Note: Descriptions are shown in the official language in which they were submitted.
CA 02308639 2000-04-25
WO 99/22720 PCT/US98/22246
1
METHOD OF USING CYCLOOXYGENASE-2 INHIBITORS IN
MAINTAINING THE FETAL DUCTUS ATERIOSUS DURING TREATMENT
AND PREVENTION OF PRETERM LABOR
Field of the Invention
This invention is iri the field of the prevention
and treatment of preterm labor. More specifically, this
invention relates to the use of cyclooxygenase-2
inhibitors or derivatives thereof in preventing and
treating preterm labor.
Background of the Invention
Prostaglandins play a major role in the
inflammation process and the inhibition of prostaglandin
production, especially production of PGG2, PGH2 and
PGE2~ has been a common target of anti-inflammatory drug
discovery. However, common non-steroidal anti-
inflammatory drugs (NSAID's) that are active in reducing
the prostaglandin-induced pain and swelling associated
with the inflammation process are also active in
affecting other prostaglandin-regulated processes not
associated with the inflammation process. Thus, use of
high doses of most common NSAID's can produce severe
side effects, including life threatening ulcers, that
limit their therapeutic potential. An alternative to
NSAID's is the use of corticosteroids, which also
produce adverse effects, especially when long term
therapy is involved.
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98/Z2246
2
NSAIDs have been found to prevent the
production of prostaglandins by inhibiting
enzymes in the human arachidonic
acid/prostaglandin pathway, including the enzyme
cyclooxygenase (COX). The recent discovery of an
inducible enzyme associated with inflammation
(named "cyclooxygenase-2 (COX-2)" or
"prostaglandin G/H synthase II") provides a
viable target of inhibition which more
effectively reduces inflammation and produces
fewer and less drastic side effects.
Spontaneous preterm labor during pregnancy is an
important and increasing problem confronting the medical
community. Few advances have been made in the
understanding of causes of preterm labor, in the early
detection of preterm labor and in its general
management. The ability to safely stop preterm labor and
thereby to allow a pregnancy to advance towards term has
thus far eluded the medical and scientific community.
Preterm delivery accounts for a major proportion of
perinatal deaths and significant proportion of postnatal
and childhood defects and therefore, maintaining the
fetus in utero is preferred to allowing preterm
delivery. Preterm labor also has proven to be a
limiting factor for types of fetal intervention.
The onset of labor appears to depend on multiple
factors. Normal progression of pregnancy until the term
requires relaxation of uterine smooth muscle until
parturition, but the mechanism that maintains uterine
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98/ZZ246
3
relaxation during pregnancy is unknown. Normal
parturition typically begins with labor. Labor consists
of a series of rhythmic, progressive contractions of the
uterus that cause effacement and dilation of the uterine
cervix. In normal pregnancy, labor usually begins within
two weeks before estimated delivery.
Once preterm labor is diagnosed, the risks and
benefits of labor inhibition must be weighed against
those of allowing delivery to occur. The risks from
labor inhibition are primarily related to the side
effects of the labor inhibiting drugs. Once preterm
labor is diagnosed and the gestational age is
established as appropriate for labor inhibition,
contraindications such as eclampsia, preeclampsia,
ruptured placenta, dead or anomalous fetus, fetal
distress or chorioammionitis to premature delivery is
determined and the particular available tocolytic agent
is selected.
Different pharmacological approaches using the
above tocolytic drugs have been tried to control preterm
labor. Currently used tocolytic agents most often used
include ~i-adrenoreceptor stimulants such as epinephrine
or its synthetic analogs and derivatives salbutamol,
terbutaline, isoxsuprine, ritodrine, and fenoterol,
magnesium sulfate, prostaglandin inhibitors such as
aspirin, indomethacin and naproxen, ethanol and calcium
channel-blocking agents such as nipedifine or
nicardipine.
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99122720 PCT/US98/22246
4
Even the best tocolytic regimen available currently
is unsatisfactory for prevention or inhibition of
preterm labor. Additionally to proving ineffective, such
standard tocolytic regimen had potentially serious
harmful effects on both mother and fetus. Halogenated
inhalation anesthesia needed to achieve uterine
relaxation had been shown to produce significant
myocardial depression in both mother and fetus. Finally,
it is becoming obvious that the aggressive treatment of
postoperative labor with maximal doses of magnesium and
betamimetics is quite toxic for the mother and attempts
to avoid maternal pulmonary edema in this clinical
setting led to maternal hypovolemia with documented
reversal of diastolic flow in the uterine arteries.
NSAIDs have been studied in the treatment and
prevention of preterrn labor. Specifically, indomethacin
and sulindac have been clinically evaluated. However,
use of these compounds is significantly limited because
od side effects including constriction of the fetal
ductus arteriosus, and tricuspid regurgitation which can
lead to significant right-heart failure in the fetus,
among others. Such side effects limit the use of
NSAIDs, especially in the all important last trimester.
Recently, an increase of cyclooxygenase-2 has been
observed during labor (Zuo et al. J. Clin. Endoc.
Metab., 79, 894-9 (1994), Slater et al., Am. J. Obstet.
Gynecol., 172, 77-82 (1995)). In addition, COX-2 plays
a role in spontaneous abortion or preterm labor caused
by maternal infection (Silver et al., J. Clin. Invest.,
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98122246
95, 725-31 (1995)). Sawdy et al. described the use of
nimesulide to prevent preterm delivery (The Lancet, 350,
265-6 (1997)). W094/26731, published November 24, 1994,
describes the use of thiophene COX-2 inhibitors for the
5 treating premature labor. W097/31631, published Sep. 4,
1997 describes the use of COX-2 inhibitors for managing
labor and uterine contractions.
Prostaglandins have been indicated in the control
of the closure of the ductus arteriosus during the last
trimester.
Compounds which selectively inhibit
cyclooxygenase-2 have been described in U.S.
patents 5,380,738, 5,394,991, 5,393,790,
5,439,178, 5,474,995, 5, 510,368 and WO documents
W096/06840, W096/03388, W096/03387, W096/25405,
W095/15316, W094/15932, W094/27980, W095/0050I,
W094/13635, W094/20480, and W094/26731.
[Pyrazol-1-yl]benzenesulfonamide have been
described as inhibitors of cyclooxygenase-2 and have
shown promise in the treatment of inflammation,
arthritis, and pain, with minimal side effects in pre-
clinical and clinical trials. Their use for treating
inflammation has been described in U.S. Patent No.
5,466,823. However, their use for treating or preventing
preterm labor has not been previously described.
The present invention is the use of compounds that
selectively inhibit COX-2 to treat and prevent preterm
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99122720 PCT/US98/22246
6
labor while maintaining circulatory flow through the
fetal ductus arteriosus.
Detailed Description of the Invention
The present invention provides a method for
treating or preventing preterm labor while maintaining
circulatory flow through the fetal ductus arteriosus in
a subject in need of such treatment or prevention, the
20 method comprises treating the subject having or
susceptible to said preterm labor with a
therapeutically-effective amount of a compound of
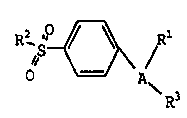
Formula I
R, '~ ~ ~ R1
~S
R3
wherein A is a 5- or 6-member ring substituent
selected from partially unsaturated or unsaturated
heterocyclo and carbocyclic rings;
wherein R1 is at least one substituent
selected from heterocyclo, cycloalkyl, cycloalkenyl
and aryl, wherein R1 is optionally substituted at a
substitutable position with one or more radicals
selected from alkyl, haloalkyl, cyano, carboxyl,
' 25 alkoxycarbonyl, hydroxyl, hydroxyalkyl, haloalkoxy,
amino, alkylamino, arylamino, nitro, alkoxyalkyl,
alkylsulfinyl, halo, alkoxy and alkylthio;
wherein R2 is selected from alkyl, and amino; _
and wherein R3 is a radical selected from halo,
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/Z2720 PCTIUS98/2Z246
7
alkyl, alkenyl, alkynyl, oxo, cyano, carboxyl,
cyanoalkyl, heterocyclooxy, alkyloxy, alkylthio,
alkylcarbonyl, cycloalkyl, aryl, haloalkyl,
heterocyclo, cycloalkenyl, aralkyl,
heterocycloalkyl, acyl, alkylthioalkyl,
hydroxyalkyl, alkoxycarbonyl, arylcarbonyl,
aralkylcarbonyl, aralkenyl, al.koxyalkyl,
arylthioalkyl, aryloxyalkyl, aralkylthioalkyl,
aralkoxyalkyl, alkoxyaralkoxyalkyl,
alkoxycarbonylalkyl, aminocarbonyl,
aminocarbonylalkyl, alkylaminocarbonyl, N-
arylaminocarbonyl, N-alkyl-N-arylaminocarbonyl,
alkylaminocarbonylalkyl, carboxyalkyl, alkylamino,
N-arylamino, N-aralkylamino, N-alkyl-N-
aralkylamino, N-alkyl-N-arylamino, aminoalkyl,
alkylaminoalkyl, N-arylaminoalkyl, N-
aralkylaminoalkyl, N-alkyl-N-aralkylaminoalkyl, N-
alkyl-N-arylaminoalkyl, aryloxy, aralkoxy,
arylthio, aralkylthio, alkylsulfinyl,
alkylsulfonyl, aminosulfonyl, alkylaminosulfonyl,
N-arylaminosulfonyl, arylsulfonyl, N-alkyl-N
arylaminosulfonyl; or a pharmaceutically-
acceptable salt thereof.
The invention would be useful for, but not limited
to treatment and prevention of preterm labor.
The invention also would be useful for, but not
limited to prevention of closure of the ductus
arteriosus and maintaining circulatory flow through the
fetal ductus arteriosus during preterm labor therapy.
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99122720 PCT/US98I22246
8
Besides being useful for human treatment,
these compounds are also useful for veterinary
treatment of mammals, including companion animals
and farm animals, such as, but not limited to,
horses, dogs, cats, cows, sheep and pigs.
The term "treatment" includes partial or total
inhibition of the preterm labor.
The term "prevention" includes either preventing
the onset of clinically evident preterm labor altogether
or preventing the onset of a preclinically evident stage
of preterm labor in individuals at risk.
The phrase "therapeutically-effective" is intended
to qualify the amount of each agent which will achieve
the goal of improvement in severity and the frequency of
incidence over treatment of each agent by itself, while
avoiding adverse side effects typically associated with
alternative therapies.
The term "subject" for purposes of treatment
includes any human or animal subject who is experiencing
preterm labor, and preferably is a human subject. For
methods of prevention, the subject is any human or
animal subject, and preferably is a human subject who is
currently pregnant and at risk for experiencing preterm
labor.
SU9STITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCTNS98122246
9
Inhibitors of the cyclooxygenase pathway in
the metabolism of arachidonic acid used in the
prevention and treatment of preterm labor may
inhibit enzyme activity through a variety of
mechanisms. By the way of example, the inhibitors
used in the methods described herein may block
the enzyme activity directly by acting as a
substrate for the enzyme. The use of
cyclooxygenasse-2 selective inhibitors is highly
advantageous in that they minimize the gastric
side effects that can occur with non-selective
NSAID's, especially where prolonged prophylactic
treatment is expected.
The term "cyclooxygenase-2 inhibitor"
denotes a compound able to inhibit
cyclooxygenase-2 without significant inhibition
of cyclooxygenase-1. Preferably, it includes
compounds which have a cyclooxygenase-2 IC5p of
less than about 0.2 uM, and also have a
selectivity ratio of cyclooxygenase-2 inhibition
over cyclooxygenase-1 inhibition of at least 50,
and more preferably of at least 100. Even more
preferably, the compounds have a cyclooxygenase-1
ICSp of greater than about 1 ~,M, and more
preferably of greater than 10 uM.
The present invention provides a novel method for
control, treatment, management and prevention of preterm
labor while maintaining circulatory flow through the
fetal ductus. The method comprises administering to a
SUBSTITUTE SHEET (i3ULE 26)
CA 02308639 2000-04-25
WO 99122720 PCTIUS98l22246
pregnant woman experiencing preterm labor a composition
consisting essentially of a compound of Formula I, alone
or in combination with other tocolytic agents in an
amount effective to inhibit or counter the onset of
5 uterine contractions. Such tocolytic agents include p-
adrenoreceptor stimulants such as epinephrine or its
synthetic analogs and derivatives salbutamol,
terbutaline, isoxsuprine, ritodrine, and fenoterol,
magnesium sulfate, ethanol, activin antagonists, cardiac
10 antiarrhythmics such as lidocaine or ocainide, nitric
oxide donors such as S-nitroso-N-acetylpenicillamine,
nitric oxide nucleophiles and adducts, nitroglycerin,
hydroxylamine, sodium azide, diethylamino nitric oxide
and analogs, and nitric oxide precursors such as L-
arginine, and calcium channel-blocking agents such as
nipedifine or nicardipine.
Derivatives are intended to encompass any compounds
which are structurally related to the cyclooxygenase-2
inhibitors or which possess the substantially equivalent
biologic activity. By way of example, such inhibitors
may include, but are not limited to, prodrugs thereof.
A preferred class of compounds which inhibit
cyclooxygenase-2 consists of compounds of Formula I
wherein A is selected from oxazolyl, isoxazolyl,
thienyl, dihydrofuryl, furyl, pyrrolyl, pyrazolyl,
thiazolyl, imidazolyl, isothiazolyl, cyclopentenyl,
phenyl, and pyridyl; wherein R1 is selected from
S- and 6-membered heterocyclo, lower cycloalkyl,
lower cycloalkenyl and aryl selected from phenyl,
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98/Z2246
11
biphenyl and naphthyl, wherein R1 is optionally
substituted at a substitutable position with one or
more radicals selected from lower alkyl, lower
haloalkyl, cyano, carboxyl, lower alkoxycarbonyl,
hydroxyl, lower hydroxyalkyl, lower haloalkoxy,
amino, lower alkylamino, phenylamino, nitro, lower
alkoxyalkyl, lower alkylsulfinyl, halo, lower
alkoxy and lower alkylthio; wherein R2 is selected
from lower alkyl and amino; and wherein R3 is a
radical selected from halo, lower alkyl, oxo,
cyano, carboxyl, lower cyanoalkyl, heteroaryloxy,
lower alkyloxy, lower cycloalkyl, phenyl, lower
haloalkyl, 5- or 6-membered heterocyclo, lower
hydroxylalkyl, lower aralkyl, acyl, phenylcarbonyl,
lower alkoxyalkyl, heteroaryloxy, alkoxycarbonyl,
aminocarbonyl, alkylaminocarbonyl, alkylamino,
aminoalkyl, alkylaminoalkyl, aryloxy, and aralkoxy;
or a pharmaceutically-acceptable salt thereof.
A more preferred class of compounds which
inhibit cyclooxygenase-2 consists of compounds of
Formula I wherein A is selected from oxazolyl,
isoxazolyl, dihydrofuryl, imidazolyl, and
pyrazolyl; wherein R1 is selected from 5- and 6-
membered heterocyclo, lower cycloalkyl, lower
cycloalkenyl and aryl selected from phenyl,
biphenyl and naphthyl, wherein R1 is optionally
substituted at a substitutable position with one or
more radicals selected from lower alkyl, lower
haloalkyl, cyano, carboxyl, lower alkoxycarbonyl,
hydroxyl, lower hydroxyalkyl, lower haloalkoxy,
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98122246
12
amino, lower alkylamino, phenylamino, nitro, lower
alkoxyalkyl, lower alkylsulfinyl, halo, lower
alkoxy and lower alkylthio; wherein R2 is amino;
and wherein R3 is a radical selected from oxo,
cyano, carboxyl, lower alkoxycarbonyl, lower
carboxyalkyl, lower cyanoalkyl, halo, lower alkyl,
lower alkyloxy, lower cycloalkyl, phenyl, lower
haloalkyl, 5- or 6-membered heterocyclo, lower
hydroxylalkyl, lower aralkyl, acyl, phenylcarbonyl,
lower alkoxyalkyl, 5- or 6-membered heteroaryloxy,
aminocarbonyl, lower alkylaminocarbonyl, lower
alkylamino, lower aminoalkyl, lower
alkylaminoalkyl, phenyloxy, and lower aralkoxy; or
a pharmaceutically-acceptable salt thereof.
An even more preferred class of compounds
which inhibit cyclooxygenase-2 consists of
compounds of Formula I wherein A is selected from
oxazolyl, isoxazolyl, imidazolyl, and pyrazolyl;
wherein R1 is phenyl optionally substituted at a
substitutable position with one or more radicals
selected from methyl, ethyl, isopropyl, butyl,
tert-butyl, isobutyl, pentyl, hexyl, fluoromethyl,
difluoromethyl, trifluoromethyl, chloromethyl,
dichloromethyl, trichloromethyl, pentafluoroethyl,
heptafluoropropyl, fluoromethyl, difluoroethyl,
- difluoropropyl, dichloroethyl, dichloropropyl,
cyano, carboxyl, methoxycarbonyl, hydroxyl,
hydroxymethyl, trifluoromethoxy, amino, N-
methylamino, N,N-dimethylamino, N-ethylamino, N,N-
dipropylamino, N-butylamino, N-methyl-N-ethylamino,
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98/22246
13
phenylamino, nitro, methoxymethyl, methylsulfinyl,
fluoro, chloro, bromo, methoxy, ethoxy, propoxy, n-
butoxy, pentoxy, and methylthio: wherein R2 is
amino: and wherein R3 is a radical selected from
oxo, cyano, carboxyl, methoxycarbonyl,
ethoxycarbonyl, carboxypropyl, carboxymethyl,
carboxyethyl, cyanomethyl, fluoro, chloro, bromo,
methyl, ethyl, isopropyl, butyl, tert-butyl,
isobutyl, pentyl, hexyl, fluoromethyl,
difluorornethyl, trifluoromethyl, chloromethyl,
dichloromethyl, trichloromethyl, pentafluoroethyl,
heptafluoropropyl, fluoromethyl, difluoroethyl,
difluoropropyl, dichloroethyl, dichloropropyl,
methoxy, ethoxy, propoxy, n-butoxy, pentoxy,
cyclohexyl, phenyl, pyridyl, thienyl, thiazolyl,
oxazolyl, furyl, pyrazinyl, hydroxylmethyl,
hydroxylpropyl, benzyl, formyl, phenylcarbonyl,
methoxymethyl, furylmethyloxy, aminocarbonyl, N-
methylaminocarbonyl, N,N-dimethylaminocarbonyl,
N,N-dimethylamino, N-ethylamino, N,N-dipropylamino,
N-butylamino, N-methyl-N-ethylamino, aminomethyl,
N,N-dimethylaminomethyl, N-methyl-N-
ethylaminomethyl, benzyloxy, and phenyloxy; or a
pharmaceutically-acceptable salt thereof.
A family of specific compounds of particular
interest within Formula I consists of compounds and
pharmaceutically-acceptable salts thereof as
follows:
3-(3,4-difluorophenyl)-4-(4-methylsulfonylphenyl)-
2-(5H)-furanone;
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCTlUS98122246
14
3-phenyl-4-4-methylsulfonylphenyl)-2-(5H)-furanone;
4-[5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-
pyrazol-1-yl]benzenesulfonamide;
4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-
pyrazol-1-yl]benzenesulfonamide;
4-(5-(3-fluoro-4-methoxyphenyl)-3-(difluoromethyl)-
1H-pyrazol-1-yl]benzenesulfonamide;
3-[1-[4-(methylsulfonyl)phenyl]-4-trifluoromethyl-
1H-imidazol-2-yl]pyridine:
2-methyl-5-[1-[9-(methylsulfonyl)phenyl]-4-
trifluoromethyl-1H-imidazol-2-yl]pyridine;
4-[2-(5-methylpyridin-3-yl)-9-(trifluoromethyl)-1H-
imidazol-1-yl]benzenesulfonamide;
4-[5-methyl-3-phenylisoxazol-4-
yl]benzenesulfonamide:
9-[5-hydroxyethyl-3-phenylisoxazol-4-
yl]benzenesulfonamide;
[2-trifluoromethyl-5-(3,4-difluorophenyl)-4-
oxazolyl]benzenesulfonamide;
4-[2-methyl-9-phenyl-5-oxazolyl]benzenesulfonamide:
and
4-[5-(3-fluoro-4-methoxyphenyl-2-trifluoromethyl)-
4-oxazolyl]benzenesulfonamide.
Within Formula I there is a subclass of compounds
of high interest represented by Formula II:
Rs
R5
O O
~s~ N ~ II
H zN ~N R4
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98/22246
I5
wherein R4 is selected from hydrido, alkyl,
haloalkyl, alkoxycarbonyl, cyano, cyanoalkyl, carboxyl,
aminocarbonyl, alkylaminocarbonyl,
cycloalkylaminocarbonyl, arylaminocarbonyl,
carboxyalkylaminocarbonyl, carboxyalkyl,
aralkoxycarbonylalkylaminocarbonyl, aminocarbonylalkyl,
alkoxycarbonylcyanoalkenyl and hydroxyalkyl;
wherein R5 is selected from hydrido, alkyl, cyano,
hydroxyalkyl, cycloalkyl, alkylsulfonyl and halo: and
wherein R6 is selected from aralkenyl, aryl,
cycloalkyl, cycloalkenyl and heterocyclic; wherein R4 is
optionally substituted at a substitutable position with
one or more radicals selected from halo, alkylthio,
alkylsulfonyl, cyano, nitro, haloalkyl, alkyl, hydroxyl,
alkenyl, hydroxyalkyl, carboxyl, cycloalkyl, alkylamino,
dialkylamino, alkoxycarbonyl, aminocarbonyl, alkoxy,
haloalkoxy, sulfamyl, heterocyclic and amino;
or a pharmaceutically-acceptable salt or
derivative thereof.
A class of compounds of particular interest
consists of those compounds of Formula II wherein R4 is
selected from lower haloalkyl; wherein R5 is hydrido;
and wherein R6 is phenyl optionally substituted at a
substitutable position with one or more radicals
selected from halo, lower alkyl, and lower alkoxy; or a
pharmaceutically-acceptable salt or derivative thereof.
SUBSTITUTE SHEET (RULE 25)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98/22246
16
A class of compounds of more particular interest
consists of those compounds of Formula II wherein R4 is
selected from trifluoromethyl and difluoromethyl;
wherein R5 is hydrido; and wherein R6 is phenyl
optionally substituted at a substitutable position with
one or more radicals selected from fluoro, chloro,
methyl, and methoxy; or a pharmaceutically-acceptable
salt or derivative thereof.
A family of specific compounds of particular
interest within Formula II consists of compounds,
pharmaceutically-acceptable salts and derivatives
thereof as follows:
9-[5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[5-phenyl-3-(trifluoromethyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[5-(4-fluorophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[5-(4-methoxyphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[5-(4-chlorophenyl)-3-(difluoromethyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[3-(difluoromethyl)-5-(4-methylphenyl)-1H-pyrazol-1-
- yl]benzenesulfonamide;
4-[3-(difluoromethyl)-5-phenyl-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[3-(difluoromethyl)-5-(4-methoxyphenyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PGT/US98lZ2246
17
~-E3-tdifluoromethyl)-5-(3-fluoro-4-methoxyphenyl)-1H-
pyrazol-1-yl]benzenesulfonamide; and
4-[5-(3-fluoro-9-methoxyphenyl)-3-(trifluoromethyl)-IH-
pyrazol-1-yl]benzenesulfonamide.
5
A family of specific compounds of more particular
interest within Formula II consists of compounds and
pharmaceutically-acceptable salts or derivatives thereof
as follows:
4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-I-
yl]benzenesulfonarnide;
4-[5-(9-chlorophenyl)-3-(difluoromethyl)-1H-pyrazol-1-
yl]benzenesulfonamide; and
4-[5-(3-fluoro-4-methoxyphenyl)-3-(difluoromethyl)-1H-
pyrazol-I-yl]benzenesulfonamide.
The term "hydrido" denotes a single hydrogen
atom (H). This hydrido radical may be attached,
for example, to an oxygen atom to form a hydroxyl
radical or two hydrido radicals may be attached to
a carbon atom to form a methylene (-CH2-) radical.
Where used, either alone or within other terms such
as "haloalkyl", "alkylsulfonyl", "alkoxyalkyl" and
"hydroxyalkyl", the term "alkyl" embraces linear or
branched radicals having one to about twenty carbon
atoms or, preferably, one to about twelve carbon
atoms. More preferred alkyl radicals are "lower
alkyl" radicals having one to about ten carbon
atoms. Most preferred are lower alkyl radicals
having one to about six carbon atoms. Examples of
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCTIUS98/2224b
18
such radicals include methyl, ethyl, n-propyl,
isopropyl, n-butyl, isobutyl, sec-butyl, tert-
butyl, pentyl, iso-amyl, hexyl and the like. The
term "alkenyl" embraces linear or branched radicals
S having at least one carbon-carbon double bond of
two to about twenty carbon atoms or, preferably,
two to about twelve carbon atoms. More preferred
alkenyl radicals are "lower alkenyl" radicals
having two to about six carbon atoms. Examples of
alkenyl radicals include ethenyl, propenyl, allyl,
propenyl, butenyl and 4-methylbutenyl. The term
"alkynyl" denotes linear or branched radicals
having at least one carbon-carbon triple bond, and
having two to about twenty carbon atoms or,
preferably, two to about twelve carbon atoms. More
preferred alkynyl radicals are "lower alkynyl"
radicals having two to about ten carbon atoms.
Most preferred are lower alkynyl radicals having
two to about six carbon atoms. Examples of such
radicals include propargyl, butynyl, and the like.
The terms "alkenyl" and "lower alkenyl", embrace
radicals having "cis" and "traps" orientations, or
alternatively, "E" and "Z" orientations. The term
"cycloalkyl" embraces saturated carbocyclic
radicals having three to about twelve carbon atoms.
More preferred cycloalkyl radicals are "lower
- cycloalkyl" radicals having three to about eight
carbon atoms. Examples of such radicals include
cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl. The term "cycloalkenyl" embraces
partially unsaturated carbocyclic radicals having
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98122246
19
three to twelve carbon atoms. More preferred
cycloalkenyl radicals are "lower cycloalkenyl"
radicals having four to about eight carbon atoms.
Examples of such radicals include cyclobutenyl,
cyclopentenyl and cyclohexenyl. The term "halo"
means halogens such as fluorine, chlorine, bromine
or iodine. The term "haloalkyl" embraces radicals
wherein any one or more of the alkyl carbon atoms
is substituted with halo as defined above.
Specifically embraced are monohaloalkyl,
dihaloalkyl and polyhaloalkyl radicals. A
monohaloalkyl radical, for one example, may have
either an iodo, bromo, chloro or fluoro atom within
the radical. Dihalo and polyhaloalkyl radicals may
have two or more of the same halo atoms or a
combination of different halo radicals. "Lower
haloalkyl" embraces radicals having one to six
carbon atoms. Examples of haloalkyl radicals
include fluoromethyl, difluoromethyl,
trifluoromethyl, chloromethyl, dichloromethyl,
trichloromethyl, trichloromethyl, pentafluoroethyl,
heptafluoropropyl, difluorochloromethyl,
dichlorofluoromethyl, difluoroethyl,
difluoropropyl, dichloroethyl and dichloropropyl.
The term "hydroxyalkyl" embraces linear or branched
alkyl radicals having one to about ten carbon atoms
- any one of which may be substituted with one or
more hydroxyl radicals. More preferred
hydroxyalkyl radicals are "lower hydroxyalkyl"
radicals having one to six carbon atoms and one ar
more hydroxyl radicals. Examples of such radicals
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCTIUS98I22246
include hydroxymethyl, hydroxyethyl, hydroxypropyl,
hydroxybutyl and hydroxyhexyl. The terms "alkoxy"
and "alkyloxy" embrace linear or branched oxy-
containing radicals each having alkyl portions of
5 one to about ten carbon atoms. More preferred
alkoxy radicals are "lower alkoxy" radicals having
one to six carbon atoms. Examples of such radicals
include methoxy, ethoxy, propoxy, butoxy and tert-
butoxy. The term "alkoxyalkyl" embraces alkyl
IO radicals having one or more alkoxy radicals
attached to the alkyl radical, that is, to form
monoalkoxyalkyl and dialkoxyalkyl radicals. The
"alkoxy" radicals may be further substituted with
one or more halo atoms, such as fluoro, chloro or
15 bromo, to provide haloalkoxy radicals. More
preferred haloalkoxy radicals are "lower
haloalkoxy" radicals having one to six carbon atoms
and one or more halo radicals. Examples of such
radicals include fluoromethoxy, chloromethoxy,
20 trifluoromethoxy, trifluoroethoxy, fluoroethoxy and
fluoropropoxy. The term "aryl", alone or in
combination, means a carbocyclic aromatic system
containing one, two or three rings wherein such
rings may be attached together in a pendent manner
or may be fused. The term "aryl" embraces aromatic
radicals such as phenyl, naphthyl,
- tetrahydronaphthyl, indane and biphenyl. Aryl
moieties may also be substituted at a substitutable
position with one or more substituents selected
independently from alkyl, alkoxyalkyl,
alkylaminoalkyl, carboxyalkyl, alkoxycarbonylalkyl,
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98/Z2246
21
aminocarbonylalkyl, alkoxy, aralkoxy, hydroxyl,
amino, halo, nitro, alkylamino, acyl, cyano,
carboxy, aminocarbonyl, alkoxycarbonyl and
aralkoxycarbonyl. The term "heterocyclo" embraces
saturated, partially unsaturated and unsaturated
heteroatom-containing ring-shaped radicals, where
the heteroatoms may be selected from nitrogen,
sulfur and oxygen. Examples of saturated
heterocyclo radicals include saturated 3 to 6-
membered heteromonocylic group containing 1 to 4
nitrogen atoms (e. g. pyrrolidinyl, imidazolidinyl,
piperidino, piperazinyl, etc.); saturated 3 to 6-
membered heteromonocyclic group containing 1 to 2
oxygen atoms and 1 to 3 nitrogen atoms (e. g.
morpholinyl, etc.); saturated 3 to 6-membered
heteromonocyclic group containing 1 to 2 sulfur
atoms and 1 to 3 nitrogen atoms (e. g.,
thiazolidinyl, etc.). Examples of partially
unsaturated heterocyclo radicals include
dihydrothiophene, dihydropyran, dihydrofuran and
dihydrothiazole. The term "heteroaryl" embraces
unsaturated heterocyclo radicals. Examples of
heteroaryl radicals include unsaturated 3 to 6
membered heteromonocyclic group containing 1 to 4
nitrogen atoms, for example, pyrrolyl, pyrrolinyl,
imidazolyl, pyrazolyl, pyridyl, pyrimidyl,
pyrazinyl, pyridazinyl, triazolyl (e. g., 4H-1,2,4-
triazolyl, 1H-1,2,3-triazolyl, 2H-1,2,3-triazolyl,
etc.) tetrazolyl (e.g. 1H-tetrazolyl, 2H-
tetrazolyl, etc.), etc.; unsaturated condensed
heterocyclo group containing 1 to 5 nitrogen atoms,
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98/22246
22
for example, indolyl, isoindolyl, indolizinyl,
benzimidazolyl, quinolyl, isoquinolyl, indazolyl,
benzotriazolyl, tetrazolopyridazinyl (e. g.,
tetrazolo[1,5-b]pyridazinyl, etc.), etc.;
unsaturated 3 to 6-membered heteromonocyclic group
containing an oxygen atom, for example, pyranyl,
furyl, etc.; unsaturated 3 to 6-membered
heteromonocyclic group containing a sulfur atom,
for example, thienyl, etc.; unsaturated 3- to 6-
membered heteromonocyclic group containing 1 to 2
oxygen atoms and 1 to 3 nitrogen atoms, for
example, oxazolyl, isoxazolyl, oxadiazolyl (e. g.,
1,2,4-oxadiazolyl, 1,3,4-oxadiazolyl, 1,2,5-
oxadiazolyl, etc.) etc.; unsaturated condensed
heterocyclo group containing 1 to 2 oxygen atoms
and 1 to 3 nitrogen atoms (e. g. benzoxazolyl,
benzoxadiazolyl, etc.); unsaturated 3 to 6-membered
heteromonocycTic group containing 1 to 2 sulfur
atoms and 1 to 3 nitrogen atoms, for example,
thiazolyl, thiadiazolyl (e. g., 1,2,4- thiadiazolyl,
1,3,4-thiadiazolyl, 1,2,5-thiadiazolyl, etc.) etc.;
unsaturated condensed heterocyclo group containing
1 to 2 sulfur atoms and 1 to 3 nitrogen atoms
(e.g., benzothiazolyl, benzothiadiazolyl, etc.) and
the like. The term "heteroaryl"also embraces
radicals where heterocyclo radicals are fused with
aryl radicals. Examples of such fused bicyclic
radicals include benzofuran, benzothiophene, and
the like. Said "heterocyclo group" may have 1 to 3
substituents such as alkyl, hydroxyl, halo, alkoxy,
oxo, amino and alkylamino. The term "alkylthio"
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98/22246
23
embraces radicals containing a linear or branched
alkyl radical, of one to about ten carbon atoms
attached to a divalent sulfur atom. More preferred
alkylthio radicals are "lower alkylthio" radicals
having alkyl radicals of one to six carbon atoms.
Examples of such lower alkylthio radicals are
methylthio, ethylthio, propylthio, butylthio and
hexylthio. The term "alkylthioalkyl" embraces
radicals containing an alkylthio radical attached
through the divalent sulfur atom to an alkyl
radical of one to about ten carbon atoms. More
preferred alkylthioalkyl radicals are "lower
alkylthioalkyl" radicals having alkyl radicals of
one to six carbon atoms. Examples of such lower
alkylthioalkyl radicals include methylthiomethyl.
The term "alkylsulfinyl" embraces radicals
containing a linear or branched alkyl radical, of
one to about ten carbon atoms, attached to a
divalent -S(=O)- radical. More preferred
alkylsulfinyl radicals are "lower alkylsulfinyl"
radicals having alkyl radicals of one to six carbon
atoms. Examples of such lower alkylsulfinyl
radicals include methylsulfinyl, ethylsulfiny~,
butylsulfinyl and hexylsulfinyl. The term
"sulfonyl", whether used alone or linked to other
terms such as "alkylsulfonyl", denotes a divalent
radical, -S02-. "Alkylsulfonyl" embraces alkyl
radicals attached to a sulfonyl radical, where
alkyl is defined as above. More preferred
alkylsulfonyl radicals are "lower alkylsulfonyl"
radicals having one to six carbon atoms. Examples
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98/22246
24
of such lower alkylsulfonyl radicals include
methylsulfonyl, ethylsulfonyl and propylsulfonyl.
The "alkylsulfonyl" radicals may be further
substituted with one or more halo atoms, such as
fluoro, chloro or bromo, to provide
haloalkylsulfonyl radicals. The terms "sulfamyl",
"aminosulfonyl" and "sulfonamidyl" denote NH2O2S-.
The term "acyl" denotes a radical provided by the
residue after removal of hydroxyl from an organic
acid. Examples of such acyl.radicals include
alkanoyl and aroyl radicals. Examples of such
lower alkanoyl radicals include formyl, acetyl,
propionyl, butyryl, isobutyryl, valeryl,
isovaleryl, pivaloyl, hexanoyl, trifluoroacetyl.
The term "carbonyl", whether used alone or with
other terms, such as "alkoxycarbonyl", denotes -
(C=O)-. The term "aroyl" embraces aryl radicals
with a carbonyl radical as defined above. Examples
of aroyl include benzoyl, naphthoyl, and the like
and the aryl in said aroyl may be additionally
substituted. The terms "carboxy" or "carboxyl",
whether used alone or with other terms, such as
"carboxyalkyl", denotes -C02H. The term
"carboxyalkyl" embraces alkyl radicals substituted
with a carboxy radical. More preferred are "lower
carboxyalkyl" which embrace lower alkyl radicals as
defined above, and may be additionally substituted
on the alkyl radical with halo. Examples of such
lower carboxyalkyl radicals include carboxymethyl,
carboxyethyl and carboxypropyl. The term
"alkoxycarbonyl" means a radical containing an
SUBSTITUTE SI~iEET (RULE 26)
CA 02308639 2000-04-25
WO 99!22720 PCT/US9$/22246
alkoxy radical, as defined above, attached via an
oxygen atom to a carbonyl radical. More preferred
are "lower alkoxycarbonyl" radicals with alkyl
porions having one to six carbons. Examples of
5 such lower alkoxycarbonyl (ester) radicals include
substituted or unsubstituted methoxycarbonyl,
ethoxycarbonyl, propoxycarbonyl, butoxycarbonyl and
hexyloxycarbonyl. The terms "alkylcarbonyl",
"arylcarbonyl" and "aralkylcarbonyl" include
10 radicals having alkyl, aryl and aralkyl radicals,
as defined herein, attached to a carbonyl radical.
Examples of such radicals include substituted or
unsubstituted methylcarbonyl, ethylcarbonyl,
phenylcarbonyl and benzylcarbonyl. The term
15 "aralkyl" embraces aryl-substituted alkyl radicals
such as benzyl, diphenylmethyl, triphenylmethyl,
phenylethyl, and diphenylethyl. The aryl in said
aralkyl may be additionally substituted with halo,
alkyl, alkoxy, halkoalkyl and haloalkoxy. The
20 terms benzyl and phenylmethyl are interchangeable.
The term "heterocycloalkyl" embraces saturated and
partially unsaturated heterocyclo-substituted alkyl
radicals, such as pyrrolidinylmethyl, and
heteroaryl-substituted alkyl radicals, such as
25 pyridylmethyl, quinolylmethyl, thienylmethyl,
furylethyl, and quinolylethyl. The heteroaryl in
- said heteroaralkyl may be additionally substituted
with halo, alkyl, alkoxy, halkoalkyl and
haloalkoxy. The term "aralkoxy" embraces aralkyl
radicals attached through an oxygen atom to other
radicals. The term "aralkoxyalkyl" embraces
SUBSTITUTE SHEET (RULE 26~
CA 02308639 2000-04-25
WO 99122720 PCT/US98/22246
26
aralkoxy radicals attached through an oxygen atom
to an alkyl radical. The term "aralkylthio"
embraces aralkyl radicals attached to a sulfur
atom. The term "aralkylthioalkyl" embraces
aralkylthio radicals attached through a sulfur atom
to an alkyl radical. The term "aminoalkyl"
embraces alkyl radicals substituted with amino
radicals. More preferred are "lower aminoalkyl"
radicals. Examples of such radicals include
aminomethyl, aminoethyl, and the like. The term
"alkylamino" denotes amino groups which are
substituted with one or two alkyl radicals.
Preferred are "lower alkylamino" radicals having
alkyl porions having one to six carbon atoms.
I5 Suitable lower alkylamino may be monosubstituted N-
alkylamino or disubstituted N,N-alkylamino, such as
N-methylamino, N-ethylamino, N,N-dimethylamino,
N,N-diethylamino or the like. The term "arylamino"
denotes amino groups which are substituted with one
or two aryl radicals, such as N-phenylamino. The
"arylamino" radicals rnay be further substituted on
the aryl ring portion of the radical. The term
"aralkylamino" embraces amino groups which are
substituted with one or two aralkyl radicals. The
terms "N-arylaminoalkyl" and "N-aryl-N-alkyl-
aminoalkyl" denote aminoalkyl groups which are
substituted with one aryl radical or one aryl and
one alkyl radical, respectively. Examples of such
radicals include N-phenylaminomethyl and N-phenyl-
N-methylaminomethyl. The term "aminocarbonyl"
denotes an amide group of the formula -C(=O)NH2.
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/Z2720 PCTILJS98/ZZ246
27
The term "alkylaminocarbonyl" denotes an
aminocarbonyl group which has been substituted with
one or two alkyl radicals on the amino nitrogen
atom. Preferred are "N-alkylaminocarbonyl" and
"N,N-dialkylaminocarbonyl" radicals. More
preferred are "lower N-alkylaminocarbonyl" and
"lower N,N-dialkylaminocarbonyl" radicals with
lower alkyl portions as defined above. The term
"alkylaminoalkyl" embraces radicals having one or
more alkyl radicals attached to an aminoalkyl
radical. The term "aryloxyalkyl" embraces radicals
having an aryl radicals attached to an alkyl
radical through a divalent oxygen atom. The term
"arylthioalkyl" embraces radicals having an aryl
radicals attached to an alkyl radical through a
divalent sulfur atom.
The compounds utilized in the methods of the
present invention may be present in the form of free
bases or pharmaceutically acceptable acid addition salts
thereof. The term "pharmaceutically-acceptable salts"
embraces salts commonly used to form alkali metal salts
and to form addition salts of free acids or free bases.
The nature of the salt is not critical, provided that it
is pharmaceutically-acceptable. Suitable
pharmaceutically-acceptable acid addition salts of
compounds of Formula I may be prepared from an inorganic
acid or from an organic acid. Examples of such inorganic
acids are hydrochloric, hydrobromic, hydroiodic, nitric,
carbonic, sulfuric and phosphoric acid. Appropriate
organic acids may be selected from aliphatic,
cycloaliphatic, aromatic, araliphatic, heterocyclic,
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98I22Z46
28
carboxylic and sulfonic classes of organic acids,
example of which are formic, acetic, propionic,
succinic, glycolic, gluconic, lactic, malic, tartaric,
citric, ascorbic, glucuronic, malefic, fumaric, pyruvic,
aspartic, glutamic, benzoic, anthranilic, mesylic, 4-
hydroxybenzoic, phenylacetic, mandelic, embonic
(pamoic), methanesulfonic, ethanesulfonic,
benzenesulfonic, pantothenic, 2-hydroxyethanesulfonic,
toluenesulfonic, sulfanilic, cyclohexylaminosulfonic,
stearic, algenic, (i-hydroxybutyric, salicylic,
galactaric and galacturonic acid. Suitable
pharmaceutically-acceptable base addition salts of
compounds of Formula I include metallic salts made from
aluminum, calcium, lithium, magnesium, potassium, sodium
and zinc or organic salts made from N,N'-
dibenzylethylenediamine, chloroprocaine, choline,
diethanolamine, ethylenediamine, meglumine (N-
methylglucamine) and procaine. All of these salts may
be prepared by conventional means from the corresponding
compound of Formula I by reacting, for example, the
appropriate acid or base with the compound of Formula I.
GENERAL SYNTHETIC PROCEDURES
The cyclooxygenase-2 inhibitor compounds of
the invention can be synthesized according to the
following procedures of Schemes I-X, wherein the
- Rl-R3 substituents are as defined for Formula I,
above, except where further noted.
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
wo 99n2~zo Pc~rn~s9snzza6
2 9 _.
Scheme I
R1
O Base
II ~ 3
R3-CCH3 RlcozcH3 R O O
1 2
EtOH, D S ~z
R2
O R2
!I _
R2~S~i O~~S ~-
O
R3
N ~ + N.~R3
Nw
Ri
R1
4
3
Synthetic Scheme I shows the preparation of
cyclooxygenase-2 inhibitor compounds, as
described in U.S. patent No. 5,521,207 and
WQ95/15316, which are incorporated by reference,
embraced by Formula I . In step 1, ketone 1 is
treated with a base, preferably NaOMe or NaH, and
an ester, or ester equivalent, to form the
intermediate diketone 2 (in the enol form) which
is used without further purification. In step 2,
diketone 2 in an anhydrous protic solvent, such
as absolute ethanol or acetic acid, is treated
with the hydrochloride salt or the free base of a
substituted hydrazine at reflux to afford a
mixture of pyrazoles 3 and 4. Recrystallization
or chromatography affords 3 usually as a solid.
SUBSTfTUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCTIUS98/22246
Similar pyrazoles can be prepared by methods
described in U.S. Pat. Nos. 4,196,721, 5,051,518,
5,134,142 and 4,919,121 which also are
incorporated by reference.
5
Scheme II
z 1) Base O R
z
R1 ~ I SR 2I R'CO-X ~ Ri ~ / SR
O O
5 6
Ra~z
SO R2 SRz
z
Rl ~ ~ Oxidize R
\\
R3 .N R3 ~ N.N
1J R' a
Ra
8 7
10 Scheme II shows the four step procedure for
forming cyclooxygenase-2 inhibitor pyrazoles 8 as
described in U.S. patent No. 5,480,534 (where Ra
is hydrido or alkyl) from ketones 5. In step 1,
ketone 5 is reacted with a base, such as lithium
15 bis(trimethylsilyl)amide or lithium
diisopropylamide (LDA) to form the anion. In
step 2, the anion is reacted with an acetylating
reagent to provide diketone 6. In step 3, the
reaction of diketone 6 with hydrazine or a
20 substituted hydrazine, gives pyrazole 7. In step
4, the pyrazole 7 is oxidized with an oxidizing
reagent, such as Oxone~ (potassium
peroxymonosulfate), 3-chloroperbenzoic acid
(MCPBA) or hydrogen peroxide, to give a mixture
25 of the desired 3-(alkylsulfonyl)phenyl-pyrazole 8
and the 5-(alkylsulfonyl)phenyl-pyrazole isomer.
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99!22720 PCT/US98/22246
31
The desired pyrazole 8, usually a white or pale
yellow solid, is obtained in pure form either by
chromatography or recrystallization.
Alternatively, diketone 6 can be formed from
ketone 5 by treatment with a base, such as sodium
hydride, in a solvent, such as dimethylformamide,
and further reacting with a nitrile to form an
aminoketone. Treatment of the aminoketone with
acid forms the diketone 6. Similar pyrazoles can
IO be prepared by methods described in U.S. Pat. No.
3,984,431 which is incorporated by reference.
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99122720 PCT/US98/22246
32 w
Scheme III
o / so2Rz
Rb0 ORb
T~ t R1
0 o Base
o "'
g 10 0 0
11
Cu, O
N
aq. NaOH,
SOZRZ 0
R1
ORb
T
O
12
HO
v O
13
/ ~ Cu, D
N
2
SOzR~
R1
R3
T
14
5 Cyclooxygenase-2 inhibitor diaryl/heteroaryl
- thiophenes (where T is S, and Rb is alkyl) can be
prepared by the methods described in U.S. Patent
Nos. 4,427,693, 4,302,461, 4,381,311, 4,590,205,
and 4,820,827, and PCT documents WO 95/00501 and
10 W094/15932, which are incorporated by reference.
Similar pyrroles (where T is N), furanones and
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98/Z2246
33
furans (where T is O) can be prepared by methods
described in PCT documents WO 95/00501 and
W094/15932, and in EP799,823.
Scheme IV
RI NaH ~, I \ ~ R
R2S ~ / ~O TBSCl 2 ~ OTBS
R S
17
16
MCPBA
OIi TBSO
Ri \ R1
\ H+ I
R20 S ~ ~ O H20 R20zS / O
19
18
R3COC1
Base
0
R3 Ri
O N
NH~OAc
\ R1 HOAc ~ R3
0
RzO2S ~ O
R20zS
20 21
Cyclooxygenase-2 inhibitor diaryl/heteroaryl
oxazoles can be prepared by the methods described
in LJ.S. Patent Nos. 3,743,656, 3,644,499 and
3,647,858, and PCT documents WO 95/00501 and
_ W094/27980, which are incorporated by reference.
Equivalent oxazole compounds can be prepared via
W096/19463 and W096/19462.
S theme V
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99!22720 PCT/US98IZ2246
34
NOH ~ -O Ra
Rl 1) 2 eq. n-BuLi R1 OOH
2) (R3C0)20
\ \
22 23
i) clso3H
a) NH4oH
N-O
R
Ri
SOZNHz
24
Cyclooxygenase-2 inhibitor diaryl/heteroaryl
isoxazoles can be prepared by the methods
described in United States No. 5,633,272, PCT
documents W092/05162, and W092/19604, and
European Publication EP 26928 which are
incorporated by reference. Sulfonamides 24 can
be formed from the hydrated isoxazole 23 in a two
step procedure. First, hydrated isoxazole 23 is
treated at about 0 °C with two or three
equivalents of chlorosulfonic acid to form the
corresponding sulfonyl chloride. In step two,
the sulfonyl chloride thus formed is treated with
concentrated ammonia to provide the sulfonamide
derivative 24.
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98I22246
Scheme VI
Alkylaluminum
R1CN + S ~ ~ ~ Reagent
z y
R2 Solvent R
25 26
27 SOzR2
Rb
R3
Alkylationf
p base
R3
R3
N OH
N Rb ~~ b
Dehydration 1 N R
R
R N
/
SOZRz
SOZRz
29 28
Scheme VI shows the three step preparation
5 of the cyciooxygenase-2 inhibitor imidazoles 29
of the present invention. In step 1, the reaction
of substituted nitriles {R1CN) 25 with primary
phenyiamines 26 in the presence of alkylaluminum
reagents such as trimethylaluminum,
10 triethylaluminum, dimethylaluminum chloride,
diethylaluminum chloride in the presence of inert
solvents such as toluene, benzene, and xylene,
gives amidines 27. In step 2, the reaction of
amidine 27 with 2-haloketones (where X is Br or
15 C1) in the presence of bases, such as sodium
bicarbonate, potassium carbonate, sodium -
carbonate, potassium bicarbonate or hindered
SU9STtTUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/Z2720 PCT/US98I22246
36
tertiary amines such as N,N'-
diisopropylethylamine, gives the 4,5-
dihydroimidazoles 28 (where Rb is alkyl). Some of
the suitable solvents for this reaction are
isopropanol, acetone and dimethylformamide. The
reaction may be carried out at temperatures of
about 20°C to about 90°C. In step 3, the 4,5-
dihydroimidazoles 28 may be dehydrated in the
presence of an acid catalyst such as 4-
toluenesulfonic acid or mineral acids to form the
1,2-disubstituted imidazoles 29 of the invention.
Suitable solvents for this dehydration step are
e.g., toluene, xylene and benzene.
Trifluoroacetic acid can be used as solvent and
catalyst for this dehydration step.
In some cases (e.g., where R3 = methyl or
phenyl) the intermediate 28 may not be readily
isolable. The reaction, under the conditions
described above, proceeds to give the targeted
imidazoles directly.
Similarly, imidazoles can be prepared having
the sulfonylphenyl moiety attached at position 2
and R1 attached at the nitrogen atom at position
1. Diaryl/heteroaryl imidazoles can be prepared
by the methods described in U.S. Patent Nos.
4,822,805, U.S. application Serial No. 08/282,395
and PCT document WO 93/14082, which are
incorporated by reference.
Scheme VII
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/Z2720 PCT/US98I22Z46
37 -
0 oTl~s
R1J~H TMSCN ~/~cN
R H
catalyst
30 31 1) Base
0
2 ) H ~ ~ SRz
1 ) Base
32
x / ~ sRz
O ,.- / SRz
O
Rl \
OH
33
Oxidizing
agent
SRz
O
R1
O
34
NH~OAc, HOAc
R CHO
SR2 SOzR2
/ ~ /
Ra'-~ ~ R3--~
N 1 OXldatlOri \N z
R ~ R
H H
3S 36
The subject imidazole cyclooxygenase-2
inhibitor compounds 36 of this invention may be
synthesized according to the sequence outlined in
Scheme VII. Aldehyde 30 may be converted to the
protected cyanohydrin 31 by reaction with a
trialkylsilyl cyanide, such as trimethylsilyl
cyanide (TMSCN) in the presence of a catalyst
such as zinc iodide (ZnI2) or potassium cyanide
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98/22246
38
(KCN). Reaction of cyanohydrin 31 with a strong
base followed by treatment with benzaldehyde 32
(where R2 is alkyl) and using both acid and base
treatments, in that order, on workup gives
benzoin 33. Examples of strong bases suitable
for this reaction are lithium diisopropylamide
(LDA) and lithium hexamethyldisilazane. Benzoin
33 may be converted to benzil 34 by reaction with
a suitable oxidizing agent, such as bismuth oxide
or manganese dioxide, or by a Swern oxidation
using dimethyl sulfoxide (DMSO) and
trifluoroacetic anhydride. Benzil 34 may be
obtained directly by reaction of the anion of
cyanohydrin 31 with a substituted benzoic acid
halide. Any of compounds 33 and 34 may be used
as intermediates for conversion to imidazoles 35
(where R2 is alkyl) according to chemical
procedures known by those skilled in the art and
described by M. R. Grimmett, "Advances in
Imidazole Chemistry" in Advances in Heterocyclic
Chemistry, 12, 104 (1970). The conversion of 34
to imidazoles 35 is carried out by reaction with
ammonium acetate~and an appropriate aldehyde
(R3CH0) in acetic acid. Benzoin 36 may be
converted to imidazoles 38 by reaction with
formamide. In addition, benzoin 36 may be
converted to imidazoles by first acylating with
an appropriate acyl group (R3C0-) and then
treating with ammonium hydroxide. Those skilled
in the art will recognize that the oxidation of
the sulfide (where R2 is methyl) to the sulfone
may be carried out at any point along the way
beginning with compounds 35, and including
oxidation of imidazoles 38, using, for examples,
reagents such as hydrogen peroxide in acetic
acid, m-chloroperoxybenzoic acid (MCPBA) and
potassium peroxymonosulfate (OXONE~).
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98/22246
39
Diaryl/heteroaryl imidazoles can be
prepared by the methods described in U.S. Patent
Nos. 3,707,475, 4,686,231, 4,503,065, 9,472,422,
4,372,969, 4,576,958, 3,901,908, U.S.
application Serial No. 08/281,903 European
publication EP 372,445, and PCT document WO
95/00501, which are incorporated by reference.
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 pCf/US98/22246
Scheme VIII
R2S0z ~ ~ Br 1.n-BuLi. THF, -78°C RZgp2 \ / ZriCl
2. ZnCl2
37 38
Br
R
Pd°
R
Br
39
SOZRZ SOzR2
ClZn i.n-BuLi, THF, -78°C Br
E
2. ZnClz
R3 R3 R3 R3
41 40
Pd° RlBr
So2R2
Ri
R3 R3
42
Diaryl/heteroary'1 cyclopentene
5 cyclooxygenase-2 inhibitors can be prepared by
the methods described in U.S. Patent No.
5,344,991, and PCT document WO 95/00501, which
are incorporated by reference.
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98I22246
41
Scheme IX
2
SOZRZ
Pd~, PhCH3.
I
Br Na2C03, D
+ R1~B (OH) 2
\ / R' R'
R3 R3
44
43
Similarly, Synthetic Scheme IX shows the
procedure for the preparation of 1,2-
diarylbenzene cyclooxygenase-2 inhibitor agents
44 from 2-bromo-biphenyl intermediates 43
(prepared similar to that described in Synthetic
Scheme VIII) and the appropriate substituted
IO phenylboronic acids. Using a coupling procedure
similar to the one developed by Suzuki et al.
[Synth. Commun., 11, 513 (1981)], intermediates
43 are reacted with the boronic acids in
toluene/ethanol at reflux in the presence of a
Pd° catalyst, e.g.,
tetrakis(triphenylphosphine)palladium(0), and 2M
sodium carbonate to give the corresponding 1,2-
diarylbenzene antiinflammatory agents 44 of this
invention. Such terphenyl compounds can be
prepared by the methods described in PCT patent
document W096/16934, which is incorporated by
reference.
SUBSTITUTE SHEET (RULE 26~
CA 02308639 2000-04-25
WO 99/22720 PCT/US98/22246
42
Scheme X
Rz~ o
p~Yt
RZ.S
H N~R3 CH~CN, Et0 ~
z
~ y~ ~~R3
R N
43 46 47
Diaryl/heteroaryl thiazole cyclooxygenase-2
inhibitors can be prepared by the methods
described in U.S. Patent No. 9,051,250,
4,632,930, European Application EP 592,664, and
PCT documents W096/03392 and WO 95/00501, which
are incorporated by reference. Isothiazoles can
be prepared as described in PCT document WO
95/00501.
Diaryl/heteroaryl pyridine cyclooxygenase-2
inhibitors can be prepared by the methods described
in U.S. Patent Nos. 5,169,857, 4,011,328,
4,533,666, and W096/24584 and W096/24585, which are
incorporated by reference.
Biological Evaluation
The efficacy of cyclooxygenase-2 inhibitors in
treatment of preterm labor is established in the
following models:
A human fetal membrane model is performed with
materials, reagents and procedures essentially as
described by Slater et al. [Am. J. Obstet. Gynecol.,
172, 77-82 (1995)]. A COX-2 inhibitor should be active
at a dose of 20 mg/kg.
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCT/US98I22246
43 -
The efficacy of cyclooxygenase-2 inhibitors in
preventing closure of the ductus arteriosus is
established in the following model:
S A sheep model is performed with materials, reagents
and procedures essentially as described by Velvis et al.
[Pediatr. Res., 30, 62-8 (1991)]. A COX-2 inhibitor
should be active at a dose of 20 mg/kg.
Materials and Methods
The active compounds of the present invention may
be administered by any suitable route known to those
skilled in the art, preferably in the form of a
pharmaceutical composition adapted to such a route, and
in a dose effective for the treatment intended. The
active compounds and composition may, for example, be
administered orally, intravascularly, intraperitoneally,
intranasal, intrabronchial, subcutaneously, intra-
muscularly or topically (including aerosol).
The administration of the present invention may be
for either prevention or treatment purposes. The methods
and compositions used herein may be used alone or in
conjunction with additional therapies known to those
skilled in the art in the prevention or treatment of
preterm labor. Alternatively, the methods and
compositions described herein may be used as adjunct
therapy. By way of example, the cyclooxygenase-2
inhibitor may be administered alone or in conjunction
with other agents that are useful for treating or
preventing preterm labor.
The phrase "adjunct therapy" (or "combination
therapy"), in defining use of a cyclooxygenase-2
inhibitor agent and another pharmaceutical agent, is -
intended to embrace administration of each agent in a
SUBSTITUTE SHEET (RULE 26~
CA 02308639 2000-04-25
WO 99/22720 PCT/US98/Z2246
49
sequential manner in a regimen that will provide
beneficial effects of the drug combination, and is
intended as well to embrace co-administration of these
agents in a substantially simultaneous manner, such as
in a single formulation having a fixed ratio of these
active agents, or in multiple, separate formulations for
each agent. The present invention also comprises a
pharmaceutical composition for the adjunct prevention
and treatment of preterm labor, comprising a
therapeutically-effective amount of a compound of
Formula I in association with at least one
pharmaceutically-acceptable carrier, adjuvant or diluent
(collectively referred to herein as "carrier" materials)
and, other agents or other growth inhibiting agents or
other drugs or nutrients.
For oral administration, the pharmaceutical
composition may be in the form of, for example, a
tablet, capsule, suspension or liquid. The pharma-
ceutical composition is preferably made in the form of a
dosage unit containing a particular amount of the active
ingredient. Examples of such dosage units are capsules,
tablets, powders, granules or a suspension, with
conventional additives such as lactose, mannitol, corn
starch or potato starch; with binders such as
crystalline cellulose, cellulose derivatives, acacia,
carp starch or gelatins; with disintegrators such as
corn starch, potato starch or sodium carboxymethyl-
cellulose; and with lubricants such as talc or magnesium
stearate. The active ingredient may also be
administered by injection as a composition~wherein, for
- example, saline, dextrose or water may be used as a
suitable carrier.
For intravenous, intramuscular, subcutaneous, or
intraperitoneal administration, the compound may be -
combined with a sterile aqueous solution which is
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99/22720 PCTIUS98122246
preferably isotonic with the blood of the recipient.
Such formulations may be prepared by dissolving solid
active ingredient in water containing physiologically
compatible substances such as sodium chloride, glycine,
5 and the like, and having a buffered pH compatible with
physiological conditions to produce an aqueous solution,
and rendering said solution sterile. The formulations
may be present in unit or mul i-dose containers such as
sealed ampoules or vials.
Formulations suitable for parenteral administration
conveniently comprise a sterile aqueous preparation of
the active compound which is preferably made isotonic.
Preparations for injections may also be formulated by
suspending or emulsifying the compounds in non-aqueous
solvent, such as vegetable oil, synthetic aliphatic acid
glycerides, esters of higher aliphatic acids or
propylene glycol.
Formulations for topical use include known gels,
creams, oils, and the like. For aerosol delivery, the
compounds may be formulated with known aerosol
exipients, such as saline, and administered using
commercially available nebulizers. Formulation in a
fatty acid source may be used to enhance
biocompatibility.
For rectal administration, the active ingredient
may be formulated into suppositories using bases which
are solid at room temperature and melt or dissolve at
body temperature. Commonly used bases include cocoa
butter, glycerinated gelatin, hydrogenated vegetable
oil, polyethylene glycols of various molecular weights,
and fatty esters of polyethylene stearate.
The dosage form and amount can be readily -
established by reference to known preterm labor
SUBSTITUTE SHEET (RULE 26)
CA 02308639 2000-04-25
WO 99122720 PCT/US98/Z2246
46
treatment or prophylactic regiments. The amount of
therapeutically active compound that is administered and
the dosage regimen for treating a disease condition with
the compounds and/or compositions of this invention
depends on a variety of factors, including the age,
weight, sex and medical condition of the subject, the
severity of the disease, the route and frequency of
administration, and the particular compound employed, as
well as the pharmacokinetic properties of the individual
treated, and thus may vary widely. The dosage will
generally be lower if the compounds are administered
locally rather than systemically, and for prevention
rather than for treatment. Such treatments may be
administered as often as necessary and for the period of
time judged necessary by the treating physician. One of
skill in the art will appreciate that the dosage regime
or therapeutically effective amount of the inhibitor to
be administrated may need to be optimized for each
individual. The pharmaceutical compositions may contain
active ingredient in the range of about 0.1 to 2000 mg,
preferably in the range of about 0.5 to 500 mg and most
preferably between about 1 and 200 mg. A daily dose of
about 0.01 to 100 mg/kg bady weight, preferably between
about 0.1 and about 50 mg/kg body weight and most
preferably from about 1 to 20 mg/kg body weight, may be
appropriate. The daily dose can be administered in one
to four doses per day.
All documents referenced herein are incorporated by
reference.
Although this invention has been described with
respect to specific embodiments, the details of
these embodiments are not to be construed as
limitations.
SUBSTITUTE SHEET (RULE 26)