Note: Descriptions are shown in the official language in which they were submitted.
CA 02310700 2000-06-06
SPECIFICATION
Title of the Invention
MICROCHIP FOR CAPILLARY GEL ELECTROPHORESIS AND
THE METHOD FOR FABRICATING THE SAME
Background of the Invention
Field of The Invention
The present invention relates to a microchip for
capillary gel electrophoresis and the method for fabricating
the same, and more particularly to a microchip for capillary gel
electrophoresis used suitably for separating nucleic acids
including a variety of sizes of DNA fractions; organic molecules
such as amino acids, peptides, and proteins; metal ions and the
like in a small experimental scale; as well as to the method for
fabricating the same.
Description of The Related Art
Heretofore, it has been known that a capillary channel is
defined on a chip composed of planar glass by means of
microfabrication in accordance with photolithographic
technique to constitute a chipfor capillary electrophoresis,
and that when the chip for capillary electrophoresis thus
fabricated is used, it becomes possible to perform high-quality
and high-speed electrophoretic separations.
In these circumstances, many researches have been made
with respect to the above described chip for capillary
electrophoresis wherein a chip prepared from a silicon material
CA 02310700 2000-06-06
such as glass, and Si/SiOZ is employed, and a capillary channel
is defined thereon in accordance with microfabrication.
In this respect, however, etching and bonding processes
having complicated operational steps must be carried out for
forming a capillary channel used in electrophoretic
separations, and for sealing positively the resulting capillary
channel in the case where glass or silicon is used as a material
of a chip for capillary electrophoresis .
Accordingly, a chip for capillary electrophoresis which
is prepared from a glass material or a silicon material becomes
inevitably expensive as a result of increasing its
manufacturing cost. Thus, discarding of such microchip as
described above for electrophoretic separations after only one
time application thereof did not pay from the viewpoint of cost.
For this reason, in a chip for capillary electrophoresis
wherein glass or silicon is used as its material, such a gel
which is difficult to remove from a capillary channel after
having been filled therewith is not employed as a separation
material ( molecular s ieves ) to be f filled into the capillary
channel for the sake of making the same possible to use in
repeating electrophoretic separations, but it is necessary for
using a buffer solution which can be allowed to freely flow away
from a capillary channel after having been filled therewith, or
an easily replaceable high molecular ( polymer ) solution such as
linear polyacrylamide, and hydroxypropyl cellulose, so that an
electrophoretic separation has been carried out by the use of
these molecular sieves.
- 2 -
CA 02310700 2000-06-06
However, in the case where electrophoretic separations
are carried out by using a buffer solution or a polymer solution
as molecular sieves in a chip for capillary electrophoresis
prepared from a glass or silicon material, it is required to use
high voltage as well as to control delicately an electric field
in order to prevent occurrence of diffusion or convection which
arises in application of voltage. Accordingly, there is such a
problem that electrical equipment and detection devices become
complicated, resulting in high cost, and further there is also
such a problem that since a long capillary channel is necessary
for increasing a degree of separation, a chip must be inevitably
large-sized.
Objects and Summary of The Invention
The present invention has been made in view of a variety
of problems involved in the prior art as described above, and an
object of the invention is to provide a disposable microchip for
capillary gel electrophoresis which can be fabricated in low
cost and suitable for discarding thereof after having been only
once used for electrophoresis as well as to provide a method for
fabricating the same.
Another object of the present invention is to provide a
microchip for capillary gel electrophoresis by which it becomes
possible to use a gel as molecular sieves, whereby
electrophoretic separations can be effected with low voltage,
but not high voltage, and further occurrences of diffusion and
convection at the time of applying an voltage are suppressed,
whereby a simplification of electrical equipment and detection
- 3 -
CA 02310700 2000-06-06
devices is intended, so that it becomes possible to remarkably
reduce its cost as well as to provide a method for fabricating
the same.
A still further object of the present invention is to
provide a microchip for capillary gel electrophoresis by which
it becomes possible to use a gel as molecular sieves, whereby it
is intended to improve a degree of separation in electrophoretic
separations, reduction of a separation distance, and reduction
of a separation time, so that it becomes possible to improve
separating performance in electrophoretic separations and to
effect electrophoretic separations in high separation
resolution as well as to provide a method for fabricating the
same.
In order to achieve the above described objects,
according to the present invention, minute processing is
applied to a microchip prepared from a high-molecular (polymer)
material such as PDMS ( polydimethyl siloxane ) , and a capillary
channel is defined on the microchip.
In this case, although silicon such as glass and Si/SiOZ
has been used heretofore as a material of microchip for def fining
a capillary channel in accordance with minute processing, it is
preferred to use a polymer material in view of low cost as
compared with that of glass or Si/SiOz, besides the latter is
less fragile than the former.
Particularly, PDMS being a kind of silicone elastomer
included in polymer materials is a material used suitably for
molding in microscale, by the use of which a capillary channel
- 4 -
CA 02310700 2000-06-06
being a microstructure for electrophoretic separations can be
processed and molded in low cost.
In other words, when a microchip prepared from PDMS is
employed in case of forming capillary channels, such capillary
channels can be easily formed in accordance with simple and
inexpensive molding and sealing manners without accompanying
such etching and bonding processes which require complicated
operations.
As a result, a microchip for capillary gel
electrophoresis according to the present invention can be
inexpensively provided, so that it becomes possible to apply a
so-called disposable use in which a microchip is discarded after
it had been once used in an electrophoretic separation by the use
of the microchip for capillary gel electrophoresis of the
present invention.
In addition, when a microchip is disposable as described
above, it is possible to use no buffer solution or no polymer
solution which can be removed from a capillary channel after
having been filled therewith as molecular sieves being a
separation material with which the capillary channel is to be
filled.
For this reason, in the present invention, a capillary
channel defined on a microchip for capillary gel
electrophoresis is filled partially with a gel ( for example,
agarose gel ) , whereby separation resolution can be improved as
compared with electrophoretic separations carried out on a
microchip wherein a buffer solution or a polymer solution, which
has been heretofore used as molecular sieves, is employed,
- 5 -
CA 02310700 2000-06-06
besides a longer length of the capillary channel required for
separations can be reduced.
Thus, according to a microchip for capillary gel
electrophoresis of the present invention, since a gel may be
used as molecular sieves to be filled in a capillary channel, it
becomes possible to effect electrophoretic separations with
high resolution without requiring extension of a capillary
channel and control of a delicate electric field for the sake of
suppressing problems of occurring diffusion and convection.
As a result, it becomes possible to separate DNA
molecules having a variety of sizes with simple equipment by the
use of a microchip for capillary gel electrophoresis prepared
from inexpensive PDMS for electrophoretic separations in
accordance with the present invention.
As mentioned hereinafter, it has been succeeded in
experiments by the present applicant that DNA molecules are
electrically separated in reality on a microchip for capillary
gel electrophoresis prepared from PDMS according to the present
invention, whereby a band corresponding to molecular weight is
formed. More specifically, in the experiments by the present
applicant which will be described hereunder, agarose is first
employed as a gel for molecular sieves in order to exhibit
suitability of a microchip for capillary gel electrophoresis
prepared from PDMS according to the present invention, and DNA
ladders labelled with FITC are separated.
- 6 -
CA 02310700 2000-06-06
More specifically, a microchip for capillary gel
electrophoresis of the present invention is composed of a planar
substrate prepared from a polymer material and a planar surface
plate to be disposed on the top surface of the substrate, a
capillary channel constituting a flow path having a
predetermined contour being defined on the top surface of the
substrate, and the capillary channel being sealed with the
surface plate.
In this case, the surface plate may be prepared from, for
example, PMMA (polymethyl methacrylate), PDMS (polydimethyl
siloxane) , glass or the like.
On one hand, the substrate may be prepared from, for
example, PDMS (polydimethyl siloxane).
Furthermore, a surface of the capillary channel defined
on the top surface of the substrate may be made, for example, to
be hydrophilic.
Moreover, in case of making the surface of the capillary
channel defined on the top surface of the substrate hydrophilic,
it may be made the surface of the capillary channel defined on
the top surface of the substrate hydrophilic by means of, for
example, oxidation with oxygen plasma.
On the other hand, a method for fabricating a microchip
for capillary gel electrophoresis of the present invention
comprises a first treatment for printing a layout pattern of a
capillary channel in a microchip for capillary gel
electrophoresis on a transparent film to prepare a
photolithographic mask; a second treatment for forming a
_ 7 _
CA 02310700 2000-06-06
negative photoresist on a silicon wafer; a third treatment for
transferring the layout pattern printed on the mask prepared by
the aforesaid first treatment to the negative photoresist
prepared by the aforesaid second treatment, and developing the
same to prepare a master; a fourth treatment for treating the
master prepared by the aforesaid third treatment with
fluorocarbon; a fifth treatment for pouring a mixed solution
consisting of a PDMS prepolymer and a curing agent over the
master treated with fluorocarbon in the aforesaid fourth
treatment, and curing the same at a predetermined temperature
for a predetermined period of time; a sixth treatment for
peeling a PDMS replica away from the master after completing the
curing for a predetermined period of time in the aforesaid fifth
treatment, so that the PDMS replica is obtained as a substrate on
which a capillary channel has been defined; a seventh treatment
for covering the substrate obtained in the aforesaid sixth
treatment by a surface plate, and attaching the latter to the
former to seal the substrate with the surface plate; and an
eighth treatment for making a surface of the capillary channel
defined on the PDMS substrate to which the surface plate has been
attached hydrophilic.
The treatment for making the surface of the capillary
channel defined on the PDMS substrate in the aforesaid eighth
treatment hydrophilic may be, for example, the one wherein the
PDMS substrate to which the surface plate has been attached in
the aforesaid seventh treatment is oxidized with oxygen plasma,
so that the capillary channel def fined on the substrate is
oxidized with oxygen plasma, whereby the surface of the
_8_
CA 02310700 2000-06-06
capillary channel is made to be hydrophilic .
With respect to the above described hydrophilic
treatment, other manners for achieving hydrophilic treatment
than the oxidation with oxygen plasma as described above may be
suitably utilized.
Brief Description of The Drawings
The present invention will become more fully understood
from the detailed description given hereinafter and the
accompanying drawings which are given by way of illustration
only, and thus are not limitative of the present invention, and
wherein:
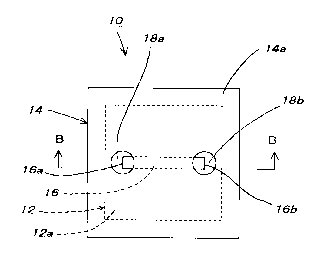
FIGS . 1 ( a ) and ( b ) show an example of a preferred
embodiment of a microchip for capillary gel electrophoresis
according to the present invention wherein FIG. 1 ( a ) is a
schematic view taken in the direction of the arrow along the line
A of FIG. 1 ( b ) , and FIG. 1 ( b ) is a sectional view taken along the
line B-B of FIG. 1 ( a ) ;
FIGS. 2 (a), (b), (c), (d) and (e) are schematic
explanatory views each illustrating a process for fabricating a
microchip lOfor capillary gel electrophoresis;
FIG. 3 is a constitutional explanatory view showing a
system for detecting fluorescence in DNA labelled with FITC;
FIG. 4 is a scanning electron micrograph of a capillary
channel defined on a substrate of the microchip for capillary
gel electrophoresis shown in FIG. 1;
FIGS . 5 ( A ) , ( H ) and ( C ) are micrographs each s howing a
_ 9 _
CA 02310700 2000-06-06
situation of introduction and separation of DNA molecules;
FIG. 6 is a graphical representation indicating changes
in electrophoresis of a microchip for capillary gel with time
wherein a capillary channel 16 is filled with agarose gel
containing DNA size standards of 100 by to 1000 bp; and
FIG. 7 is a scanning electron micrograph of a capillary
channel having another shape defined on a substrate of a
microchip for capillary gel electrophoresis.
Detailed Description of The Preferred Embodiments
An example of a preferred embodiment of a microchip for
capillary gel electrophoresis as well as a method for
fabricating the same according to the present invention will be
described in detail hereinafter in conjunction with the
accompanying drawings.
FIGS . 1 ( a ) and ( b ) show an example of a preferred
embodiment of a microchip for capillary gel electrophoresis
according to the present invention wherein FIG. 1 ( a ) is a
schematic view taken in the direction of the arrow along the line
A of FIG. 1 ( b ) , and FIG. 1 ( b ) is a sectional view taken along the
line B-B of FIG. 1 (a) .
In these figures, a microchip 10 for capillary gel
electrophoresis is composed of a planar substrate 12 prepared
from PDMS, and a planar surface plate 14 prepared from PMMA
(polymethyl methacrylate) which is disposed on the top surface
12a of the substrate 12.
-10-
CA 02310700 2000-06-06
Further, a capillary channel 16 is formed on the top
surface 12a of the substrate 12 so as to define a so-called
I-shaped flow pass.
Namely, the capillary channel 16 defined on the top
surface 12a of the substrate 12 is sealed with the surface plate
14.
Two ports 18a and 18b being openings each of which is
formed so as to pass through a bottom surface 14b from the top
surface 14a of the surface plate 14 are bored on the surface
plate 14 for introducing a sample and mounting electrodes,
respectively.
In this case, two ports 18a and 18b as well as the
capillary channel 16 are defined and arranged to have each size
in such that opposite ends 16a and 16b of the capillary channel
16 are located over a part of each port 18a or 18b so that the
port 18a and the port 18b are communicated with the end 16a and
the end 16b, respectively.
Moreover, a length of the capillary channel 16 is
determined, for example, to be 14 mm, a width of the capillary
channel 16 is determined, for example, to be 400 ,~ m, and a depth
of the capillary channel 16 is determined, for example, to be 40
,u m, respectively.
It is to be noted that a length of the capillary channel
16 is not specifically limited, but it may be arbitrarily
determined according to need, a width of the capillary channel
16 is also not particularly restricted, but it may be
arbitrarily determined as occasion demands, for example, it may
be determined to be any optional value ranging from 10 ,~ m to 800
- il -
CA 02310700 2000-06-06
a m, and a depth of the capillary channel 16 is not specifically
limited, but it may arbitrarily be determined as circumstances
demand, for example, it may be determined to be any arbitrary
value ranging from 5 ~ m to 150 a m.
A fabricating process which will be described by
referring to FIGS . 2 ( a ) , ( b ) , ( c ) , ( d ) and ( a ) is applied for
fabricating the above described microchip 10 for capillary gel
electrophoresis. In this respect, first, a layout pattern of
the capillary channel 16 in the microchip 10 for capillary gel
electrophoresis has been printed on a transparent film at high
resolution, e.g., 4064 dpi prior to the fabricating process in
order that the printed layout pattern is utilized as a mask for
photolithography.
In the following, a process for fabricating the microchip
for capillary gel electrophoresis containing the above
described substrate 12 prepared from PDMS will be described in
detail.
An outline of a fabrication process of the microchip 10
for capillary gel electrophoresis is illustrated in FIGS. 2(a),
(b), (c), (d) and (e).
A 20 mm x 20 mm silicon (Si) wafer is dried in an oven
( FIG. 2 ( a ) ) , and the negative photoresist SU-8 is spin-coated at
2,500 rpm for 20 seconds, thereafter the resulting photoresist
is baked in the oven at 90°C for 30 minutes ( FIG. 2 ( b ) ) .
In the present embodiment, such a process wherein the
negative photoresist SU-8 is spin-coated at 2, 500 rpm for 20
seconds, and baked in the oven at 90°C for 30 minutes was repeated
-i2-
CA 02310700 2000-06-06
twice or so in order to prepare a capillary channel structure
having 40 ~ m depth.
Then, the layout pattern in the microchip 10 for
capillary gel electrophoresis which has been printed on a mask
was transferred to a silicon wafer coated with SU-8 in
accordance with a photolithographical manner by the use of a
mask aligner ( for example, "PEM-800; Union Optical Co. , Tokyo,
Japan" may be employed as a mask aligner), and the resulting
material was developed in 1-methoxy-2-propyl acetate for 20
minutes (FIG. 2(c)).
The master thus fabricated was washed in isopropyl
alcohol, and consecutively distilled water.
The master had been treated with fluorocarbon by the use
of RIE ( Reactive Ion Etching ) system before pouring a PDMS
prepolymer.
The fluorocarbon treatment is useful for removing the
PDMS replica after molding.
Then, the PDMS prepolymer and a curing agent ( for
example, "Sylgard 184 " : Dow Corning Co . , MI may be used as a
curing agent) were admixed at a ratio of 10 : 1, the admixture was
stirred thoroughly, and then, degassed in vacuum for only 15
minutes to prepare a prepolymer mixed solution. The prepolymer
mixed solution thus prepared was poured over the master and
cured at 65°C for 1 hour, thereafter 135°C for 15 minutes (FIG.
2(d)).
After the above described curing, when the PDMS replica
was peeled off from the master, the PDMS substrate 12 was
obtained. Then, the PDMS substrate 12 was attached to the PMMA
- 13 -
CA 02310700 2000-06-06
surface plate 14 on which had been bored the ports 18a and 18b so
as to cover the PMMA surface plate 14, whereby the capillary
channel 16 (FIG. 2(e) ) is sealed therewith.
In the present preferred embodiment, an expression "to
seal the capillary channel 16" does not mean to seal completely
the capillary channel 16, but to arrange the opposite ends 16a
and 16b of the capillary channel 16 as well as two ports 18a and
18b in such that the former members communicate with the latter
members, respectively.
Furthermore, the PDMS substrate 12 attached to the PMMA
surface plate 14 was oxidized with oxygen plasma by employing
RIE system, whereby the capillary channel 16 was oxidized with
oxygen plasma to achieve surface hydrophilicity of the
capillary channel 16.
It is to be noted that a manner for achieving surface
hydrophilicity of the capillary channel 16 is not limited to the
above described oxidizing manner with oxygen plasma, but the
other manners may suitably be utilized.
In the following, a preparation of a gel used for
electrophoretic separations in the microchip lOfor capillary
gel electrophoresis will be described.
First, agarose powder ( for example, "SeaKem GTG agarose;
FMC BioProducts, ME" may be employed as such agarose powder) was
dissolved into 1 time larger volume of TBE (tris borate EDTA)
buffer solution while heating them in an oven to prepare an
agarose solution. The resulting agarose solution was kept in
the oven at 65°C, then, it was introduced into the capillary
- 14 -
CA 02310700 2000-06-06
channel 16 from the ports 18a and 18b in the microchip 10 for
capillary gel electrophoresis by utilizing capillary action,
and the resulting member was allowed to stand at room
temperature for 5 minutes to cure the agarose solution .
Next, preparation of a sample used for experiments, its
sample loading, and its electrophoretic separations will be
described. First, DNA size standards in every 100 bps each
labeled with FITC (fluorescein isothiocyanate) (for example,
such DNA size standards in every 100 bps each labeled with FITC
may be purchased from "Bio-Rad Co . " ) were maintained at 4°C .
Thereafter, 2 a m of a DNA ladder ( s ize standard )
solution was placed in the port 18b .
Sample loading into the agarose gel was carried out by
applying 100 V to the capillary channel 16 through platinum
electrodes mounted in the ports 18a and 18b .
In the following, experimental results obtained by
applying electrophoretic separations to the sample introduced
into the capillary channels 16 as described above will be
described.
Fluorescence of FITC labeled DNA was detected by a system
composed of an inverted fluorescence microscope (for example,
"DIAPHOT-TMD; Nikon Co. , Japan" may be used as such inverted
fluorescence microscope) 100, an ICCD camera (for example,
"C2400-8; Hamamatsu Photonics Co. , Japan may be employed as such
ICCD camera ) 102 into which light from the inverted fluorescence
microscope 100 is input, and a video camera 104 by which image
signals output from the ICCD camera 102 are recorded.
- 15 -
CA 02310700 2000-06-06
The inverted fluorescence microscope 100 is composed of
an xenon lamp 106, a band pass filter 108 allowing the light
irradiated from the xenon lamp 106 to selectively pass through
the same, a dichroic mirror 110 which allows the light
transmitted from the band pass filter 108 to pass through the
same and to output the light to the microchip 10 for capillary
gel electrophoresis, and at the same time, reflects the light
reflected by the microchip 10 for capillary gel
electrophoresis, and a band pass filter 112 which allows the
light reflected by the dichroic mirror 110 to selectively pass
through the same and to input the light into the ICCD camera 102.
In this case, fluorescein was activated at 488 nm,
emission was around 517 nm, and the image of electrophoresis was
recorded by the video camera 104 .
After the experiments, the image of electrophoresis was
digitized by the use of an image analysis program ( for example,
"NIH image 1 .62a" may be used as such image analysis program) .
Moreover, changes of electrophoresis with time was obtained by
processing the digitized data with the use of a prescribed
computer program.
FIG. 4 shows a scanning electron micro graph of the
capillary channel 16 defined on the substrate 12 prepared from
PDMS. As is clearly shown in FIG. 4, when the substrate 12
prepared from PDMS is used, a photoresist structure on the
master which has been prepared from silicon can be transferred
with high reproducibility.
- 16 -
CA 02310700 2000-06-06
The surface of a molded PDMS substrate 12 by using a
master prepared from silicon is intrinsically hydrophobic which
prevents capillary action between the capillary channel 16 and a
gel solution.
Under the circumstances, in the present embodiment, a
surface treatment with oxygen plasma was carried out for 2
minutes for the sake of filling the capillary channel 16 with an
agarose solution as described above. As a result, a contact
angle of water with respect to the PDMS substrate 12 changed from
108° to 32°, so that surface hydrophilicity of the substrate 12,
i.e., surface hydrophilicity of the capillary channel 16 could
be achieved.
Further, the cured PDMS substrate 12 could adhere to the
PMMA surface plate 14 at ordinary temperatures without
requiring any elaborate bonding manner. In addition, a master
prepared from silicon containing the photoresist pattern can be
utilized many times with just fluorocarbon treatment before
molding.
Taking such fabricating processes into consideration, it
is possible to fabricate the microchip 10 for capillary gel
electrophoresis at extremely low cost, so that it is suitable
for disposable use wherein the microchip is discarded after
having been used for electrophoretic separations only one time.
Although agarose gel has been used as molecular sieves in
the present embodiment, it has been generally known to be
difficult to prepare homogeneously and stably a capillary
- 17 -
CA 02310700 2000-06-06
channel filled with a gel.
In this connection, gel instability during
electrophoresis, i.e., bubble formation and clogging of pores
in gels restricts electric field strength and separating
performance of a gel.
In the present embodiment, however, the agarose can be
easily introduced into the surface treated capillary channel 16
having hydrophilicity, and gelled, besides the whole operations
can be completed within 10 minutes .
Moreover, neither bubbleformation nor morphological
change of agarose gel was observed during the electrophoresis
wherein even 300 V was applied.
FIG. 5 (A) , (B ) , and (C ) show situations of introduction
and separation of DNA molecules, respectively.
More specifically, a sample plug could be formed by
applying 100 V for 1 second ( FIG. 5 (A) ) .
Further, the separation process was visualized by the
system shown in FIG. 3, and movements of bands could be clearly
observed ( FIG. 5 ( B ) and FIG. 5 ( C ) ) . The separation was attained
by applying an electric field of 71 .4 V/cm to 2.0$ agarose gel
wherein a TBE buffer solution is used. This electrical field
strength is in a lower level than that of ordinary
microfabricated capillary electrophoresis, i.e., several kV.
FIG. 6 indicates changes in electrophoresis of a
microchip 10 for capillary gel with time wherein a capillary
- i8 -
CA 02310700 2000-06-06
channel 16 is filled with agarose gel containing DNA size
standards of 100 by to 1000 bp. As shown in the graph of FIG. 6,
separation of DNA molecules ranging from 100 by to 1000 by was
completed in 2 minutes which is ten to twenty times faster than
conventional slab gel electrophoresis.
As explained, as to the microchip 10 for capillary gel
electrophoresis prepared from PDMS according to the present
invention in the above description, the microchip 10 for
capillary gel electrophoresis can be easily fabricated in
accordance with a simple and inexpensive molding method as well
as a sealing method. It has been found that the microchip 10 for
capillary gel electrophoresis is sufficiently available for DNA
separation.
While in the above described preferred embodiment, the
capillary channel 16 having a contour of a so-called I-shaped
flow path has been defined on the substrate 12, the invention is
not limited thereto as a matter of course. In this connection,
for example, a capillary channel having a contour of a so-called
cross-shaped flow path may be defined on the substrate 12. In
the case where a capillary channel of a cross-shaped flow path
has been defined on the substrate 12, four ports are bored on the
surface plate 14 so as to correspond to four opposed ends of the
capillary channel having a cross-shaped flow path,
respectively.
- 19 -
CA 02310700 2000-06-06
Since the present invention has been constituted as
described above, it is possible to provide a microchip for
capillary gel electrophoresis which can be fabricated at low
cost, and is suitable for disposable use, i.e., the microchip is
discarded after having been used only one time in
electrophoretic separation as well as to provide a method for
fabricating such a microchip.
Furthermore, since the present invention has been
constituted as described above, it is possible to provide a
microchip for capillary gel electrophoresis by which it becomes
possible to use a gel as molecular sieves, so that
electrophoretic separations can be made at low voltage without
employing any high voltage, and occurrence of diffusion and
convection at the time of applying a voltage is prevented,
whereby it is intended to simplify electrical equipment and a
detection device, so that remarkable reduction of cost can be
achieved as well as to provide a method for fabricating such a
microchip.
Moreover, since the present invention has been
constituted as described above, it is possible to provide a
microchip by which a gel can be used as molecular sieves, whereby
it is intended to improve a degree of separation, to reduce a
separation distance, and to reduce a separation time, so that it
becomes possible to improve separation performance in
electrophoretic separations and to achieve an electrophoretic
separation of high resolution as well as to provide a method for
fabricating such a microchip.
- 20 -
CA 02310700 2000-06-06
It will be appreciated by those of ordinary skill in the
art that the present invention can be embodied in other specific
forms without departing from the spirit or essential
characteristics thereof.
The presently disclosed embodiments are therefore
considered in all respects to be illustrative and not
restrictive. The scope of the invention is indicated by the
appended claims rather than the foregoing description, and all
changes that come within the meaning and range of equivalents
thereof are intended to be embraced therein.
The entire disclosure of ,7apanese Patent Application No.
11-345050 filed on December 3, 1999 including specification,
claims, drawings and summary are incorporated herein by
reference in its entirety.
-2~-