Note: Descriptions are shown in the official language in which they were submitted.
CA 02309973 2000-OS-15
WO 99/32464 PCT/EI°98/08335
-1 -
Thiazole isothiazole and thiadiazole-derivatives having microbicidaf and plant
immunizin4
activities.
The invention relates to a method for protecting and immunizing plants against
attack by
phytopathogenic microorganisms by applying to the plants, to parts of the
plants and/or to
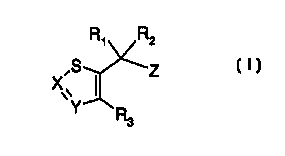
the locus of the plants a compound of formula I
R, R2
~Z
\Y R
3
wherein
a) X is CR4 and Y is N; or
b) X is N and Y is CRS; or
c) X and Y are N; and wherein
Z is a C,-group to which 1-3 halogen atoms or 1-3 unsubstituted or substituted
hetero atoms
selected from the group O, S and N are bonded;
R, and Rz are independently H, OH, SH, CN, COOH, N02, NH2, halogen, C,-
Csalkyl,
haloC,-Csalkyl, alkoxyC,-Csalkyl, aminoC,-Csalkyf, alkoxaminoC,-Csalkyl, C,-
Csalkoxy, halo-
C,-Csalkoxy, C,-Csalkanoyloxy, aroyloxy, C,-Csalkoxycarbonyi, aryloxycarbonyl,
benzyloxycarbonyl, C,-Csalkylcarbonyl, arylcarbonyl, benzylcarbonyl,
aminocarbonyl,
C,-Csalkylaminocarbonyl, C,-Csdialkylaminocarbonyl, C,.Csalkylthio, haloC,-
Csalkylthio,
C,-Csalkylsulfinyl, haloC,-Csalkylsulfinyl, C,-Csalkylsulfonyl, haloC,-
Csalkylsulfonyl,
arylsulfinyl, arylsulfonyl, C2-Csalkenyl, haloC2-Csalkenyl, CZ-Csalkinyl,
carboxyC,-Csalkyl,
alkoxycarbonylC,-Csalkyl, haloalkoxycarbonylC,-Csalkyl, C3-Cscycloalkyl,
alkanoylC,-Csalkyl,
alkylcarbonyloxyC,-Csalkyl, phenylcarbonyloxyC,-Csalkyl, C,-Csalkylamino, C,-
Csdialkylamino, C2-Csalkenylamino, C,-Csalkanoylamino, C,-
Csalkoxycarbonylamino,
benzylamino, benzoylamino, benzyioxyarbonylamino, phenyl, phenoxy, benzyl or
phenethyl,
wherein all the aromatic groups are unsubstituted or substituted from 1 to 5
substituents
independently selected from halogen, hydroxy, C,-C4alkyl, halo-C,-C2alkyl, C,-
C2alkoxy,
halo-C,-C2alkoxy and nitro; or optionally substituted heterocyclyl; or tri(C,-
Csalkyl)silyl or
tri(C,-Csalkyl)silyloxy;
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-2-
with the proviso that R, and R2 are not simultaneously a group selected from
OH, SH, N02,
NH2, C,-Csalkylamino, C,-Cgdialkylamino and C2-Cealkenylamino; or
R, and R2 together are =O or =S; or
R, and R2 together with the carbon atom to which they are bonded are an
unsubstituted or
substituted 3 to 8 membered isocyclic or heterocyclic ring; or
R2 and Z together with the carbon atom to which they are bonded are an
unsubstituted or
substituted 3 to 7 membered lactone, lactame, thiolactone or thiolactame,
which ring may
have 1 to 2 additional hetero atoms selected from the group O, S and N;
R3, R, and RS are independently H, OH, SH, CN, N02, NH2, halogen, C,-Ceaikyl,
haloC,-Csalkyl, hydroxyC,-Cealkyl, alkoxyC,-Csalkyl, aminoC,-Ceaikyl,
alkoxaminoC,-Csalkyl,
C,-Csalkoxy, C,-Cgalkylthio, haloC,-Cealkylthio, C,-Cealkylsulfinyl, haloC,-
Cealkylsulfinyl,
C,-Csalkylsulfonyl, haloC,-Ceaikylsulfonyl, halo-C,-Cealkoxy, CZ-Cealkenyl,
haloC2-Csalkenyl,
C2-Csalkinyl, carboxyC,-Cgalkyl, C,-Cgalkanoyl, C,-Csalkoxycarbonyl,
alkoxycarbonylC,-CBalkyl, haloalkoxycarbonylC,-Cealkyl, C3-CBcycloalkyl,
alkanoylC,-Csalkyl,
alkylcarbonyloxyC,-Csalkyl, phenylcarbonyloxyC,-Cealkyl, C,-CBalkylamino,
C,-Cgdialkylamino, C2-CBalkenylamino, C,-Cealkanoylamino, C,-
CBalkoxycarbonylamino,
benzylamino, benzoylamino, phenyl, phenoxy, benzyl or phenethyl, the phenyl
rings of
which are unsubstituted or substituted from 1 to 3 substituents independently
selected from
halogen, hydroxy, C,-C,alkyl, halo-C,-CZalkyl, C,-C2alkoxy, halo-C,-C2alkoxy
and vitro; or
optionally substituted heterocyclyl.
The invention relates also to new compounds of formula i, to the preparation
of those
compounds, to new intermediates and to agrochemical compositions comprising at
least
one of those compounds as active ingredient.
Thiazole and thiadiazole derivatives having plant-fungicidal activities are
known from
EP-A-395,174, US 5,135,927, WO 96/17840, and WO 96/29871.
EP-A-757,987 and WO 97/20465 discloses thiazole and thiadiazole derivatives
exhibiting
plant immunizing activities. These compounds have no or very weak direct
activity against
fungi and bacteria, but protect the plants from phytopathogenic microorganisms
by
activation and stimulation of the plant's own defence system (immunisation).
That mode of
action has also become known by the name 'Systemic Activated Resistance'
('SAR"). Such
compounds and methods are ecologically advantageous and are complementary to
current
methods in crop protection. It is therefore desirable to provide more
compounds and
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-3-
methods for protecting plants by immunizing them against attack by
phytopathogenic
microorganisms.
Surprisingly it has now been found that compounds of formula I can be used for
protecting
and immunizing plants against attack by microorganisms, such as
phytopathogenic fungi,
bacteria and viruses and for improving the qualities of the plants.
The formula I embraces all stereoisomeric forms and mixtures thereof, such as
enantiomeric and diastereomeric pure forms and mixtures thereof.
The compounds of formula I and, where appropriate, their tautomers can be in
the form of
salts. Compounds of formula I that have at least one basic centre can form
acid addition
salts. Furthermore, compounds of formula I having at least one acid group can
form salts
with bases. Preference is given to agrochemicaily advantageous salts.
Z is a C,-group which means that no additional carbon atoms are directly
attached to this
group. Examples for the group Z are trihalomethyl, dihalomethyl or halomethyi
as
chloromethyl; formyl or an acetal or thioacetal thereof; a carboxylic acid or
derivatives
thereof, as nitrite, esters, anhydrides, thioesters, amides, amidines, imidic-
,hydrazonic- and
hydroxamic-acids or derivatives thereof; or heterocyclyl, as 2-imidazolyl, 2-
pyrimidinyl and 2-
thiazolyl.
Compounds of formula I wherein R, and R2 are simultaneously a group selected
from OH,
SH, N02, NH2, C,-Cealkylamino, C,-Cediaikylamino and C2-Cgalkenylamino are not
stable in
general and are thus not part of this invention.
Unless defined otherwise, the general terms used hereinbefore and hereinafter
have the
meanings given below:
Hydrocarbon radicals may be saturated or unsaturated, open-chained or cyclic,
or mixed
open-chained and cyclic, for example cyclopropylmethyl or benzyl.
Alkyl groups are straight-chained or branched and are, for example, methyl,
ethyl, n-propyl,
isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, sec-amyl, tert-amyl, 1-
hexyl or 3-hexyl.
Unsaturated hydrocarbon radicals are alkenyl, alkynyl or alkenynyl groups
having not more
than three multiple bonds, for example butadienyl, hexatrienyl or 2-penten-4-
ynyl.
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-4-
Alkenyl is straight-chained or branched aikenyl, for example allyl, methallyl,
1-methylvinyl or
but-2-en-1-yl. Preference is given to alkenyl radicals having a chain length
of 2 to 4 carbon
atoms.
Alkynyl may be straight-chained or branched, for example propargyl, but-1-yn-i-
yl or but-1-
yn-3-yl. Propargyl is preferred.
Cyclic unsaturated hydrocarbon radicals may be aromatic, for example phenyl
and
naphthyi, or non-aromatic, for example cyclopentenyl, cyclohexenyl,
cycloheptenyl and
cyclooctadienyl, or partially aromatic, for example tetrahydronaphthyl and
indanyl.
Halogen, or halo, is fluorine, chlorine, bromine or iodine, preferably
fluorine, chlorine or
bromine.
Haloalkyl may contain identical or different halogen atoms, for example
fluoromethyl,
difluoromethyl, difluorochloromethyl, trifluoromethyl, chloromethyl,
dichloromethyl, trichloro-
methyl, 2,2,2-trifluoroethyl, 2-fluoroethyl, 2-chloroethyl, 2,2,2-
trichloroethyl, 3,3,3-trifluoro-
propyl.
Alkoxy is, for example, methoxy, ethoxy, propoxy, isopropoxy, n-butoxy,
isobutoxy, sec-
butoxy and tert-butoxy, preferably methoxy and ethoxy.
Haloalkoxy is, for example, difluoromethoxy, trifluoromethoxy, 2,2,2-
trifluoroethoxy, 1,1,2,2-
tetrafluoroethoxy, 2-fluoroethoxy, 2-chloroethoxy and 2,2-difluoroethoxy.
Cycloalkyl is cyciopropyl, cyclobutyl, cyclopentyl or cyclohexyl.
Alkanoyl is either straight-chained or branched. Examples are formyl, acetyl,
propionyl,
butyryl, pivaloyl and octanoyl.
A heterocyclyl radical is to be understood as being a 3 to 7-membered,
aromatic or non-
aromatic ring having hetero atoms N, O andlor S. Furthermore, an unsubstituted
or substitu-
ted benzo group may be fused onto such a heterocyclyl radical bonded to the
rest of the
molecule. Examples of heterocyclyl groups are pyridyl, pyrimidinyl,
imidazolyl, thiazolyl,
1,3,4-thiadiazolyl, triazolyl, thienyl, furanyl, pyrrolyl, morpholinyl,
oxazolyl and the correspon-
ding partially or completely hydrogenated rings. Examples of heterocyclyl
groups to which a
benzo group is fused are quinolyl, isoquinofyl, benzoxazolyl, quinoxaiinyl,
benzothiazolyl,
benzimidazolyl, indolyl and indolinyl.
Aryl is phenyl, naphthyl, phenanthryl or fluorenyl, in particular phenyl.
The hydrocarbyi groups, as alkyl, alkenyl, alkynyl, and the haloalkyl,
haloalkenyl, haloalkoxy
and alkanoyl groups mentioned hereinabove and hereinbelow can be substituted
by aryl,
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-5-
hetaryl, aryloxy, hetaryloxy, arylsulfenyl, arylsulfinyl, arylsulfonyl,
heterarylsulfenyl,
hetarylsulfinyl or heterarylsulfonyl, each of which is unsubstituted or
additionally substituted.
All the aryl, hetaryi and heterocyclyl groups mentioned hereinabove and
hereinbelow can be
mono- or polysubstituted, for example by halogen, C,-C,,alkyl, C2-C4alkenyl,
C2-C4alkynyl,
C,-C4alkoxy, C,-C,alkylthio, C,-C~haloalkyl, C2-Cehaloalkenyi, C2-C4-
haloalkynyl,
C,-C,haloalkoxy, halogen, cyano, cyano-C,-CZalkyl, cyano-C,-CZalkoxy, OH, N02,
SCN,
thiocyanomethyl, Si(CH3)3, NH2, NH(C,-C,alkyl), N(C,-C,alkyl)2, C,-
Cealkoxymethyl,
C,-C,,haloalkylcarbonyl, C,-C4haloalkyloxycarbonyl, C,-C4alkylcarbonyl, C,-C4-
alkoxycarbonyl, aminocarbonyl, C,-Cealkylaminocarbonyl, bis(C,-
C,alkylamino)carbonyl,
arylaminocarbonyl, arylaminothiocarbonyl, C,-C,aikoximinomethyl, -CSNHZ, -SH,
C,-
C4alkylthiomethyl, C2-C4alkenyloxy, C2-C4alkynyloxy, CZ-C,haloalkenyloxy, C,-
C4alkylsulfinylmethyl, C,-C,alkyisulfonylmethyl, phenylsulfinylmethyl,
phenylsulfonylmethyl,
trifluoromethyfsulfonyl, C3-Cgcycloalkyl, C,-C4haloalkylcarbonyloxy, C,-
C,alkylcarbonyloxy,
C,-C,alkoxycarbonyloxy, haloalkoxycarb~onyloxy, aminocarbonyfoxy,
C,-C4alkylaminocarbonyloxy, bis(C,-C,alkylamino)carbonyloxy,
arylaminocarbonyloxy,
arylaminothiocarbonyloxy.
Amongst the compounds and methods of their use the following groups are
preferred:
(1 ) Compounds of formula
R~ R2
S
LA .
R 4 _"C~
N R
3
The compounds of the formula
R ~ OMe
CO-NH-CHZ CHZ ~ ~ OMe
CI -~~
N CI T
wherein
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-6- '
a) R, is OCO-CH3 and T is Br,
b) R, is OH and T is Br,
c) R, is OH and T is H,
are known from WO 96/17840 as fungicides, but no indication is given therein
to plant
immunizing properties of these compounds ; these compounds are thus part of
the
invention only as far as the method for immunizing plants is concerned.
(2) Compounds of formula
R~ R2
.S Z
_ LB.
R3
Rs
(3) Compounds of formula
R~ R2
,S
'Z LC.
~R
3
(4) Compounds of formula I, wherein
Z is CN, CO-A, CS-A or CH{OR,o)2,;
A is hydrogen, halogen, ORs, SR,, N(Re)Ra, ON(R")R,2 or N(R,3)OR,4;
Rs to R" are independently hydrogen, an unsubstituted or substituted, open-
chained,
saturated or unsaturated hydrocarbon radical containing up to 8 carbon atoms,
an
unsubstituted or substituted, cyclic, saturated or unsaturated hydrocarbon
radical containing
up to 10 carbon atoms, unsubstituted or substituted benzyl or phenethyl, an
unsubstituted
or substituted acyl group containing up to 8 carbon atoms, an unsubstituted or
substituted
benzoyl group, or an unsubstituted or substituted heterocyclyl radical; or
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
_7_
R8 and R9, or R" and R,2, together with the nitrogen atom to which they are
bonded, form a
5- or 6-membered, unsubstituted or substituted heterocycle having 1 to 3
hetero atoms
selected from O, S and/or N;
R,o are identical or different and are C,-CBalkyl that is unsubstituted or
substituted by
phenyl, C,-C2alkoxy, phenoxy or by benzyloxy; or
two substituents OR,o, together with the carbon atom to which they are bonded,
form a
cyclic acetal group that is unsubstituted or substituted by C,-C3alkyi,
phenyl, benzyl,
hydroxy or by C,-C3hydroxyalkyl.
(5) Compounds of formula I, wherein
Z is CO-A or CS-A;
A is OR6, SR,, N(R8)R9, ON(R")R,2 or N(R,3)OR,4;
R6 to R,4 are independently H, C,-CBalkyl, haloC,-Cgalkyl, C,-
Csalkoxycarbonyl,
C,-C4alkanoylC,-C4alkyl, C3-Cgcycloalkyl, C3-Cscycloalkylmethyl, phenyl,
benzyl, phenethyl,
the phenyl rings of which are unsubstituted or substituted from 1 to 5
substituents
independently selected from halogen, C,-C4alkyl, halo-C,-C2alkyl, C,-CZalkoxy,
haio-
C,-C2alkoxy and C,-C2alkylenedioxy.
(6) Compounds of formula I, wherein
R3 is H, OH, C,-CBalkyl, C3-CBCycloalkyl, haloC,-Csalkyl, C,-Caalkoxy or
haloC,-Csaikoxy.
(7) Compounds of formula I, wherein
R, is H, OH, NH2, halogen, COOH, C,-C,afkyl, haloC,-C4alkyl, C,-C,alkoxy,
C,-C,alkanoyloxy, aroylyloxy, C,-C,alkoxycarbonyl, aryloxycarbonyl,
benzyloxycarbonyl,
C,-C,alkylcarbonyl, arylcarbonyl, benzylcarbonyl, aminocarbonyl, C,-
C,alkylaminocarbonyl,
C,-C,dialkylaminocarbonyl, alkanoylC,-C4alkyl, alkylcarbonyloxyC,-C,alkyl, C2-
C4alkenyl,
haloC2-C,alkenyl, C,-C,,alkylamino, C,-C4dialkylamino, C,-C,alkanoylamino,
C,-C,alkoxycarbonylamino, benzylamino, benzoylamino, phenyl, phenoxy, benzyl
or
phenethyl, the phenyl rings of which are unsubstituted or substituted from 1
to 3
substituents independently selected from halogen, hydroxy, C,-C,alkyl, halo-C,-
CZalkyl,
C,-CZalkoxy, halo-C,-Czalkoxy and vitro;
~RZ is H, OH, C,-C,,alkyl, C,-Cdalkoxy or phenyl; or
R, and RZ together are a group selected from
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-g_
R,a R,~ R,e
N_R ,s
or
R2+Z together are a group selected from
R" R,a R" R,a H H
O O n Rn
O O R ,e HO \O
O O
O O
wherein R", R,e and R,s are independently H or C,-C4alkyl;
R3 is H, halogen, C,-Csalkyl, haloC,-Cealkyl, C3-Ca-cycloalkyl, C,-C,
alkoxycarbonyl, phenyl
which is unsubstituted or substituted from 1 to 3 substituents independently
selected from
halogen, C,-C4alkyl, halo-C,-C2alkyl, C,-CZalkoxy, halo-C,-C2alkoxy, amino,
C,-C,alkylamino, C,-C,dialkylamino, benzylamino, C,-C,alkanoylamino,
benzoylamino,
C,-C4alkoxycarbonylamino, formyl, or a 4-7-membered cyclic or C,-C4alkyl open-
chained
acetal or thioacetal thereof;
R4 is H, OH, halogen, amino, C,-CBalkyl, C,-C<alkylamino, C,-C4alkenylylamino,
C,-C4dialkylamino, benzylamino, C,-C4alkanoylamino, benzoylamino,
C,-C4alkoxycarbonylamino.
(8) Amongst group (7) those, wherein
R3 is H, OH, halogen, C,-Cealkyl, haloC,-Cgalkyl, C3-Ca-cycloalkyl, C,-
Caalkoxy,
alkoxycarbonylC,-CBalkyl, phenyl, benzyl, the phenyl rings of which are
unsubstituted or
substituted from 1 to 3 substituents independently selected from halogen, C,-
C,alkyl, halo-
C,-C2alkyl, C,-C2alkoxy and halo-C,-C2alkoxy; or formyl, or a 4-7-membered
cyclic or
C,-C4alkyl open-chained acetal or thioacetal thereof;
R4 is H, OH, halogen, amino, C,-Cgalkyl, C,-C4alkylamino, C,-C4alkenylylamino,
C,-C,dialkylamino, benzylamino, C,-C,alkanoylamino, benzoylamino, or
C,-C4alkoxycarbonylamino.
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-g-
(g) Amongst group (6) those, wherein
Z is CO-A or CS-A;
A is hydrogen, halogen, ORs, SRS, N(Re)R9, ON(R")R,2 or N(R,3)OR,4;
R6 to R9 and R" to R,4 are independently hydrogen, an unsubstituted or
substituted, open-
chained, saturated or unsaturated hydrocarbon radical containing up to 8
carbon atoms, an
unsubstituted or substituted, cyclic, saturated or unsaturated hydrocarbon
radical containing
up to 10 carbon atoms, unsubstituted or substituted benzyl or phenethyl, an
unsubstituted
or substituted acyl group containing up to 8 carbon atoms, an unsubstituted or
substituted
benzoyl group, or an unsubstituted or substituted heterocyclyl radical; or
R8 and R9, or R" and R,2, together with the nitrogen atom to which they are
bonded, form a
5- or 6-membered, unsubstituted or substituted heterocycle having 1 to 3
hetero atoms
selected from O, S and/or N;
R,o are identical or different and are C,-Cgalkyl that is unsubstituted or
substituted by
phenyl, C,-C2alkoxy, phenoxy or by benzyloxy; or
two substituents OR,o, together with the carbon atom to which they are bonded,
form a
cyclic acetal group that is unsubstituted or substituted by C,-C3alkyl,
phenyl, benzyl,
hydroxy or by C,-C3hydroxyalkyl.
(10) Amongst group (9) those, wherein
A is OR6, SR,, or N(RB)R9;
R6, R,, R8 R9 are independently H, C,-Csalkyl, haloC,-CBalkyl, C,-
C4alkoxycarbonyl,
alkoxycarbonylC,-CBalkyl, C,-C,alkanoylC,-C,alkyl, C3-CBcycloalkyl, C3-
Cscycioalkylmethyl,
phenyl, benzyl, or phenethyl, the phenyl rings of which are unsubstituted or
substituted from
1 to 3 substituents independently selected~from halogen, C,-Cealkyl, halo-C,-
CZalkyl,
C,-C2alkoxy, halo-C,-C2alkoxy.
(11 ) Amongst group (g) those, wherein
Z is CO-A;
A is OR6 or N(RB)R9;
R, is H, OH, halogen, C,-C<alkyl, haloC,-C4alkyl, C,-C,alkoxy, halo-C,-
C,,alkoxy, amino,
C,-C4alkylamino, C,-C4dialkylamino, benzylamino; phenyl, benzyl, or phenethyl,
the phenyl
rings of which are unsubstituted or substituted from 1 to 2 substituents
independently
selected from halogen, halo-C,-C2alkyl, C,-C2alkoxy, halo-C,-C2alkoxy;
CA 02309973 2000-OS-15
WO 99/32464 PC'T/EP98/08335
-10-
R2 is H, OH, halogen, C,-C4alkyl, haloC,-Csaikyl, or phenyl,
R3 is H, OH, C,-Csalkyl, C3-Cscycloalkyl, haloC,-Csalkyl, C,-Csalkoxy or
haloC,-Csalkoxy,
R4 is H or CI,
Rs, Re and R9 are independently H, C,-Csalkyl, haloC,-Csalkyl, C,-Csaikoxy,
haloC,-Csalkoxy, C,-C4alkoxycarbonyl, C,-C4alkanoylC,-Caalkyl, C3-
Cscycloalkyl,
C3-Cscycfoalkylmethyl, phenyl, benryl, or phenethyl, the phenyl rings of which
are
unsubstituted or substituted from 1 to 3 substituents independently selected
from halogen,
C,-C<alkyl, halo-C,-C2alkyl, C,-Czalkoxy, halo-C,-CZalkoxy.
(12) Amongst group (11 ) those, wherein
R, is H, OH, halogen, C,-C,alkyl;
RZ is H.
R3 is H, cyclopropyl or CF3,
R4 is CI.
(13) Amongst group (9) those, wherein
R3 is H, halogen, C,-C,alkyl, haloC,-Caalkyl, C3-Cs-cycloalkyl, C,-C,
alkoxycarbonyl, formyl,
or a 4-7-membered cyclic or C,-C,alkyl open-chained acetal or thioacetal
thereof.
(14) Compounds of formula
R~ R2
S
Z LA
R 4.-'~~ I
N R
3
Z IS CO-A;
A is hydrogen, ORs, SRS, N(R8)R9;
R, is H, OH, halogen or C,-C4alkyl,
RZ is H;
R3 is H, OH, C,-Csalkyl, C3-Cscycloalkyl, haloC,-Csalkyl, C,-Csalkoxy, haloC,-
Csalkoxy,
formyl, or a 4-7-membered cyclic or C,-Cealkyl open-chained acetal or
thioacetal thereof;
R4 is CI;
Rs, R,, Re and R9 are independently H, C,-Csalkyl, haloC,-Csalkyl, C,-
Csalkoxy,
haloC,-Csalkoxy, C,-C4alkoxycarbonyl, C,-C,,alkanoylC,-C,alkyl, C3-
Cscycloalkyl,
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
C3-Cgcycloalkylmethyl, phenyl, benryl, or phenethyl, the phenyl rings of which
are
unsubstituted or substituted from 1 to 3 substituents independently selected
from halogen,
C,-C,alkyl, halo-C,-C2alkyl, C,-CZalkoxy, halo-C,-C2alkoxy.
(15) Amongst group (14) those, wherein
A is ORs or SRS;
R, and R2 are H;
R3 is C,-Cgalkyl, C3-Cgcycloalkyl, CF3 or formyl;
R, is CI;
R6 and R, are independently H, C,-CBalkyl, phenyl, benzyl, or phenethyl, the
phenyl rings of
which are unsubstituted or substituted with 1 to 2 substituents independently
selected from
halogen, C,-Coalkyl, halo-C,-CZalkyl, C,-CZalkoxy and halo-C,-C2alkoxy.
Also preferred are the compounds of the tables.
The compounds of formula I may be prepared as outlined in the following
reaction
schemes.
Abbreviations:
position to which the rest of the molecule is attached
S
Het: X'
Y R
3
Hal: halogen
L: leaving group, preferably chlorine, bromine, mesylate or tosylate.
R: a group which is inert under the reaction conditions
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-12- _
Scheme 1
Het-COOK Het-CH3 Het-H
halogenation Lithiation Lithiation
reduction e.g.N-halosuccinimid e.9.butyllithium
e.g.butyllithium
e.g. LiAIH4 alomethyl~ation
~e g.HCI/CHZO
Het-CH20H Het-CHZ-Li Het-Li
hatogenation L-CH2-COOR
e.g.SOCl2 OHC-COOK
"Amdt- ' ert" C02
carbonylation
Het-CH2-Hal Het-CH2 COOR ~ duction Het-CH(OH)-COOR
e.g. CO/cat: cobalt-carbonyl e.g. H~/cat
Of particular importance is the reaction step
~S CH2CI ~S CH2 COOH
X I
X ~ ~ ---,~ ~ Y
Y
Ra R3
11.1
1.1
wherein X, Y and R3 are as defined for formula I,
which comprises reaction of a compound of formula 11.1 with carbon monoxide
under
pressure of 2-20 bars, preferably 5-10 bars, in presence of a catalyst, for
example cobalt
carbonyl and optionally a phase transfer catalyst.
Scheme 2
H H R 1-L/base H R ~ R2-L/base R 2 R ,
Het~z ----~ ---- ~
Het Het' \z
z
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/Ot3335
_ ~3 _ _
Scheme 3
NXS_ H X RNH2 H NHR
Het z e.g.N-Bromo ~ ~
Het"z Het"z
succinimide
Scheme 4
O R
H ~-R
OH ~ H O- \
,~OH R R ~O
Het - ~ -~ Het'
O O
H NHR 'R~
OH R R HN ' R
Het'~ H O
O Het
O
Scheme 5
~ . Base
O
2.
RO~O~R
O
HO R2 O
O
. iH ~, gee O~'R Oxidation ~ ~/ R
Het Het ,~ Het
2. O O O
R2~O~R
O
Nu
Het~H ~. Base p O~R HO
Nu - R
O Het "'~' Het
O (nucleophil) O
'~ R
O
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
_~4_ _
The functional groups of the compounds of formula I can be converted by known
methods.
For example, carboxylic acid derivatives can be converted as follows:
Scheme 6
RZ R~ A RZ R, NH3 R2 R,
Het'' 'CO I
Het C
CO-A Het CONHZ
thionating agent reduction _H20
e.g. Lawesson reagent e~9~ scat e.g. SOCIz
R2 R~ RZ R~
~ ~ Het ~CN
Het'~C~A Het''
CHO
acetalisation
with alcohol or glycol
R 2~ ~
Het CH(ORto)2
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
- _
Of importance are the syntheses of schemes 7 and 8
Scheme 7
t-Bu-0NO
CI COOR NH2 CS-NHZ S COOR CuCl2
H2N \\ I ---
O R3 N Rs
COOR S CH2COOR
CI----~ I CI-~ I
N Rs N R3
ROH
reduction 1. reduction, e.g. LiAIH4
e.g.H~/Pd/C 2~ Halogenation; e.g.SOCl2
3. Carbonylation,
e.g CO/Co2(CO)8
S COOR or S CH2COOR
H~\ ~ Cyanation/alcohoiysis H~ I
N Ra N R3
S COOR S CH2COOR
RO--~ I RO--~ I
N
R3 N R3
CA 02309973 2000-OS-15
WO 99/32464 ' PCT/EP98/08335
_~s_ _
Scheme 8
S COOR S COOR o~~tion S COOR
CI---<~ ~ ~ogenation ~ CI ---~ CI
N e.g. bromination ~N ~ Ha!
CH 3 with NBS v e.g. Mn02 or CHO
N-methylmorpholine-
N-oxide
COOR S 2
acetalis~n Ci S reduction CH -OH
I O CI-~ I O
N O~E e.g.:l.iAIH4 N jE
O
chlorination CI ,S I CHzCI ca~i~ S CH2COOH
e.g. SOCI2 or --~N O\E CI~ I O
Ph3P/CCI4 O/ e.g.CO/Co2(CO)8 N ~E
O
CI~S I CO-A hydrolysis CI--~S I CO-A
N ~ N CHO
O-E
E: optionally substituted C2-CSmethylene
Of particular importance is the reaction step
g CH2CI g CHZ COOH
~ 4--C
N
R3 R3
ILA.1 LA.1
which comprises reaction of a compound of formula ILA.1 with carbon monoxide
under
pressure of 2-20 bars, preferably 5-10 bars, in presence of a catalyst, for
example cobalt
carbonyl and optionally a phase transfer catalyst.
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/pg335
-17- _
Particulartly preferred is this reaction with compounds wherein R3 is C,-
Csalkyl, CF3 or an
acetal group, and R4 is CI.
Suitable bases, leaving groups, solvents and catalysts are known to the
skilled person.
The thiazoles, isothiazoles and thiadiazoles can be synthesized by known
methods or in
analogy thereto according to the following references:
111 1.3-Thiazoles
Ahluwalia V. K. et al, Heterocycles, 32, (1991 ), 907.
Fukatsu H. et al, Heterocycles, 29, (1989) 1517.
Byers J. R.et al, Org. Synthesis II, (1943) 31.
1.2 1.2-Isothiazoles
R. G. Micetich, Can J. Chem.; (1970), 48, 2006.
Adams A., Slack, J. Chem. Soc. (1959) 3061.
Buttimore D. et al, J. Chem. Soc. (1963) 2032.
Wooldrige K.R.H. Adv. Het. Chem. (1972), 14, 1.
1.3 1.2.3-Thiadiazoles
Hurd C. D., Mori E. J., J. Am. Chem. Soc., (1995), 5359.
Ramsky S. !. et al., Acta Pharm. Suecica 10, (1973), 285.
Scheitauer S., Mayer R. Chem. Ber. 100, (1967), 1413.
R. Raap, Can. J. Chem. (1968), 46. 2255.
The compounds of the invention can be used in the agricultural sector and
related fields
preventively and/or curatively. Besides their microbicidal properties, the
compounds exhibit
plant immunizing properties, i.e. plants can be protected by activation and
stimulation of the
plant's own defense system (immunization) which is known as "Systemic
Activated
Resistance" ("SAR").
Accordingly, with the compounds and methods of the invention, it is possible
to control plant
diseases on the one hand by strengthening the plant by activating its own
defence system
and on the other hand by additionally controlling the pathogens directly. The
compounds
offer a long lasting protection against a variety of pathogenes in different
crops.
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-18-
The compounds I can also be used as dressings in the treatment of seed (fruit,
tubers,
grains) and plant cuttings to provide protection against fungus infections as
well as against
phytopathogenic fungi which occur in the soil.
The compounds I are effective, for example, against phytopathogenic fungi
belonging to the
following classes: Fungi imperfecti (e.g. Botrytis, Pyricularia,
Helminthosporium, Fusarium,
Septoria, Cercospora and Altemaria) and Basidiomycetes (e.g. Rhizoctonia,
Hemileia,
Puccinia). Moreover, they are effective against the classes of the Ascomycetes
(e.g.
Venturia and Erysiphe, Podosphaera, Monilinia, Uncinula) and Oomycetes (e.g.
Phyto-
phthora, Pythium, Plasmopara).
Target crops to be protected within the scope of the present invention
comprise e.g. the
following species of plants: cereals (wheat, barley, rye, oats, rice, maize,
sorghum and
related species); beet (sugar beet and fodder beet); pomes, stone fruit and
soft fruit
(apples, pears, plums, peaches, almonds, cherries, strawberries, raspberries
and black-
berries); leguminous plants (beans, lentils, peas, soybeans); oil plants
(rape, mustard,
poppy, olives, sunflowers, coconut, castor oil plants, cocoa beans,
groundnuts); cucumber
plants (marrows, cucumber, melons); fibre plants (cotton, flax, hemp, jute);
citrus fruit
(oranges, lemons, grapefruit, mandarins); vegetables (spinach, lettuce,
asparagus,
cabbages, carrots, onions, tomatoes, potatoes, paprika); lauraceae (avocados,
cinnamon,
camphor); and plants such as tobacco, nuts, coffee, aubergines, sugar cane,
tea, pepper,
vines, hops, bananas and natural rubber plants, as well as ornamentals.
The compounds I are generally used in the form of compositions and can be
applied to the
crop area or plant to be treated, simultaneously or in succession, with
further compounds.
These further compounds can be, for example, fertilisers or micronutrient
donors or other
preparations that influence plant growth. They can also be selective
herbicides as well as
insecticides, fungicides, bactericides, nematicides, molluscicides or mixtures
of several of
these preparations, if desired together with further carriers, surfactants or
other application-
promoting adjuvants customarily employed in formulation technology.
Suitable solvents, carriers and adjuvants are known to the skilled person.
CA 02309973 2000-OS-15
WO 99/31464 PCT/EP98/08335
-19-
A preferred method of applying a compound of formula !, or an agrochemical
composition
comprising at least one of those compounds, is application to the leaves
(foliar application).
The frequency and rate of application depend upon the risk of infestation by
the correspon-
ding pathogen. The compounds I can, however, also penetrate the plant through
the roots
via the soil (systemic action) if the locus of the plant is impregnated with a
liquid formulation
or if the substances are introduced in solid form into the soil, e.g. in the
form of granules
(soil application). In paddy rice crops, such granules can be applied in
metered amounts to
the flooded rice field. In order to treat seed, the compounds I can, however,
also be applied
to the seeds (coating), either by impregnating the grains or tubers with a
liquid formulation
of the active ingredient, or by coating them with a solid formulation.
Advantageous rates of application are normally from 5 g to 2 kg of active
ingredient (a.i.)
per hectare (ha), preferably from 10 g to 1 kg a.i./ha, especially from 20 g
to 600 g a.i./ha.
When the compounds are used as seed dressings, dosages of from 10 mg to 1 g of
active
ingredient per kg of seed are advantageously employed.
The agrochemical compositions generally comprise 0.1 to 99 % by weight,
preferably 0.1 to
95 % by weight, of a compound of formula 1, 99.9 to 1 % by weight, preferably
99.8 to 5
by weight, of a solid or liquid adjuvant and 0 to 25 % by weight, preferably
0.1 to 25 % by
weight, of a surfactant.
Whereas commercial products will preferably be formulated as concentrates, the
end user
will normally employ dilute formulations.
The compositions may also comprise further auxiliaries, such as stabilisers,
antifoams,
viscosity regulators, binders or tackifiers, as well as fertilisers or other
active ingredients for
obtaining special effects.
The compounds of formula I can be mixed with other fungicides, producing in
some cases
unexpected synergistic effects.
Especially preferred mixing partners are
azoles, as azaconazole, bitertanol, bromuconazole, cyproconazole,
difenoconazole,
diniconazole, epoxiconazole, fenbuconazole, fluquinconazole, flusilazole,
flutriafol,
hexaconazole, imazalil, imibenconazole, ipconazole, metconazole, myclobutanil,
pefurazoate, penconazole, pyrifenox, prochloraz, propiconazole, tebuconazole,
tetraconazole, triadimefon, triadimenol, triflumizole, triticonazole;
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-20-
pyrimidinyl carbinoies, as ancymidol, fenarimol, nuarimol;
2-amino-pyrimidines, as bupirimate, dimethirimol, ethirimol;
morpholines, as dodemorph, fenpropidin, fenpropimorph, spiroxamin, tridemorph;
anilinopyrimidines, as cyprodinil, mepanipyrim, pyrimethanil;
pyrroles, as fenpiclonil, fludioxonil;
phenylamides, as benalaxyl, furalaxyl, metalaxyl, R-metalaxyl, ofurace,
oxadixyi;
benzimidazoles, as benomyl, carbendazim, debacarb, fuberidazole,
thiabendazole;
dicarboximides, as chlozolinate, dichlozoline, iprodione, myclozoline,
procymidone,
vinclozolin;
carboxamides, as carboxin, fenfuram, flutolanil, mepronil, oxycarboxin,
thifluzamide;
guanidines, as guazatine, dodine, iminoctadine;
strobilurines, as azoxystrobin, kresoxim-methyl, SSF-126 (metominostrobin or
fenominostrobin; SSF-129 (a-methoximino-N-methyl-2-[(2,5-
dimethylphenoxy)methyl}-
benzeneacetamide), trifloxystrobin (2-[a-([(a-methyl-3-trifluormethyl-
benzyl)imino}-oxy}-o-
tolyl] -glyoxylsaure-methylester-O-methyloxim);
dithiocarbamates, as ferbam, mancozeb, maneb, metiram, propineb, thiram,
zineb, ziram;
N-halomethylthiodicarboximides, as captafol, captan, dichlofluanid,
fluoromide, folpet,
tolyfluanid;
copper compounds, as Bordeaux-mixture, copper hydroxide, copper oxychloride,
copper
sulfate, cuprous oxide, mancopper, oxine-copper;
nitrophenol-derivatives, as dinocap, nitrothal-isopropyl;
organo-P-derivatives, as edifenphos, iprobenphos, isoprothiolane, phosdiphen,
pyrazophos,
tolclofos-methyl;
other compounds, as acibenzolar-S-methyl, anilazine, blasticidin-S,
chinomethionat,
chloroneb, chtorothalonil, cymoxanil, dichlone, diclomezine, dicloran,
diethofencarb,
dimethomorph, dithianon, etridiazole, famoxadone, fentin, ferimzone,fluazinam,
flusulfamide, fenhexamid, fosetyl-aluminium, hymexazol, kasugamycin,
methasulfocarb,
pencycuron, phthalide, polyoxins, probenazole, propamocarb, pyroquilon,
quinoxyfen,
quintozene, sulfur, triazoxide, tricyclazole, triforine, validamycin.
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-21 -
Examples
A. Preparation Examples
Abbreviations: Me = methyl; Et = ethyl; Pr = n-propyl; i-Pr = isopropyl; Bu =
n-butyl; i-Bu =
isobutyl; sec-Bu = sec-butyl; t-butyl = tert-butyl, Ph = phenyl; Ac = acetyl,
THF =
tetrahydrofuran; TPP = triphenylphosphine; Val = valine; m.p. = melting point
1. Compound No.l.1 lEl
0 0
CI~S ~ S CI NaAIH2(OCH2CHZOCH3)z CI-.(S ~ OH
OOH S~ C ~I
N F N F N F
F F F F C F F
A g
SOCI2
S O
S
CI~ ~ OOH ~ C02 / Coz(CO)e CI~ ~ SCI
~~N F N F
F F F F
E D
A mixture of thiazols A (synthesized according to EP 0279239) (25.5 g, 0.11
Mol) and
thionyl chloride (26.2 g, 0.22 Mol) in 25m1 of toluene, is held at reflux for
1.5 hours. After,
evaporation of the toluene under reduced pressure, 24 g of the acid chloride B
(b.p 90-92°,
45mbar) is distilled through a Vigreux column.
To the acid chloride B (97.5 g, 0.39 Mol) in 11 dry tetrahydrofuran at -
70°C under nitrogen
atmosphere, NaAIH2(OCH2CH20CH3)2 (commercial solution 3.5M in toluene,
0.429Mo1),
diluted in 300m1 of toluene, is added dropwise. After 45min of stirring at -
70°C, the cooling
bath is removed and the reaction is quenched with 370 ml of 3.5N HCI is added.
The
organic phase extracted with ethyl acetate, dried over sodium sulfate,
concentrated under
reduced pressure and flash-chromatographed to afford 67.5g of the alcohol C as
an oil.
A mixture of alcohol C (60 g, 0.276 Mol) and thionyl chloride (98.5 g, 0828
Mol) in 400 ml of
dichloromethane containing 0.1 ml of dimethyl formamide is stirred at reflux
for 8 hours:
Another portion of thionyl chloride (16.4 g, 0.138 Mol) is then added and the
reaction
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
heated for additional 16 hours. After the reaction is cooled down to room
temperature, the
solvent is removed under reduced pressure (60°C, 200 mbar) and the
resulting crude yellow
oil is distilled through a 5 cm Vigreux column (92-95°C, 20 mbar) to
give 53.5 g of
compound D as an colorless oil.
A mixture of compound D (125 g, 1.059 Mol), benzyitriethylammonium chloride
(4.8 g, 0.042
Mol), cobalt carbonyl (7.2 g, 0.042 Mol), sodium carbonate (101 g, 2.4 Mol),
1.5 I of water
and 1.361 of dichloromethane is stirred under carbon monoxide pressure (10
bars) for 24
hours at room temperature. The biphasic mixture is then filtered over celite,
extracted two
times with dichloromethane. The water phase is acidified with105 ml of
concentrated HCI
and extracted with ethyl acetate. The organic layer is then washed with brine,
dried over
sodium sulfate, treated with active charcoal at 60°C, filtered over
celite and concentrated in
vacuo to give 114 g of the acid E which is used without further purification.
2. Compound No 2 5 (J) lby Amdt-Eistert-reaction)
CI S OH 1. (COCI)2 S CH
---_-~. CI C-NzTMS PhCH 20H S
N HZ _~ CI--( ~ COOCHZPh
2. Me3SICH N
z 2 80 C N
G
H
To compound G (2.0 g, 10.44 mmol) dissolved in 20 ml of dichloromethane at
0°C, is added
oxalyl chloride (0.9 ml, 10.5 mmol}. After the development of carbon dioxide
stopped, 0.1 ml
of dimethylformamide is added and the yellow suspension is stirred at room
temperature for
3 hours. The resulting yellow solution is then concentrated under reduced
pressure,
dissolved in a mixture of 10 ml tetrahydrofuran and 10 ml acetonitrile, cooled
down to 0°C,
and successively treated with triethylamine (1.8 ml),
trimethylsilyldiazomethane (commercial
2N solution in hexane) (6.05 ml, 13.05 mmol). After 12 hours of stirring at
0°C, the solvents
are evaporated under reduced pressure, and the intermediate diazoketone G is
rearanged
in a mixture of 13 ml of benzylalcohol and 13 ml of trimethylpyridine at
180°C for 8 min. The
dark mixture is cooled down to room tempature, diluted with ethyl acetate,
washed 3 times
with citric acid (10% aqueous solution). The ethyl acetate layer is dried over
magnesium
sulfate, filtered, evaporated under reduced pressure, and flash-
chromatographed to give
the compound J as an oil.
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-23-
3. Compound No.1 2
S ~ COOCH3
c.--.y
N CF3
2-Chloro-4-trifluoromethyl-5-acetic acid (10 g, 40.71 mmol) in f 00 ml of
methanol is heat-
refluxed in presence of concentrated sulfuric acid (4 g, 40.71 mol) for
l2hours. After cooling
at room temperature, the methanol is distilled off under reduced pressure, the
residue is
dissolved in ethyl acetate, and successively washed with a saturated solution
of sodium
bicarbonate (3 times) and brine. After drying over magnesium sulfate, the
ethyl acetate
layer is filtered, evaporated under reduced pressure and flash-chromatographed
on silica-
gel to give 10.368 of the the title compound as a pate red oil.
4. Compound No.1 21
S NCO-NH-CH(COOMe)-CH(CH3)z
C!--~\ I
N CFa
The acid chloride of comp. 1.2 (78.5 g, 0.2973 Mol) and L-Valine methyl ester
hydrochloride are suspended in 600 ml of toluene, and heated at 110°C
for 25 minutes. The
resulting clear solution is cooled down to room temperature, extracted
successively with
water, a saturated solution of sodium bicarbonate, brine. The organic layer is
then dried
over sodium sulfate and the solvent removed under reduced pressure to give a
crude oil
which is chromatographed on silica-gel to afford 1028 of the title compound
(mp:51-53°C).
5. Compound No 1 76
Br
S ~COOCH3
c!---~\ 1
N CF3
A mixture of compound 1.2 (9 g, 34.7 mmol) and N-Bromosuccinimide (15.49 g, 87
mmol) in
200 ml of carbon tetrachloride, irradiated w'tth a 150W quartz lamp is heated
at reflux
temperature. After 1.5 hours of stirring, the mixture is cooled down to room
temperature and
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-24-
filtered over celite. After removal of the solvent under reduced pressure, the
filtrate is
suspended in hexane at 60°C and the solid filtered over celite. The
hexane evaporated
under reduced pressure to give a red oil, which after distillation
(150°C, 0.13mbars) gives
10g of the title compound as a pale red oil.
6. Compound No. 1 97
NH-CH2 Ph
~ COOCH3
cl--~\ I
N CFs
A mixture of compound 1.76 (1 g, 2.9 mmol) and (0.63 g, 5.9 mmol) of
benzylamin is stirred
at room temperature for 4 hours. After completion of the reaction, the
reaction mixture is
removed under reduced pressure, and chromatographed on silica to give 0.8 g of
the title
compound as an oil.
7. Compound No 1 82
CH3
\ COOCH3
CI--<\ (
N CF3
Compound 1.2 (1 g, 3.8 mmol) is added to a suspension of sodium hydride (55%
in mineral
oil) (0.17g, 4,2mmol) in tetrahydrofuran at -50°C, and the resulting
red solution stirred for
1 hour at -35°C. After this period, methyl iodide (0.7 g, 5 mmol) is
rapidly added. After 2
hours of stirring, the reaction is quenched with a saturated aqueous ammonium
chloride
solution, and extracted with ethyl acetate. The organic layer is dried over
magnesium
sulfate, evaporated under reduced pressure and chromatographed on silica to
give 0.72 g
of the title compound as a pale yellow oil.
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-25-
8. Compound No.1.92
OCH3
~COOCH3
cl--~~
N CF3
Compound 1.2 (1 g, 3.8 mmol) is added to a suspension of sodium hydride (55%
in mineral
oil) (0.17 g, 4,2 mmol) in dry tetrahydrofuran at -50°C, and the
resulting red solution stirred
for 1 hr at -35°C. Methyl chloroformiate ( 6 mmol) is then added, and
after 2hours of stirring,
the reaction is quenched with a saturated aqueous ammonium chloride solution,
and heated
up to room temperature. After extraction with ethyl acetate, the organic layer
is dried over
magnesium sulfate, filtered and evaporated under reduced pressure. The
reaction mixture
is purified on silica to afford 0.79g of the title compound as a white solid.
9. Compound No. 1.99
HsC CHs
~ COOCH3
cl--~~
N CF3
To a suspension of sodium hydride (55% in mineral oil)(0.61 g, 25.4 mmol) at -
50°C, is
added compound 1.2 (3 g, 11.55 mmol). After 2.5 hours of stirring at -
30°C, the red mixture
is cooled down to -78°C, treated with methyiiodide (4.92 g, 34,65
mmol), and slowly heated
up to -20C over a period of l.5hrs. After hydrolysis with a saturated aqueous
ammonium
chloride solution, the reaction mixture is extracted with ethyl acetate, dried
over magnesium
sulfate, and after evaporation of the solvent under reduced pressure, the
resulting crude
material is purified by flash-chromatography to give 2.06g of the title
compound as an oil.
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-26-
10. Compound No.1.100
OH
~COOEt
CI---~\
N CFs
To a tetrahydrofuran solution kept at -78°C of lithium
diisopropylamide, prepared at 0°C
from diisopropylamine (0.83 ml, 5.9 mmol) and n-butyl lithium (3.33 ml, 5.3
mmol), 2-chloro-
4-trifluoromethyl-thiazole is slowly added (1 g, 5.33 mmol). After 2 hours of
stirring, the
green solution is transferred, via a canula to a flask containing a solution
of ethylglyoxylate
(50% toluene commercial solution) (15m1, 10.6mmol) in tetrahydrofuran kept at -
78°C. After
minutes, the mixture is treated with a saturated aqueous ammonium chloride
solution,
extracted with ethyl acetate and concentrated in vacuo. The resulting crude
residue is then
purified by chromatography on silica-gel to give 0.28 g of the title compound
as an oil.
11. Compound No.1.106
HO CHs
~COOEt
CI--~\
N CF3
2-chloro-4-trifluoromethyl-thiazole (0.5 g, 2,66 mmol) dissolved in
tetrahydrofurane is
treated at -78°C with lithium hexamethyldisilyamide (commercial
solution, 1 M in
tetrahydrofuran, 2,67m1), stirred for 1.5 hours, followed by the addition of
ethylpyruvate
(0.305 ml, 2.9 mmol). After the reaction is completed, it is quenched with a
saturated
aqueous ammonium chloride solution, extracted with ethyl acetate. The organic
layer is
dried over magnesium sulfate, concentrated under reduced pressure and
chromatographed
on silica-gel to afford 0.730 g of the title compound as a yellow oil.
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-27-
12. Compound No. 1.113
COOEt
Lr3
2-Chloro-4-trifluoromethyl-thiazole (2 g, 10.66 mmol) dissolved in
tetrahydrofuran is treated
at -78°C with lithium hexamethyldisilyamide {commercial solution, 1 M
in tetrahydrofuran,
2,67 ml), stirred for 1.5 hours, followed by the addition of
ethylbromopyruvate {1.79 ml,
12.79 mmol). After the reaction is completed, it is quenched with a saturated
aqueous
ammonium chloride solution and heated up to room temperature. The reaction
mixture is
then extracted with ethyl acetate, the organic layer dried over magnesium
sulfate, the
solvent removed under reduced pressure, and the crude material chromatographed
on
silica-gel to afford 0.2548 of 2.088 of the title compound as oils.
13. Compounds Nos. 2.18 (Dl. 2.19 (R). 2.25 S) !Scheme 13)
(a) A mixture of compound K (179 g, 0.871 Mol) NBS (159.8 g, 0.871 Mol) and
Azoisobutyronitrile (AIBN) (14.6 g, 87 mmol) in 600 ml of CCI4 is heated at
reflux for 16 hrs.
After cooling, the crude mixture is filtered, concentrated under reduced
pressure and flash-
chromatographed to afford 190 g of compound L contaminated with the starting
material K.
(b) To a solution of compound L (189.4 g, 0.666 Mot) in 1.5 I of acetonitrile,
is added
0.3 I of 4A molecular sieves followed by N-methylmorpholine-N-oxide {139.2 g,
0.99 Mol).
After 2.5 hrs. of stirring at room temperature, the mixture is filtered on
silicagel,
concentrated in vacuo and purified by flasf~-chromatography to give 92 g of
aldehyde M.
(c) A solution of aldehyde L (91.25 g, 0.415 Mol), ethylene glycol (29 ml, 0.5
Mol) and
p-totuenesulfonic acid (9.12 g, 41 mmol) is heated for 16 hrs. at reflux in
300 ml of benzene
while water is destilled off. After cooling, the crude mixture is extracted
with water and ether,
the organic phase is then dried over MgS04, concentrated under reduced
pressure and
purified by flash-chromatography to give 65 g of the dioxolane N.
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
_28_ _
Scheme 13
COOEt COOEt g COOEt
CI~\ I S
CI~
N (a) ~N Br (b) \N ~ CHO
K L M
COOEt
CHZOH
CI~\
O
..__". N ~ ~ O
(~) N
p
N O O
COOH
I
_'~' CI"-~\ ~ O -.~ N O
(e) N
p
O p
P
_~ CI S COOMe CI S COOMe
'_"~\ ~
(9) N O (h) N CHO
pJ
R
(d) To a suspension of LiAIHa (1.78 g, 45.4 mmol) in 220 ml of dry THF at
0°C, is
added dropwise compound N (10 g, 37.9 mmol) dissolved in 100 ml of THF. After
5 min of
stirring the reaction is completed. The mixture is successively treated with
1.78 ml of water,
1.78 ml of NaOH (15% aqueous solution) and 5.34 ml of water. The suspension is
then
filtered over celite, extracted 3 times with ethylacetate and water. The
combined organic
phases are concentrated under reduced pressure and chromatographed on silica
to afford
31 g of the alcohol O.
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-29-
(e) To a solution of compound O ( 9.85 g, 44.46 mmol) in 180 ml of CC14 is
added
triphenlyphosphine (11.8 g, 44.46 mmol). The mixture is stirred at 85°C
for 24 hrs. After
cooling, the crude solution is concentrated under reduced pressure and
purified by
chromatography on silica to afford 6.4 g of compound P.
(f) A mixture of compound P {7.43 g, 30.9 mmol), benzyltriethylammonium
chloride
(283 mg, 1.24 mmol), cobalt carbonyl (423 mg, 1.24 mmol), sodium carbonate
(5.83 g, 69.4
mmol), 68 ml of water and 62 ml of dichloromethane is stirred under carbon
monoxide
pressure (10 bars) for 24 hours at room temperature. The biphasic mixture is
then filtered
over celite, extracted two times with dichloromethane. The water phase is
acidified to pH 2
with concentrated HCI and extracted with ethyl acetate. The organic layer is
then washed
with brine, dried over sodium sulfate, filtered and concentrated in vacuo to
give 3.2 g of the
acid D which is used without further purification.
(g) A solution of the acid Q in THF is carefully treated at room temperature
with an
ether solution diazomethane. The reaction is monitored by tlc. After
completion of the
reaction, the crude mixture is concentrated under reduced pressure and
chromatographed
to 2 g of the methylester R.
(h) Compound R (1.61 g, 6.47 mmol) is stirred for 40 min in 19 ml of THF, 19
ml of
water and 9 ml of trifluoroacetic acid. After evaporation of the solvent, the
crude mixture is
diluted with ether and washed with NaHC03 (sat. aqueous solution). The ether
phase is
concentrated under reduced pressure to afford the aldehyde S which is an oil.
The aldehyde group may be converted by known methods into many different other
groups.
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-30
Table 1.A
Table 1 R , R 2
~ CO-A
CI~\
N CF3
No. R, RZ A Phys. data
m.p °C
1.1. H H OH 122-123°C
1.2. H H OMe oil
1.3. H H oil
o-~co~Ec
1.4. H H Of Bu oil
1.5. H H OCH2CH=CH2 oil
1.6. H H ocHZ~ oil
'~ /0
1.7. H H OCH2Ph oil
1.8. H H oil
OCH= \ ~ OMe
1.9. H H oMe solid
OCHz \ ~ OMe
1.10. H H o~ oil
ocHz ~ ~ oMe
OMe
1.11. H H M°O OMe oil
ocH2 ~ ~ oMg
1.12. H H o\ oil
ocH2 ~ 00/
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-31
No. R, R2 A Phys. data
m.p °C
1.13. H H M°° solid
ocH2 ~ ~ oMe
1.14. H H oil
OCHZ \ ~ OCF~
1.15. H H solid
OCHz \ / NOZ
1.16. H H oil
OCHz \ ~ OCHzPh
1.17. H H oil
OCHi ~ ~N
1.18. H H 90-91
ocHZ
N
1.19. H H cl oil
oCH~ ~ ~-CI
N
1.20. H H OCH2C4Me oil
1.21. H H CoZMe 51-53
NH---
1.22. H H NMe2
1.23. H H NHMe
1.24. H H NHEt
1.25. H H NHn-Bu
1.26. H H NHt-Bu
1.27. H H cl 127-8
NHCHz \ ~ CI
1.28. H H 142-3
NHCHz ~ ~ CI
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-32
No. R, R2 A phys. data
m.p °C
1.29. H H ~I 122-3
NHCHz \ ~ CI
1.30. H H G solid
NHCHz \
1.31. H H F 105-6
NHCHZ ~ ~ F
1.32. H H F 51-2
NHCHz
F
1.33. H H F solid
NHCH~ \
1.34. H H F 85-6
NHCH2
F
1.35. H H F solid
NHCHs ~ ~ F
1.36: H H F 115-6
NHCHz \
1.37. H H 110-20
NHCHi ~ ~ F
1.38. H H F 123-4
NHCH2 ~ ~ Br
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-33- -
No. R, R2 A Phys. data
m.p °C
1.39. H H ~F~ 98
NHCHz
1.40. H H ~F~ 97-101
NHCHz
1.41. H H 102-4
NHCHZ ~ ~ CF3
1.42. H H ~F3 106-7
NHCHs \
CFA
1.43. H H 99-101
NHCH2
Me
1.44. H , H 120
NHCH2 ~ ~ Me
1.45. H H solid
NHCHZ \ ~ NOz
1.46. H H NHCH2Ph 110-1
1.47. H H 126-30
NHCHz ~ ~ OMe
1.48. H H Mao 115
NHCH2 \
1.49. H H oMe 107-8
NHCHZ \
1.50. H H oMe 132-3
NHCHz \ ~ OMe
CA 02309973 2000-OS-15
WO 99/32464 PC'T/EP98/08335
_~_ _
No. R, R2 A Phys. data
m.p °C
1.51. H H Meo 159-60
NHCHz ~ ~ OMe
Me0
1.52. H H oMe 140-2
NHCHZ
OMe
1.53. H H o~ 130-1
NHCHs ~ ~ O
1.54. H H Br 1 gg-g
NH
Br
1.55. H H Br solid
NH ~ ~ Br
Br
1.56. H Br 51
NH \ ~ OCF~
Br
1.57. H H ONHCOOCH2Ph oil
1.58. H H ONHCH(CH3)Z oii
1.59. H H ONHC(CH3)3 oii
1.60. H H ON=C(CH3)OEt oil
1.61. H H ONHCOOC(CH3)3 oil
1.62. H H ONHCOOEt oil
1.63. H H ONHS02Ph 114-20
1.64. H H NHOC(Ph)3 177
1.65. H H NHOCH2Ph 78-85
1.66. H H NHOMe 117-8
1.67. H H NHOCH2CH=CH2 73-4
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-35
No. R, R2 A Phys. data
m.p C
1.68.H H NHOC(CH3)3 solid
1.69.H H NHOPh
1.70.H H F F solid
NHOCHz \ / F
F F
1.71.H H SMe oil
1.72.H H SEt
1.73.H H SPh
1.74.H H SCH2Ph
1.75.Br H OH oil
1.76.Br H OMe oil
1.77.F H OH
1.78.F H OMe oil
1.79.CI H OMe
1.80.CI H OH
1.81.Me H OH oil
1.82.Me H OMe oil
1.83.Et H OMe oil
1.84.Et H OH oil
1.85.Pr H OH
1.86.Pr H OMe
1.87.nBu H OH
1.88.nBu H OMe
1.89.CH2CH=CH2 H OH oil
1.90.CH2Ph H OH 127-8C
1.91.CH2Ph H OMe oil
1.92.COOMe H OMe oil
1.93.CHZCOOH H OH 130-1 C
1.94.NH2 H OH 166-7C
1.95.NHZ.HCI H OMB 176-7C
1.96.NEt2 H OMe 56-8C
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-36- --
No. R, R2 A Phys. data
.
m.P C
1.97. NHCH2Ph H OMe oil
1.98. Me Me OH 137-8C
1.99. Me Me OMe oil
1.100.OH H OEt oil
1.101.OH H OH
1. OAc H OEt oil
i
02.
R' R _ _ A
1.103.a H OEt oil
oco
1.104.OMe H OEt oil
1.105.OSi-t-BuMe2 H OEt oil
1.106.OH Me OEt oil
1.107.OH CF3 OMe oil
1.108.OH Ph OEt 77-9C
1.109.OH CHZBr OEt oil
1.110.s cF, OH OEt oil
cl--~~
N CHI
1.111.s cF~ H OMe 151-2C
~
CI~~
I H
~
N CoOMe
~:
1.112.R1+R2 H OEt oil
=O
1.113.R1+RZ H OEt oil
U
1.114.rN OH OEt oil
N~/N~C~
CA 02309973 2000-OS-15
WO 99/32464 PGT/EP98/08335
Table 1.B
Compounds of the formula
R~
NiS ~ ~'CO-A
CF3
CI
wherein the R,, R2 and A have the meanings of the corresponding compounds of
Table 1.A.
Table 1.C
Compounds of the formula
R~
NiS ~ NCO-A
N CF3
wherein R,, R2 and A have the meanings of the corresponding compounds of Table
1.A.
Phys. data of compounds) of Table 1.C:
No. R, R2 A Phys. data
m.p. °C
1.C.1 H H OH oil
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
_3g_
Table 2.A
R~ RZ
NCO-A
CI--~\
N R
3
No R, RZ R3 A Phys.data
m.p.C
2.1. H H H OCH2Ph oil
2.2. H H H OH
2.3. H H Me OCHZPh oil
2.4. H H Me OH
2.5. H H Et OCHZPh oil
2.6. H H Et OMe oil
2.7. H H Et OH 191-3
2.8. H H n-Pr OMe oil
2.9. H H 2-Pr OMe oil
2.10. H H 2-Pr OH
2.11. H H cyclo-Pr OMe oil
2.12. H H t-Bu OMe oil
2.13. H H t-Bu OH
2.14. H H Ph OMe oil
2.15. H ?-I CH2Ph OMe oil
2.16. H H 2-thiophenyl OMe oil
2.17. H H COOMe OMe oil
2.18. H H ~~ ~ OH oil
0
2.19. H H ~ OMe oil
~
~
0
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-39- _
No R, R2 R3 A Phys.data
m.p.C
2.20. H H o OH
0
2.21. H H s OH
s
2.22. H H s- OH
2.23. H H /s OH
S
2.24. H H o OMe
0
2.25. H H CHO OMe oil
2.26. H H CH20H OMe solid
2.27. H H CH2CI OMe oil
2.28. H H CH2Br OMe
2.29. H H CH2F OMe
2.30. H H CH2NHC00- OMe
t-Bu
2.31. H H CH2NEtz OMe
2.32. H H CH2NH2 OMe
2.33. H H CH2NHOH OMe
2.34. H H CH=CH2 OMe
2.35. H H CH=CHCOOMe OMe
2.36. H H CH=CHMe OMe
2.37. H H CH=CBrz OMe
2.38. H H CHOHMe OMe
2.39. H H CHOHEt OMe
2.40. H H CHOHCIMe OMe
2.41. H H CHOHFMe OMe
2.42. H H CHOHBrMe OMe
CA 02309973 2000-OS-15
WO 99/32464 PC'T/EP98/08335
- 40 - -
No R, R2 R3 A Phys.data
m.p.C
2.43. H H CH=CF2 OMe
2.44. H H COEt OMe
2.45. H H CHOHMe OMe
2.46. H H CHCIMe OMe
2.47. H H CHFMe OMe
2.48. H H CHBrMe OMe
2.49. H H 4-CI-Ph OMe
2.50. H H 3-Me0-Ph OMe
2.51. H H 2,4-Me2-Ph OMe
2.52. H H - H OMe
2.53. Br H COHMe2 OCH2Ph
2.54. Br H COHEt2 OH
2.55. F H Et OCHZPh
2.56. CI H Et OH
2.57. CI H n-Pr OCH2Ph
2.58. Me H 2-Pr OMe
2.59. Et H cyclo-Pr OH
2.60. Et H t-Bu OMe
2.61. Pr H t-Bu OMe
2.62. Pr H Ph OH
2.63. nBu H CHzPh OMe
2.64. nBu H 2-thiophen OMe
2.65. CH2CH=CH2 H COOMe OH
2.66. CH2Ph H ~~ ~ OMe
0
2.67. CHZPh H ~ OMe
~
0
2.68. COOMe H OMe
~ ~
0
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-41 - -
No R, R2 R3 A Phys.data
nl.~.C
2.69. CHZCOOH H s OMe
s
2.70. NH2 H - OMe
~
r
s-
2.71. NH2.HC1 H /s\ OCH2Ph
s
2.72. NEt2 H o OH
0
2.73. NHCH2Ph H CHO OCHZPh
2.74. Me H CH20H OH
2.75. Me H CHZCI OCHZPh
2.76. OH H CHZBr OMe
2.77. OH H CH2F OH
2.78. OAc H CHCI2 OMe
2.79. _ c~ H H OMe
oco
2.80, OMe H Me OH
2.81. OSi-t-BuMez H Me OMe
2.82. OH H Et OMe
2.83. OH H Et OH
2.84. OH H Et OMe
2.85. OH H n-Pr OMe
2.86. s cF, CF3 2-Pr OMe
ci--
N
2.87. s cF, Ph 2-Pr OMe
ci-.<\
~COOMe
2.88. R,+R2 CH2Br cyclo-Pr OMe
=O
CA 02309973 2000-OS-15
WO 99/32464 PCf/EP98/08335
-42
No R, RZ R3 A Phys.data
m.p.°C
2.89. R~+RZ ~ OH t-Bu OMe
2.90. H H cyclo-Pr OCHZPh oil
Ta le 2.B
Compounds of the formula
O-A
N
3
C~
wherein R,, R2, R3 and A have the meanings of the corresponding compounds of
Table 2.A.
Table 2.C
Compounds of the formula
i O-A
N
R3
wherein R,, R2, R3 and A have
the meanings of the corresponding
compounds of Table 2.A.
Phys. data of compounds of
Table 2.C:
No. R, R2 R3 A Phys.data
m.p.C
2.C.3 H H Me OCH2Ph oil
2.C.5 H H Et OCH2Ph oil
2.C.7 H H Et OH oil
2.C.20 H H .o OH solid
Co--i
R, R2
iS ~ ~C
R
~C
N
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-43
Table 3.A
R, R2
S Z
CI-~~
N R
3
No. R, R2+ Z R3 Phys. data
m.p.°C
3.1. H CF3 159-61 °C
H
~O
O
3.2. H H CF3 OII
""~
R,+Rz+Z
I
0
0
3.3. H ,~ ~ CF3 solid
H
/O-~Me
'O
~
I
IO
3.4. H M ,,~ CF3 solid
0
r < 'o
~
0
3.5. H Me' Me CF3
~O
'NH
~
I
IO
3.s. H cF3
Me
~
N
~ ~O
X
' IO
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
_~_
No. R, Rz+ Z R3 Phys. data
m.p.C
3.7. H ~ Me CF3
o~Me
NH
0
3.8. H Me Me CF3
H
N--~Me
/
r ' 'O
~
(
I '
O
3.9. H Me a CF3
N- \~
'NH
~
(
~ I
O
3.10. H M ,,,~ CF3
N
C 'NH
~
I
'O
3.11. H Me
H
HO
O
3.12. H H H Me
"~
R1+R2+Z
I
0
0
3.13. H Me Me Et
H
~
~
O--'r Me
~ ~~O
I IO
3.14. H M ,"~ Et
0
~<'o
~
0
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-45-
No. R, R2+ 2 R3 Phys. data
m.p.C
3.15. H Me Et
Me
'
~0
'NH
x
I
IO
3.16. H H ,"~ n-Pr
N
l C 'O
X
I
'O
3.17. H Me ,,~ 2-Pr
H
~
~
/O-'( Me
'NH
x
I
IO
3.18. H Me Me 2-Pr
H
~ ~
N~Me
/
'O
N
I
IO
3.19. H Me M8 cyclo-P r
H
~ ~
N~Me
/
f ' 'NH
C
' I
O
3.20. H Me t-Bu
Me
N '
~,(
'NH
X
,
~O
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
- 46 -
Table 3.B
Compounds of the formula
R~ R2
\Z
N~ I
R3
CI
wherein R,, R2, R3 and Z have the meanings of the corresponding compounds of
Table 3.A.
Table 3.C
Compounds of the formula
S \Z
N~ i
N R
3
wherein R,, R2, R3 and Z have the meanings of the corresponding compounds of
Table 3.A.
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-47- _
Ta le 4.A
R, R2
S
R e~\
N R
3
No. R, RZ R3 R4 Z Phys.data
m.p.C
4.1. H H CF3 Ph COOMe oil
4.2. H H Me CF3 COOH
4.3. H H CF3 H COOH
4.4. H H CF3 H COOMe oil
4.5. H H Me H COOEt solid
4.6. R,+R2 Me H COOEt solid
=O
4.7. R,+R2=O Me NHCOOEt COOEt solid
4.8. R,+RZ Me NHn-Bu COOEt solid
=O
4.9. R,+R2 Me NHt-Bu COOEt solid
=O
4.10.H H Me NHMe COOEt solid
4.11.H H Me NHCH2CH=CH2 COOH solid
4.12.H H Et NH-t-Bu COOEt solid
4.13.H H Ph p-PhS02NH2 COOEt solid
4.14.H H p-CIPh NHPh COOEt solid
4.15.H H OH OH COOH solid
4.16.R,+R2 Me NH-COOEt COSMe
=O
4.17.R,+R2 Me NH-n-Bu COSMe
=O
4.18.R,+R2 Me NH-t-Bu COSMe
=O
4.19.H H CF3 CF3 COSMe
4.20.H H CF3 OMe COOMe
4.21.H H CF3 OEt COOMe
4.22.H H CF3 O-n-Pr COOMe
4.23.H H CF3 SMe COOMe
4.24.H H CF3 SEt COOMe
CA 02309973 2000-OS-15
WO 99/32464 PGT/EP98/08335
_48- _
No. R, RZ R3 R4 Z Phys.data
m.p.C
4.25.H H CF3 SPh COOMe
4.26.H H CF3 NMez COOMe
4.27.H H CF3 NEt2 COOMe
4.28.H H CF3 NHZ COOMe
4.29.H H CF3 SH COOMe
4.30.H H CF3 NH2 CSOMe
4.31.H H Me CI p solid
---~
r
_
4.32.H H CF3 Ph CSOMe
4.33.H H Me CF3 CSOH
4.34.H H CF3 H CSOH
4.35.H H CF3 H CSOMe
4.36.H H Me H CSOEt
4.37.R,+RZ Me H COOEt
=S
4.38.R,+R2 Me NHCOOEt COOEt
=S
4.39.R,+RZ Me NH-n-Bu CSOEt
=S
4.40.R,+R2 Me NH-t-Bu CSOEt
=S
4.41.H H Me NHMe CSOEt
4.42.H H Me NHCH2CH=CH2 CSOH
4.43.H H Et NHt-Bu CSOEt
4.44.H H Ph p-PhS02NH2 CSOEt
4.45.H H p-CIPh NHPh CSOEt
4.46.H H OH OH CSOH
4.47.R,+R2 Me NH-COOEt CSSMe
=O
4.48.R,+RZ Me NHn-Bu CSSMe
=O
4.49.R,+R2 Me NHt-Bu CSSMe
=S
4.50.H H Et CF3 COSMe
4.51.H H Et OMe COOMe
4.52.H H n-Pr OEt COOMe
4.53.H H i-Pr n-Pr COOMe
4.54.H H OH SMe COOMe
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-49-
No. R, R2 R3 R, Z Phys.data
m.p.C
4.55.H H OH SEt COOMe
4.56.H H CF3 SPh CONHMe
4.57.H H CF3 NMe2 CONHMe
4.58.H H CF3 NEt2 CONHMe
4.59.H H CF3 NH2 CSNHMe
4.60.H H CF3 SH CSNHMe
4.61.H H CF3 CSNHMe
Table 4.B
Compounds of the formula
" 4
wherein R,, R2, R3, R,, and Z have the meanings of the corresponding compounds
of Table
4.A.
Ta le 4.C
Compounds of the formula
R, R2
S \Z
N~ I
N R
3
wherein R,, RZ, R3 and Z have the meanings of the corresponding compounds of
Table 4.A.
Formulation Examples
for similar purposes of pesticidal use are descibed for example in WO
97/33890.
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-50-
Biological Examples
Example B.1: Immunization of Cucumis sativus L a,.giainst Collgtotrichum
lag~enarium
a) After a cultivation period of 2 weeks, cucumber plants are sprayed with a
spray mixture
prepared from a wettable powder formulation of the test compound
(concentration:
200 ppm). After 72 hours, the plants are infected with a spore suspension (1.0
x 105
spores/ml) of the fungus and incubated for 30 hours at high humidity and a
temperature of
23°C. Incubation is then continued at normal humidity and 22°C
to 23°C.
Evaluation of protective action is made 7 to 8 days after infection and is
based on fungus
infestation.
b) After a cultivation period of 2 weeks, cucumber plants are treated by soil
application with
a spray mixture prepared from a wettable powder formulation of the test
compound
(concentration: 20 ppm, based on the volume of the soil). After 72 hours, the
plants are
infected with a spore suspension (1.5 x 105 spores/ml) of the fungus and
incubated for
30 hours at high humidity and a temperature of 23°C. Incubation is then
continued at
normal humidity and 22°C.
Evaluation of protective action is made 7 to 8 days after infection and is
based on fungus
infestation.
Compounds of the Tables exhibit good activity in tests (a) and (b) and reduce
fungus
infestation to 0 tc 20 %. On the other hand, Colletotrichum infestation is 90
% on untreated
and infected control plants.
c) Comparison test: Direct action against Colletotrichum laqenarium
The fom~ulated active ingredient is mixed in various concentrations (100, 10,
1, 0.1 ppm)
with autoclaved and cooled nutrient medium containing 10 000 spores per ml and
is poured
into microtitre plates. Incubation is then carried out at 22°C in the
dark. After 2 to 3 days,
fungus growth is measured by spectrophotometry.
With compounds of the Tables, no inhibition of fungus growth is observed; on
the other
hand, when the fungicide "Benomyl" (commercial product) is used as comparison
substance
at 0.2 ppm, 50 % inhibition (ECM) of fungus growth occurs.
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-51
Example B.2: Action against Phytophthora infestans on tomato lants
a) After a cultivation period of 3 weeks, tomato plants are sprayed with a
spray mixture
prepared from a wettable powder formulation of the test compound (0.02 %
active ingre-
dient). After 72 hours, the treated plants are infected with a sporangia
suspension of the
fungus. Fungus infestation is evaluated after incubation of the infected
plants for 5 days at
90-100 % relative humidity and 20°C.
Compounds of the Tables exhibit good activityin the tests and reduce fungus
infestation to
0 to 20 %. On the other hand, Phytophthora infestation is 60 % on untreated
and infected
control plants.
Example B.3: Action against Pyricularia oryzae on rice~~lants
2-week-old rice plants are watered with a spray mixture prepared from a
wettable powder
formulation of the test compound (0.006 % active ingredient, based on the
volume of the
soil). The pots are then filled with water until the lowermost parts of the
stems of the rice
plants are standing in water. After 96 hours, the treated rice plants are
infected with a
conidia suspension of the fungus. Fungus infestation is evaluated after
incubation of the
infected plants for 5 days at 95-100 % relative humidity and approximately
24°C.
In comparison with untreated control plants (100 % infestation), fungus
infestation on rice
plants treated with a spray mixture comprising a compound of the Tables as
active ingre-
dient is only approximately 50 %.
Example B.4: Action against Cercospora nicotine on tobacco plants
a) Foliar application
Tobacco plants (13 weeks old) are sprayed with a formulated solution of the
test compound
(concentration: 0.02 % active ingredient). Four days after treatment, the
plants are inocu-
lated with a sporangia suspension of Cercospora nicotine (150 000 spores/ml),
kept for
days in the dark at 25°C and high humidity and then incubated further
under a normal
day/night sequence.
Evaluation of the symptoms in the tests is based on the leaf surface infested
with fungus.
Infestation is approximately 60 % on the control plants; on plants treated
with compounds of
the Tables, infestation is 0 to 30 %.
CA 02309973 2000-OS-15
WO 99/32464 PCT/EP98/08335
-52-
Example B.S: Action against Eryrsiphe graminis on wheat
Protective action: 18-day-old wheat plants are sprayed with a formulated
solution of the test
compound (0.02 % active ingredient). Immediately after the treatment the
plants are incuba-
ted under cylinders. 24 hours later, the plants are covered. After a further 3
days, the
treated plants are cut off above the primary leaf. The primary leaves are
arranged horizon-
tally and are inoculated in a dusting bell with Erysiphe graminis spores
(spore density:
0.2 mg/mZ). The test is carried out in a climatic chamber with 12 hours of
light (18 KLux), at
20°C and 12 hours of darkness, at 18°C. Infestation is evaluated
9 and 13 days after
inoculation.
Compounds of the Tables exhibit good activity in the tests and reduce fungus
infestation to
0 to 20 %. On the other hand, Erysiphe infestation is 70 % on untreated and
infected
control plants.