Note: Descriptions are shown in the official language in which they were submitted.
CA 02309684 2000-OS-10
SPECIFICATION
NON-AQUEOUS ELECTROLYTIC SOLUTION AND NON-AQUEOUS
ELECTROLYTIC SOLUTION SECONDARY BATTERY
TECHNICAL FIELD
The invention relates to a non=aqueous electrolytic solution that contains
an ether compound containing fluorine, more specifically a non-aqueous
electrolytic solution that provides a non-aqueous electrolytic solution
secondary battery exhibiting high safety and excellent charging/discharging
properties.
Furthermore, the invention relates to a non-aqueous electrolytic solution
secondary battery containing such non-aqueous electrolytic solution.
CONVENTIONAL TECHNOLOGY
In recent years, research has been done actively in secondary batteries
making use of the oxidation reduction reaction of alkali metal or the doping
and dedoping reaction of alkali metal ions as high-energy-density secondary
batteries. Especially a battery which uses a carbon material capable of
doping and dedoping lithium ions for the negative electrode and a complex
oxide of lithium and metal for the positive electrode are called the lithium
ion
battery Because of its small size, lightness and high energy density, the
lithium ion battery is finding a sharply expanding application areas. In the
meantime, while there have been new portable electronic devices coming into
being one after another, such as VTR with a built-in camera, cellular phones
and laptop computers, there has been a growing demand for improvements to
be made in the performance of the lithium ion battery, including higher
CA 02309684 2000-OS-10
2
energy density and higher output so that a further improvement in the
functions of those portable electronic devices is made.
Those secondary batteries utilizing the reaction of alkali metal ions
whose representative example is the lithium ion battery show a great
potential difference between positive and negative electrodes and cannot use
any electrolytic solution using water as the solvent. Because of this, a so-
called non-aqueous electrolytic solution with alkali meal salt dissolved in a
non-aqueous solvent is used for those secondary batteries.
As such non-aqueous solvent, polar organic solvents that dissolve alkali
metal salt easily and are stable electrochemically are used. Representative
examples of such polar organic solvents include carbonic acid esters such as
ethylene carbonate, propylene carbonate, dimethyl carbonate, methylethyl
carbonate and diethyl carbonate, esters such as y -butyrolactone, methyl
formate, methyl acetate and methyl propionate, and ethers such as
dimethoxyethane, tetrahydrofurane and dioxolane. Moreover, examples of
alkali metal salt which is dissolved include lithium salts such as LiPFs,
LiBF4, LiN(CFsSOs)$, LiC104 and LiCFsSOs.
It is desirable that such non-aqueous electrolytic solution should have
high ion conductivity and low viscosity so that the discharging performance
of the battery using the non-aqueous electrolytic solution will be improved.
It is also desirable that such non-aqueous electrolytic solution should be
stable chemically and electrochemically against the positive and negative
electrodes so that the performance of the battery using the non-aqueous
electrolytic solution will not decline when the charging and discharging of
the battery is repeated.
Furthermore, the non-aqueous electrolytic solution is required not to
burn readily so that the safety of the battery is secured. Zb this end, the
CA 02309684 2000-OS-10
3
addition of phosphoric ester to the non-aqueous electrolytic solution
(Japanese Laid-open Patent Application HEI 8-22839) and the use of a
halogen compound (Japanese Laid-open Patent Application SHO 63-248072)
have been proposed. The use of a chemically-stable fluorine-containing
alkoxyethanes as the electrolytic solution solvent of the lithium battery has
also be proposed (Japanese laid-open Patent Application HEI 1-117838).
Further, the lithium battery is~generally equipped with a protective
circuit so that there will be no abnormal release of heat due to the
malfunctioning of the battery such as an external short circuit and
overcharging. However, if any abnormal release of heat can be prevented
by controlling the chemical reaction itself between electrolytic solution and
electrodes, the safety of the battery will be improved. Consequently,
reducing the reactivity between electrolytic solution and electrodes provides
an effective means for improving the safety of the battery
In view of the circumstances as described above, the inventors made a
serious study to resolve the above issue of improving the safety of the
battery
and completed the invention after finding that there is a correlation between
the reactivity of electrolytic solution and electrodes and the heat release
rate
of the reaction, and that it is possible to reduce the heat release rate by
use of
a non-aqueous solvent containing a particular fluorine-containing ether
compound.
The invention is intended to provide a non-aqueous electrolytic solution
showing excellent safety and battery properties and a non-aqueous
electrolytic solution secondary battery containing this non-aqueous
electrolytic solution.
The invention provides a non-aqueous electrolytic solution containing a
fluorine-containing ether compound and a non-aqueous electrolytic solution
CA 02309684 2000-OS-10
4
secondary battery containing this non-aqueous electrolytic solution.
DISCLOSURE OF THE INVENTION
The non-aqueous electrolytic solution of the invention comprises a non-
aqueous solvent containing a fluorine-containing ether compound
represented by the following general formula (1) or (2), and an electrolyte:
RO-(AO)~-CHz-X ' (1)
X-CHz-(AO)"-CHz-X (2)
(In formulas (1) and (2), R represents a hydrocarbon group having 1 to 20
carbon atoms; X represents a fluorine atom-substituted hydrocarbon group
having 1 to 10 carbon atoms; and A represents an alkylene group having 2 to
4 carbon atoms. Furthermore, n is a whole number of 1 to 30.)
Furthermore, it is desirable that the aforesaid non-aqueous electrolytic
solution should contain cyclic carbonic acid ester and/or chain carbonic acid
ester, and it is also desirable that the aforesaid cyclic carbonic acid ester
should be a cyclic carbonic acid ester compound containing an alkylene group
having 2 to 5 carbon atoms and the aforesaid chain carbonic acid ester
should be a chain carbonate compound containing a hydrocarbon group
having 1 to 5 carbon atoms.
Moreover, it is desirable that the electrolyte should be at least one
selected from the group of LiPFs, LiBF4, LiOSOzRI, LiN(SOaRz)(S02R3),
LiC(SOZR4)(SO2R6)(SOaRs) and LiN(SOzOCH2R~)(SOzOCH2R8) (wherein Rl to
R8, which may be equal or different, are perfluoroalkyl groups having 1 to 6
carbon atoms).
The non-aqueous electrolytic solution secondary battery of the invention
comprises the aforesaid non-aqueous electrolytic solution, a negative
electrode which contains any of metal lithium, lithium-containing alloy,
CA 02309684 2000-OS-10
carbon material capable of doping or dedoping lithium ions, tin oxide capable
of doping or dedoping lithium ions, silicon capable of doping or dedoping
lithium ions and titanium oxide capable of doping or dedoping lithium ions
as the active negative electrode material, and a positive electrode which
5 contains a complex oxide of lithium and transition metal as the active
positive electrode material.
BRIEF DESCRIPTION OF THE DRAWINGS
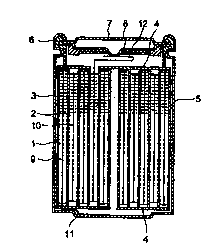
Fig. 1 is a schematic cross-sectional view showing the non-aqueous
electrolytic solution secondary battery representing of the present invention.
SPECIFIC EMBODIMENTS OF THE INVENTION
Given below is a specific explanation of the non-aqueous electrolytic
solution and non-aqueous electrolytic solution secondary battery of the
invention.
[Non-aqueous Electrolytic Solution]
The non-aqueous electrolytic solution of the invention comprises a non-
aqueous solvent containing a fluorine-containing ether compound as the
indispensable component, and an electrolyte.
First, the components of the non-aqueous electrolytic solution are
explained below
[Fluorine-containing Ether Compound]
In the invention, a compound represented by the following general
formula (1) or (2):
RO-(AO)n-CHz-X (1)
X-CHz-(AO)~-CHz-X (2)
(In formulas (1) and (2), R represents a hydrocarbon group having 1 to 20
CA 02309684 2000-OS-10
6
carbon atoms; X represents a fluorine atom-substituted hydrocarbon group
having 1 to 10 carbon atoms; and A represents an alkylene group having 2 to
4 carbon atoms. Furthermore, n is a whole number of 1 to 30.)
As the hydrocarbon group having 1 to 20 carbon atoms, an alkyl group
having 1 to 20 carbon atoms and an aryl group having 6 to 20 carbon atoms
can be cited. Specifically methyl group(-CHs), ethyl group(-C2Hs), n-propyl
group(-CHzCHzCHs), isopropyl group(-CH(CHs)z), butyl group(-CaH9), phenyl
group, p-t-butyl phenyl group, p-octyl phenyl group, p-nonyl phenyl group
can be cited. Among them preferred are methyl group(-CHs), ethyl group(-
CzHs), n-propyl group(-CHzCH2CHs), isopropyl group(-CH(CHs)z), p-octyl
phenyl group and p-nonyl phenyl group
The fluorine-substituted hydrocarbon group having 1 to 10 carbon atoms
is the group in which at least one hydrogen atom of a hydrocarbon group is
substituted by fluorine atom. There can be cited as specific examples
monofluoromethyl group(-CH2F), difluoromethyl group(-CHFz),
trifluoromethyl group(-CFs), 2-fluoroethyl group(-CH2CHzF), 2,2-
difluoroethyl group(-CHsCHFz), 2,2,2-trifluoroethyl group(-CHaCFs),
1,1,1,2,2-pentaffuoroethyl group(-CFzCFs), 3-fluoropropyl group(-
CHaCHzCHzF), 3,3,-difluoropropyl group(-CHzCH2CHFz), 3,3,3-
trifluoropropyl group(-CH2CHz~Fs), 2,2,3,3,3=pentaffuoropropyl group(-
CHZCFzCFs), -(CFz)z-CFs, -CHz(CFz)z-CFs, -CHz(CFz)sCFs, -(CHz)CFsCFs, -
(CHz)z(CFz)sCFs, and -CHz(CFz)7CFs. Among them, preferred are
monofluoromethyl group(-CHZF), difluoromethyl group(-CHFz),
trifluoromethyl group(-CFs), 2-fluoroethyl group(-CH2CHzF) and 1,1,1,2,2-
pentafluoroethyl group(-CFzCFs).
As specific examples of the alkylene group, there can be cited -CH2CHz-, -
CHaCH(CHs)-, -CH2CH(CH2CHz)-, -CH2CHzCHz-, -CHzCHzCH$CHz- and
CA 02309684 2000-OS-10
CH2CH(CHzOCHs)-. Preferable examples are -CHaCHz- and -CHzCH(CHs)-.
In the formula, n is a whole number of 1 to 30, preferably 1 to 10, more
preferably 1 to 3.
As the fluorine-containing ether compound represented by the formula [lJ
and [2], there can be cited the following compounds.
CHsO-CHzCH20-CH2CHzF,
CHsCHzO-CHZCHzO-CHzCHzF,
CHsO-CHaCH20-CHzCFs,
CHsCHzO-CHzCHaO-CHaCFs,
CHsO-CH$CH(CHs)O-CHzCHaF,
CHsCH20-CHzCH(CHs)O-CHaCHzF,
CHsO-CHzCH(CHs)O-CH2CFs,
CHsO-CH2CH(CHs)O-CHaCFs,
CHsO-(CHzCHaO)z-CH2CH2F,
CHsCHzO-(CHaCH20)z-CH2CHzF,
CHsO-(CH2CH20)z-CH2CFs,
CHsCH20-(CHaCH20)z-CHzCFs,
FCH2CHa0-CHzCHz-O-OCHzCHzF,
CFsCH20-CHzCHz-O-CHzCFs,
FCH2CHz0-CHaCH(CHs)-OCHzCHzF,
CFsCHzO-CHaCH(CHs)-O-CHzCFs,
CFaCH20-(CHzCHaO)z-CHzCFs,
FCH2CHz0-(CH2CHz0)z-CH2CH2F,
CFsCHzO-(CH2CHz0)z-CH2CFs,
FCH2CHz0-(CH$CH(CHs)O)z-CHzCH2F,
FCHzCHzO-(CH2CH(CHs)O)z-CHzCFs,
C9H~s-CsH4-O-CHaCHzO-CH2CHzF (-CsHa- stands for 1,4-phenylene group.),
CA 02309684 2000-OS-10
8
CsH~s-CsH4-O-CHaCHaO-CH2CFs (-CsH4- stands for 1,4-phenylene group.),
CaHls-CsH4-O-CHzCH(CHs)O-CHaCH2F (-CsH4- stands for 1,4-phenylene
group .),
CsH~s-CsH4-O-CHaCH(CHs)O-CHzCFs (-CsH4- stands for 1,4-phenylene
group.),
CsH~s-CsH4-O-(CHzCHzO)z-CHzCH2F (-CsH4- stands for 1,4-phenylene
group.), and
CsHi9-CsHa-O-(CH2CHa0)z-CHZCFs (-CsH4- stands for 1,4-phenylene group.).
Out of these, preferable fluorine-containing ether compounds are
CHs-CH2CHa0-CHaCHaF,
CHsO-CHzCH20-CHaCFs,
CHsO-CHaCH(CHs)O-CHaCHaF,
CHsO-CH2CH(CHs)O-CHZCF3,
FCHzCHzO-CHzCHz-O-OCHzCHsF,
FCH2CHz0-CHzCH(CHs)-OCHaCHzF,
CFsCHaO-(CHzCHzO)z-CH2CFs,
CFsCH20-CHaCH(CHs)-O-CHzCFs,
CsH~s-CsHa-O-CHzCH20-CHaCFs (-CsH4- stands for 1,4-phenylene group.)
and
CsH~s-CsH4-O-CHzCH(CH3)O-CHzCFs (-CsH4- stands for 1,4-phenylene
group.).
The number of the fluorine atoms in the fluorine-containing ether
compound is required to be at least not less than one, preferably 1 to 10,
more
preferably 1 to 6. The number of fluorine atoms being in a range of 1 to 10
offers the advantage of the fluorine-containing ether compound showing
satisfactory compatibility with non-aqueous solvents such as cyclic carbonic
acid ester and chain carbonic acid ester as described below.
CA 02309684 2000-OS-10
9
Such fluorine-containing ether compound has the properties of being safe
physically, being not readily decomposed thermally and being nonflammable
and resistant to electrochemical oxidation and reduction.
[Non-aqueous Solvents]
The aforesaid fluorine-containing ether compound may be used single as
the non-aqueous solvent for the non-aqueous electrolytic solution. However,
it may also be used as a mixed solvent with another non-aqueous solvent
such as carbonic acid ester. In this case, it is desirable for the sake of
improving the safety of the battery that the aforesaid fluorine-containing
ether compound should be contained in the amount of 0.1 to 100 wt%,
preferably 1 to 90 wt%, more preferably 20 to 70 wt%, in the non-aqueous
solvent.
In the non-aqueous electrolytic solution of the invention, it is desirable for
the sake of improving its ion conductivity that especially a mixed solvent
containing the aforesaid fluorine-containing ether compound and cyclic
carbonic acid ester and/or chain carbonic acid ester should be used.
[Cyclic Carbonic Acid Ester]
As an example of the cyclic carbonic acid ester used in the invention, the
carbonates represented by the following general formula (3) can be cited:
O [3J
p ~ R1o
In the above formula, R9 and R1~, which may be equal or different,
represent hydrogen atom, a straight-chain, branched or cyclic alkyl group, or
a halogen-substituted alkyl group having the aforesaid alkyl group any part
CA 02309684 2000-OS-10
or whole of the hydrogen of which has been substituted with at least one of
chlorine and bromine.
As such straight-chain alkyl group, straight-chain alkyl groups having 1
to 4 carbon atoms such as the methyl group, ethyl group, propyl group and
5 butyl group are preferable.
As such branched alkyl group, branched alkyl groups having 3 to 10
carbon atoms such as the isopropyl group, isobutyl group, sec-butyl group
and tert-butyl group are preferable.
As such cyclic alkyl group, cyclic alkyl groups having 5 to 10 carbon atoms
10 such as the cyclopentyl group, cyclohexyl group and 1-methyl-cyclohexyl
group are preferable.
Furthermore, as such cyclic carbonic acid ester, the 6-membered cyclic
compound as well as the 5-membered cyclic compound represented by the
aforesaid formula (3) may be used.
As such cyclic carbonic acid ester represented by the above formula [3],
specifically there can be cited ethylene carbonate, propylene carbonate, 1,2-
butylene carbonate, 2,3-butylene carbonate, 1,3-butylene carbonate, 2,4-
pentylene carbonate, 1,3-pentylene carbonate and vinylene carbonate.
Moreover, a halongen-substituted cyclic carbonic acid ester having a
methyl group, such as the aforesaid propylene carbonic acid ester, any part
or whole of the hydrogen of which has been substituted with at least any of
fluorine, chlorine and bromine, may be used as such cyclic carbonic acid
ester.
In the invention, cyclic carbonic acid esters containing an alkylene group
having 2 to 5 carbon atoms, particularly ethylene carbonate and propylene
carbonate, are preferable as the cyclic carbonic acid ester.
These cyclic carbonic acid esters may be used in a combination of two or
CA 02309684 2000-OS-10
11
more.
[Chain Carbonic Acid Ester]
As an example of the chain carbonic acid ester, the carbonic acid esters
represented by the following formula (4) can be cited:
O
/ R12
O O ,
In the above formula, R11 and R12, which may be equal or different,
represent a straight-chain, branched or cyclic alkyl group, or a halogen-
substituted alkyl group having the aforesaid alkyl group any part or whole of
the hydrogen of which has been substituted with at least any of fluorine,
chlorine and bromine.
As such straight-chain alkyl group, straight-chain alkyl groups having 1
to 4 carbon atoms such as methyl group, ethyl group, propyl group and butyl
group are preferable.
As such branched alkyl group, branched alkyl groups having 3 to 10
carbon atoms such as isopropyl group, isobutyl group, sec-butyl group and
tert-butyl group are preferable.
As such cyclic alkyl group, cyclic alkyl groups having 5 to 10 carbon atoms
such as cyclopentyl group, cyclohexyl group and 1-methyl-cyclohexyl group
are preferable..
As such chain carbonic acid ester; specifically there can be cited dimethyl
carbonate, diethyl carbonate, di-n-propyl carbonate, dibutyl carbonate,
diisopropyl carbonate, methylethyl carbonate, etc.
Out of these chain carbonic acid esters, preferred are the chain carbonic
acid esters having 1 to 15 carbon atoms, especially dimethyl carbonate,
CA 02309684 2000-OS-10
12
methylethyl carbonate, and diethyl carbonate.
[Solvent Composition]
In the case of using the fluorine-containing ether compound or a mixed
solvent of the fluorine-containing ether compound and the aforesaid cyclic
carbonic acid ester and/or the chain carbonic acid ester as the non-aqueous
solvent, it is desirable that the fluorine-containing ether compound should be
contained in an amount of 0.1 to 100 wt%, preferably 1 to 90 wt%, especially
preferably 20 to 70 wt%, in the non-aqueous solvent; and it is also desirable
that the aforesaid cyclic carbonic acid ester and/or chain carbonic acid ester
should be contained in an amount of 0 to 99.9 wt%, preferably 10 to 99 wt%,
especially preferably 30 to 80 wt%, in the non-aqueous solvent.
The non-aqueous electrolytic solution using the non-aqueous solvent of
the composition as described above shows low reactivity between positive
electrode and electrolytic solution, thus improving the safety of the battery
Specifically, the non-aqueous electrolytic solution containing the non-
aqueous solvent of the composition as described above shows a decline of
approximately not more than 1/10 in the maximum heat release rate when
mixed with a positive electrode in a fully charged state, compared with the
non-aqueous electrolytic solution containing no fluorine-containing ether
compound.
Further, the maximum heat release rate represents the maximum heat
release rate in exothermic reaction (the reaction between positive electrode
and non-aqueous electrolytic solution in the invention). When the
maximum heat release rate is measured under the same conditions, the rise
in temperature is gentle and safe if the maximum heat release rate is low.
The maximum heat release rate is measured by use of the accelerating
rate calorimeter (hereinafter referred to as "ARC"). Further, ARC is a
CA 02309684 2000-OS-10
13
technique for assessing the hazardousness of reactive chemical substances
(Thermochimica Acta, 37(1980), 1-30). ARC is a technique in which a
reactive substance is slowly heated, and when any heat of reaction released
from the reactive substance is detected, the ambient temperature is raised to
keep pace with the temperature rise of the reactive substance so that the
reactive substance is put in a pseudo-thermally-insulated state, thereby
faithfully reproducing the self heft-release decomposition. Further, in the
case of using the fluorine-containing ether compound or a mixed solvent of
the fluorine-containing ether compound and the aforesaid cyclic carbonic
acid ester and/or chain carbonic acid ester as the non-aqueous solvent in the
invention, the ratio between the aforesaid cyclic carbonic acid ester and the
aforesaid chain carbonic acid ester is 0:100 to 100:0, preferably 20:80 to
80:20
(weight ratio).
[Other Solvents]
The non-aqueous electrolytic solution of the invention may contain any
solvents other than mentioned above as the non-aqueous solvent. As the
other solvents, there can be cited cyclic esters such as y -butyrolactone, y -
valerolactone, 3-methyl- y -butyrolactone and 2-methyl- y -butyrolactone;
chain esters such as methyl formate, ethyl formate, methyl acetate, ethyl
acetate, propyl acetate, methyl propionate, methyl butyrate and methyl
valerate; cyclic ethers such as 1,4-dioxane, 1,3-dioxane, tetrahydrofuran, 3-
methyl-1,3-dioxolane and 2-methyl-1,3-dioxolane; chain ethers such as 1,2-
dimethoxy ethane, 1,2-diethoxy ethane, diethyl ether, dimethyl ether and
dipropyl ether; sulfur-containing compounds such as sulforane and dimethyl
sulfate; phosphorus-containing compounds such as trimethyl phosphate and
triethyl phosphate.
These solvents can be used either individually or in a combination of 2 or
CA 02309684 2000-OS-10
14
more. Furthermore, the electrolytic solution of the invention may be gelated
by adding a high-molecular-weight gelling agent to it.
A preferable embodiment of the non-aqueous electrolytic solution
comprises a non-aqueous solvent containing a fluorine-containing ether
compound represented by the following general formula (1) or (2) and a
carbonic acid ester, and an electrolyte:
RO-(AO)n-CHz-X ~ ( 1)
X-CHz-(AO)~-CHz-X (2)
(In formulas (1) and (2), R represents a hydrocarbon group having 1 to 20
carbon atoms; X represents a fluorine atom-substituted hydrocarbon group
having 1 to 10 carbon atoms; and A represents an alkylene group having 2 to
4 carbon atoms. Furthermore, n is a whole number of 1 to 30.)
As another example of a preferable embodiment of the non-aqueous
electrolytic solution of the invention, a non-aqueous electrolytic solution
comprising a non-aqueous solvent containing a fluorine-containing ether
compound represented by the following general formula (1) or (2), and an
electrolyte, can be cited:
RO-(AO)n-CHz-X (1)
X-CHz-(AO)n-CHz-X (2)
(In formulas (1) and (2), R represents a hydrocarbon group having 1 to 20
carbon atoms; X represents a fluorine atom-substituted hydrocarbon group
having 1 to 10 carbon atoms; A represents an alkylene group having 2 to 4
carbon atoms; and n is a whole number of 1 to 30. Furthermore, in formula
(1), when X is CFs and R is CHs, CzHs or CH(CHs), Ais an alkylene group
having 3 or 4 carbon atoms.)
[Electrolyte]
As the electrolyte used in the invention, any electrolytes may be used
CA 02309684 2000-OS-10
without being limited to any particular type, so long as they are normally
used as the electrolyte for non-aqueous electrolytic solutions.
Specifically there can be cited lithium salts such as LiPFs, LiBFa, LiC104,
LiAsFs, LiAlCls, LizSiFs, LiOSOzRI, LiN(S02Rz)(SOzR3),
5 LiC(SOzR4)(SOzRs),(SOzRs) and LiN(SOaOCHzR~)(SOzOCHzRB), wherein Rl-
R8 stand for perfluoroalkyl groups having 1 to 6 carbon atoms which may be
equal or different, and alkali metal salts formed by substitute lithium of the
above lithium salts by an alkali metal.
Among these salts, preferred are LiPFs, LiBF4, LiOSOzRI,
10 LiN(S02Rz)(SOzRg), LiC(SOzR4)(SOzRs),(SOzRs) and
LiN(SOZOCH2R~)(SOzOCHzRs).
It is desirable that such electrolyte should be contained in a concentration
of normally 0.1 to 3.0 mol/liter, preferably 0.5 to 2.0 mol/liter, in the non-
aqueous electrolytic solution.
15 [Non-aqueous Electrolytic Solution Secondary Battery)
The non-aqueous electrolytic solution secondary battery of the invention
comprises the aforesaid non-aqueous electrolytic solution, a negative
electrode and a positive electrode.
A preferable example of such negative electrode is a negative electrode
containing any of metal lithium, lithium-containing alloy, carbon material
capable of doping or dedoping lithium ions, tin oxide capable of doping or
dedoping lithium ions, silicon capable of doping or dedoping lithium ions and
titanium oxide capable of doping or dedoping lithium ions as the active
negative electrode material.
A preferable example of such positive electrode is a positive electrode
containing a complex oxide of lithium and transition metal as the active
positive electrode material.
CA 02309684 2000-OS-10
16
Such non-aqueous electrolytic solution secondary battery may be applied
to a non-aqueous electrolytic solution secondary battery of the cylindrical
shape. Such non-aqueous electrolytic solution secondary battery of the
cylindrical shape is so constructed that as shown in Fig. 1, Negative
Electrode 1 comprising Negative Electrode Collector 9 coated with an active
negative electrode substance and Positive Electrode 2 comprising Positive
Electrode Collector 10 coated with an active positive electrode substance are
wound via Separator 3 which is filled with the non-aqueous electrolytic
solution and housed in Battery Can 5 with the Insulating Board 4 mounted
on the top and bottom of the wound assembly Battery Cover 7 is attached
to Battery Can 5 by caulking it by means of Sealing Gasket 6, with each
connected electrically to Negative Electrode 1 or Positive Electrode 2 via
Negative Electrode Lead 11 and Positive Electrode Lead 12 so that each
functions as the negative electrode and positive electrode of the battery. In
this battery, Positive Electrode Lead 12 is so arranged that it is connected
electrically to Battery Cover 7 via Current Shutoff Plate 8.
As such active negative electrode substance making up Negative
Electrode 1, any of metal lithium, lithium alloy and carbon materials capable
of doping and dedoping lithium ions reversibly may be used. Out of these,
carbon materials capable of doping and dedoping lithium ions are preferable.
For such carbon materials, either graphite or non-crystalline carbon may be
used, and any carbon materials may be used including activated carbon,
carbon fiber, carbon black and mesocarbon microbeads.
Further, examples of the substances that nay be used as the active
positive electrode substance making up Positive Electrode 2 include complex
oxides comprising lithium and transition metal such as LiCoOz, LiMnOz,
LiMnz04, LiNiOz and LiNixCo~~.x>Oz , and VaOs.
CA 02309684 2000-OS-10
17
Moreover, the non-aqueous electrolytic solution secondary battery of the
invention contains the non-aqueous electrolytic solution as explained above
as an electrolytic solution, and the shape or form of the battery is not
limited
to the example shown in Fig. 1 and may be of the coil shape, the rectangular
shape, etc.
EXAMPLES
The invention will be understood more readily with reference to the
following examples. However these examples are not to be construed to limit
the scope of the invention.
Preparation of a non-aqueous electrolytic solution:
A non-aqueous electrolytic solution was prepared by dissolving LiPFs in a
non-aqueous solvent in which propylene carabonate (PC), dimethyl
carbonate (DMC) and the fluorine-containing ether compound represented
by the following formula (A) had been so blended that PC:DMC:fluorine-
containing ether compound (weight ratio) is 15:15:70 in such manner that
the LiPFs concentration was 1 mol/liter.
Formula (A) CHsO-CHaCHaO-CHzCHzF
Preparation of a positive electrode:
LiCoOz, vinylidene fluoride resin and graphite were so blended that they
were in a weight ratio of 91:3:6, brought into a slurry state by use of NMP
and coated onto aluminum foil. After the coating was dried, a positive
electrode was prepared. Using the positive electrode thus prepared, an Li
negative electrode and an non-aqueous electrolytic solution charging in
which LiPFs had been dissolved in a solvent containing propylene carbonate
and dimethyl carbonate having been blended at a volume ratio of 1:1 in such
CA 02309684 2000-OS-10
18
manner that the LiPFs concentration was 1 mol/liter, constant-voltage
charging at 4.4V was carried out. The electric potential after 2 hours from
the charging was 4.38V This electrode was washed adequately with
dimethyl carbonic acid ester and dried for the removal of dimethyl carbonic
acid ester.
This electrode was cut into an approximately 2-mm rectangular length,
and thus a positive electrode for the measurement of maximum heat release
rate was obtained.
The maximum heat release rate of the positive electrode was measured by
the method as described below. Results are shown in Table 1.
Measurement of maximum heat release rate:
0.3 ml of the non-aqueous electrolytic solution prepared as described
above and 1.00 g of the positive electrode for the measurement of maximum
heat release rate were mixed in an argon atmosphere.
The measurement was made by the normal method using ARCTM
(accelerating reaction calorimeter) available from Columbia Scientific. The
measurement temperature range was set at 40°C to 35°C.
Further, the heat release rate represents the temperature rise of the
sample per unit time, and the maximum heat release rate represents the
maximum value of the heat release rate in the measurement period.
Preparation of a cylindrical battery:
A carbon material having properties close to those of glass-like carbon
was obtained by crosslinking petroleum pitch with oxygen and sintered it at
1,000°C in an inactive gas current. This carbon material was crushed,
and
the carbon material powder thus obtained was used as the active negative
electrode material. A negative electrode compound was obtained by
blending 90 wt% of the active negative electrode material and 10 wt% of
CA 02309684 2000-OS-10
19
vinylidene fluoride resin as the binder. This negative electrode compound
was brought into a slurry state by dispersing it by using N-
methylpyrrolidone as the solvent. Using copper foil in the strip form as the
negative electrode collector, a negative electrode compound slurry was coated
on both sides of the collector. After drying and removing the solvent, the
collector was compress-molded, and as a result, Negative Electrode 1 was
obtained.
A positive electrode compound was prepared by blending 91 wt% of LiCoz
as the active positive electrode material, 6 wt% of graphite as the conductive
material and 3 wt% of vinylidene fluoride resin as the binder and brought
into a slurry state by getting it dispersed in N-methylpyrrolidone. Next, the
positive electrode compound slurry thus obtained was coated on both sides of
aluminum foil in the strip form which was the positive electrode collector.
After the solvent was dried and removed, the collector was compression-
molded, and as a result, Positive Electrode 2 was obtained.
Using porous polypropylene film as Separator 3 to be intervened between
Negative Electrode 1 and Positive Electrode 2, which were prepared as
described above, a four-layer laminate of Negative Electrode 1, Separator 3,
Positive Electrode 2 and Separator 3 in this order was prepared. This
laminate was wound many times in the form of vortex along the lengthwise
direction in such manner that Negative Electrode 1 is positioned outside
Positive Electrode 2. This wound object was fixed at the place of the wound
end by means of adhesive tape comprising polyester film as the substrate on
which silicone-based adhesive had been coated so that the wound object
would not become loose. As a result, a wound electrode was obtained.
This wound electrode was put in Battery Can 5 made of nickel-plated iron.
Electrical Insulator 4 was placed on both of the top and bottom of the wound
CA 02309684 2000-OS-10
electrode. In order to connect Negative Electrode 1 and Battery 5
electrically, Nickel Negative Electrode Lead 11 was taken out of the negative
electrode collector and was welded to Battery Can 5. Zb connect Positive
Electrode 2 and Battery Cover 7 electrically, Aluminum Positive Electrode 12
5 was taken out of the positive electrode collector and welded to Battery
Cover
7. The non-aqueous electrolytic solution prepared as described above was
poured into the battery case housing the wound electrode, and Current
Shutoff Plate 8 and Battery Cover 7 were fixed by caulking the opening
section of the battery case by means of Sealing Gasket 6 so that the inside of
10 the battery would be air-tight. Through the process as described above, a
cylindrical battery was prepared.
External short-circuit test on the battery:
The cylindrical battery prepared as described above was charged for 7
hours at a constant current of lA with the ceiling voltage set at 4.2V. After
15 that, the positive electrode and the negative electrode was short-circuited
by
connecting Battery Cover 7 and Battery Can 5 by use of copper wire. The
highest temperature of the battery that was reached due to the external
short-circuit was measured by recording the change in the temperature of
the outside walls of the battery at that time. Results are shown in Table 1.
The non-aqueous electrolytic solution was prepared and maximum heat
release rate was measured in the same manner as described in Example 1
except that the fluorine-containing ether compound shown in Table 1 was
used. Results are shown in Table 1.
Co~narative Example 1
The non-aqueous electrolytic solution was prepared and maximum heat
release rate was measured in the same manner as described in Example 1
CA 02309684 2000-OS-10
21
except that the solvent having the composition shown in Table 1 was used.
Results are shown in Table 1.
CA 02309684 2000-OS-10
22
U
t~,cCc~
'
~ .
a~
v ~
'f a o
an ,.
a~
o
d
0
~+~+~
v V ~ ~ ~ a~~ ~ ~ ~ v
U ~ ~ ~ ~ ~ ~ wl~ ~ ~
~a~ o
~ s~ z
b
0
0 0 0 0 ~ p
a
...,U p
H o ~ ~ ~ ~ covcostcy c goto
q
s
b
~!7~cJ~fJ~JO O O O O p
.-.~.-ar-ar-1GV CSICV CJCV
v
U
w w U
~' ~ x ~ M x x
w w w
U U U U U U U
x w ~ x x x x o 0
U U U U U U '~
V
x ~ O O O O O U U
U U x x x x x x x
O O V V ~ ~ U U U
x x x o
x x x x x
~, ~ ~ *
U U , , U U , U
~ U U
v o U U ,"~,'x ~ ~ tj w
' U V U
O O 0 O
v ~ U U ~ m U U ~
U U
Fs;~ , U U z
P1 M d~tc~COC- c007 t~..-i
s
w w w w w w w w w caw
CA 02309684 2000-OS-10
23
POSSIBILITY OF INDUSTRIAL UTILIZATION
Since the non-aqueous electrolytic solution of the invention contains a
fluorine-containing ether compound and uses a non-aqueous solvent of a
particular composition, the rate of heat release due to the reaction between
the electrolytic solution and the positive electrode is low, which implies
excellent safety Furthermore, such non-aqueous electrolytic solution shows
a commercial level of ion conductivity, and yet entails no separation of the
electrolyte.
This non-aqueous electrolytic solution can be used suitably as the
electrolytic solution for lithium ion secondary batteries.