _
CA 02307853 2000-04-27
_ WO 99/23087 PCTICA98/00998 _ -
Table 1
1
R'
O
Example R1 R2 R~ Ar
a CH3 CHZCH3 CH3
b NH2 CH2CH3 CH3
c ~3 CH2~3 ~3
F
d CH3 (S)-CHZCH3 CH3
F
a CH3 (R)-CHZCH3 CH3
F
f CH3 CH2CH3 CH3 F
-20-
CA 02307853 2000-04-27
WO 99/23087 PCT/CA98/00998 _
Table 1 (continued)
S02R~
O
Example R1 R_2 R3 Ar
g CH3 CHZCH3 CH3 F
F
h CH3 (R)-CH2CH3 CH3 F
F
i CH3 (S)-CH2CH3 CH3 F
F
j NH2 CH2CH3 CH3 F
F
-21-
CA 02307853 2000-04-27
WO 99/23087 PCTICA98/00998
1
R
fable 1 (continued)
Example R 1 R2 R3 A r
k CH3 CH2CH3 CH3
/ CI
1 ~3 CH2~3 CH3
/ Br
m CH3 CH2CH3CH3 CH3
n CH3 CHZCH2CH3 CH3
/ F
o CH3 CH2CH2CH3 CH3 F
p CH3 CH2CH2CH3 CH3 F
/ F
q NH2 CHZCH2CH3 CH3
/ F
-22-
CA 02307853 2000-04-27
WO 99/23087 PCT/CA98/00998 _ -
S02R~
R
O fable 1 (continued)
Ex~ R_1 R2 R3 Ar
r NH2 CH2CH2CH3 CH3 F
F
s CH3 CH(CH3)2 CH3
/ F
t* CH3 CH2CH3 CH2CH3
a * CH3 CH2CH3 CH2CH3
/ F
v * NH2 CH2CH3 CHZCH3
w* NH2 CH2~3 CH2CH3
/ F
x CH3 CH2CH3 CF3 F
- ~ / F
CA 02307853 2000-04-27
WO 99/23087 PCT/CA98/00998
R~
Table 1 (continued)
Example R_1 R2 R_3 Ar
y CH3 CH2CH3 CHZF F
/ F
z CH3 CHZCH3 CHF2 F
/ F
as CH3 CHZCH3 CH3 N-
bb CH3 CH2CH3 CH3 - N
cc CH3 CH2CH3 CH3
~N
dd CH3 ~ CH3 F
/ F
CA 02307853 2000-04-27
_ WO 99/23087 PCT/CA98100998 _ .
R~
R
O Table 1 (continued)
Example R1 R2 R3 Ar
CH3 CH2/f CH3
~ F
Compounds of Formula I can be tested using the following
assays to determine their COX-2 inhibiting activity.
INHIBITION OF CYCLOOXYGENASE ACTIVITY
Whole cell assays for COX-2 and COX-1 using CHO transfected cell
lines
Chinese hamster ovary (CHO) cell lines which have been
stably transfected with an eukaryotic expression vector pCDNAIII
containing either the human COX-1 or COX-2 cDNA's are used for the
assay. These cell lines are referred to as CHO [hCOX-1 ] and CHO
[hCOX-2], respectively. For cyclooxygenase assays, CHO[hCOX-lJ
cells from suspension cultures and CHO[hCOX-2] cells prepared by
trypsinization of adherent cultures are harvested by centrifugation (300
x g, 10 min) and washed once in HBSS containing 15 mM HEPES, pH
7.4, and resuspended in HBSS, 15 mM HEPES, pH 7.4, at a cell
concentration of 1.5 x 106 cells/ml. Drugs to be tested are dissolved in
DMSO to 66.7-fold the highest test drug concentration. Compounds are
typically tested at 8 concentrations in duplicate using serial 3-fold serial
dilutions in DMSO of the highest drug concentration. Cells (0.3 x 106
-25-
CA 02307853 2000-04-27
_ WO 99/23087 PCT/CA98/00998 _
cells in 200 ~.1) are preincubated with 3 ~.1 of the test drug or DMSO
vehicle for 15 min at 37°C. Working solutions of peroxide-free AA
(5.5 ~tM and 110 ~.M AA for the CHO [hCOX-1 ] and CHO [COX-2]
assays, respectively) are prepared by a 10-fold dilution of a
concentrated AA solution in ethanol into HBSS containing 15 mM
HEPES, pH 7.4. Cells are then challenged in the presence or absence of
drug with the AA/HBSS solution to yield a final concentration of 0.5
~,M AA in the CHO[hCOX-I] assay and a final concentration of 10 ~,M
AA in the CHO[hCOX-2] assay. The reaction is terminated by the
addition of 10 ~tl 1 N HCl followed by neutralization with 20 ~,l of 0.5
N NaOH. The samples are centrifuged at 300 x g at 4°C for 10 min,
and an aliquot of the clarified supernatant is appropriately diluted for
the determination of PGE2 levels using an enzyme-linked immunoassay
for PGE2 (Correlate PGE2 enzyme immunoassay kit, Assay Designs,
Inc.). Cyclooxygenase activity in the absence of test compounds is
determined as the difference in PGE2 levels of cells challenged with
arachidonic acid versus the PGE2 levels in cells mock-challenged with
ethanol vehicle. Inhibition of PGE2 synthesis by test compounds is
calculated as a percentage of the activity in the presence of drug versus
the activity in the positive control samples.
Assay of C(JX-1 Activity from U937 cell microsomes
U 937 cells are pelleted by centrifugation at 500 x g for 5
min and washed once with phosphate-buffered saline and repelleted.
Cells are resuspended in homogenization buffer consisting of 0.1 M
Tris-HCI, pH 7.4, 10 mM EDTA, 2 ~,g/ml leupeptin, 2 ~.g/ml soybean
trypsin inhibitor, 2 ~,g/ml aprotinin and 1 mM phenyl methyl sulfonyl
fluoride. The cell suspension is sonicated 4 times for 10 sec and is
centrifuged at 10,000 x g for 10 min at 4°C. The supernatant is
centrifuged at 100,000 x g for 1 hr at 4°C. The 100,000 x g
microsomal pellet is resuspended in 0.1 M Tris-HCI, pH 7.4, 10 mM
EDTA to approximately 7 mg protein/ml and stored at -80°C.
Microsomal preparations are thawed immediately prior to
use, subjected to a brief sonication, and then diluted to a protein
-26-
CA 02307853 2000-04-27
WO 99/23087 PCTICA98I00998 _ -
concentration of 125 ~.g/ml in 0.1 M Tris-HCl buffer, pH 7.4 containing
mM EDTA, 0.5 mM phenol, 1 mM reduced glutathione and 1 ~,M
hematin. Assays are performed in duplicate in a final volume of 250 ~.1.
Initially, 5 ~,l of DMSO vehicle or drug in DMSO are added to 20 p.l of
5 0.1 M Tris-HCl buffer, pH 7.4 containing 10 mM EDTA in wells of a
96-deepwell polypropylene titre plate. 200 ~,1 of the microsomal
preparation are then added and pre-incubated for 15 min at room
temperature before addition of 25 ~.1 of 1 M arachidonic acid in 0.1 M
Tris-HCl and 10 mM EDTA, pH 7.4. Samples are incubated for 40 min
10 at room temperature and the reaction is stopped by the addition of 25 ~.1
of 1 N HCI. Samples are neutralized with 25 ~.1 of 1 N NaOH prior to
quantitation of PGE2 content by radioimmunoassay (Dupont-NEN or
Amersham assay kits). Cyclooxygenase activity is defined as the
difference between PGE2 levels in samples incubated in the presence of
arachidonic acid and ethanol vehicle.
Assay of the activit~Qf purified human COX-2
The enzyme activity is measured using a chromogenic assay
based on the oxidation of N,N,N',N'-tetramethyl-p-phenylenediamine
(TMPD) during the reduction of PGG2 to PGH2 by COX-2 (Copeland
et al. ( 1994) Proc. Natl. Acad. Sci. 91, 11202-11206).
Recombinant human COX-2 is purified from Sf9 cells as
previously described (Percival et al. ( 1994) Arch. Biochem. Biophys.
I5, 111-118). The assay mixture (180 ~,L) contains 100 mM sodium
phosphate, pH 6.5, 2 mM genapol X-100, 1 ~,M hematin, 1 mg/ml
gelatin, 80-100 units of purified enzyme (One unit of enzyme is defined
as the amount of enzyme required to produce an O.D. change of
0.001/min at 610 nm) and 4 ~,L of the test compound in DMSO. The
mixture is pre-incubated at room temperature (22°C) for 15 minutes
prior to initiation of the enzymatic reaction by the addition of 20 ~,L of
a sonicated solution of 1 mM arachidonic acid {AA) and 1 mM TMPD
in assay buffer (without enzyme or hematin). The enzymatic activity is
measured by estimation of the initial velocity of TMPD oxidation over
the first 36 sec of the reaction. A non-specific rate of oxidation is
-2'7-
CA 02307853 2000-04-27
WO 99123087 PCT/CA98/00998 _ -
observed in the absence of enzyme (0.007 - 0.010 O.D. /min) and is
subtracted before the calculation of the % inhibition. ICSp values are
derived from 4-parameter least squares non-linear regression analysis of
the log-dose vs % inhibition plot.
HUMAN WHOLE BLOOD ASSAY
Human whole blood provides a protein and cell-rich milieu
appropriate for the study of biochemical efficacy of anti-inflammatory
compounds such as selective COX-2 inhibitors. Studies have shown that
normal human blood does not contain the COX-2 enzyme. This is
consistent with the observation that COX-2 inhibitors have no effect on
PGE2 production in normal blood. These inhibitors are active only
after incubation of human whole blood with LPS, which induces COX-
2. This assay can be used to evaluate the inhibitory effect of selective
COX-2 inhibitors on PGE2 production. As well, platelets in whole
blood contain a large amount of the COX-1 enzyme. Immediately
following blood clotting, platelets are activated through a thrombin-
mediated mechanism. This reaction results in the production of
thromboxane BZ {TxB2) via activation of COX-1. Thus, the effect of
test compounds on TxB2 levels following blood clotting can be
examined and used as an index for COX-1 activity. Therefore, the
degree of selectivity by the test compound can be determined by
measuring the levels of PGE2 after LPS induction (COX-2) and TxB2
following blood clotting (COX-1 ) in the same assay.
Method
A. SOX-2 (LPS-induce ~~GE2 production)
Fresh blood is collected in heparinized tubes by
venipuncture from both male and female volunteers. The subjects have
no apparent inflammatory conditions and have not taken any NSAIDs
for at least 7 days prior to blood collection. Plasma is immediately
obtained from a 2 mL blood aliquot to use as blank (basal levels of
PGE2). The remaining blood is incubated with LPS { I00 pg/ml final
concentration, Sigma Chem, #L-2630 from E. coli; diluted in 0.1% BSA
-28-
CA 02307853 2000-04-27
WO 99/23087 PCTICA98/00998 _
(Phosphate buffered saline) for S minutes at room temperature. Five
hundred ~..iL aliquots of blood are incubated with either 2 ~.L of vehicle
(DMSO) or 2 ~,L of a test compound at final concentrations varying
from 10 nM to 30 ~,M for 24 hours at 37°C. At the end of the
incubation, the blood is centrifuged at 12,000 x g for 5 minutes to
obtain plasma. A 100 ~,L aliquot of plasma is mixed with 400 ~,L of
methanol for protein precipitation. The supernatant is obtained and is
assayed for PGE2 using a radioimmunoassay kit (Amersham, RPA#530)
after conversion of PGE2 to its methyl oximate derivative according to
the manufacturer's procedure.
B. COX-1 (Clotting-induced ~B2 production)
Fresh blood is collected into vacutainers containing no
anticoagulants. Aliquots of 500 ~,L are immediately transferred to
siliconized microcentrifuge tubes preloaded with 2 ~,L of either DMSO
or a test compound at final concentrations varying from IO nM to 30
~.M. The tubes are vortexed and incubated at 37°C for 1 hour to allow
blood to clot. At the end of incubation, serum is obtained by
centrifugation (12,000 x g for S min.). A 100 ~.L aliquot of serum is
mixed with 400 ~.L of methanol for protein precipitation. The
supernatant is obtained and is assayed for TxB2 using a enzyme
immunoassay kit {Cayman, #519031) according to the manufacturer's
instruction.
RAT PAW EDEMA ASSAY
Male Sprague-Dawley rats (150-200 g) are fasted overnight
and are given, po, either vehicle ( 1 % methocel or 5 % Tween 80) or a
test compound. One hr Later, a line is drawn using a permanent marker
at the level above the ankle in one hind paw to define the area of the
paw to be monitored. The paw volume (VO) is measured using a
plethysmometer (Ugo-Basile, Italy) based on the principle of water
displacement. The animals are then injected subplantarly with 50 ~.1 of
1 % carrageenan solution in saline (FMC Corp, Maine} into the paw
using an insulin syringe with a 25-gauge needle (i.e. 500 ~,g carrageenan
-29-
CA 02307853 2000-04-27
_ WO 99123087 PCTICA98100998 _
per paw). Three hr later, the paw volume (V3) is measured and the
increases in paw volume (V3 - VO) are calculated. The animals are
sacrificed by C02 asphyxiation and the absence or presence of stomach
lesions scored. Data is compared with the vehicle-control values and
percent inhibition calculated. All treatment groups are coded to
eliminate observer bias.
NSAID-INDUCED GASTROPATHY IN RATS
The major side effect of conventional NSAIDs is their
ability to produce gastric lesions in man. This action is believed to be
caused by inhibition of Cox-1 in the gastrointestinal tract. Rats are
particularly sensitive to the actions of NSAIDs. In fact, rat models have
been used commonly in the past to evaluate the gastrointestinal side
effects of current conventional NSAIDs. In the present assay, NSAID-
induced gastrointestinal damage is observed by measuring fecal SlCr
excretion after systemic injection of ~ I Cr-labeled red blood cells. Fecal
51 Cr excretion is a well-established and sensitive technique to detect
gastrointestinal integrity in animals and man.
ethods
Male Sprague Dawley rats ( 150 - 200 g) are administered
orally a test compound either once (acute dosing) or b.i.d, for 5 days
(chronic dosing). Immediately after the administration of the last dose,
the rats are injected via a tail vein with 0.5 mL of 51 Cr-labeled red
blood cells from a donor rat. The animals are placed individually in
metabolism cages with food and water ad lib. Feces are collected for a _
48 h period and 5 I Cr fecal excretion is calculated as a percent of total
injected dose. SICr-labeled red blood cells are prepared using the
following procedures. Ten mL of blood is collected in heparinized
tubes via the vena cava from a donor rat. Plasma is removed by
centrifugation and replenished with equal volume of HBSS. The red
blood cells are incubated with 400 ~,Ci of sodium Slchromate for 30
min. at 37°C. At the end of the incubation, the red blood cells are
washed twice with 20 mL HBSS to remove free sodium Slchromate. -
-30-
CA 02307853 2000-04-27
_ WO 99/23087 PCT/CA98/00998
The red blood cells are finally reconstituted in 10 mL HBSS and 0.5 mL
of the solution (about 20 ~,Ci) is injected per rat.
PROTEIN-LOSING GASTROPATHY IN SQUIRREL MONKEYS
Protein-losing gastropathy (manifested as appearance of
circulating cells and plasma proteins in the GI tract) is a significant and
dose-limiting adverse response to standard non-steroidal anti-
inflammatory drugs (NSAIDs). This can be quantitatively assessed by
intravenous administration of SICrCl3 solution. This isotopic ion can
avidly bind to cell and serum globins and cell endoplasmic reticulum.
Measurement of radioactivity appearing in feces collected for 24 h after
administration of the isotope thus provides a sensitive and quantitative
index of protein-losing gastropathy.
ods
Groups of male squirrel monkeys (0.8 to 1.4 kg) are
treated by gavage with either 1% methocell or 5% Tween 80 in H20
vehicles, (3mL/kg b.i.d.) or test compounds at doses from 1 - 100
mg/kg b.i.d. for 5 days. Intravenous 51 Cr (5 ~.Ci/kg in 1 ml/kg
phosphate buffer saline (PBS)) is administered 1 h after the last
drug/vehicle dose, and feces collected for 24 h in a metabolism cage and
assessed for excreted 5lCr by gamma-counting. Venous blood is
sampled 1 h and 8 h after the last drug dose, and plasma concentrations
of drug measured by RP-HPLC.
LPS-Induced Pvrexia in Conscious Rats
Male Sprague-Dawley rats ( 150 - 200 g) were fasted for 16
- 18 h before use. At approximately 9:30 a.m., the animals were placed
temporarily in plexiglass restrainers and their baseline rectal
temperature was recorded using a flexible temperature probe (YSI
series 400) connected to a digital thermometer (Model 08502, Cole
Parmer). The same probe and thermometer were used for all animals
to reduce experimental error. The animals were returned to their cages
after the temperature measurements. At time zero, the rats were
-31-
CA 02307853 2000-04-27
WO 99/23087 PCT/CA98/00998
injected intraperitoneally with either saline or LPS (2 mg/kg, Sigma
Chem) and the rectal temperature was remeasured at 5, 6 and 7 h
following LPS injection. After the measurement at 5 h, when the
increase in temperature had reached a plateau, the LPS-injected rats
were given either the vehicle ( 1 % methocel) or a test compound orally
to determine whether the compound could reverse the pyrexia. Percent
reversal of the pyrexia was calculated using the rectal temperature
obtained at 7 h in the control (vehicle-treated) group as the reference
(zero reversal) point. Complete reversal of pyrexia to the pre-LPS
baseline value is taken as 100%.
LPS-Induced Pvrexia in Conscious Sciuirrel Monkeys
Temperature probes were surgically implanted under the
abdominal skin in a group of squirrel monkeys (Saimiri sciureus) ( 1.0 -
1.7 kg). This allows for the monitoring of body temperature in
conscious, unrestrained monkeys by a telemetric sensing system (Data
Sciences International, Minnesota). The animals were fasted and were
placed in individual cages for acclimatization 13 - 14 h before use.
Electronic receivers were installed on the side of the cages which pick
up signals from the implanted temperature probes. At approximately
9:00 a.m. on the day of the experiment, the monkeys were restrained
temporarily in training chairs and were given a bolus LV. injection of
LPS, (6 mg/kg, dissolved in sterile saline). The animals were
returned to their cages and body temperature was recorded continuously
every 5 min. Two h after injection of LPS, when the body temperature
had increased by 1.5 - 2°C, the monkeys were dosed orally with either
vehicle ( 1 % methocel) or a test compound (3 mg/kg). One hundred
minutes later, the difference between the body temperature and the
baseline value was determined. Percent inhibition was calculated taking
the value in the control group as 0% inhibition.
Acute Inflammator~yperalgesia Induced by C'arrageenan in Rats
Experiments were performed using male Sprague Dawley
rats (90- I l Og). Hyperalgesia to mechanical compression of the hind
-32-
CA 02307853 2000-04-27
_ WO 99123087 PCT/CA98/00998
paw was induced by intraplantar injection of carrageenan {4.5 mg into
one hind paw) 3 h previously. Control animals received an equivalent
volume of saline (0.15 ml intraplantar). A test compound (0.3-30
mg/kg, suspended in 0.5% methocel in distilled water) or vehicle (0.5%
methocel) was administered orally {2ml/kg) 2 h after carrageenan. The
vocalisation response to compression of the hind paw was measured 1 h
later using a Ugo Basile algesiometer.
Statistical analysis for carrageenan-induced hyperalgesia
was performed using one-way ANOVA {BMDP Statistical Software
Inc.). Hyperalgesia was determined by subtracting the vocalisation
threshold in saline injected rats from that obtained in animals injected
with carrageenan. Hyperalgesia scores for drug-treated rats were
expressed as a percentage of this response. ID50 values {the dose
producing 50% of the maximum observed response) were then
calculated by nonlinear least squares regression analysis of mean data
using GraFit (Erithacus Software).
Adjuvant-Induced Arthritis in Rats
Seventy, 6.5-7.5 week old, female Lewis rats (body weight
146-170 g) were weighed, ear marked, and assigned to groups (a
negative control group in which arthritis was not induced, a vehicle
control group, a positive control group administered indomethacin at a
total daily dose of 1 mg/kg and four groups administered with a test
compound at,total daily doses of 0.10-3.0 mg/kg) such that the body
weights were equivalent within each group. Six groups of 10 rats each
were injected into a hind paw with 0.5 mg of Mycobacterium butyricum
in 0.1 ml of light mineral oil (adjuvant), and a negative control group of
10 rats was not injected with adjuvant. Body weights, contralateral paw
volumes (determined by mercury displacement plethysmography) and.
lateral radiographs (obtained under Ketamine and Xylazine anesthesia)
were determined before (day -1 ) and 21 days following adjuvant
injection, and primary paw volumes were determined before (day -1 )
and on days 4 and 21 following adjuvant injection. The rats were
anesthetized with an intramuscular injection of 0.03 - 0.1 ml of a
-33-
CA 02307853 2000-04-27
WO 99123087 PCT/CA98/00998 _ -
combination of Ketamine ($7 mg/kg) and Xylazine (13 mg/kg) for
radiographs and injection of adjuvant. The radiographs were made of
both hind paws on day 0 and day 21 using the Faxitron (45 kVp, 30
- seconds) and Kodak X-GMAT TL film, and were developed in an
automatic processor. Radiographs were evaluated for changes in the
soft and hard tissues by an investigator who was blinded to experimental
treatment. The following radiographic changes were graded
numerically according to severity: increased soft issue volume {0-4),
narrowing or widening of joint spaces (0-5) subchondral erosion (0-3),
periosteal reaction (0-4), osteolysis (0-4) subluxation (0-3), and
degenerative joint changes (0-3). Specific criteria were used to establish
the numerical grade of severity for each radiographic change. The
maximum possible score per foot was 26. A test compound at total
daily doses of 0.1, 0.3, 1, and 3 mg/kg/day, Indomethacin at a total daily
dose of 1 mg/kg/day, or vehicle (0.5% methocel in sterile water) were
administered per os b.i.d. beginning post injection of adjuvant and
continuing for 21 days. The compounds were prepared weekly,
refrigerated in the dark until used, and vortex mixed immediately prior
to administration.
Two-factor ( 'treatment' and 'time' ) analysis of variance
with repeated measures on 'time' were applied to the % changes for
body weight and foot volumes and to the rank-transformed radiographic
total scores. A post hoc Dunnett's test was conducted to compare the
effect of treatments to vehicle. A one-way analysis of variance was
applied to the thymic and spleen weights followed by the Dunnett's test
to compare the effect of treatments to vehicle. Dose-response curves
for % inhibition in foot volumes on days 4, 14 and 21 were fitted by a
4-parameter logistic function using a nonlinear least squares' regression.
ID50 was defined as the dose corresponding to a 50% reduction from
the vehicle and was derived by interpolation from the fitted 4-parameter
equation.
-34-
CA 02307853 2000-04-27
_ WO 99123087 PCTICA98/00998
PHARMACOKINETICS IN RATS
Per Os Pharmacokinetics in Rats - Procedure:
The animals are housed, fed and cared for according to the
Guidelines of the Canadian Council on Animal Care. Male Sprague
Dawley rats (325-375 g) are fasted overnight prior to each PO blood
level study. The rats are placed in the restrainer one at a time and the
box firmly secured. The zero blood sample is obtained by nicking a
small (1 mm or less) piece off the tip of the tail. The tail is then stroked
with a firm but gentle motion from the top to the bottom to milk out the
blood. Approximately 1 mL of blood is collected into a heparinized
vacutainer tube.
Compounds are prepared as required, in a standard dosing
volume of 10 mL/kg, and administered orally by passing a 16 gauge, 3"
gavaging needle into the stomach. Subsequent bleeds are taken in the
same manner as the zero bleed except that there is no need to nick the
tail again. The tail is cleaned with a piece of gauze and milkedlstroked
as described above into the appropriately labelled tubes.
Immediately after sampling, blood is centrifuged,
separated, put into clearly marked vials and stored in a freezer until
analysed. Typical time points for determination of rat blood levels after
PO dosing are 0, 1 Smin, 30min, 1 h, 2h, 4h, and 6h.
After the 4 hr time point bleed, food is provided to the rats
ad libitum. Water is provided at all times during the study.
The following vehicles may be used in PO rat blood level
determinations:
PEG 200/3001400: restricted to 2 mL/kg
Methocel 0.5% - 1.0%: IOmL/kg
Tween 80: IOmL/kg
Compounds for PO blood levels can be in suspension form.
For better dissolution, the solution can be placed in a sonicator for
approximately 5 minutes.
-35-
CA 02307853 2000-04-27
_ WO 99/23087 PCT/CA98/00998 _
For analysis, aliquots are diluted with an equal volume of
acetonitrile and centrifuged to remove protein precipitate. The
supernatant is injected directly onto a C-18 HPLC column with UV
detection. Quantitation is done relative to a clean blood sample spiked
with a known quantity of drug.
Intravenous Pharmacokinetics in Rats - Procedure
The animals are housed, fed and cared for according to the
Guidelines of the Canadian Council on Animal Care. Male Sprague
Dawley (325-375 g) rats are placed in plastic shoe box cages with a
suspended floor, cage top, water bottle and food. The compound is
prepared as required, in a standard dosing volume of 1 mL/kg.
Rats are bled for the zero blood sample and dosed under
C02 sedation. The rats, one at a time, are placed in a primed C02
chamber and taken out as soon as they have lost their righting reflex.
The rat is then placed on a restraining board, a nose cone with C02
delivery is placed over the muzzle and the rat restrained to the board
with elastics. With the use of forceps and scissors, the jugular vein is
exposed and the zero sample taken; followed by a measured dose of
compound which is injected into the jugular vein. Light digital pressure
is applied to the injection site, and the nose cone is removed. The time
is noted. This constitutes the zero time point.
The 5 min bleed is taken by nicking a piece (1-2 mm) off
the tip of the tail. The tail is then stroked with a firm but gentle motion
from the top of the tail to the bottom to milk the blood out of the tail.
Approximately 1 mL of blood is collected into a heparinized collection
vial. Subsequent bleeds are taken in the same fashion, except that there is
no need to nick the tail again. The tail is cleaned with a piece of gauze
and bled, as described above, into the appropriate labelled tubes.
Typical time points for determination of rat blood levels after LV.
dosing are either a) 0, 5 min, l5min, 30min, lh, 2h, 6h, or b) 0, 5 min,
30min, lh, 2h, 4h, fih.
The following vehicles may be used in IV rat blood level
determinations:
-36-
CA 02307853 2000-04-27
WO 99/23087 PC'r/CA98/00998 _ -
Dextrose: 1 mL/kg
Moleculosol 25%: 1mL/kg
- DMSO (dimethylsulfoxide): Restricted to a dose volume of
0.1 mL per animal
PEG 200: Not more than 60% mixed
with 40% sterile water -
1 mL/kg
With Dextrose, either sodium bicarbonate or sodium carbonate can be
added if the solution is cloudy.
For analysis, aliquots are diluted with an equal volume of
acetonitrile and centrifuged to remove protein precipitate. The
supernatant is injected directly onto a C-18 HPLC column with UV
detection. (~uantitation is done relative to a clean blood sample spiked
with a known quantity of drug. Bioavailability (F') is assessed by
comparing area under the curve (AUC) i.v. versus p.o.
F - AUCpo x D SEiv x 100%
AUCiv DOSEpo
Clearance rates are calculated from the following relation:
CL = DOSEiv(mg/kg)
AUCiv
The units of CL are mL/h~kg (milliliters per hour kilogram)
Compounds of the present invention are inhibitors of COX-
2 and are thereby useful in the treatment of COX-2 mediated diseases as
enumerated above. The activities of the compounds against
cyclooxygenase may be seen in the representative results shown below.
In the assay, inhibition is determined by measuring the amount of
prostaglandin E2 (PGE2) synthesized in the presence of arachidonic
acid, COX-1 or COX-2 and a putative inhibitor. The IC50 values
-37-
CA 02307853 2000-04-27
WO 99123087 PCT/CA98/00998 _
represent the concentration of putative inhibitor required to lower
PGE2 synthesis to 50% of that obtained as compared to the uninhibited
control.
The following Tables illustrate the in vitro activity, COX-
1/COX-2 selectivity and a pharmacokinetic parameter, the half life in
rats. These data are required to show the advantage of the claimed
class of compounds.
Table 2 consists of selected examples showing good in vitro
activity, and half lives in rats ranging from 1 h to 5 h.
Compounds shown in Table 3 are comparison compounds
having similar in vitro activity to the class described in Table 2 but have
extended half lives in rats of > 24 . Compound T3-1 is 3-(4-fluorophenyl)-
5,5-dimethyl-4-[4-(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone (WO
95/00501, Example 12), and compound T3-2 is 3-(3,4-difluorophenyl)-5,5-
dimethyl-4-[4-(methylsulfonyl)phenyl]-2,5-dihydro-2-furanone
(W095/00501, Example 58).
Therefore the class of compounds described in Table 2 will
provide therapeutic benefits similar to the class of compounds described
in Table 3 but without the possible adverse effects associated with long
lasting (or unmetabolised) drugs. In general, it is undesirable to have a
foreign agent in circulation for longer than is necessary to accomplish
its beneficial mission. And in particular, should any toxicity due to a
medication be manifested in a particular patient, a prolonged half life
would result in on-going exposure to the detrimental effects, and thus
would increase the risk/benefit ratio. A half life of under 24 h also
permits a more rapid, deliberate and controllable variation of the dose
level in patients than would be possible with a medication with a much
longer half life. It is to be noted that the vast majority of the currently
used antiinflammatory and analgesic agents have half lives of less than
24 h (see A. Mukherjee et al. Inflamm. Res. vol. 45, pp. 531-540 (1996)).
Table 4 consists of compounds wherein R2=R3=Et. They are
listed for comparative purposes to illustrate the superior in vitro activity
of compounds from Table 2 over compounds in which R2=R3.
-38-
CA 02307853 2000-04-27
WO 99/23087 PCT/CA98100998 _ -
The results for inhibition of PGE2 production may be seen
in the following tables (compound references are from Table 1):
- Biological activity of compounds of the present invention
T b~ le 2
Compound CHO HWB U937 HWB T 1/2
Cox-2 Cox-2 Cox-1 Cox-1 in Rats
(wM) (~.M) (~.M) (wM) (h)
a < 0.37 3-10 > 30
c 0.022 0.59 7.4 > 30 3
d 0.057 0.31 3.2 8.3
a 0.020 0.13 6.2 46
f 0.48 > 10 > 30
h 0.051 1.55 24.8 76 3.6
i 0.042 0.53 13.4 46.4 4.8
n 0.194 2.51 > 10 > 90 ~ 1
0 0.692 3.55 >10
0.237 2.44 >10
~~le 3 Comparison Compounds
Compound CHO HWB U937 HWB T 1/2
Cox-2 Cox-2 Cox-1 Cox-1 in Rats
(~M) (~,M) (~,M) (~.M) (h)
T3-1 0.042 < 0.37 5.8 86.1 > 24
T3-2 0.57 7.4 > 30 > 24
-39-
CA 02307853 2000-04-27
WO 99/23087 PCTICA98100998 _
Comparison compounds t and a show the following
activities
Table 4 Comparison Compounds
Compound CHO HWB U937
Cox-2 Cox-2 Cox-1
(p.M) (~,M) (~,M)
t > 5 12.2 >10
a > 5 13.9 >10
The invention will now be illustrated by the following non-
limiting examples in which, unless stated otherwise:
(i) all operations were carried out at room or ambient
temperature, that is, at a temperature in the range 18-25°C,
(ii) evaporation of solvent was carried out using a rotary
evaporator under reduced pressure (600-4000 pascals: 4.5-30 mm. Hg)
with a bath temperature of up to 60°C,
(iii) the course of reactions was followed by thin layer
chromatography (TLC) and reaction times are given for illustration
only;
(iv) melting points are uncorrected and 'd' indicates
decomposition; the melting points given are those obtained for the
materials prepared as described; polymorphism may result in isolation
of materials with different melting points in some preparations;
(v) the structure and purity of all final products were
assured by at least one of the following techniques: TLC, mass
spectrometry, nuclear magnetic resonance (NMR) spectrometry or
microanalytical data;
(vi) yields are given for illustration only;
(vii) when given, NMR data is in the form of delta (d)
values for major diagnostic protons, given in parts per million (ppm)
relative to tetramethylsilane (TMS) as internal standard, determined at
300 MHz or 400 MHz using the indicated solvent; conventional
-40-
CA 02307853 2000-04-27
WO 99123087 PCTJCA98100998 _
abbreviations used for signal shape are: s. singlet; d. doublet; t. triplet;
m. multiplet; br. broad; etc.: in addition "Ar" signifies an aromatic
signal;
(viii) chemical symbols have their usual meanings; the
following abbreviations have also been used v (volume), w (weight),
b.p. (boiling point), m.p. (melting point), L (litre(s)), mL (millilitres),
g (gram(s)), mg (milligrams(s)), mol (moles), mmol (millimoles), eq
(equivalent(s)).
EXAMPLE 1 (a)
5-Ethyl-5-methyl-4-[4-(methylsulfonyl)phenyl]-3-phenyl-2,5-dihydro-
2-furanone
Step 1: -methyl-1-f4-(methvlthio)phenyll-1-butanone
A 500 mL RBF equipped with a mechanical stirrer was charged with
A1C13 (35.4 g) and CHC13 {300 mL) and cooled in an ice bath. Then 2-
methylbutanoyl chloride (31.4 g) was added over 0.5 h to the ice-cold
suspension. Keeping the internal temperature <10°C, thioanisole (31.2
mL) was added dropwise over I h. After completion of addition, the
resulting mixture was stirred at r.t. for 2 h. The resulting suspension
was poured onto an ice-water mixture and stirred until decoloraton.
The organic layer was separated, washed with water, dried over Na2S04
and concentrated to dryness. The resulting white solid was swished in
hexane and collected by filtration to give the title compound as a white
solid.
1H NMR,(CD3COCD3): S 0.88 (3H, t), 1.12 (3H, d), 1.47 (1H, m), 1.78 (1H,
m), 2.57 (3H, s), 3.49 (1H, m), 7.37 (2H, d), 7.93 (2H, d).
Step 2: ~y roxy-2-methyl-1-f4-(meth lv thio)phenvll-1-butanone
To a solution of the ketone from step 1 (33 g) in toluene
(48 mL), CC14 (23.5 mL) and Aliquat, 336 (10.5 mL), was added NaOH
pellets ( 14.5 g) and the resulting mixture was vigorously stirred for 2
days. The reaction mixture was diluted with SN HCI, aqueous NH4Cl
-41-
CA 02307853 2000-04-27
WO 99123087 PCTICA98/00998 _ -
and extracted with Et20. The organic layer was dried over Na2S04
and concentrated. The crude product was purified by flash
chromatography eluted with EtOAc/Hexane 9% to give a yellow syrup.
Step 3: 2-Hydroxy-2-methyl-1-[4-(methylsulfonyl)phenyl]-1-
butanone
To an ice-cold solution of the methylthio compound obtained
from step 2 (21.0 g) in CH2C12 (3I5 mL) and MeOH (35 mL) was added
MMPP (54.0 g of 80% pure). The resulting mixture was stirred at r.t. for
40 min. The reaction was diluted with CH2Cl2 (200 mL) filtered through
silica gel, and washed with NaHC03 (200 mL). The mixture was shaken
vigorously, layers separated, the organic layer was dried over MgS04
and concentrated to give a yellow syrup which was used without
purification.
1-Methyl-1- [4-(methylsulfonyl)benzoyl] propyl-2-
g~.enylacetate
A mixture of tertiary alcohol from Step 3 (2.13 g), phenyl
acetic acid (4.3 g), 1-cyclohexyl-3-(2-morpholinoethyl)carbodiimide
metho p-toluenesulfonate (9.8 g) and DMAP (0.41 g) in CH2Cl2 (50 mL)
was heated at 60°C for 2 h. The reaction mixture was allowed to cool to
r.t., diluted with EtOAc (250 mL) and washed twice with aqueous
NaHC03. The organic layer was dried over MgS04 and concentrated.
The resulting crude product was purified by flash chromatography.
(40%--X50% ETOAc in hexane) to give a colorless gum.
Ste .~5: 5-Ethyl-5-methyl-4-[4-(methylsulfonyl)phenyl]-3-phenyl-
dihvdro-2-furanone
To an ice-cold solution of the ester from step 4 (2.5 g) in
acetonitrile (60 mL) was added DBU (1.5 mL) over 3 min. and the
resulting mixture was stirred at r.t. for 2 h. Solvents were removed
under reduced pressure and the resulting residue was applied as such
on a flash chromatography column (40%~50% -X60% EtOAc in hexane)
and then crystallized in 40 mL of 1:2 EtOAc/hexane to give white needles.
-42-
CA 02307853 2000-04-27
_ WO 99123087 PCTJCA98/00998 _ ..
1H NMR(CD3COCD3): b 0.98 (3H, t), 1.63 (3H, s), 1.96 (2H, m), 3.16 (3H,
s), 7.29 (2H, m), 7.35 (2H, m), 7.63 (2H, d), 8.02 (2H, d).
EXAMPLE 2 lc)
5-Ethyl-3-(4-fluorophenyl)-5-methyl-4-[(methylsulfonyl) phenyl]-2,
5-dihvdro-2-furanone
Using the procedures described in example 1 and replacing
in Step 4 phenylacetic acid with p-fluorophenylacetic acid, the title
compound was obtained as a white solid.
1H NMR(CD3COCD3): 8 0.98 (3H, t), 1.63 {3H, s), 1.96 (2H, m), 3.17 (3H,
s), 7.06 (2H, t), 7.41 (2H, dd), 7.64 (2H, d), 8.04 (2H, s).
(5R)-5-Ethyl-3-(4-fluorophenyl)-5-methyl-4-[4-(methyl-sulfonpl)phen.
~, 5-dihvdro-2-furanone
Step: (2S, 5S)-2-(tert-bu,~vl)-5-methyl-1,3-daoxolan-4-one
A mixture of L-Lactic acid (106 g of 85% in H20),
pivalaldehyde (220 mL), p-toluene sulfonic acid mono hydrate (2.0 g) and
H2S04 conc. (8 drops) in pentane (800 mL) was refluxed with azeotropic
removal of the water formed using a Dean-Stark trap. The supernatant
was decanted, washed with water, dried over MgS04 and concentrated.
The resulting residue was dissolved in hexane (800 mL) and cooled to -
80°C for 20 h. Crystals were collected by filtration in a cold room
(5°C)
and washed with cold hexane (-?8°C). The crystals were dissolved in
Et20, dried over MgS04 and concentred to give the title compound as a
colorless oil.
1H NMR(CD3COCD3): 8 0.95 (9H, s), 1.38 (3H,d), 4.47 (1H, m), 5.24 (IH,
s).
X0064 CA 02307853 2000-04-27
Step 2: (2S, 5R)-2-(fert--butyl)-5-ethyl-5-methyl-1,3-dioxolan-4-one
To a solution of diisopropylamine (44 mL) in THF (700 mL) at -
30°C was added a solution of 1.6M n-butyllithium in hexane (190 mL).
The
resulting solution was stirred at -10°C for 30 min. and cooled to -
78°C. Then
a solution of dioxolanone obtained from Step 1 (44.5 g) in THF (100 mL) was
added to the reaction mixture with a double-tipped needle. After 1.5 h,
iodoethane (33 mL) was added. The reaction mixture was stirred at -78°C
for
1 h and then allowed to warm to -10°C over 1 h. The reaction mixture
was
diluted with half-saturated aqueous NH4C1 (1 L) and extracted with EtOAc (1
L). The organic layer was washed with brine, dried over MgS04 and
concentrated. The crude product was purified by distillation. Fraction
passing at 85-92°CI10 mm Hg (1.33 SI Units) was collected (lift. 110116
mm
Hg) 1 H NMR(CD3COCD3); b 0.94 (9H, s), 0.97 (3H, t), 1.37 (3H, s), 1.77
(2H, m), 5.29 (1 H, s).
St- ep 3: (2S, 5S)-2-(tert-butyl)-5-ethyl-5-methyl-4-[4-(methylthio) hen I -
1,3-dioxolan-4-of
A solution of 4-bromothioanisole (48.5 g) in THF (600 mL) was
cooled to -70°C and a 2.5M solution of n-butyllithium in hexane (96 mL)
was
slowly added over 20 min. The resulting suspension was stirred for 1 h
allowing the temperature of the cooling bath to raise to -50°C. It was
then
cooled back to -70°C and a solution of dioxolanone from Step 2 (29.8 g)
in
THF (100 mL) was added dropwise over 30 min. The reaction was allowed to
proceed for another 30 min. and then quenched with AcOH (23 mL) always at
-70°C. The reaction mixture was allowed to warm to r.t., diluted with a
25%
aqueous solution of NH40Ac and extracted with EtOAc (2 L). The organic
layer was dried over MgS04 and concentrated. The resulting crude product
was purified by flash chromatography (2-~3-~5->7% EtOAc in hexane) to
give a light yellow solid.
1 H NMR(CD3COCD3): mixture of diastereomers b 0.50 (0.5H, m), 0.68
(1.5H, s), 0.72 (1.5H, t), 0.97 (1.5H, t), 0.98 (9H, s), 1.38 (1.5H, s), 1.52
(0.5H,
-44-
AiIII'I'~JU~L S! i~~T
IP~P,i~.P
CA 02307853 2000-04-27
_ WO 99/23087 PCT/CA98/00998
m), 1.68 (0.5H, m), 2.10 (0.5H, m), 2.48 (3H, 2s), 4.85 (1H, s, OH), 5.01
(0.5H, s), 5.32 (0.5H, s), 7.22 - 7.30 (2H, m), 7.50 (2H, dd).
-Step 4: (2R)-2-Hydroxy-2-methyl-1-[4-(methylsulfonyl)
~yllbutan-1-one
A mixture of the dioxolanol from Step 3 (46.2 g) in water (50
mL) with p-toluenesulfonic acid mono hydrate (1.3 g) was refluxed for 1
hour. The reaction was allowed to cool to r.t. and extract twice with
EtOAc (200 + 150 mL). To this solution was added t-BuOH (175 mL) and
Aliquat0 336. This solution was cooled to 10°C and then a solution
of
OXONE~ (130 g) in water (800 mL) was added over 30 min. The
resulting mixture was stirred at r.t. for 20 h. The reaction mixture was
neutralized by carefull and portionwise addition of aqueous saturated
NaHC03 and extracted with EtOAc. The organic layer was dried over
MgS04 and concentrated to give a yellow syrup.
1H NMR(CD3COCD3): 8 0.90 (3H, t), 1.49 (3H, s), 1.81 (1 H, m), 1.95 (1H,
s, OH), 1.99 (lH, m), 3.17 (3H, s), 8.02 (2H, d), 8.32 (2H, d).
Step 5: (5R)-5-Ethyl-3-(4-fluorophenyl)-5-methyl-4-[4-
(methylsulfon~ h~ye 11-2 5-dihydro-2-furanone
Using the procedures described in Example I, Step 4 and
Step 5, replacing phenylacetic acid with p-fluorophenylacetic acid and
replacing 2-hydroxy-2-methyl-1-[4-(methylsulfonyl)phenyl]-1-butanone
with (2R)-2-hydroxy-2-methyl-1-[4-(methylsulfonyl)phenyl]butan-1-one
from Step 4, the title compound was obtained as a white solid.
M.S. (+APCI) m/z 375 (M + H)+, [a]D +32° (c = 1.0, acetone), m.p.
120-
121°C.
- 45 -
CA 02307853 2000-04-27
WO 99/23087 PCT/CA98/00998 _
(5S)-5-Ethyl-3-(4-fluorophenyl)-5-methyl-4-[4-(methyl-sulfon~nhenvll-
-~5-dihvdro-2-furanone
Using the procedure described in Example 3, Step 5 and
replacing (2R)-2-hydroxy-2-methyl-1-[4-(methylsulfonyl)phenyl]butan-1-
one with (2S)-2-hydroxy-2-methyl-1-[4-(methyl-sulfonyl)phenyl]butan-1-
one the title compound was obtained as a white solid.
M.S. (CI, CH4) m/z 375 (M+H)+,[a]D -33° (C = 1.0, acetone), m.p.
119-
120°C.
EXAMPLE 5 (f)
5-Ethyl-3-(3-fluorophenyl)-5-methyl-4-[4-(methylsulfonyl)phenyl]-2,
5-dihvdro-2-furanone
Using the procedures described in Example 1 and replacing
in Step 4 phenylacetic acid with m-fluorophenylacetic acid, the title
compound was obtained as a white solid.
1H 1VMR(CD3COCD3): 8 0.99 (3H, t), 1.64 (3H, s), 1.97 (2H, m), 3.18 (3H,
s), 7.14-7.23 (2H, m), 7.20 (1H, d), 7.32 (1H, m), 7.66 (2H, d), 8.05 (2H, d).
3-(4-fluorophenyl)-5-methyl-4-[4-(methylsulfonyl)phenyl]-5-propyl-~
dihvdro-2-furanone
Using the procedures described in Example 1, replacing in
Step 1 2-methylbutanoyl chloride with 2-methylpentanoyl chloride and
replacing in Step 4 phenylacetic acid with p-fluorophenylacetic acid, the
title compound was obtained as a white solid.
M.S. (CI, CH4) m/z 389 (M+H)+, m.p. 112-113°C.
CA 02307853 2000-04-27
WO 99/23087 PCT/CA98/00998 _
EXAMPLE 7 f h)
(5R)-3-(3,4-difluorophenyl)-5-ethyl-5-methyl-4-[4-(methylsulfonyl)
nhen l~l-,5-dihydro-2-furanon,~
Using the procedures described in Example 3 and replacing
in Step 5 p-fluorophenylacetic acid with 3,4-difluorophenylacetic acid,
the title compound was obtained as a white solid.
m.p. 144°C, [a]D +32.1° (c = 1.3, CHC13).
EXAMPLE 8 (i)
(5S)-3-(3,4-difluorophenyl)-5-ethyl-5-methyl-4-[4-(methylsulfonyl) phen.
2, 5-dihvdro-2-furanone
Using the procedure described in Example 4 and replacing
p-fluorophenylacetic acid with 3,4-difluorophenylacetic acid, the title
compound was obtained as a white solid.
m.p. 142 - 143°C, [a]D -32.7° (c = 1.1, CHCIg)
EXAMPLE 9Sp)
3-(3,4-difluorophenyl)-5-methyl-4-[4-(methylsulfonyl)phenyl]-5-prowl-2.5-
,vdro-2-furanone
Using the procedure described in Example 6 replacing p-
fluorophenylacetic acid with 3,4-difluorophenylacetic acid, the title
compound was obtained as a white solid.
M.S. (+APCI) m/z 407 (M+H)+, m.p. 89-90°C.
-47-
CA 02307853 2000-04-27
WO 99/23087 PCTICA98/00998 _ .
E~11~PLE 10 (o)
3-(3-fluorophenyl)-5-methyl-4-[4-(methylsulfonyl)phenyl]-5-propyl-2_,~5-
dihvdro-2-furanone
Using the procedure described in Example 6 replacing p-
fluorophenylacetic acid with m-fluorophenylacetic acid, the title
compound was obtained as a white solid.
M.S. (CI, CH4) m/z 389 (M+H)+, m.p. 92-93°C.
-48-