Note: Descriptions are shown in the official language in which they were submitted.
CA 02310290 2000-OS-30
COMPOSITION FOR AND METHOD OF PREVENTING OR TREATING
BREAST CANCER
Field of the Invention
The present invention relates to a composition containing a selective estrogen
receptor modulator and at least one isoflavone, and a method of treating
breast cancer
while inhibiting selective estrogen receptor modulator induced uterotrophic
effects.
Back round of the Invention
Breast cancer is one of the leading causes of cancer mortality among Western
women, and is predicted to become a leading cause of cancer death in Oriental
women in
countries such as Japan in the near future. The American Cancer Society
estimates that 1
in 9 women face a lifetime risk of this disease, which will prove fatal for
about one-
quarter of those afflicted with the disease.
Tamoxifen (Fig. 1), a synthetic nonsteroidal selective estrogen receptor
modulator, has been used effectively in the treatment of breast cancer for
over twenty
years. Tamoxifen is one of the most widely prescribed antineoplastic agents in
the
United States and Great Britain, and is one of the initial hormonal treatments
of choice in
both premenopausal and postmenopausal women with estrogen receptor positive
metastatic disease. Furthermore, adjuvant therapy studies show a substantial
reduction of
contralateral primary breast carcinoma in tamoxifen treated women, which
indicates that
tamoxifen may be of use in breast cancer prevention.
Tamoxifen has tissue-specific estrogenic and antiestrogenic effects. Estrogen,
an
ovarian hormone, increases the risk of breast and endometrial cancer by
inducing an
estrogen receptor mediated increase in the frequency of breast and endometrial
cell
division. Cell division is essential in the complex process of genesis of
human cancer
since it per se increases the risk of genetic error - particularly genetic
errors such as
inactivation of tumor suppressor genes.
Tamoxifen has antiestrogenic effects in breast tissue. Tamoxifen's
antiestrogenic
effect in breast tissue is a primary mechanism by which tamoxifen inhibits the
EI193646463US
CA 02310290 2000-OS-30
proliferation of breast cancer cells. Tamoxifen competes with estrogen for
binding to
cytoplasmic estrogen receptors ("ER"), with subsequent inhibition by the
tamoxifen/ER
complex of many of the activities of endogenous estrogen within tumor cells.
Endogenous estrogen binds with ERs to promote cellular activities such as
estrogen/ER-
mediated gene transcription, DNA synthesis, cancer cell growth, and increases
in
autocrine polypeptides such as transforming growth factor-alpha, epidermal
growth
factor, insulin-like growth factor-II, and other growth factors that may be
involved in cell
proliferation. Competitive inhibition of estrogen binding to ERs by tamoxifen
reduces or
prevents such cancer growth inducing cellular activities. As a result of
tamoxifen's
antiestrogenic activity in breast tissue, tamoxifen prevents the transition of
breast cancer
cells from the early G1 phase to the mid-G1 phase of the cell cycle and
exhibits a
cytostatic effect on breast cancer cells. Tamoxifen has been shown to reduce
distant
breast cancer metastasis as well as local-regional recurrence of such cancers
in both node-
negative and node-positive women.
Tamoxifen, however, has an estrogenic effect on uterine tissues when
endogenous
estrogen levels are low, which occurs in postmenopausal women and
oopherectimized
women. Uterine epithelial cell heights are significantly increased by the
estrogenic effect
of tamoxifen in postmenopausal and oopherectimized women, leading to uterine
hypertrophy. Tamoxifen also causes marked uterine eosinophilia. These effects
have
been associated with endometrial carcinoma, and long term use of tamoxifen is
linked to
an increased risk of endometrial cancer, up to a fivefold excess of risk
relative to women
not treated with tamoxifen therapy. Therefore, application of tamoxifen for
long term
breast cancer prevention and long term treatment of breast cancer has
significant
associated risks.
Efforts have been made to develop new selective estrogen receptor modulators
("SERMS") which act in a mechanism similar to that of tamoxifen in breast
tissue, while
avoiding the risks caused by the estrogenic effects of tamoxifen in uterine
tissue. Several
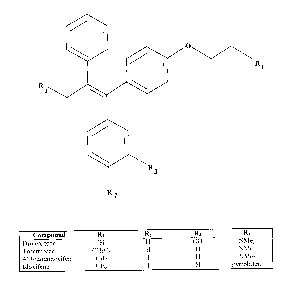
of these SERMS are triphenylethylene tamoxifen analogs. As shown in Fig. 2,
droloxifene is a tamoxifen analog in which a 3'-hydroxyphenyl moiety is
substituted in
place of a phenyl moiety of tamoxifen. Droloxifene has a binding affinity for
the
CA 02310290 2000-OS-30
estrogen receptor which is ten times that of tamoxifen, has been shown to have
antiestrogenic .activity in breast tissue and to be efficacious in treatment
of advanced
breast cancer, yet has lower estrogenic effects in uterus tissue than
tamoxifen.
Droloxifene a New Estrogen AntagonistlA~onist Prevents Bone Loss in
Ovariectomized
Rats, Ke at al., Endocrinology 136:2435-2441 (1995).
Toremifene, shown in Fig. 2, is a tamoxifen analog having a 4-chloro
substituent.
Pharmacologically toremifene has quite similar effects as tamoxifen on breast
tissue,
acting as potent antiestrogen. Toremifene also exhibits anti-tumor cytolytic
effects at
high doses which are independent of its antiestogenicity, effects which do not
occur with
high doses of tamoxifen. Antiestro~,enic Potency of Toremifene and Tamoxifen
in
Postmenopausal Women, Homesley et al., Am. J. Clin. Onc., 16(2):117-122
(1993).
4-Iodotamoxifen, shown in Fig. 2, is another tamoxifen analog, having a 4'-
iodophenyl substituent in place of a phenyl substituent of tamoxifen.
Iodination of
tamoxifen at the 4'-phenyl postion reduces estrogenic activity, mimicking the
high
antiestrogenic activity of the tamoxifen metabolite 4'-hydroxytamoxifen, while
giving the
compound a longer duration of action in vivo by blocking formation of the
rapidly
metabolized 4'-hydroxytamoxifen metabolite. Pyrrolidino-4-iodotamoxifen and 4-
Iodotamoxifen New Analogues of the Antiestro~,en Tamoxifen for the Treatment
of
Breast Cancer, Chander et al., Cancer Research, 51:5851-5858 (November 1,
1991);
Idoxifene~ Resort of a Phase I Study in Patients with Metastatic Breast
Cancer, Coombes
et al, Cancer Research, 55:1070-1074 (March 1, 1995). 4-Iodotamoxifen has been
shown
to have less estrogenic agonist activity in uterine tissue than tamoxifen,
and, therefore, is
less likely to cause endometrial cancer when administered over a long term.
Idoxifene, also known as pyrrolidino-4-iodotamoxifen, shown in Fig. 2, is
another
tamoxifen analog, and is modeled on the 4'-iodotamoxifen analog. Idoxifene has
the
same general molecular structure as 4'-iodotamoxifen, except that the N,N-
dimethylamino moiety of 4'-iodotamoxifen is replaced with a pyrrolidino
moiety.
Substitution of the pyrrolidino group for the dimethylamino group reduces
possible toxic
side effects by inhibiting the metabolization of the compound by the liver to
a desmethyl
metabolite with the concomitant release of potentially toxic formaldehyde.
Idoxifene has
CA 02310290 2000-OS-30
a 2.5 to 5 fold higher affinity for ERs than tamoxifen, and is 1.5-fold more
efffective in
inhibiting the growth of MCF-7 breast cancer cells. Idoxifene also has less
uterotrophic
estogenic effects than tamoxifen and 4'-iodotamoxifen, and produced
uterotrophic effects
comparable to that of tamoxifen only at a dose which was ten times greater.
I'yrrolidino-
4 Iodotamoxifen and 4-Iodotamoxifen New Analogues of the Antiestro~en
Tamoxifen
for the Treatment of Breast Cancer, Chander et al., Cancer Research, 51:5851-
5858
(November 1991); Idoxifene~ Report of a Phase I Study in Patients with
Metastatic Breast
Cancer, Coombes et al., Cancer Research, 55:1070-1074 (March l, 1995).
Other SERMS which are not tamoxifen analogs have shown effectiveness in
preventing or minimizing the development of breast cancer. Raloxifene (Fig.
3), a
benzothiophene derivative, has shown potent antiestrogenic inhibition of
estradiol
binding to the ER and significantly inhibits estrogen dependent proliferation
of MCF-7
cells derived from human mammary tissue. Raloxifene, unlike tamoxifen and its
analogs,
exhibits an antiestrogenic effect in uterine tissue, and provides a nearly
complete
blockade of uterotrophic responses to estrogen as well as tamoxifen. Selective
Estrogen
Receptor Modulators, Kauffman & Bryant, DN&P, 8(9) 531-539 (November 1995).
It is desirable to utilize these SERMS to develop new compositions which may
be
used to improve the SERMS' prevention or minimization of the development of
breast
cancer while reducing their uterotrophic activity, if any.
Summary of the Invention
In one aspect, the present invention is a composition for preventing or
minimizing
the development or growth of breast cancer. The composition comprises a
combination
of a selective estrogen receptor modulator selected from at least one of
raloxifene,
droloxifene, toremifene, 4-iodotamoxifen, and idoxifene, and at least one
isoflavone
selected from genistein daidzein, biochanin A, formononetin, or their
respective naturally
occuring glucosides or glucoside conjugates.
In another aspect, the present invention is a method for preventing or
minimizing
the development or growth of breast cancer in a human. A selective estrogen
receptor
modulator and an isoflavone are co-administered to a human to prevent or
minimize the
CA 02310290 2000-OS-30
development or growth of breast cancer. The selective estrogen receptor is
selected from
at least one of raloxifene, droloxifene, toremifene, 4'-iodotamoxifen, and
idoxifene. 'f he
isoflavone is selected from at least one of genistein, daidzein, biochanin A,
formononctin,
or their naturally occuring glucosides or glucoside conjugates.
Brief Description of the Drawings
Fig. 1 is a molecular representation of tamoxifen.
Fig.2 is a molecular representation of the selective estrogen receptor
modulators
droloxifene, toremifene, 4'iodotamoxifen, and idoxifene.
Fig. 3 is a molecular representation of the selective estrogen receptor
modulator
raloxifene.
Fig. 4 is a molecular representation of genistein, daidzein, biochanin A, and
formononetin.
Fig. 5 is a molecular representation of the naturally occuring glucosides of
genistein and
daidzein.
Description of the Preferred Embodiments
As used herein, the term "ER" refers to "estrogen receptor". The term "breast
cancer" means any cancer having its origin in breast cells, and includes
metastatic and
local forms of breast cancer (node negative and node positive), as well as ER
positive and
ER negative forms of breast cancer. The term "uterotrophic effect" means the
proliferation of uterine epithelial cells, which frequently is a side effect
of administration
of selective estrogen receptor modulators to women, and which appears to be
directly
related to development of endometrial cancer. As used herein "Mal" represents
"malonyl' and "Ac" represents "acetyl". The term "minimize", or a derivative
thereof,
includes a complete or partial inhibition of a specified biological effect
(which is apparent
from the context in which the term minimize is used). The term "isoflavone"
may mean
both a single isoflavone or plural isoflavones when the isoflavone is defined
as at least
one of a selected group of isoflavones. "SERM" means a selective estrogen
receptor
modulator and its physiologically acceptable salts, other than tamoxifen,
which is a
CA 02310290 2002-03-26
compound which produces estrogen antagonist effects in one or more desired
target tissues
(e.g. breast tissue and uterine tissue), while producing either estrogen
agonist effects or
minimal agonism in other non-target tissues.
The present invention resides in the discovery that the combination of
selected
SERMs with certain isoflavones can be used to treat or prevent breast cancer
in a woman
having or predisposed to breast cancer, and the isoflavones will augment the
SERM
induced prevention, minimization, or reversal of the development or growth of
breast
cancer, as well as prevent or minimize uterotrophic effects associated with
some SERMs.
The SERMs which are useful in the compositions and methods of the present
invention are
droloxifene, toremifene, 4'-iodotamoxifen, idoxifene, and raloxifene. The
isoflavones
which are useful in the compositions and methods of the present invention are
genistein,
daidzein, glycitein, biochanin A, formononetin, their naturally occuring
glycosides and
their naturally occuring glycoside conjugates, shown in Figs. 4 and 5.
Materials
The selective estrogen receptor modulator compounds used in the compositions
and
methods of the present invention can be chemically synthesized according to
known
methods, and include the salt forms of each of the compounds. Raloxifene, 6-
hydroxy-2-
(4-hydroxyphenyl)-3-[4-(2-piperdinoethoxy)benzoyl]benzo[b]thiophene (Fig. 3),
and its
physiologically acceptable salts may be produced according to the methods
described in
U.S. Patent Nos. 4,418,068 and 4,133,814, each of which may be referred to for
further
details. Droloxifene, E-1-[4'-(2-dimethylaminoethoxy)phenyl]-1-(3'-
hydroxyphenyl)- 2-
phenyl-1-butene (Fig. 2), and its physiologically acceptable salts may be
produced
according to the methods described in U.S. Patent No. 5,047,431, which may be
referred
to for further details. Toremifene, 4-chloro-1,2-diphenyl-1-{4-[2-(N,N-
dimethylamino)ethoxy]-phenyl}-1-butene (Fig. 2), and its physiologically
acceptable salts
may be produced by the methods described in U.S. Patent No. 4,696,949, which
may be
referred to for further details. 4'-Iodotamoxifen, E-1-{4-[2-
(dimethylamino)ethoxy]phenyl}-1-(4-iodophenyl)-2-phenyl-1-butene (Fig. 2), and
its
physiologically acceptable salts may be produced according to combined methods
6
CA 02310290 2002-03-26
described in Stereoselective Olefin Formation from the Dehydration of 1-(p-
Alkoxyphenyl)-1,2-diphenylbutan-1-ols: Application to the Synthesis of
Tamoxifen,
McCague, J. Chem. Soc. Perkin Trans., 1:1011-1015 (1987); and Derivatives of
Tamoxifen. Dependence of Antiestro e~ty on the 4-Substituent, McCague et al.,
J.
Med. Chem., 32(12):2527-2533 (1989), each of which may be referred to for
further
details. Idoxifene, E-1-(4-iodophenyl)-1-[4-(2-pyrrolidinoethoxy)phenyl]-2-
phenyl-1-
butene (Fig. 2), may be produced according to combined methods described in
the
references above that provide methods for producing 4'-iodotamoxifen.
The isoflavone compounds used in the compositions and methods of the present
invention are naturally occurring substances which may be found in plants such
as
legumes, clover, and the root of the kudzu vine (pueraria root). Common legume
sources
of these isoflavone compounds include soy beans, chick peas, and various other
types of
beans and peas. Clover sources of these isoflavone compounds include red
clover and
subterranean clover. Soy beans are a particularly preferred source of the
isoflavone
compounds (except biochanin A which is not present in soy).
The isoflavone compounds may be isolated from the plant sources in which they
naturally occur, or may be synthetically prepared by processes known in the
art. For
example, daidzein may be isolated from red clover as disclosed by Wong (J.
Sci. Food
Agr., Vol. 13, p. 304 (1962)) or may be isolated from the mold Micromonospora
halophytica as provided by Ganguly and Sarre CChem. & Ind. (London), p. 201
(1970)),
both references of which may be referred to for further details. Daidzein may
be
synthetically prepared by the methods provided by Baker et al. (J. Chem. Soc.,
p. 274
(1933)), Wesley et al. (Ber. Vol. 66, p. 685 (1933)), Mahal et al. (J. Chem.
Soc., p. 1769
(1934)), Baker et al. (J. Chem. Soc., p. 1852 (1953)), or Farkas (Ber. Vol.
90, p. 2940
( 1957)), each reference of which may be referred to for further details. The
isoflavone
glucoside daidzin may be synthetically prepared by the method of Farkas et al.
(Ber., Vol.
92, p. 819 ( 1959)), which may be referred to for further details. The
daidzein isoflavone
glucoside conjugates 6'-0-Mal daidzin and 6'-0-Ac daidzin can be prepared by a
conventional saponification of daidzin with a malonyl or an acetyl anhydride,
respectively.
7
CA 02310290 2002-03-26
Genistein may be synthetically prepared by the methods provided by Baker et
al.
(J. Chem. Soc., p. 3115 (1928)); Narasimhachari et al. (J. Sci. Ind. Res.,
Vol. 12, p. 287
(1953)); Yoder et al., (Proc. Iowa Acad. Sci., Vol. 61, p. 271 (1954)); and
Zemplen et al.
(Acta. Chim. Acad. Sci. Hung., Vol. 19, p. 277 (1959)), each reference of
which may be
referred to for further details. The isoflavone glucoside genistin may be
synthetically
prepared by the method of Zemplen et al. (Ber. Vol 76B, p. 1110 (1943)), which
may be
referred to for further details. The isoflavone glucoside conjugates of
genistein, 6'-0-Mal
genistin and 6'-0-Ac genistin, can be prepared by a conventional
saponification of genistin
with a malonyl or an acetyl anhydride, respectively.
Biochanin A can by synthetically prepared by the method provided by Baker et
al.
(Nature 169:706 ( 1952)), which may be referred to for further details.
Biochanin A can
also be separated from red clover by the method provided by Pope et al. CChem.
& Ind.
(London) p. 1092 (1953)), which may be referred to for further details.
Formononetin can
be synthetically prepared by the methods disclosed by Wessely et al. (Ber.
66:685 (1933))
and Kagel et al. (Tetrahedron Letters, p. 593 ( 1962)), both references of
which may be
referred to for further details. Formononetin can be isolated from soybean
meal by the
method of Walz (Ann. 489:118 (1931)) or can be isolated from clover species by
the
method of Bradbury et al. (J. Chem. Soc. p. 3447 ( 1951 )), both references of
which may
be referred to for further details.
It is preferred to extract the isoflavones useful in the compositions and
methods of
the present invention from the plant materials in which they naturally occur.
A preferred
method of isolating the isoflavone compounds is to extract the plant materials
with an
alcohol, preferably methanol or ethanol, or an aqueous solution, preferably an
aqueous
alkaline solution, to remove the isoflavones from the plant material. It is
preferred to
comminute the plant material before extracting the isoflavone compounds to
maximize
recovery of the isoflavone compounds from the plant material. The isoflavone
compounds
can be isolated from the extract by conventional separation procedures such as
reverse
phase high performance liquid chromatography ("HPLC").
In a preferred embodiment, the isoflavone compounds genistein, genistin, 6'-0-
Mal
genistin, 6'-0-Ac genistin, daidzein, daidzin, 6'-0-Mal daidzin, 6'-0-Ac
daidzin,
8
CA 02310290 2000-OS-30
glycitein, glycitin, and 6'-O-Mal glycitin are isolated from a soy material,
preferably a
commercially available soy material. Soy materials from which the isoflavone
compounds can be isolated include: soy beans, dehulled soy beans, soy meal,
soy flour,
soy grits, soy flakes (full fat and defatted), soy cotyldeons, soy molasses,
soy protein
concentrate, soy whey, soy whey protein, and soy protein isolate. In one
embodiment,
the isoflavones are extracted from soy beans, dehulled soy beans, soy meal,
soy flour, soy
grits, soy flakes, soy protein concentrate, soy whey protein, or soy protein
isolate,
preferably soy meal, soy flour, soy grits, or soy flakes, with a low molecular
weight
organic extractant, preferably an alcohol, ethyl acetate, acetone, or ether,
and most
preferably aqueous ethyl alcohol or methyl alcohol. Most preferably the
extractant has a
pH at about the isoelectric point of soy protein (about pH 4 to pH 5) to
minimize the
amount of soy protein extracted by the extractant.
The extractant containing the isoflavones is separated from the insoluble soy
materials to form an isoflavone enriched extract. If desired, an isoflavone
enriched
material may be recovered by concentrating the extract to remove the solvent,
thereby
producing a solid isoflavone enriched material.
In a more preferred embodiment the isoflavone compounds are further purified
from other soy materials soluble in the extract by contacting the extract with
a material
which adsorbs the isoflavones in the extract, and eluting the adsorbed
isoflavones out of
the adsorbent material with a solvent which causes the isoflavones to be
differentially
eluted from the adsorbent material.
In a preferred embodiment, the isoflavones are separated from impurities in
the
extract by a conventional reverse phase HPLC separation. After extraction of
the
isoflavones from the soy material and separation of the extract from the
insoluble soy
materials, the extract is filtered to remove insoluble materials that could
plug an HPLC
column. An HPLC column is prepared by packing a conventional commercially
available HPLC column with a particulate adsorbent material which will
releasably bind
the isoflavones and impurities in the extract in a compound specific manner.
The
adsorbent material may be any reverse phase HPLC packing material, however, a
preferred packing material may be chosen by the criteria of load capacity,
separation
CA 02310290 2000-OS-30
effectiveness, and cost. One such preferred packing material is Kromasil C18
l6pm
100A beads available from Eka Nobel, Nobel Industries, Sweden.
The filtered extract is passed through the packed HPLC column until all the
binding sites of the column are fully saturated with isoflavones, which is
detected by the
appearance of isoflavones in the effluent from the column. The HPLC column may
then
be eluted with a solvent to effect the separation. In a preferred embodiment,
the eluent is
a polar solvent such as ethanol, methanol, ethyl acetate, or acetonitrile, and
preferably is
an aqueous alcohol having an alcohol content of between about 30% and about 90
%,
most preferably about 50%, and most preferably the alcohol is ethanol.
The isoflavone compounds and impurities are separately collected from the
column effluent. The isoflavone fractions of the eluent may be identified from
other
eluent fractions in accordance with conventional HPLC and analytical chemistry
techniques. In a preferred embodiment the eluent fractions containing the
aglucone
isoflavones are collected separately since the aglucone isoflavones are
believed to be
particularly active tyrosine kinase inhibitors and anti-angiogenesis agents
which inhibit
the development or progression of breast cancer. Of the aglucone isoflavone
materials,
the fraction of effluent containing daidzein elutes from the column first,
followed by a
glycitein fraction, followed by the more polar genistein.
The isoflavone fractions of the eluent may be collected from the column, and
the
volatile content of the solvent (e.g. alcohol) can be removed by evaporation.
The
isoflavone compounds can be recovered directly if all of the solvent is
removed by
evaporation, or may be recovered by chilling the remaining solvent (e.g.
water) to
crystallize the isoflavones and centrifuging or filtering the remaining
solvent away from
the crystallized isoflavones.
In a particularly preferred embodiment the soy isoflavone glucosides and
isoflavone glucoside conjugates -- 6'-O-Mal genistin, 6'-O-Ac genistin, 6'-O-
Mal
daidzin, 6'-O-Ac daidzin, 6'-O-Mal glycitin, genistin, daidzin, and glycitin --
are
converted to their respective aglucone isoflavone forms -- genistein,
daidzein, and
glycitein. The conversion of the isoflavone glucoside conjugates and the
isoflavone
glucosides to the aglucone isoflavones can be effected in the substrate from
which the
to
CA 02310290 2000-OS-30
isoflavones are to be extracted prior to the extraction, or may be effected in
the isoflavone
enriched extract after separation of the extract from the insoluble materials.
The aglucone
isoflavone compounds are especially desirable in the compositions and methods
of the
present invention since, as noted above, they are believed to be particularly
active in
inhibiting angiogenesis and inhibiting tyrosine kinase activity.
The isoflavone glucoside conjugates 6"-O-Mal genistin, 6"-O-Ac genistin, 6"-O-
Mal daidzin, 6"-O-Ac daidzin, and 6"-O-Mal glycitin can be converted to their
respective
glucosides genistin, daidzin, and glycitin by forming an aqueous alkaline
solution of the
substrate containing the isoflavones having a pH of about 6 to about 13,
preferably about
pH 9 to about pH 11, and treating the aqueous alkaline solution at a
temperature of about
2°C to about 121 °C, preferably about 25°C to about
75°C, for a period of time sufficient
to effect the conversion, preferably about 30 minutes to about 5 hours, more
preferably
about 30 minutes to about 1.5 hours. The isoflavone glucosides genistin,
daidzin, and
glycitin can be converted to their respective aglucone forms genistein,
daidzein, and
glycitein by contacting the isoflavone glucosides with an enzyme capable of
cleaving a
1,4-13-glucoside bond -- preferably a commercially available beta-glucosidase
enzyme, an
alpha- or beta-galactosidase enzyme, a pectinase enzyme, a lactase enzyme, or
a gluco-
amylase enzyme -- at a pH at which the enzyme is active, typically from about
pH 3 to
about pH 9, and at a temperature of about 25°C to about 75°C,
more preferably about
45°C to about 65°C, for a period of time sufficient to effect
the conversion, typically
about 1 hour to about 24 hours, preferably about 1 hour to about 3 hours.
The aglucone isoflavones can be separated from the substrate using
conventional
separation procedures. For example, the aglucone isoflavones may be extracted
from the
substrate with a low molecular weight alcohol. The aglucone isoflavones may be
separated from the extract by conventional recrystallization processes, or by
HPLC. In a
particularly preferred embodiment, an isoflavone composition isolated from a
soy
substrate for formulation into a composition for use in the method of the
present
invention includes at least 40% genistein, at least 1 S% daidzein, and at
least 1 % glycitein.
In another particularly preferred embodiment of the invention, an isoflavone
composition
isolated from a soy substrate for formulation into a composition for use in
the method of
CA 02310290 2000-OS-30
the present invention contains at least 85% genistein, at least 5% daidzein,
and at least
0.5% glycitein. In yet another preferred embodiment, each isoflavone is
recovered
separately in pure form.
Several of the isoflavone compounds are commercially available, and may be
purchased for formulation into compositions provided in the present invention,
or used in
the methods of the present invention. For example, genistein, daidzein, and
glycitein are
commercially available and may be purchased, for example, from Indofine
Chemical
Company Inc., P.O. Box 473, Somerville, New Jersey 08876, and biochanin A is
available from Aldrich Chemical Company, Inc., 940 West Saint Paul Avenue,
Milwaukee, Wisconsin 53233.
Methods
In one aspect the present invention is a method for preventing or minimizing
the
development or growth of breast cancer in a human by co-administering at least
one
SERM selected from raloxifene, droloxifene, toremifene, 4-iodotamoxifen, and
idoxifene,
and at least one isoflavone selected from genistein, daidzein, biochanin A,
formononetin,
their respective glucosides, and their respective glucoside conjugates. The
SERM and
isoflavone may be co-administered prophylactically to prevent the development
of breast
cancer in women susceptible of developing breast cancer, or the SERM and
isoflavone
may be co-administered to treat breast cancer by preventing, minimizing, or
reversing the
growth and development of the cancer. The SERM may be obtained for use in
accordance with the method the present invention as described above, or,
preferably, may
be provided in a composition of the present invention, as described below. The
isoflavone may be obtained for use in accordance with the method of the
present
invention as described above, or, preferably, may be provided in a composition
of the
present invention, as described below.
The SERM and the isoflavone may be co-administered either concurrently or
sequentially within a specified period of time, preferably daily, on a
periodic basis. Most
preferably the SERM and the isoflavone are co-administered concurrently in a
composition of the present invention, as described below, on a periodic basis,
preferably
12
CA 02310290 2000-OS-30
daily. Alternatively, the SERM and the isoflavone are administered
sequentially as
separate components. "Sequentially" as used herein is intended to mean
administration
of desired amounts of the SERM and isoflavone individually within a specified
periodic
period of time, for example daily, and is not intended to be limited to
immediate
consecutive administration of the SERM and isoflavone.
The SERM is administered in an amount sufficient to prevent or treat the
development or growth of breast cancer in combination with the isoflavone. The
amount
of SERM sufficient to prevent or treat the development or growth of breast
cancer in
combination with the isoflavone is dependent on the particular SERM utilized,
the
amount and activity of the isoflavone utilized, the size of the patient to
which the SERM
is administered, whether the SERM is administered prophylatically or to treat
breast
cancer, and if used in treatment, the extent of the cancer. The amount of SERM
sufficient
to prevent the development of breast cancer in a woman predisposed to breast
cancer is
preferably at least 0.5 mg per day, more preferably from about 0.5 mg to about
100 mg
per day, and most preferably from about 5 mg to about 50 mg per day. The
amount of
SERM sufficient to treat the development or growth of breast cancer to
prevent,
minimize, or reverse the development or growth of the cancer is preferably at
least 0.5 mg
per day, more preferably from 0.5 mg to about 500 mg per day, and most
preferably from
about 40 mg to about 400 mg per day. The SERM may be administered in several
doses
per day to achieve the daily amount of the SERM sufficient to prevent or treat
breast
cancer, however, it is preferred that the daily required amount of SERM be
administered
in one or two doses.
The isoflavone is co-administered to the human in an amount sufficient to
prevent
or treat the development or growth of breast cancer in combination with the
SERM. The
amount of isoflavone sufficient to prevent or treat the development or growth
of breast
cancer in combination with the SERM is dependent on the particular isoflavone
utilized,
the amount and activity of the co-administered SERM, the size of the patient,
whether the
isoflavone is administered prophylatically or to treat breast cancer, and if
used in
treatment, the extent of the cancer. The amount of isoflavone sufficient to
prevent the
development of breast cancer in a woman predisposed to breast cancer in the
present
~3
CA 02310290 2000-OS-30
method is preferably at least 1 mg per day, more preferably from about 10 mg
to about
200 mg per day. The amount of isoflavone sufficient to treat the development
or growth
of breast cancer to prevent, minimize, or reverse the development or growth of
the cancer
is preferably at least 1 mg per day, more preferably from about 1 mg to about
1000 mg
per day, and most preferably from about 50 mg to about 500 mg per day.
The isoflavones utilized in the method of the present invention prevent,
minimize,
or reverse the growth of breast cancer by several mechanisms, which in
combination with
the anti-estrogenic activity of the SERM in breast tissue, increase the
relative anti-breast
cancer activity of each compound. First, the isoflavones are anti-estrogenic
in breast
tissue, and serve to competitively inhibit estrogen induced cancerous breast
cell division
by binding to the ER of the cell, where the isoflavone/ER complex inhibits
cancer cell
growth in much the same manner as tamoxifen and the SERMs (e.g. daidzein halts
cell
growth in the G 1 phase of the cell cycle, and genistein halts cell growth in
the G2 phase
of the cell cycle). Second, some of the isoflavones, particularly genistein
and biochanin
A, and to a lesser extent daidzein and formononetin, are tyrosine kinase
inhibitors which
inhibit enzymatic tyrosine kinase activity. Tyrosine kinase activity is
necessary for
cancerous cells to produce proteins required for cellular differentiation and
growth.
Third, the isoflavones inhibit angiogenesis, thereby preventing a cancerous
cell mass
from developing the network of blood vessels necessary to support the cell
mass, limiting
the sustainable growth of the cell mass. Fourth, the isoflavones decrease
endogenous
estrogen levels by interfering with pituitary and hypothalmus gland feedback
mechanisms
which regulate the release of gonadotropins such as estradiol. The effect of
the combined
mechanisms of action is to further prevent or minimize the development or
growth of
breast cancer when co-administered with a SERM effective to prevent or
minimize the
growth of breast cancer.
In a particularly preferred embodiment of the method of the present invention,
the
isoflavone is co-administered with the SERM in an amount sufficient to prevent
or
minimize SERM induced uterotrophic effects. Atlhough the SERMs utilized in the
present invention are less uterotrophic than tamoxifen, each of the SERMs
except
raloxifene induces uterotrophic effects at relatively high doses. The
isoflavones utilized
14
CA 02310290 2000-OS-30
in the present method have an antiestrogenic effect in uterine tissues when
concenrations
of estrogen or an estrogen agonist SERM are relatively high. One mechanism by
which
the isoflavones likely cause an antiestrogenic effect in uterine tissue in the
presence of
uterine estrogen agonist SERMs is by binding to uterine cell ERs and
competitively
inhibiting the estrogen agonist SERMs from binding to the ERs. Unlike uterine
tissue
estrogen agonist SERMs, the isoflavones do not cause an estrogemc response
upon
binding to uterine cell ERs, therefore, the isoflavones prevent, inhibit, or
minimize the
uterotrophic effects caused by uterine endothelial cell ER/SERM complexes.
Preferably
the isoflavone is co-administered with the SERM to prevent or minimize
uterotrophic
effects in a weight/weight ratio of isoflavone:SERM of about 0.25:1 to about
100:1, and
more preferably from about 0.5:1 to about 20:1.
In a particularly preferred embodiment of the method, co-administration of the
isoflavone with a uterine tissue estrogen agonist SERM in an amount sufficient
to prevent
or minimize uterotrophic effects is also effective to prevent or minimize the
development
of endometrial cancer when the SERM is used to prevent or treat breast cancer.
As noted
above, tamoxifen and uterine tissue estrogen agonist SERMs cause an increased
risk of
the development of endometrial cancer as a result of estrogen-like activity in
uterine
tissue and its uterotrophic effects. Co-administration of the isoflavone
together with an
uterine tissue estrogen agonist SERM prevents or minimizes the development of
endometrial cancer by preventing or minimizing SERM induced uterotrophic
effects.
Compositions
In another aspect, the present invention is a composition useful for
preventing or
minimizing the development or growth of breast cancer. The composition
includes
combination of a selective estrogen receptor modulator selected from at least
one of
raloxifene, droloxifene, toremifene, 4-iodotamoxifen, and idoxifene, and at
least one
isoflavone selected from genistein, daidzein, biochanin A, formononetin, their
respective
naturally occuring glucosides and glucoside conjugates. These SERM and
isoflavone
materials necessary to form compositions in accordance with the present
invention may
be obtained as described above. The composition contains from about 1% to
about 99%
is
CA 02310290 2000-OS-30
SERM, by weight of biologically active ingredients, and from about 1 % to
about 99%
isoflavone, by weight of biologically active ingredients.
The SERM is present in the composition in an amount sufficient to prevent,
minimize, or reverse the development or growth of breast cancer in a woman
when co-
administered with the isoflavone. Preferably at least 0.5 mg of the SERM is
present in
the composition, more preferably from about 0.5 mg to about 500 mg, and most
preferably from about 5 mg to about 100 mg. Most preferably, the SERM is
present in
the composition in an amount sufficient to prevent, minimize, or reverse the
development
or growth of breast cancer by itself.
Preferably at least 1 mg of the isoflavone is present in the composition, more
preferably from about 1 mg to about 1000 mg, and most preferably from about 10
mg to
about 200 mg. In a preferred embodiment the isoflavone is present in the
composition in
an amount sufficient to augment the composition's SERM induced prevention or
minimization of development or growth of breast cancer when the composition is
administered to a woman. In a more preferred embodiment, the isoflavone is
present in
the composition in an amount sufficient to prevent, minimize, or reverse the
development
or growth of breast cancer by itself.
In another preferred embodiment, the isoflavone is present in the composition
in
an amount sufficient to prevent or minimize the composition's SERM induced
uterotrophic effects when the composition is administered to a woman. The
isoflavone
should be present in a ratio of isoflavone:SERM of from about 0.25:1 to about
100:1 by
weight, and more preferably from about 0.5:1 to about 50:1 by weight, to be
present in
the composition in an amount sufficient to prevent or minimize the
composition's SERM
induced uterotrophic effects. In a most preferred embodiment, the isoflavone
is present
in the composition in an amount sufficient to augment the composition's SERM
induced
prevention or minimization of the development or growth of breast cancer and
to prevent
or minimize the composition's SERM induced uterotrophic effects when the
composition
is administered to a woman.
A composition in accordance with the present invention containing a SERM and
an isoflavone can be prepared by conventional procedures for blending and
mixing
16
CA 02310290 2000-OS-30
compounds. Preferably, the composition also includes an excipient, most
preferably a
pharmacuetical excipient. Compositions containing an excipient and
incorporating the
SERM and isoflavone can be prepared by procedures known in the art. For
example, the
SERM and the isoflavone can be formulated into tablets, capsules, powders,
suspensions,
solutions for parenteral administration including intravenous, intramuscular,
and
subcutaneous administration, and into solutions for application onto patches
for
transdermal application with common and conventional carriers, binders,
diluents, and
excipients.
Inert pharmaceutically acceptable carriers useful to form pharmaceutical
compositions in accordance with the present invention include starch,
mannitol, calcium
sulfate, dicalcium phosphate, magnesium stearate, silicic derivatives, and/or
sugars such
as sucrose, lactose, and glucose. Binding agents include carboxymethyl
cellulose and
other cellulose derivatives, gelatin, natural and synthetic gums including
alginates such as
sodium alginate, polyethylene glycol, waxes and the like. Diluents useful in
the
invention include a suitable oil, saline, sugar solutions such as aqueous
dextrose or
aqueous glucose, and glycols such as polyethylene or polypropylene glycol.
Other
excipients include lubricants such as sodium oleate, sodium acetate, sodium
stearate,
sodium chloride, sodium benzoate, talc, and magnesium stearate, and the like;
disintegrating agents including agar, calcium carbonate, sodium bicarbonate,
starch,
xanthan gum, and the like; and adsorptive carriers such as bentonite and
kaolin.
Coloring and flavoring agents may also be added to the pharmaceutical
compositions.
The following non-limiting formulations illustrate pharmaceutical compositions
of the present invention.
FORMULATIONS
The following Formulations 1-4 illustrate pharmaceutical formulations
including
a SERM and an isoflavone.
Formulation 1
Gelatin capsules
Hard gelatin capsules are prepared using the following ingredients: SERM 0.5-
100 mg/capsule; Isoflavone 0.1-1000 mg/capsule; Starch, NF 0 - 600 mg/capsule;
Starch
t7
CA 02310290 2000-OS-30
flowable powder 0 - 600 mg/ capsule; Silicone fluid 350 centistokes 0 -20
mg/capsule.
The ingredients are mixed, passed through a sieve, and filled into capsules.
Formulation 2
Tablets
Tablets are prepared using the following ingredients: SERM 0.5-100 mg/tablet;
Isoflavone 0.1-1000 mg/ tablet; Microcrystalline cellulose 20-300 mg/tablet;
Starch 0-50
mg/tablet; Magnesium stearate or stearate acid 0-15 mg/tablet; Silicon
dioxide, fumed 0-
400 mg/tablet; silicon dioxide, colloidal 0-1 mg/tablet, and lactose 0-100
mg/tablet. The
ingredients are blended and compressed to form tablets.
Formulation 3
Suspensions
Suspensions are prepared using the following ingredients: SERM 0.5-100
mg/Sml; Isoflavone 0.1-1000 mg/Sml; Sodium carboxymethyl cellulose 50-700
mg/Sml;
Sodium benzoate 0-10 mg/Sml; Purified water 5 ml; and flavor and color agents
as
needed.
Formulation 4
Parenteral solutions
A parenteral composition is prepared by stirring 1.5% by weight of active
ingredients (SERM and isoflavone wtlwt ratio of from 10:1 to 1:10) in 10% by
volume
propylene glycol and water. The solution is made isotonic with sodium chloride
and
sterilized.
The above description is intended to be illustrative of the present invention,
and is
not intended to be limiting. Other embodiments are within the claims.
18