Note: Descriptions are shown in the official language in which they were submitted.
CA 02310990 2000-06-27
-1-
A USE OF GALANTA11~I)~1E FOR THE TREATMENT OF ALZHEIMER'S
DISEASE; TAF;GETIN~G THE UNDERLYING CAUSE OF THE DISEASE
The present invention relates to the use of an effective amount of galantamine
for the
treatment of Alzheimer's disease, wherein said treatment provides both
symptomatic
relief and also treats the underlying cause of the disease.
BACKGROUND CIF THE INVENTION
Galantamine is known to be a reversible cholinesterase inhibitor that can be
isolated
from a number of different. plant sources, including daffodil bulbs.
Galantamine
interacts competitively with the enzyme, acetylcholinesterase, and
demonstrates a 10
to SO fold selectivity for acetyl vs. butyryl cholinesterase.
1 S Galantamine has bef:n used for the treatment of a number of chronic
diseases, where
life-long treatment may be necessary. For example Galantamine has been shown
to be
effective in the treatment of Alzheimer's Disease (United States Patent
4,663,318).
The etiology of nf;uronal degeneration and associated cognitive impairment in
Alzheimer's disease is not fully understood. However, three consistent
neuropathological hallmarks have been identified: amyloid-rich senile plaques,
tau-
positive neurofibrill.ary tanl;les, and neuronal cell death. Methods of
treating
Alzheimer's disease include the use of therapeutic drugs for symptomatic
relief of the
cognitive symptom;; of the: disease. US Patent 5,962,535 acknowledged the
shortcomings of thf: prior treatments and proposed the use of a pharmaceutical
composition for treating or preventing Alzheimer's disease which comprises
idebenone
in combination with a compound having acetylcholinesterase inhibitory
activity.
According to this prior art, this combination of compounds produced clinical
benefits
not seen in previous treatments.
CA 02310990 2000-06-27
-2-
Thus, there is a need for other therapeutic drugs for treating, preventing and
inhibiting
the progression of Alzheimer's disease by targeting the underlying cause of
the disease.
SUMMARY OF THE INVENTION
Thus, according to the present invention there is provided a use of
galantamine for
treating, preventing and inhibiting the progression of Alzheimer's disease by
targeting
the underlying cause of the disease.
BRIEF DESCRIPTION O:E THE DRAWINGS
These and other features of the invention will become more apparent from the
following description in which reference is made to the appended drawings
wherein:
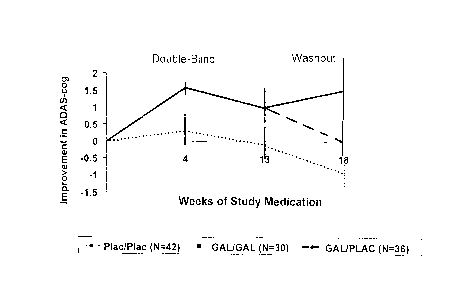
FIGURE 1 shows t:he mean change (~SE) in ADAS-cog/11 from baseline to the end
of the treatment period to the end of the washout period.
FIGURE 2 shows the prior girt results with donepezil hydrochloride in a
similar study.
DESCRIPTION O:E PREFERRED EMBODIMENT
The present invention relatEa to the use of an effective amount of galantamine
for
treating, preventing and inhibiting the progression of Alzheimer's disease by
targeting
the underlying cause: of the disease.
Galantamine, a tertiary alkaloid, has been isolated from the bulbs of the
Caucasian
snowdrops Galanta~nus woronowi (Proskurnina, N. F. and Yakoleva, A. P. 1952,
Alkaloids of Galanthus woronowi. II. Isolation of a new alkaloid. (In
Russian.)
Zh.Obschchei Khim. (J. Gen. Chem.) 22, 1899-1902). It has also been isolated
from
the common snowdrop Gala.nthus nivalis (Boit, 1954). Galantamine is a well-
known
acetylcholinesterase inhibitor which is active at nicotinic receptor sites but
not at
muscarinic receptor sites. It is capable of passing the blood-brain barrier in
humans,
and presents no severe side effects in therapeutically effective dosages.
Galantarnine has been used extensively as a curare reversal agent in
anaesthetic
practice in Eastern bloc countries (cf. review by Paskow, 1986) and also
CA 02310990 2000-06-27
-3-
experimentally in the West (cf. Bretagne and Valetta, 1965: Wislicki, 1967;
Consanitis. 1971 ).
Galantamine has been marketed by the company Waldheim (Sanochemia Gruppe) as
NivalinT"' in Germany and Austria since the 1970s for indications such as
facial
neuralgia.
In the present invention when we refer to galantamine we include within this
term
galantamine itself, derivatives and salts thereof, such as halides, for
example
galantamine hydrobromide.
For the purposes of the present invention galantamine and derivates and salts
thereof
may be formulated according to convention methods of pharmacy, together where
appropriate with one or more pharmaceutically acceptable carriers, excipients
or
diluents, as is known in the: art. Such formulations can take the form of
tablets,
capsules, solutions, or lozenges, pessaries, creams, suppositories or
transdermal
formulations, depending on the route of administration.
According to the prcaent invention, it has been found that galantamine, for
example
galantamine hydrobromide, acts on the cholinergic system in two ways - by
modulating
the release of acelylcholine nicotinic receptors and inhibiting
acetylcholinesterase.
Galantamine hydrob romide is thought to increase the release of acetylcholine
through
a mechanism called allosteric modulation of the nicotinic receptors. Although
not
wanting to be bound to any particular theory, it is thought that galantamine
hydrobromide binds to a site on the presynaptic nicotinic receptor, that is
distinct from
the acetylcholine-binding site, and evokes a change in the shape of the
receptor. This
triggers the openin~; of ion channels in the presynaptic membrane, thus making
cholinergic neurons more excitable. This results in an increased release of
acetylcholine, which can then further stimulate the presynaptic nicotinic
receptors to
mediate the release of more a.cetylcholine. Such modulation is self
regulating, which
produces overstimulation. The increased production of acetylcholine
competitively
CA 02310990 2000-06-27
-4-
inhibits the enzyme responsible for its breakdown, acetylcholinesterase. In
addition
to increasing the amount of a~cetylcholine released, stimulation of
presynaptic nicotinic
receptors also increases the; release of other neurotransmitters (such as
glutamate)
thought to play important roles in cognitive function.
In the present invention, the impact of drug withdrawal was used as an
indication of
the impact of the dmg on cognitive function. The expectation was that patients
treated
with galantamine, and then withdrawn from the drug would return to levels of
cognitive function similar to those documented in the placebo group. As is
shown
below, prior art studies h;~ve demonstrated that following the withdrawal of a
cholinesterase inhibitor, in the treatment of Alzheimer's disease, the
symptomatic
improvement diminished rapidly as a function of the pharmacokinetic
elimination of
the inhibitor. However, according to the present invention it was found that
patients
treated with galantamine enjioyed some sustained benefit, even after being
removed
from the drug.
According to the preaent invention, a safe and effective amount of galantamine
can be
used for treating, preventing and inhibiting the progression of Alzheimer's
disease by
targeting the underlying cau<,;e of the disease.
Precise dosage rates and regimes can be determined empirically by the medical
practitioner, depending on individual circumstances. For example, if the
compound
is delivered orally, a daily dose of from about 1 mg to about 100 mg is
effective. In
a further example th.e compound can be delivered at about 5 mg to about 50 mg
per
day. In yet a further examplf: the compound can be delivered at about 16 mg to
about
32 mg per day. Precise dail~~ dosages can be selected from 16 mg, 18 mg, 24 mg
or
32 mg per day. It is preferred that the daily dosage be divided into two or
three equal
dosages.
It has been found flat the tolerability or safety of the drug can be improved
if the
patient is introduced to the drug slowly over a number of weeks.
CA 02310990 2000-06-27
-$-
In one embodiment. of the present invention the patient is introduced to
galantamine
slowly from about :~ weeks to about 10 weeks, wherein the dose is increased
over this
period.
S In one embodiment: of the present invention the patient received a dose of
about 8
mg/day for about 1 week, followed by a dose of about 16 mg/day for about 1
week,
followed by a maintenance dose of about 24 mg/day thereafter.
In one embodiment of the present invention the patient received a dose of
about 8
mg/day for about 1 week, followed by a dose of about 16 mg/day for about 1
week,
followed by a dose of about 24 mg/day for about 1 week, followed by a
maintenance
dose of about 32 ml;/day thereafter.
In one embodiment of the present invention the patient received a dose of
about 8
mg/day for from about 2 weeks to about 4 weeks, followed by a dose of about 16
mg/day for from about 2 weeks to about 4 weeks, followed by a maintenance dose
of
about 24 mg/day thE;reafter.
In one example of this embodiment the patient received a dose of about 8
mg/day for
about 4 weeks, followed by a. dose of about 16 mg/day for about 4 weeks,
followed by
a maintenance dose of about 24 mg/day thereafter.
In a further embodiment of the present invention the patient received a dose
of about
8 mg/day for from about 2 weeks to about 4 weeks, followed by a maintenance
dose
of about 16 mg/day thereafter. In one example of this embodiment the patient
received
a dose of about 8 mg/day for about 4 weeks, followed by a maintenance dose of
about
16 mg/day thereafter.
The present invention is illustrated by the following example, which is not to
be
construed as limiting;.
CA 02310990 2000-06-27
-6-
EXAMPLES
A six-week randomized withdrawal study compared withdrawal from a three month
treatment with galantamine, to continued treatment with 24 mg or 32 mg
galantamine
(given in two equal doses), or continued treatment with a placebo.
Patients who received placebo in the earlier study continued to receive
placebo
(PLA/PLA). Patients who received galantamine in the earlier study were
randomized
into one of two groups. The withdrawal group (GAL/PLA~ were removed from the
galantamine treatment and received placebo for 6 weeks. The other group
(GAL/GAL)
continued to receive the same dose of galantamine, 12 mg or 16 mg bid (twice
daily).
Saftey was assessed by ECG, periodic physical examination, laboratory tests
and
reports of adverse events. The Alzheimer's Disease Assessment Scale (ADAS)
ADAS-
cognitive scale (ADAS-cog) was used to evaluate efficacy during treatment
(Rosen,
W.G. et al., Amer. J. Psychiatry, 141: 1356- 1364, 1984).
All patients were monitored throughout the study, with follow-up and cognitive
evaluation at six weeks after the start of the withdrawal study.
The primary efficacy endpoints were the change from baseline ADAS-cog/11. The
ADAS consists of two parts -- a cognitive subscale and a behavioral subscale.
The
behavioral subscale was not b~e used in this study. The cognitive subscale,
the ADAS--
cog-11 comprises the following 11 items, with a score ranging from 0 to 70:
Word Recall (0 to 10;>
Word Recog~ution memory tests (0 to 12)
Object and Finger Naming (0 to 5)
Commands (l) to 5)
Construction,~l Praxis (0 to 5)
Ideational Praxis (0 to 5)
Orientation (l) to 8)
Remembering; Test Instructions (0 to 5)
CA 02310990 2000-06-27
Spoken language Ability (0 to 5)
Comprehension of Spoken language (0 to 5) and
Word Finding Difficulty (0 to 5).
To reduce variability due to circadian fluctuations in cognitive status the
ADAS was
done always at the same tirr~e of the day, preferably before noon.
The ADAS-cog/13 was used as a secondary variable. The ADAS-cog/13 subscale
consisted of the 11 items in~, the ADAS-cog/11 subscale plus two new items,
which
were added to provide more :information regarding the patient's status. This
secondary
variable, with a score ranging from 0 to 85 contains the additional two items:
Concentration and Distraction (0 to 5) and
Delayed Word Recall (0 to 10).
Additional secondary variables included the ADAS-cog/10 and ADAS-cog/mem
tests.
The items in the AI)AS-cog,~l0 analysis are:
Object and hinger Naming (0 to 5)
Commands (0 to 5)
Constructional Praxis (0 to 5)
Ideational Praxis (0 to 5)
Orientation (0 to 8)
Remembering Test h~structions (0 to 5)
Spoken language Ability (0 to 5)
Comprehension of Spoken language (0 to 5)
Word Finding Difficulty (0 to 5) and
Concentration and Diistraction (0 to 5).
The memory ADAS cognitive subscale is the sum of the following three items
(score
range 0 to 32):
Word Recall (0 to 10)
Word Recognition memory tests (0 to 12) and
Delayed Word Recall. (0 to 10).
CA 02310990 2000-06-27
_g_
The MMSE is a very brief to;st of cognitive functions including orientation to
time and
place, instantaneous recall, short-term memory, and ability to perform serial
subtractions or reverse spelling, constructional capacities and the use of
language. The
MMSE score was derived from the sum of the points assigned to each completed
task.
A total possible score is 30. The MMSE was performed at visit 1(screening).
Descriptive statistics for all patients were provided for demographic
variables and
baseline characteristics (obtained at baseline visit before the administration
of
galantamine). Similar summaries were provided for the three treatment groups
(PLA/PLA, GAL/PhA, GAL/GAL).
The comparability between treatment groups at baseline was evaluated with
respect to
demographic and baseline variables. A two-way analysis of variance (ANOVA)
model
with factors for treatment group, investigator, and their interaction term
(when
appropriate) was used for continuous variables. The Van Elteren test
controlling for
investigator was used for the between-treatment group comparison of ordinal
categorical
variables. For nominal catel;orical variables, the Cochran-Mantel-Haenszel
test for
general association controlling for investigator was used.
The results for medi<;al and surgical history updates since the beginning of
randomized
withdrawal phase were sumrr~arized by the number and percent of abnormality of
each
system. No formal statistical comparison was done.
Concomitant and psychotropic medications used were summarized by the number
and
percentage of patients presenting per treatment.
The primary efficacy parameter was the total score of the 11 cognitive items
from the
original ADAS cognitive subscale (ADAS-cog/11). The secondary efficacy
parameters
were ADAS cluster scores AI)AS-cog/13, ADAS-cog/10, and ADAS-cog/mem, as well
as the percentage of responders.
CA 02310990 2000-06-27
-9-
All efficacy data were summarized and compared to the initial visit (the end
of the initial
treatment phase) to evaluate the changes that occurred during the 6 weeks of
withdrawal.
The entire efficacy ;profile' from the beginning of the preceding study to the
end of this
withdrawal trial was also evaluated by methods for analyzing continuous
repeated
S measures.
The primary efficacy comparisons were made between the GAL/PLA group
regardless
of galantamine dose, and the PLA/PLA group, unless specified otherwise.
Within-group comparisons for changes from the initial visit and changes from
baseline
were performed using the paired t-test or Wilcoxon signed rank test depending
on the
distribution of the data. For the continuous data, changes from the initial
visit were
compared among two treatment groups (PLA/PLA, GAL/PLA). An analysis of
covariance model (ANCOVA,) with treatment, investigator, and their interaction
(if found
to be significant at p=0.1 significance level) as factors and the initial
value as covariate
was used. Following ANCOVA, pairwise comparisons were subsequently performed
by
Fisher's least significant difference procedure. If parametric methods were
deemed
inappropriate, that i;s, if the data did not follow a normal distribution,
nonparametric
methods such as two-way AiVOVA on ranked data or Van Elteren test controlling
for
investigator were used.
For ordinal categorical variables, the Van Elteren test controlling for
investigator was
used for the between-treatment group comparison. For the nominal data, the
Cochran-
Mantel-Haenszel test for general association controlling for investigator was
used. -
All analyses indicated in the protocol were conducted as above. However, for
continuous
data, a one-way ANOVA with treatment as factor was applied at all time points
instead
of the ANCOVA model as described in the protocol. The center effect was not
included
in the ANOVA model due to the small center sizes in this trial. The
longitudinal analysis
using a mixed model approach was not performed since the GAL/PLA group changed
treatment in this trial, a simple mixed effect model would not address this
issue.
CA 02310990 2000-06-27
-10-
The safety of the drug was also monitored throughout the study. All patients
who had
taken at least one dose of trial medication in this withdrawal trial were
included in the
safety summary. For these patients, the entire safety profile from the
beginning of the
preceding study to t:he end o:f this withdrawal trial (last day trial
medication was taken
plus 3 days) was also summarized. Serious adverse events were recorded up to
30 days
after the last day of trial medication.
The type and incidence of adverse events that patients experienced during the
preceding
trial and this withdrawal trial were summarized by treatment group. Separate
tabulations
were prepared for severity of treatment emergent adverse events, drug-related
adverse
events, serious adverse event:;, and other significant adverse events, such as
those leading
to the patient's discontinuation from treatment.
For the clinical laboratory data, descriptive statistics and pre-versus within-
and post-
treatment cross-tabulations (with classes for below, within and above the
normal range
of the central laboratory, Cwance Central Laboratory Services, Inc.,
Indianapolis,
Indiana) were generated for all tests performed.
In addition, potentially clinically important values were tabulated for all
biochemistry
tests and for all urine parameters. The parameters were assessed before
(reference or
baseline value), during or after treatment. Criteria limits for potentially
clinically
important values for :most biochemical tests were as defined by Lippert and
Lehmann (SI
Units in Medicine. Baltimore-Munich; Urban & Schwarzenberg;1978). For enzymes,
the
lower criteria limit is defined as zero, and the upper criteria limit as twice
the upper limit
of normal.
This study was conducted on a total of 118 patients that had completed an
earlier study,
receiving a placebo, 24 mg/clay or 32 mg/day of galantamine for three months.
The
placebo group, 47 patients, continued receiving the placebo. The galantamine
group
were divided into two groups. One group, 39 patients, were given a placebo in
this
CA 02310990 2000-06-27
-11-
withdrawal test, whereas the other group, 32 patients, continued receiving the
dose of
galantamine, 24mg/day or 32 mg/day, given in the earlier completed study.
Ninety
four percent of the patients completed the trail.
Table 1 summarizes the demographics and baseline characteristics (from intake
into the
initial study) for all patients participating in this trial. Slightly more
women (59%) than
men (41 %) participated in the; trial. The maj ority (91 %) of patients were
white, with an
average age of 75 ye~~rs and are average weight of 69 kg.1'hese factors were
well balanced
across treatment groups, and no statistically significant differences were
found among the
three treatment groups.
CA 02310990 2000-06-27
-12-
Table 1. Demographic and baseline disease characteristics for all patients
Characteristic or PLA/PLA GAL/PLA GAL/GAL Total
variable .
n=47 n=39 n=32 N=118
Sex: N (%)
Male 19 (40.4%)19 (48.7%)11 (34.4%)49 (41.5%)
Female 28 (59.6%)20 (51.3%)21 (65.6%)69 (58.5%)
Race: N (%)
White 42 (89.4%)34 (87.2%)31 (96.9%)107 (90.7%)
Black 0 (0%) I (2.6%) 1 (3.1%)2 (1.7%)
Hispanic 3 (6.4%) 2 (5.1%) 0 (0%) 5 (4.2%)
Oriental I (2. l 1 (2.6%) 2 ( 1.7%)
%)
Other 1 (2. ( 1 (2.6%) 0 (0%) 2 ( 1.7%)
%)
0 (0%)
Age (mean t SE) years.74 t 1.17 76.5 t 75.3 75.2 t
1.26 t 1.12 0.7
Weight (mean t SE) 67.99 t 69.41 t 70.13 69.04 ~
kl; 2.47 2.63 t 2.72 1.50
Smoker: yes n (%) 1 (2.1 4 (10.3 0 (0.0%)5 (4.2%)
%) %)
Age at onset of cognitive
problem (mean t SE) 71.4 ~ 73.4 t 72.3 72.3 ~
1.23 1.37 f 1.32 0.76
Years since cognitive
problem
diagnosis (mean t 3.62 ~ 4.18 t 4.11 3.94 t
SE) ~', 0.35 0.44 t 0.41 0.23
Age at diagnosis
of probable
AD (mean ~ SE) 73.8 ~ 76.4 t 75.5 75.1 t
1.2 1.33 t 1.15 0.72
Years since probable
AD
diagnosis (mean t 1.1 ~ 0.211.1 I t 0.83 1.03 t
SE) 0.21 t 0.12 0.11
Relatives) with AD:
N (%) 1 I (23%) 12 (31%) 8 (25%) 31 (26%)
Cholinomimetics trial
participant: N (%) 0 (0%) 2 (5. I 1 (3.1 3 (2.5%)
%) %)
MMSE score
(mean t SE) 20.3 t 20 t 0.51 19.9 20.1 t
0.47 t 0.78 0.33
ADAS-cog/11 score 22.3 t 24.9 t 23.6 23.5 f
(mean t 1.39 1.45 t 2.18 0.94
SE)
CA 02310990 2000-06-27
-13-
All 118 patients in this trial were treated with at least one dose.
The primary analysis was performed on ADAS data collected at Week 6
(traditional
observed case) and compared to ADAS data collected at initial visit (primary)
and
baseline visit (secondary). Patients missing data for Week 6 were not included
in the
analysis. The primary comparison was between the PLAJPLA group and the GAL/PLA
group in change from initial visit at Week 6.
Five patients, 1 in the; PLA.lPLA group, 3 in the GAL/PLA group, and 1 in the
GAL/GAL
group, did not have any ADAS data collected at the initial visit. Eight
patients, 5 in the
PLAJPLA group, 1 in the C.TA.I,/PLA group, and 2 in the GAL/GAL group, did not
have
ADAS data collected at Week: 6. In a traditional LOCF (last observation
carried forward)
analysis, available ADAS scores from the initial visit for these patients were
used for the
Week 6 visit. These results did :not differ from results obtained in the
traditional observed
case analysis.
ADAS-cog/11 (Alzlueimer's lDisease Assessment Scale - Cognitive Subscale) was
the
total score of 11 cognitive items on the ADAS. The total score ranges from 0-
70, with
a higher score indicating a worsening of cognitive function. ADAS-cog/11 score
was
calculated only when all 11 items were available. Missing ADAS-cog/11 scores
were not
replaced or imputed due to the low missing rate.
The primary analysis was the; change from initial visit versus Week 6. These
data are
summarized in Table2.
CA 02310990 2000-06-27
-14-
Table 2. ADAS-col;/11 mean scores and change from initial visit for observed
case
data
Timepoint PLA/PLA GAL/PLA GAL/GAL
n Mean n Mean n Mean
t t t
SE SE SE
Initial 46 22.6 36 23.8 31 23.1
t t t
1.43 1.46 2.65
Week 6a 41 23.0 36 25.2 29 22.3
t t t
1.41 1.77 2.76
Change from 0.8 I .4 -0.9
initial t t t
visit 0.83 0.89 1.02
a:
Patients
with
ADAS-c:og/11
score
at
initial
visit
(start
of
this
trial)
At the beginning of this trial, initial mean ADAS-cog/11 scores were slightly
higher for
patients who had been receiving galantamine as compared to patients who had
been
receiving placebo. A.t the end of the 6-week withdrawal period, patients in
the placebo
group through both treatment and withdrawal (PLAJPLA) had slightly higher mean
ADAS scores compared to scores at the initial visit, indicating a decline in
cognitive
function. Patients in the galantamine group through both treatment and
withdrawal group
(GAL/GAL) had slil;htly lower mean ADAS scores, thus maintaining a trend
towards
improved cognitive function. Patients in the group that had received
galantamine during
treatment and who v,rere withdrawn to placebo in this trial (GAL/PLA), had
improved
while on active treatment, and declined upon withdrawal of medication.
Upon entry into the initial study, baseline ADAS-cog/11 scores were slightly
higher for
the GAL/PLA and GAL/GAL groups as compared to PLA./PLA, but were not
statistically
significantly different between groups (p=0.491). These data, along with mean
change
from baseline, are shown in Table 3.
CA 02310990 2000-06-27
-15-
Table 3. ADAS-cogJl l mean scores and change from baseline visit for observed
case
data
Timepoint PLA/PLA GAL/PLA GAL/GAL
n Mean n Mean n Mean
t t t
SE SE SE
Baseline 47 22.3 39 24.9 31 23.6
t t t
1.39 1.45 2.18
Week 6a 42 22.8 38 24.8 29 21.9
t t t
1.39 1.70 2.70
Change from 0.9 0.1 -1.5
baseline t t t
0.97 0.79 1.16
a: rarients wnn al~A~-<;og/11 score at m~tial msit (start of this trial)
Patients in the PLA/PLA group, who received placebo throughout both trials,
ended the
withdrawal phase (Week 6) with slightly higher mean ADAScog/11 scores,
indicating
gradual deterioration of mental function. Patients in the GAL/GAL group, who
received
galantamine throughout both trials, ended the withdrawal phase (Week 6) with
slightly
lower mean ADAScog/11 scores, demonstrating a sustained symptomatic effect.
Patients
in the GAL/PLA group, who had received galantamine for 3 months and then were
withdrawn from active treatment, had returned to near original baseline values
after 6
weeks without galantamine.
The mean change in .ADAS-cog/11 scores and standard errors are displayed in
Figure 1.
In addition to ADAS-cog/11 score, three cluster scores were identified and
analyzed as
secondary efficacy p<~rameters. Table 4 presents means and mean change from
initial visit
(beginning of this trial) for ADAS cluster scores at Week 6.
CA 02310990 2000-06-27
-16-
Table 4. Mean score and change from initial visit for ADAS cluster scores at
Week
6
PLA/PLA GAL/PLA GAL/GAL
ADAS cluster scoren Mean Mean n Mean Mean ' Mean Mean
n
t SE change t change t SE change
SE
t SE t SE t SE
ADAS-cog/13 40 31.9 0.2 36 34.6 I.1 29 30.7 -1.7
t t t t t t
1.67 0.94 2.06 1.01 3.18 I.15
ADAS-cog/mem 40 21.3 0.1 36 23.7 0.4 30 20.6 -0.8
t t t t t t
0.87 0.58 0.82 0.63 1.21 0.78
ADAS-cog/10 41 10.7 0.1 37 11.5 0.9 29 10.3 -0.8
t t t ~ t t
1.02 0.50 1.61 0.78 2.18 0.75
Table 5 presents the; means and mean change from baseline (beginning of the
initial
study) for ADAS cluster scores at Week 6.
CA 02310990 2000-06-27
-17-
Table 5. Mean score and cliange from baseline for ADAS cluster scores at Week
6
PLA/PLA GAL/PLA GAL/GAL
ADAS cluster n Mean Mean n Mean Mean n Mean Mean
score
t chan t chang f SE change
SE SE
ge a t SE
t t
SE
SE
ADAS-cog/13 42 31.9 0.4 38 34.2 -0.2 30 30.3 -2.3
~ t t t
1.09 t 0.93 3.09 1.39
I 1.98
.61
ADAS-cog/mem 42 21.4 -0.6 38 23.4 0.2 31 20.4 -0.8
t t t
t t t 0.59 1.18 0.73
0.84 0.63 0.81
ADAS-cog/10 42 10.5 I.1 39 11.4 -0.1 30 10.1 -1.4
t t t t
t 0.80 f 0.75 2.12 0.87
1.00 1.53
Table 6 shows the mean changes from initial visit on ADAS individual item
scores at
Week 6. In general, no change from initial visit was seen in all of the
groups.
CA 02310990 2000-06-27
-18-
Table 6. Mean change from initial visit for individual ADAS item scores at
Week
6: observed case
PLA/PLA GAL/PLA GAL/GAL
ADAS item (score n Mean changen Mean changen Mean change
range) t SE t SE t SE
Word recall (0-10) 41 -0.15 t 37 0.30 t 30 0.10 t
0.14 0.24 0.22
Naming objects & 41 -0.15 t 37 0.05 t 30 -0.07
fingers (0-5) 0.08 0.09 f 0.1
I
Delayed word recall 40 -0.50 t 36 -0.17 30 -0.50
(0-10) 0.16 t 0.22 t 0.35
Commands (0-5) 41 -0.10 t 37 0.27 t 30 -0.13
0.13 0.17 t 0.17
Constructional praxis41 0.05 t 37 -0.19 29 -0.07
1,0-5) 0.13 t 0.12 t 0.14
Ideational praxis 41 0.22 t 37 0.24 f 29 -0.07
(0-5) 0.13 0.16 t 0.14
Orientation (0- 8) 41 0.24 t 37 0 t 0.22 30 -0.13
0.29 ~ 0.23
Word recognition 41 0.73 t 36 0.31 t 30 0.37 t
test y0-12) 0.51 0.55 0.58
Remember test instruction41 -0.27 t 37 0.24 t 30 0.10 t
(o-s) 0.17 0.18 0.09
Spoken language ability41 0.02 t 37 0.03 t 30 -0.13
(0-5) 0. I 1 0.13 t 0.13
Word fording difficult'41 0.17 t 37 0.24 f 30 -0.10
(0-5) 0.11 0.13 t 0.15
Comprehension (0-5) 41 0 t 0.12 37 0.03 t 30 0.07 t
0.14 0.12
Concentration/distractibility41 -0.05 t 37 0 t 0.16 30 -0.27
(0-5) 0.13 t 0.15
Four definitions for ADAS-cog/11 response were used to summarize ADAS-cog/11
response data. Responder (0) was defined for those patients with no change or
better
(zpoints improvement) on ~?.DAS-cog/11 score. Similarly, responder (4) was for
z4
points improvement, respondesr (7) for s 7 points improvement and responder (
10) for z 10
points improvement.
The percentages are based on total number of patients who had an evaluation at
the
specific visit. The response data, from initial visit, at Week 6 shows that
about 61 % of
PLA/PLA patients had no change or were improved in ADAS-cog/11 score, as
compared
CA 02310990 2000-06-27
- 19-
to 47% in the GAL/l?LA group and 55% in the GAL/GAL group. The use of higher
cut-
off points demonstrate that patients who received galantamine were higher
responders
than patients who only received placebo. Only fifteen percent of PLA/PLA group
patients
had z4 points improvement iin ADAS-cog/11 score at Week 6, as compared to 19%
in
the GAL/PLA group and 28°ro in the GAL/GAL group. Five percent of
PLA/PLA group
patients had z7 points improvement in ADAS-cog/11 score at Week 6, as compared
to
6% in the GAL/PLA group and 14% in the GAL/GAL group. Only patients in the
GAL/GAL group had any responders (7%) with z 10 points improvement.
In summary, response data for patients withdrawn from galantamine to placebo
were
intermediate between patients who remained on placebo and patients who
remained on
galantamine, for all Four cutoff points.
At the initial visit of this withdrawal trial, patients treated with
galantamine for 3 months
in a preceding double blind, flexible dose trial tended to have improved
cognitive
function (as measured by a reduction in ADAS-cog/11 score from baseline)
compared
to patients who had received placebo.
At the final Week 6 visit of thiis withdrawal trial, ADAS-cog/ 11 scores for
patients in the
GAL/PLA group, who had received galantamine for 3 months, then were withdrawn
and
placed on placebo fir 6 weeks, were back to approximately the level of the
original
double-blind baseline. Patients in the PLA/PLA group, who had received placebo
during
both the original treatment period and the withdrawal period, had ADAS-cog/11
scores
that continued to increase, indicating a worsening in cognitive function.
Patients in the
GAL/GAL group, who had received galantamine during both the original treatment
and
the withdrawal period, had ADAS-cog/11 scores that continued to decrease,
indicating
an improvement in cognitive function.
All adverse side effects were monitored throughout the 6 week withdrawal
period.
Serious side effects were few in this 6-week trial, and occurred with similar
frequency
across the treatment groups. None were considered to be related to trail
medication.
CA 02310990 2000-06-27
-20-
There were no adverse events reported that could be related to central or
peripheral
reduction in cholinexgic tonf; after sudden withdrawal of galantamine (detail
results nt
shown).
As noted above the results of the present invention contrast the results
available in the
prior art in a study using another cholinesterase inhibitor, donepezil
hydrochloride.
In the prior art study patients received either Smg/day or 10 mg/day for a
treatment
period of 24 weeks, followed by a 6 week placebo washout period. At the end of
the
24 week treatment period statistically significant improvements from baseline
were
demonstrated. However after removal from the drug, the treatment group was
indistinguishable from the group that received only placebo for the 30 week
period.
These results are shown in Figure 2 and demonstrate that donepezil
hydrochloride does
not treat the underlying cause of the disease.
All scientific publications and patent documents are incorporated herein by
reference.
The present invention has been described with regard to preferred embodiments.
However, it will be obvious to persons skilled in the art that a number of
variations
and modifications can be made without departing from the scope of the
invention as
described in the following claims.