Note: Descriptions are shown in the official language in which they were submitted.
CA 02311539 2000-OS-19
WO 99/20203 PCTNS97/19445
METHOD OF FORMING A MEMBRANE. ESPECIALLY A LATEX OR
POLYMER MEMBRANE., INCLUDING MULTIPLE DISCRETE LAYERS
Technical Field
The present invention relates to membranes formed from materials including
latex, polyurethane, polyethylene, rubber, and other polymers and elastomers.
Background Art
Known applications of such membranes include surgical and examination
gloves, condoms, diaphragms, dressings, sheaths, slippers, overshoes, sterile
bands,
catheters, tubing, drapes, gut openings, mouth pieces, baby nipples, infra
gastric
nasal tubes, nasal gastric tubes, kidney shunts, eye and brain shunts, dental
dams,
dental braces, sub-clavian vein and artery shunts, and colostomy bags.
Typically,
1 S such membranes in use contact a person's or animal's skin or other
tissues.
In recent years, there has been a growing interest in improving such
membranes to provide increased protection against the transmission of viruses
such
as hepatitis and HIV, as well as other pathogens and harmful agents.
Disclosure Of Invention
The present invention discloses several embodiments which provide
membranes with an improved resistance to transmission of viruses and other
harmful
agents, and capabilities to disinfect needles and other membrane piercing
objects,
and also discloses the provision of one or more indicating layers to detect
and
indicate membrane breach and the presence of viruses, and other pathogens, as
well
as harmful chemicals.
In one aspect of the invention, a membrane of multi-layer construction
includes one or more inner-layers, which serve as reservoirs for substances or
agents
such as biocides, lubricants, or indicators, which can pass through one or
more
permeable or semi-permeable outer layers to make the reservoir substance
available
SUBSTITUTE SHEET (RULE 26)
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
2
on the outside of the membrane, or to alternatively prevent exterior
transmission of
reservoir substances while allowing exterior substances to pass at least
partially
through the membrane. As an alternative to permeability, substantially
impermeable
layers may transmit the substance or agent upon rupture or piercing and
completely
S contain the substances at all other times.
According to another aspect of the invention, a membrane of mufti-layer
construction includes one or more inner-layers, which serve as reservoirs for
substances or agents such as biocides, lubricants, or indicators, which can
contact or
otherwise interface with substances passing through one or more outer layers
of the
membrane and which may react with, indicate the presence of, or otherwise
respond
to the presence of the substance originally outside the membrane. Such mufti-
layer
membranes can be used to provide anti-microbial, disinfectant, or other
killing or
disabling action to infectious agents, microbes, viruses, or bacteria, through
selective
or controlled flow of the inner substances or agents to the surface of the
membrane.
1 S According to another aspect of the invention, a mufti-layer membrane
provides a site for indicating materials such as a DNA probe based reaction
such as
Chiron's "Branched DNA Probe", Hoffrnan-LaRoche's "Polymerase Chain Reaction"
technique, or conventional color change indicator reactions, titration
reactions,
reactions to detect Ph, and reactions to detect chemicals, viruses or other
pathogens.
The-mufti-layer membrane includes one or more permeable or semi-permeable
layers to allow migration of a material to be detected through one or more
outer
layers and into contact with an indicating material or system. One or more of
the
layers may be impermeable to prevent migration beyond the indicators or other
reservoir materials.
In another aspect of the invention, indicators to detect chemicals, viruses,
or
other pathogens are admixed with the material of the membrane, or with a layer
of
a mufti-layer membrane, or coated on an outer layer of the membrane or mufti-
layer
membrane.
CA 02311539 2000-OS-19
WO 99/Z0203 PCT/US97/19445
3
Another aspect of the invention involves the provision of an indicator to
indicate a breach of a membrane. Indication may be provided by color change,
shade change, e.g., darker or lighter, temperature change, or tactile change,
e.g.,
stiffness or tightness. Indicators such as cobalt chloride can indicate
membrane
breach by reacting in the presence of moisture.
In another aspect of the invention, a permeable or semi-permeable membrane
of multi-layer construction includes one or more inner-layers which serve as
reservoirs for substances or agents such as biocides, lubricants, hydrogels,
or
indicators. The substances or agents can pass through the outer layer or
layers to
make the reservoir substance available on the outside of the membrane.
In another aspect of the invention, one or more semi-permeable or permeable
membrane layers permit migration of a substance in a predetermined direction.
For
example, viruses or other pathogens may migrate inwardly into contact with an
indicator or biocide. Additionally or alternatively, biocides, lubricants,
spennicides,
antiseptics, or indicators, may migrate outwardly.
In another aspect of the invention, a single or mufti-layer membrane includes
an indicator located on an inner or outer surface to detect the presence of a
virus or
other harmful material disposed exteriorly of the membrane and/or also
indicate
transmission of a harniful material either partially or entirely through the
membrane.
Another aspect of the invention includes the provision of a tactile-feeling
enhancing liquid or gel between layers of a mufti-layer membrane.
Another aspect of the invention involves the dispersion of micro-fibers or
fibers such as Kevlar within or onto a membrane forming substance prior to or
during membrane formation to increase membrane strength and penetration
resistance.
In one aspect of the invention, the membrane includes an indicator which
provides a prompt identifiable reaction in the presence of a harmful substance
to
alert a user. Example indicators include a DNA probe such as those developed
by
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
4
Chiron or Hoffman-LaRoche to detect the presence of particular viruses, or
conventional color change indicator technology such as phenolphthalein
reactions
or Ph indicator materials, chromophores, or dyes which change in the presence
of the
substance to be detected.
In one aspect of the invention, a single or mufti-layer membrane effects
transport across one or more membrane layers by capillary or wicking action.
The
membrane or one or more layers may also constitute a size selective membrane
to
limit the size of viruses or microbes passing through. Additionally or
alternatively,
one or more of the membrane layers may be chemically selective. For example,
membranes of the type used in filtration and purification procedures may be
employed.
In another aspect of the invention, a membrane including a permeable or
semi-permeable outer or inner layer includes a sealing treatment or coating to
entrap
indicators or biocide agents therein.
The invention also contemplates the provision of an indicator dispersed
throughout a membrane or restricted to a certain spot or area on or in the
membrane,
such as in or on dots or stripes. The indicator may be added to the membrane
during
formation or after completion.
In another aspect of the invention, a needle treatment substance in one or
more inner layers of a membrane functions to clean, coat, wipe, scrape,
cleanse,
disinfect, render less harmful, or otherwise treat a needle or other membrane
piercing
object.
Another aspect of the invention discloses a method of making a mufti-layered
membrane, such as a glove or condom, in which the layers are joined in one or
more
predetermined regions, such as only in a cuff or top region.
In another aspect of the invention, a method of admixing protein binding
biocides such as gentian violet with wet latex prior to membrane formation
CA 02311539 2000-OS-19
WO 99/20203 PG"T/US97/19445
increases membrane strength and\or reduces or eliminates allergic affects in
latex-
allergic or sensitive individuals.
In another aspect of the invention an adhesive backed patch includes an
antiseptic or cleansing agent which contacts a needle or catheter piercing
5 therethrough.
Brief Description Of Drawing
Figure 1 depicts a diagrammatic plan view illustrating a double glove
produced according to a method of the present invention.
Figure 2 is a cross-sectional view illustrating the double glove of Figure 1
including a biocide disposed in an intermediate reservoir formed between inner
and
outer layers of the glove.
Figure 3 is a diagrammatic plan view illustrating a double glove according
to the present invention having an indicating strip with separate detecting
regions to
indicate the presence of different pathogens or other harmful agents or
chemicals in
the.environment of the glove user. .
Figure 4 is a cross-sectional view illustrating an example mufti-layer
membrane according to the present invention.
Best Mode For Carrvin:: Out The Inve~ tion
Needles, both hollow core and solid, as well as other sharp objects such as
catheters, scalpels, wires, or bone fragments, have long been a concern for
health
care professionals and others in regard to the infection which they can
transmit.
Needles are usually in sterile condition when removed from their wrappers,
however
they can be inadvertently contaminated when they pass through a person's body
and
are potentially contaminated when they are removed from an infected patient's
body.
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
6
It is common for health care workers and hospital staff to be accidentally
stuck by needles or other sharp objects both during and after their use.
Pursuant to the present invention, a material is incorporated within a multi-
layer membrane, such as a glove, to clean, coat, wipe, scrape, cleanse,
disinfect,
render less harmful or otherwise treat a needle or other sharp object passing
therethrough.
A membrane formed from liquid latex, solvent cast membranes, liquid
polymers or other synthetics, or elastomers may be formed by dip forming, the
use
of fluidized beds, or spraying the liquid material onto a former. After
deposit of one
or more layers, a middle layer including a material having treatment
properties is
deposited. Thereafter, one or more outer layers are formed and the membrane is
cured or set according to conventional techniques.
Suitable polymers for use in producing membranes pursuant to the invention
include prepolymers, i.e. low molecular weight polymers and polymer
precursors,
prepolymers and polymer precursors dissolved in solvents, liquid monomers, and
liquid monomers dissolved in solvents. Specific examples include low molecular
weight polymers such as silicone rubber (polydimethyl siloxane: HO-(Si-(CH3)2-
O-
)~ H) with n from 2 to 200; polymer precursors such as low molecular weigh
diol,
e.g. HO-((CHZ)4-O),8)-H and low molecular weight diisocyanate, e.g. OCN-C6H6-
CHZ-C~-NCO which when mixed and polymerized form polyurethane. Example
solvents for low molecular weight polymers include xylene and n-hexane.
Suitable
solvents for polymer precursors include dimethyl formamide and dimethyl
sulfoxide.
Example liquid monomers include alpha-alkyl cyanoacrylate, where the alkyl
group
can be -methyl, -ethyl, -propyl, etc. Example solvents for liquid monomers
include
dimethyl formamide. In the context of this description, the terms prepolymer,
polymer, and polymer precursors include mixtures of one or more prepolymers,
polymers, or polymer precursors.
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
7
In one embodiment of the invention, the needle or sharp object treating layer
comprises a gummy coating such as urethane of a gum-like consistency, semi-
cured
latex, a gei, polymers, an adhesive, or a pituitous substance, with or without
an
admixed biocide, antiseptic, or sterilizing agent, as an inner layer.
As the needle or other object pierces the membrane, the treatment substance
tends to stick to it, coat it, cleanse it, or otherwise deactivate any harmful
substances
which might adhere thereto. The mechanics of the needle or sharp object
treatment
mechanism may include both chemical and mechanical aspects. For example, the
layer preferably includes a biocide or antiseptic effective against pathogens.
Additionally, the layer may also function to wipe blood and other bodily
fluids from
the needle as it passes therethrough. Alternately, abrasive materials of a fme
texture
capable of physically dislodging or scraping materials coated on the needle
may be
used individually or in combination.
Example treatment chemicals added to a biocide in the inner needle treatment
1 S layer include polyethylene oxide, and a mixture of polyethylene oxide and
glycerin.
In forming latex membranes, a first latex layer is deposited by dipping or
spraying
or by other conventional techniques. A biocide, such as a gentian violet
solution, is
thickened with a mixture of polyethylene oxide and glycerin. The thickened
mixture
is applied over the latex layer, allowed to dry to some extent, and then
coated with
one or more latex layers.
The needle treatment layer may also include adhesive or film-forming
materials which would form a physical sheath or additional membrane over the
needle or other sharp object and over other harmful agents thereon.
The needle treating layer or layers may also include a detergent or other
agent which will modify the surface tension properties of harmful agents on
the
needle rendering it possible for a subsequent layer to physically remove,
contain,
disinfect, or render less harmful the harmful agent, or for it to be less
prone to infect
or contaminate the person or animal on the opposite side of the membrane.
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
8
The aforementioned approaches may be used individually or in combination
and may be contained in the same layer or in separate layers of a multi-layer
membrane. They may be coagulants or not and the use of natural latex and man-
made latex or polymers or elastomer substitutes may be interchangeable.
Needle Treatment Patch
A needle treatment patch and a method for its use in assisting in disinfecting
a needle or other sharp object after or during its use are described below.
In one embodiment a small adhesive backed patch or disc can be attached to
the area which the needle is set to penetrate. The disc can be similar to a
squashed
or flattened vitamin E pellet which the needle pierces before piercing the
skin. The
disc includes an antimicrobial, antiseptic or cleansing ingredient which
contacts the
needle prior to piercing the skin and upon exiting the skin. The disc may be
transparent to assist a health care professional in locating a vein or other
target.
Likewise, the adhesive may be disposed only around the outer periphery of the
disc
so that it would not be carned into the puncture.
The back side of the patch may include a biocide or antiseptic adapted to
contact the skin of a patient or animal.
The disc may be constructed such that the weave or pattern allows
penetration by the needle or syringe upon entering and then creates a wiping
action
upon being withdrawn back through the disc. The patch preferably includes an
antimicrobial or disinfecting solution.
The biocide or antiseptic solution contained in both embodiments may be of
a gel-like and/or sticky consistency to help coat the needle or seal it on the
way out
of the body or patch.
The patch may also be used in conjunction with insertion or attachment of
catheters and ostomy products, and may be employed in conjunction with long
term
attachment of a catheter to inhibit growth and/or transmission of pathogens.
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
9
Suitable patch adhesives, as set forth in U.S. Patent No. 5,2345,957, the
entire disclosure of which is incorporated by reference herein, include
partially
esterified polyacrylic acid polymers, including but not limited to,
polyacrylic acid
polymers crosslinked with a polyalkenyl polyether such as those commercially
available from B.F. Goodrich, Cincinnati, Ohio, under the trademarks Carbopol
934,
934P, 940 and 941. Other suitable adhesives include natural or synthetic
polysaccharides such as cellulose derivatives such as methylcellulose,
cellulose
acetate, carboxymethylcellulose, hydroxyethylcellulose and the like. Other
suitable
adhesives are pectin, a mixture of sulfated sucrose and aluminum hydroxide,
hydrophilic polysaccharide gums such as natural plant exudates, including
karaya
gum, ghatti gum, tragacanth gum, xanthan gum, jaraya gum and the like, as well
as
seed gums such as guar gum, locust bean gum, psillium seed gum and the like.
Suitable biocides for use in membranes according to the disclosure of the
instant application include phenols, acridine dyes, gentian violet (crystal
violet),
chlorhexadine, Triclosan, Nonoxynol 9, Gluconate, dextran sulphate,
benzalkonium,
betadyne, mercurochrome, silver salts, and an extract of blue-green algae, in
addition
to a long list of other suitable biocides appended to this description
immediately
prior to the claims.
In most surgical settings the physician or surgeon does not know if the
patient has a communicable disease carried by blood borne pathogens. It would
be
beneficial, especially for those in the operating room and emergency mom, to
have
an indicator on their glove that would alert them when they are in contact
with the
patient's blood or body fluid that there is the presence of a harmful,
contagious or
potentially fatal substance in that fluid or blood.
The present invention contemplates the provision of indicating mechanisms
to multi-dipped or solvent formed membranes, including, but not limited to,
gloves
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
and condoms. The present invention discloses the provision of indicators to
alert a
user to membrane breach, as well as to alert a user of dangerous substances
present
in blood and other body fluids.
As disclosed in U.S. Patent No. 4,935,260, fluid reservoirs within a
5 membrane may include colored, fluorescent, or reactive substances which
serve as
an indicator if the membrane is breached, defective or disturbed. The present
invention contemplates the provision of a visual indication to a user of
membrane
breach by color change or appearance or other physical means.
An indicating change to a material in an inner layer of a mufti-Iayer
10 membrane can be triggered by the presence of air, moisture, body fluids,
harmful
substances, change in electrical activity, surface tension, or partial
pressure. The
indicating system responds to an exposure of the indicating element to an
outside
substance or change in the physical integrity of the surrounding membrane. Ph
change in the inner material because of exposure to substances outside the
membrane
can be an additional indicating mechanism.
Other suitable indicators for use in membranes according to the present
invention include indicating materials such as a DNA probe based reaction such
as
Chiron's "Branched DNA Probe", HofFman-LaRoche's "Polymerase Chain Reaction"
technique, or conventional color change indicator reactions, to detect
chemicals,
viruses or other pathogens. A mufti-layer membrane may include one or more
permeable or semi-permeable layers to allow migration of a material to be
detected
through the outer layers and into contact with an indicating material or
system.
Other suitable viral indicators and indicating methods are disclosed in U.S.
Patents
Nos. 4,879,21 i ; 4,942,122; 5,039,604; 5,093,230; 5,108,891; 5,149,623;
5,156,949;
5,208,321; 5,235,039; 5,260,189; 5,268,265; the entire disclosure of each of
which
are hereby incorporated by reference herein.
A method of making a membrane including a color indicator for membrane
breach is set forth below.
CA 02311539 2000-OS-19
WO 99/20203 PC'T/US97/19445
11
1. Using conventional dipping, spraying, or other sheet forming
techniques an initial layer is formed using elastomer materials such as latex,
solvent
cast membranes, liquid polymers, or elastomers, or polymer films. A second
layer
is created by conventional techniques such as coating or dipping (with or
without a
coagulant) including an indicating material such as dyes, crystals, reactants,
colored
agents, or congealing substances.
2. The dyes or indicators are selected to provide a noticeable change in
appearance, feel, (stiffness, clumpyness, consistency), or temperature, to
indicate to
the user that the membrane is compromised.
3. One or more additional elastomeric membrane layers are then formed
to at least partially contain the indicating substance.
The present invention also contemplates the provision to a mufti-layer
membrane of an indicator to detect and indicate the presence of pathogens in
blood
or other bodily fluids.
Outer membrane layers selected to be either impermeable, permeable, or
selectively permeable to a substance included in a reservoir created between
membrane layers, or to the substances, microbes or pathogens whose presence is
to
be detected. Instead of, or in addition to, the inclusion of various
substances in a
reservoir formed between membrane layers, the substances may be applied to
inner
or outer surfaces of the membrane after formation.
Mufti-layer gloves according to the present invention, in addition to or
instead of indicators, may also include one or more reservoirs disposed
between
adjacent membrane layers and containing one or more substances such as
lubricants,
biocides, spermicides, antiseptics, gels, hydrogels, pituitous substances,
cleansing
agents, surfactants, detergents, abrasives, coating agents, wiping agents,
fibers,
tactile enhancing objects, and sheet forming agents, such that said substance
is
substantially contained between the adjacent layers. One or more of the
multiple
membrane layers may be permeable or semi-permeable to allow directional
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
12
migration of ( 1 ) reservoir substances exteriorly to the membrane, or (2)
exterior
substances at least partially through the membrane.
An example multi-layer membrane 50 according to the present invention is
illustrated in Figure 4. The membrane 50 may, for example, comprise a condom.
An inner impermeable layer 52 may be formed from latex or from a polymer
material. A biocide and/or spermicide such as Nonoxynol 9 at least partially
fills a
reservoir 54 formed between the inner layer 52 and a middle or intermediate
impermeable layer 56, which may also comprise a latex or polymer material. A
lubricant fills a reservoir 58 formed between the intermediate layer 56 and an
outer
permeable layer 60 to effect a controlled release of the lubricant through the
pores
of the permeable layer 60 over time. The permeable layer 60 may comprise a
membrane with pores which open to permit the lubricant from reservoir 58 to
pass
through when stretched under pressure.
In the event of breach of the inner layer 52, seminal fluids will initially
contact the biocide/spermicide in the reservoir 54, even in the event of a
concurrent
breach of the intermediate layer 56. Similarly, in the event of breach of the
intermediate layer 56, vaginal fluids will also initially contact the
biocide/spermicide
in the reservoir 54, even in the event of a concurrent breach of the inner
layer 52.
The methods of introducing or forming the inner layer or one or more
intermediate or outer layers of multi-layer membranes pursuant to the present
invention include dip forming methods and other techniques such as spray
coating,
sheet forming techniques, fluidized bed deposition, vapor deposition,
electrical
discharge deposition, vacuum deposition, centrifugal coating, and extrusion.
Molding techniques, such as rotational molding, or other types of molding
techniques employing positive or negative mold surfaces may be employed.
Membranes or membrane layers pursuant to the invention may also include
latex, elastomer, or polymer or synthetic films where the membrane or membrane
layer is coated with a desired substance such as biocides, lubricants,
indicators,
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
13
spermicides, antiseptics, gels, hydrogels, pituitous substances, cleansing
agents,
surfactants, detergents, abrasives, coating agents, wiping agents, fibers,
tactile
enhancing objects, and sheet forming agents, and is then surface treated to
contain
the coated substance. Examples of such surface treatment methods include
treatment
with a chemical such as chlorine or bromine, coating with a sealant such as
silicone
or an acrylic, a heat treating process such as melting or glazing, treatment
by
exposure to reduced temperatures, mechanical treatment processes such as
rolling,
pressing, ultrasonics, or radio frequency heating. In each instance the common
objective is the substantial containment of a desired substance on a surface
of an
inner, intermediate, or outer membrane layer.
The surface on one side may be designed to be impervious while the
substance on the other side of the mufti-layer membrane may be designed to be
permeable or selectively permeable.
The indicator may be provided on an interior surface of single or mufti-layer
membranes, or on the outer surface, where it can be exposed to a pathogen or
harmful substance or antibody of a harmful substance.
Figure 3 illustrates an example glove 20 according to the present invention
which includes inner 14 and outer 12 layers joined at a cuff region 16. An
indicating
strip 30 bonded or otherwise attached to the outer surface of the glove 30
includes
separate indicators in boxes or regions 32, 34, and 36 for detecting and
indicating to
the user the presence of pathogens or other harmful agents or chemicals in the
user's
environment. For example the indicator bar may show the presence of Strep in
box
32, the presence of a retro-virus in box 34, and the presence of Staph in box
36.
The indicating substance may be more effectively retained by the membrane
if the membrane comprises a film selected from the family of glow discharge
treated
polymers, such as polyethylene, tetrafluoroethylene PE (TFE/PE),
polyethyleneterephthalate (PET), TFE/PET, polytetrafluoroethylene (PTFE),
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
14
ehtylene glow discharge treated PET (E/PET) and hexamethyldisiloxane glow
discharge treated PET (HMDS/PET).
The indicator preferably included in mufti-layer membranes produces an
identifiable reaction, alerting the wearer to the presence of a potentially
harmful
substance.
Indicators may be specific for any number of substances and
microorganisms, including viruses (including HIV), bacteria, yeasts,
undesirable and
harmful chemicals, etc.
Specific viral indicators may include DNA Probe based reactions such as
Chiron's "Branched DNA Probe", Hoffman-LaRoche's "Polymerase Chain Reaction"
technique, the elements of the P-24 antigen kit, the Abbot Lab preparation or
mixed
preparation for GP-120.
Additional examples of indicators include conventional color change
indicator reactions where the material to be detected (pathogen, chemical, or
other
substance) could migrate through the outer membrane and reach the indicator
system. Similarly, certain of these indicators could be admixed with the
material of
the membrane or a layer of the mufti-layer membrane or coated on the outer
layer
of the membrane or mufti-layer membrane.
For example, HIV and Hepatitis B belong to a family called the retro-virus
family. Indicators that could pick up the presence of HIV or its antigens in
blood are
the P-24 antigen kit, and the Abbot Lab preparation or mixed preparation for
GP-
120. In addition, other tests can pick up indications of the presence of a
retro-virus
or a lenti virus by reacting with a substance common to the virus family, like
the
envelope of the virus family. Indicators may also pick up anti-bodies to these
harmful substances in bodily fluids such as blood, semen, or vaginal fluid.
And it
is particularly noted that one specific substance may be picked up instead of
a family
of harmful substances. Some of these indicators may be, but are not
necessarily
limited to, the family or group of synthetic peptides and epitopes.
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
When a glove is in use, a defect can sometimes be detected by various leak
detector devices -- some utilizing changes in the electrical properties or
patterns of
the material or material surface. These are of little benefit if the wearer is
not in a
5 position to change his gloves and remove the defective glove or pull another
glove
over it.
The present invention discloses a glove constructed in a mufti-layered
manner that contains a chemical that may be triggered to be released inside
the
glove, next to the hand to protect the wearer, until the time when he or she
can take
10 proper action.
The technology applied to the membrane is similar to existing transdermal
patch technology. This technology is in use in nicotine patches and hormone
releasing patches. They can provide a sustained release or a specific release
upon
a change in the electrical properties on the surface of, or a breach in
integrity of, the
15 membrane. Examples of transdermal patches are disclosed in U.S. Patents
Nos.
4,286,592; 4,627,429; 4,839,174; 4,921,475; 4,978,531;,5,008,110; 5,087,240;
5,163,899; 5,164,189; 5,230,896; 5,234,957; 5,262,165; 5,273,756; and
5,286,490;
5,290,561; the entire disclosures of each of which are hereby incorporated by
reference herein.
The mufti-layer glove preferably contains a reservoir of
antiseptic/disinfectant which is released through the transdermal system upon
deterioration of the glove film as indicated by a change in electrical
properties, the
presence of moisture or other indications of decrease in glove integrity.
Double La~rer Membrane Dini Forming,~g~hod
In many applications, use of a double layer membrane can provide increased
protection. For example, it is now an accepted practice for surgeons and other
health
care practitioners to don two pairs of gloves, one over the other to provide
maximum
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
16
protection from infection. Indeed, some studies show that the use of two
gloves
worn together reduces the occurrence of infection.
Such a double layer membrane configuration also creates a space which can
serve as a reservoir, particularly when the layers of the membrane are joined
at a cuff
or top region. This reservoir can be used to contain a variety of materials
including
biocides, needle treating materials, tactile enhancing liquids or gels,
lubricants,
spermicides, hydrogels, indicators, and scales, discs, or other materials for
inhibiting
penetration of needles and other sharps.
Hydrogels may be of the type disclosed in U.S. Patent No. 4,499,154, the
entire disclosure of which is incorporated herein by reference. Hydrogels may
function to absorb a biocide and to hold membrane layers apart, and can
function as
a coagulant or as a lubricant.
Examples of lubricants within the scope of this application include water
soluble nontoxic chemical compounds that incorporate sodium or potassium in
varying chemical combinations with carbonates, acetates, bicarbonates, acetate
trihydrates and citrate dihydrate, as disclosed in U.S. Patent No. 4,143,423,
the entire
disclosure of which is hereby incorporated by reference herein. Other suitable
lubricants include microspheres as described in U.S. Patent No. 5,073,365, the
entire
disclosure of which is hereby incorporated by reference herein.
It is possible to form a double glove or other double membrane in a multi-dip
manufacturing process. Latex gloves and condoms are conventionally produced
using a dip forming method in which shaped formers are dipped into vats of
liquid
latex. A method of making a double layer latex membrane pursuant to the
present
invention includes the following steps:
1. Clean formers.
2. Heat formers for eight minutes at 210-220 F degrees.
3. Dip into coagulating solution such as CaC03 plus alcohol plus N03,
(or Calcium Carbonate plus alcohol plus nitrate).
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
17
4. Stand 2-3 minutes.
5. Dip into uncured latex.
6. Stand 2-3 minutes.
7. Leach with cold tap water for 15 minutes . . . stand 2 minutes.
S 8. Produce ring roll.
9. Dry in oven 6 minutes.
10. Dip into 1.5% solution gentian violet in distilled water. (May
substitute other biocides or chemicals in various solutions, or
optionally eliminate biocide dip, as it is possible to make a double
glove or other membrane without use of a biocide).
11. Dry in oven.
12. Stand 5 minutes.
13. Dip in 20% or greater concentration calcium nitrate coagulant.
14. Dip into uncured latex.
15. Stand 8 minutes.
16. Dry in oven for 30 minutes to cure by heating the membrane to dry
them to their final form.
17. Powder and strip the doublet membrane from the former.
This method produces a glove within a glove or a condom within a condom
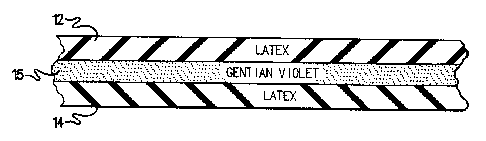
joined at the cuff or top. Figure 1 illustrates an example double glove 10
produced
according to the invention which includes a discrete separated outer layer 12
and an
inner layer 14 joined in the cuff region at a ring roll 16. As can be readily
appreciated, the inventive double glove 10 has substantial advantages in ease
of
donning compared to separate single layer gloves. The space between the two
membranes can be constructed with an additional step or steps of incorporating
different substances including but not limited to gels, biocides, chemicals,
silicones,
neutralizing chemicals, buffering chemicals, spermicides, lubricants, tactile
enhancers, and scales, discs, or other materials for inhibiting penetration of
needles
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
i8
and other sharps. For -example, as shown in Figure 2, the reservoir 15 formed
between the inner 14 and outer 12 layers of the glove may be filled with a
biocide
such as gentian violet.
It should be appreciated that this double membrane configuration can also be
made by the above method with a biocide component by dipping in biocide before
dipping in the coagulant, or by mixing the biocide or chemical with the
coagulant.
The salient steps in the above method comprise:
(a) depositing onto a former a first latex layer;
(b) treating the first layer with a material effective as a coagulant for
latex;
(c) depositing over the first layer on the former a second latex layer, with
the
coagulant effective to substantially prevent fusing of the first and second
layers; and
(d) setting or curing the first and second layers.
It is particularly preferred that the coagulant is not applied to a
circumferentially extending top or cuff region of the first layer such that
the first and
second layers will fuse in the cuff region to form a reservoir in the
remaining
regions. Prior to application of coagulant to the first layer, a substance may
be
deposited over the first layer on the former, with the substance selected from
the
group consisting of biocides, indicators, spermicides, antiseptics, gels,
hydrogels,
pituitous substances, cleansing agents, surfactants, detergents, abrasives,
discs,
scales, and other materials for inhibiting penetration of needles and other
sharps,
coating agents, wiping agents, fibers, tactile enhancing objects, and sheet
forming
agents, such that the substance is substantially contained between the first
latex layer
and the second latex layer. Such intermediate substances may also be admixes
for
application with the coagulant.
In another example method of making a double latex membrane according
to the present invention, such as "double gloves," a glove mold or former
warmed
to about 70 degrees C is first dipped in a coagulant slurry in a conventional
manner.
This coating is dried in the oven at about 80 degrees C for five minutes. The
mold
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
19
in then dipped in a latex compound, available under the designation Vultex 1-N-
4402 at 38% solids from General Latex and Chemical Corporation, with a dwell
time
of about five seconds. The deposit is then partially dried at 80 degrees C for
about
one minute. The latex deposit is then leached in warm water for about three
minutes
S and dried in the oven at 100 degrees C for five minutes or until the latex
deposit is
completely dry.
A separating agent coating of zinc stearate water emulsion at about 3-5%
solids is applied over the first latex layer on the former, except in a region
within one
to two inches of the cuff or bead area, by spraying or alternatively by
dipping. The
zinc stearate coating is then dried at about 100 degrees C for about three to
five
minutes and a powder free coagulant is applied over the first latex coating.
This
coagulant coating is then dried at about 80 degrees C for five minutes and
then the
former is again dipped into the latex compound, adjusting the dipping speeds
to
provide a uniform second layer. The second layer is then partially dried for
about
one minute at about 80 degrees C and leached in warm water for about three
minutes. The second latex layer is then cured at 125 degrees C for twenty
minutes.
A coating of corn starch powder is applied, and the glove is then stripped
from the
former.
The resulting glove has two discrete layers, except in the one to two inch
region of the cuff or bead, where the glove consists of a single layer.
The separating agent may be either applied after the second coagulant
coating, or alternatively incorporated into the second coagulant formulation
for
concurrent application therewith.
A variety of different potential separating agent may be employed, including:
zinc stearates and other stearates, hydrogel compositions, powders such as
calcium
carbonates, cornstarch, microspheres, wax emulsions such as paraffin and micro-
crystalline, silicon emulsions, gentian violet at high concentrations, silicon
oils,
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
acrylic separating compositions, separate curing of latex layers, and
chlorination of
the first latex layer before application of the second layers.
The formation of the second layer requires flexibility in regard to dipping
speeds depending upon the particular formulations of latex, separating agent,
and
5 coagulants employed. For example, different dipping speeds may be employed
in
the cuff region (not coated with the separating agent) and the main body
region,
and/or the second layer may be double dipped in the main region and single
dipped
in the cuffregion. Temperatures, speeds, and dwell times may also vary
dependent
upon the particular formulations employed.
10 Another example method of making a mufti-latex membrane according to the
present invention includes the following steps:
1. Clean formers.
2. Heat formers for eight minutes at 65 C degrees.
3. Dip into latex, while rotating formers.
1 S 4. Heat formers for three minutes at 65 C degrees.
5. Cool.
6. Dip into uncured latex, while rotating formers.
7. Heat formers for three minutes at 65 C degrees.
8. Dip into Cal-Dip solution (a stearate compound from Rubichem,
20 Inc.) using 37.4 gms of Cal-Dip per 22.6 gms isopropyl alcohol.
(water may be used as an alternative to alcohol.)
9. Heat formers for one minute at 65 C degrees.
10. Dip into latex, while rotating formers.
11. Cure for ten minutes at 65 C degrees.
12. Leach with water for three minutes.
17. Dry, powder and strip the membrane from the former.
Suitable release or detacking agents for use in the present invention include
calcium stearate, zinc stearate, and ammonium stearate as layer separating
agents.
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
21
In addition, chlorination ~of the finished membrane or of intermediate layers
during
formation may be employed as a release agent.
A method of forming a polymeric mufti-layer membrane according to the
present invention includes the steps of:
(a) depositing onto a former a material selected from the group consisting of
liquid polymers and polymers dissolved in a solvent to form a first layer;
(b) treating the first layer with a surfactant;
(c) depositing over the first layer on the former a material selected from the
group consisting of liquid polymers and polymers dissolved in a solvent to
form a
second layer, wherein the surfactant is effective to substantially prevent
fusing of the
first and second layers; and
(d) setting or curing the first and second layers.
As in the method of making latex membranes, a variety of substances may
be provided in an intermediate layer or reservoir between the first and second
layers.
Example surfactants include ionic surfactants capable of emulsifying or
destabilizing
polymers in a known manner. In the context of this application, the term
polymer
includes water based synthetic materials.
It is also believed possible to use other techniques to produce membranes
having multiple discrete latex or polymer layers. For example, temperatures of
the
mold/former and/or the latex or polymer bath, leach bath, oven, and/or a
surrounding
chamber might be varied at different stages in the process. For example, the
first
latex or polymer layer might be cooled prior to application of the second
layer to
form separate layers in the resulting membrane. Additionally, application of
sonic,
ultrasonic, or thermal shocks might be employed to separate or facilitate
separation
of layers. Irradiation with energy of various frequencies of the
electromagnetic
spectrum might also be employed.
In the case of both latex and polymer or synthetic membranes, the first and
second layers may be selectively fused or separated by selective application
and/or
CA 02311539 2000-OS-19
WO 99/Z0203 PCT/US97/19445
22
variations in the formulation of the separating agent, coagulant or
surfactant. For
example the first and second layers might be fused or joined in selected areas
of a
glove such as the palm, the back of the hand, the knuckles, finger tips.
Similar
selective joining might be employed with other multiple layer membranes such
as
S condoms. For example, multiple layers of a condom might be selectively in
the tip
and/or intermediate region. A bubble or blister effect may also be created by
fusing
two layers of a multiple layer membrane in such a manner to create discrete
sealed
chambers. Such chambers might contain a variety of different solids, liquids,
and
gasses such as lubricants, sealants, biocides, indicators, spermicides,
antiseptics,
gels, hydrogels, pituitous substances, cleansing agents, surfactants,
detergents,
abrasives, coating agents, wiping agents, fibers, tactile enhancing objects,
and discs,
scales and other materials for inhibiting penetration of needles and other
sharps. For
example, the chambers might contain liquids or gasses to provide a cushioning
effect. Alternatively, the chambers might contain materials which combine upon
rupture of the chambers to provide an indicating effect. Diverse materials
might also
be selected which combine to provide a color change, heating effect,
disinfectant or
biocide, stiffening, softening, or alteration in tactile sense.
It is also possible to vary the extent of separation or fusing of the layers
of
a multiple layer membrane by varying the chemistry of the latex or polymer,
the
chemistry of the separating agent, surfactant or coagulant, the cure times and
temperatures, the dip speed and dwell times, and through other methods, such
as
chlorination. The extent of separation or fusing of the layers can range from
completely discrete layers to layers which, although initially stuck together,
may be
peeled apart with some effort.
As an alternative to dip or spray forming of latex, synthetic, or polymer
membranes having multiple discrete layers, the techniques described above may
also
be employed in connection with conventional sheet forming and extrusion
processes
to make a variety of other multiple layer membranes. For example, a multiple
layer
CA 02311539 2000-OS-19
WO 99/Z0203 PCT/US97/19445
23
medical or other type of tubing may be formed using an extrusion process. As
in the
case of dip or spray forming techniques, the various layers of multiple layer
membranes formed by sheeting or extrusion techniques may be joined or fused in
selected regions and separated or discrete in other selected regions. Such
multiple
layer membranes find applications in applicances where an added measure of
security against rupture is desired, for example in colostomy bags.
The grain or other characteristics of the individual latex or polymer layers
of
a multiple layer membrane might be different. For example, the former might be
differently inclined and/or rotated in forming different layers to provide an
enhanced
strength membrane. Alternatively, different layers might be applied during
application of different electrostatic or magnetic fields. Further, the
various layers
might be formed of different materials such as latex, polymers, and
synthetics,
possibly treated in various different manners, such as by conventional
chlorination
treatments of latex layers.
1 S Incoy oration Of Biocide Into Pol~~rethane Films
Glow discharge treatment techniques can be used to enhance the pick-up and
retention properties of certain polymer families.
Biocides may be more effectively picked up and retained by certain polymer
films. The film may be selected from the family of glow discharge treated
polymers,
such as polyethylene, tetrafluoroethylene glow discharge treated PE{TFE/PE),
polyethyleneterephthalate) (PET), TFE/PET, polytetrafluoroethylene (PTFE),
ethylene glow discharge treated PET (E/PET) and hexamethyldisiloxane glow
discharge treated PET (HMDS/PET), or any of the other polymers treated by the
glow discharge process. The biocides may be directly applied to the glow
discharge
treated films. The resulting films may be somewhat stiffer but very strong and
therefore thinner films will be satisfactory for many applications.
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
24
Alternately, the biocides may be fed, as a gas, into a chamber and directly
deposited by creating the glow discharge or RF discharge to facilitate the
deposition.
Additionally, the biocides may be introduced on, and/or into the polymer
during the fabrication of the film in such a way as to be available to provide
disinfectant properties. This can be accomplished by conventional dipping or
mixing, with additional layers deposited by dipping, casting, spray coating,
vacuum
depositing, passing through fluidized beds, centrifugal spinning, etc. Outer
coats can
be formed by similar techniques to contain the biocide and minimize leaching
where
desirable.
Coatings within the scope of the present invention include spermicides such
as Nonoxinol-9 and one or more organopolysiloxane compounds which may be
applied to latex membranes as disclosed in U.S. Patent No. 5,304,375, the
entire
disclosure of which is hereby incorporated by reference herein. Rubber
membranes
may be provided with a transparent coating of an aqueous composition
containing
a preformed latex binder, an emulsifying agent, an inorganic fluoro-containing
compound,.and a thickening agent as described in U.S. Patent No. 5,182,142,
the
entire disclosure of which is hereby incorporated by reference herein. A
cellulosic
coating material including synthetic latex formed by emulsification of
cellulosic
polymers stabilized by surfactants and containing a water-soluble pore forming
agent
and a plasticizer may also be employed, as described in U. S. Patent No.
5,126,146,
the entire disclosure of which is hereby incorporated by reference herein.
Incoruoration Of Biocide Into Porous And on-Porous Pohrurpth~n.. Films
This can be done in the four ways described below. All except the last
require the biocide to have a low vapor pressure at room temperature (less
than -
0.013 bar). In all cases the solvents that come in contact with the biocide
must not
react with or chemically alter the biocide in such a way as to irreversibly
destroy
their anti-bacterial and anti-viral activity.
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
( 1 ) Physical entrapment of the biocide in the pores of a porous film.
A. Introduction of the biocide during fabrication of the film
Such films can be fabricated using ( 1 ) a fully polymerized polyurethane
dissolved in a suitable solvent, or (2) using a polyurethane prepolymer of
molecular
5 weight 1000 to 3000 dissolved in a solvent and subsequently vulcanized or
cured
with a crosslinking or curing agent added to the solution. In both cases, the
solvents
must be solvents for both the biocide and the polyurethane.
The solvents for this purpose will depend on the type of polyurethane:
polyether, polyester or polyester-polyamide, and on the type of biocide. Some
10 candidates are listed in the table below.
Table I. Candidate Solvents and their Solubility Parameters.
Solubility Parameter Hydrogen
Solvent ~cals/c In Bonding
Dimethyl formamide (DMF) 12.1 medium
15 Dimethyl acetamide (DMF) 10.8 medium
Tetrahydrofuran (THF) 9 .1 medium
Dimethyl sulfoxide (DMS) 12 medium
Dioxane 1,4 10 medium
Phenol strong
20 m-Cresol 10.2 strong
Formic acid 12.1 strong
Sulfuric acid strong
Methyl ethyl ketone 9.3 medium
Diethyl ketone 8.8 medium
25 Ethylene glycol monoethyl ether10.5 medium
Ethylene glycol monomethyl ether
11.4 medium
The use of water soluble polymers is also contemplated within the scope of
the instant invention.
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
26
Mixtures of these solvents with each other and with non-solvents having
solubility parameters in the range: 8 to 24 (cals/cc)'~ and medium or strong
hydrogen bonding are also candidates.
The biocide is first dissolved in such a solvent or solvent mixture, to form a
nearly saturated solution, and is then mixed with a solution of the
polyurethane or
urethane prepolymer in the same or similar solvent. In the case of solutions
containing the polyurethane prepolymer, the curing agent (typically amines or
alcohols with functionality of 2 or more) is added to the polymer plus biocide
solution just prior to casting the films. Films of the resulting mixture are
then cast
using the methods described in the Gilding patents: U.S. Patents Nos.
4,813,966 and
4,704,130, the entire disclosures of which are hereby incorporated by
reference
herein, with the following modifications.
On immersion of the cast film in the precipitation bath, and subsequently in
the solvent extraction bath, there will be a tendency for the biocide to be
leached out
of the rubber and into the bath solution. This leaching can be reduced in two
ways
(1) saturate the precipitation or extraction bath with the biocide, or (2) use
liquids of
low polarity (having solubility parameters less than -9 (cals/cc)'n and weak
hydrogen
bonding) in the precipitation and extraction baths. Since most biocides are
strongly
polar, they will tend to remain in the medium to strongly polar environment of
the
polyurethane rather than be extracted into the bath.
CA 02311539 2000-OS-19
WO 99120203 PCT/US97/19445
27
On subsequent drying and annealing, the remaining solvent is removed
leaving the biocide physically trapped in the pores of the film.
B. Introduction of the biocide subsequent to th_e fahricatinn of the f 1n,
A porous polyurethane film can be swelled with a solvent or solvent mixture
S from Table I, or with a mixture of such solvents with non-solvents,
saturated with
a biocide. In the case of a linear polyurethane, the proportion of non-solvent
must
be adjusted so that the solvent mixture swells the soft segments (polyether
segments)
of the urethane chain, but does not dissolve the polymer. During the swelling,
the
solvent/swelling agent carries the biocide through the polymer structure and
into the
pores.
After removal of the film from the biocide solution, the film is dried and
annealed, which removes the swelling agent and leaves the biocide trapped in
the
pores as well as in the polymer matrix.
(2) Adsorption of the biocide on internal pore surfaces. This method is
applicable to porous films.
The same procedures as in lA and 1B are followed except that the extraction
of the solvent or swelling agent from the rubber is accomplished mostly by
immersion of the film in a non-solvent bath of low polarity (solubility
parameter less
than 9(caUcc)'~ and weak hydrogen bonding). In this case, the non-solvent bath
is
not saturated with the biocide, as a consequence there will be a strong
tendency for
CA 02311539 2000-OS-19
WO 99/20203 PCT1US97/19445
28
the biocide to adsorb to the polar polyurethane pore surfaces (as well as
being
trapped within the rubber matrix). The non-adsorbed biocide molecules in the
pores
will be leached out into the non-solvent leaving the adsorbed biocide on the
pore
surfaces. Any solvent or on solvent remaining in the films can be removed by
drying and annealing.
(3) Precipitation of the biocide within the rubber matrix. This method is
applicable to non-porous urethane films.
The same procedures as in 1 A and 1 B are used except that the methods for
producing the pores, described in the Gilding patents are not applied.
Instead, the
films are simply cast from solution using the common film forming procedures:
dip-
coating, spraying, spinning, etc. In the case of prefabricated films the
diffusion of
the swelling agent plus biocide can be enhanced by stretching the rubber film
biaxially.
Extraction of the solvent/swelling agent is accomplished by drying and
annealing. A non-solvent bath saturated with biocide can also be used as in (
1 ) but
drying is preferred. As the concentration of the solvent/swelling agent in the
rubber
decreases, the biocide will precipitate out forming phase-separated regions
within
the rubber matrix.
(4) Chemical bonding of the biocide to functional groups on the polyurethane
chains. This method is applicable to both porous and nonporous films.
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
29
Polyurethanes possess the advantage that the urethane and urea linkages in
the chains are relatively reactive. Furthermore, the rubber can easily be
formulated
to have excess amine, -OH or isocyanate groups at chain ends or branch points,
simply by deviating slightly from the stoichiometric proportions of 1:1
isocyanate:
amine groups or isocyanate: -OH groups.
The biocide molecules can then be chemically bonded to such groups. It is
important that such bonding be accomplished in such a way that the biocidal
activity
of these molecules is not compromised.
Such binding reactions can be carried out (1) prior to fabrication of the
film,
while the polyurethane or urethane prepolymer are in solution (subsequent to
the
addition of the crosslinking or curing agent), or (2) after fabrication of the
film.
In the first case, the biocide binding reaction would take place with the
polyurethane in solution. In the second case, the biocide binding reaction
would take
place in the swelled network of the rubber. In both cases, the solvent or
swelling
agent must remain inert during the binding reaction.
After reaction, the solvent or swelling agent is removed as described in
sections (1) or (3). Even though the biocide is chemically bound to the
polyurethane
chains, it will still phase separate in the rubber matrix, but on a much finer
scale than
with the method described in section (3).
The necessity of using solvents or swelling agents may be obviated by
employing water based synthetics.
CA 02311539 2000-OS-19
WO 99/20203 PGT/US97/19445
(5) Sealing of film surfaces.
For all the above methods, application of a final coating to seal the surfaces
of the film to prevent leaching of the biocide during use or storage is highly
desirable. Such a coating can be applied by a final dip in a polyurethane
solution of
5 low viscosity (low % solids), or by plasma or vapor deposition of a thin
elastic
polymer film.
According to one aspect of the invention, gentian violet is admixed with
liquid latex prior to membrane formation by dipping or spraying techniques. A
wide
10 variety of different concentrations may be used, but a 1.5% by weight
solution of
gentian violet is preferred. Applicant has found, that in addition to biocidal
properties, the addition of gentian violet by admixing yields two unexpected
results.
The gentian violet appears to bind protein molecules in the latex, which
yields two
important benefits. First, this produces an anti-aging or anti-oxidizing
effect which
15 extends shelf life and increases tear resistance. Second, it appears to
minimize
allergic reactions in latex-allergic or sensitive users. Other proteing
binding biocides
may be employed in place of or in combination with gentian violet.
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
31
The present invention also contemplates membrane reinforcement by the
addition of microfibers during the production process. Microfibers such as
aramids,
Kevlar, fiber glass, natural fibers, nylon, and graphite may be directly
admixed with
latex or polymer membrane materials either prior to, during, or after
application.
S Such fiber reinforcement may be employed in connection with a single layer
membrane, or in one or more layers of a mufti-layer membrane. Fiber
reinforcement
may also be effected by adding one or more preformed sheet layers in a mufti-
layer
membrane.
Reinforcement might also be employed by the incorporation of
monofilament, similar to fishing line, in or between one or more layers, or by
winding monofilament in a predetermined pattern around or between one or more
layers.
Fish scale-like particles or small discs might also be employed to enhance
strength and penetration resistance of membranes. Such scales or discs might
be
incorporated into or disposed between one or more layers of a single or
multiple
layer membrane. The scales or discs might also include magnetic properties
such
that they could be oriented in a predetermined manner by application of an
electromagnetic or magnetic field during membrane formation.
LIST OF BIOCIDES THAT A_PPi.IC'ANT RF11FVE ,~RF SUIT LE
FOR
USE IN CONNECTION W'[,TH THE DISCLOSFn rNVENTION
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
32
HC BLUE NO. 1
N4, N°-Bls (2-Hydroxyethyl)-N'-Methyl-2-Nitro-p-
Phenylenedlamine
HC BLUE NO. 2
N', N4, N4-(2-Hydroxyethyl)-2-Nitro-p-Phenylenediamine
HC BLUE NO. 3
Cibalan Blue FBL
HC BLUE NO. 4
HC BLUE NO. 5
HC BROWN NO. 1
Capracyl Brown 2R
HC ORANGE NO. 1
2-Nitro-4-Hydroxydiphenylamine
HC RED NO. 1
4-Amino-2-Nitrodiphenylamine
HC RED NO. 3
N'-(2-Hydroxyethyl)-2-Nitro-p-Phenylenediamine
HC RED NO. 6
HC YELLOW NO. 2
N-(2-Hydroxyethyl)-2-Nitroaniline
HC YELLOW NO. 3
N'-Tris (Hydroxymethyl)-Methyl-4-Nitro-o-Phenylenediamine
HC YELLOW NO. 5
N'-(2-Hydroxyethyl)-4-Nitro-o-Phenylenediamine
NONOXYNOL-2
Polyoxyethylene (2) Nonyl Phenyl Ether
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
33
NONOXYNOL-4, -8 -
NONOXYNOL-9 IODINE
NONOXYNOL-I2 IODINE
PIGMENT RED 57
PIGMENT RED 57:1
PIGMENT RED 63: i
PIGMENT RED 64:1
PIGMENT RED I 12
PIGMENT VIOLET 19
PIGMENT YELLOW 1
PIGMENT YELLOW 3
PIGMENT YELLOW 12
PIGMENT YELLOW 13
PIGMENT YELLOW 73
QUINOLINE
QUINOLINE SALTS
TERPENES
TERPINEOL
VAT DYES
XANTHENE
ACID BLACK 58
CA 02311539 2000-OS-19
WO 99/20203 PC"T/US97/19445
34
Irgalan Grey B 1 -
ACID BLACK 107
Lanamid Black Bl.
ACID BLACK 131
Nigrosine
ACID BLUE 9 AMMONIUM SALT
ACID BLUE 62
ACID BROWN 46
ACID BROWN 48
ACID DYES
ACID FUCHSIN
ACID GREEN 25
ACID ORANGE 7
ACID ORANGE 24
ACID RED 33
ACID RED 35
ACID RED 51
ACID RED 52
ACID RED 87
ACID RED 92
ACID RED 95
ACID VIOLET 43
CA 02311539 2000-OS-19
WO 99120203 PCT/US97/19445
ACID YELLOW 1
ACID YELLOW 3
ACID YELLOW 23
ACID YELLOW 73
5 ACID YELLOW 73 SODIUM SALT
D & C BLUE NO. 1 ALUMINUM LAKE
Brilliant Blue Lake
D & C BLUE NO. 2 ALUMINUM LAKE
Acid Blue 74, Indigetine 1 A, Indigo Carmine
10 D & C BLUE NO. 4
Acid Blue 9 (Ammonium Salt)
D & C BLUE NO. 6
Indigo
D&CBROWNI
15 Resorcin Brown, Capracyl Brown
D & C GREEN NO. 3
Aluminum Lake. Food Green 3
D & C GREEN NO. 5
Acid Green 25
20 D & C GREEN NO. 6
Solvent Green 3
D & C GREEN NO. 8
Solvent Green 7
D & C ORANGE NO. 4
25 Acid Orange 7
D & C ORANGE NO. 5
CA 02311539 2000-OS-19
WO 99120203 PCT/US97/19445
36
Acid Orange 11. - Solvent Red 72. Dibromofluorescein
D & C ORANGE NO. 5. ALUMINUM LAKE
Dawn Orange. Manchu Orange.
D & C ORANGE NO. 5 ZIRCONIUM LAKE
Petite Orange. Dawn Orange. Acid Red 26. Ponceau R.
D & C ORANGE NO. 10
Solvent 73. Dilodofluorescein.
D & C ORANGE NO. 10 ALUMINUM LAKE
Solvent Red 73. Erythrosine G.
D & C ORANGE NO. 11
D & C ORANGE NO. 17
D & C ORANGE NO. 17 LAKE
D & C RED NO. 2 ALUMINUM LAKE
D & C RED NO. 3 ALUMINUM LAKE
D & C RED NO. 4 ALUMINUM LAKE
D&CREDN0.6
Lithol Rubin B
D & C RED NO. 6 ALUMINUM LAKE
D & C RED NO. 6 BARIUM LAKE
D & C RED NO. 7 CALCIUM LAKE
D & C RED NO. 7 ZIRCONIUM LAKE
D & C RED NO. 8
D & C RED NO. 8 BARIUM LAKE
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
37
D & C RED NO. 8 SODIUM LAKE
D & C RED NO. 9
D & C RED NO. 9 BARIUM LAKE
D & C RED NO. 9 ZIRCONIUM STRONTHIUM LAKE
S D & C RED NO. 10
D&CREDN0.17
D&CREDN0.19
Rhodamine B. Magenta
D & C RED NO. 19 BARIUM LAKE
Rhodamine B. Magenta
D & C RED NO. 19 ZIRCONIUM LAKE
D&CREDN0.21
D & C RED NO. 21 ALUMINUM LAKE
D & C RED NO. 21 ZIRCONIUM LAKE
D & C RED NO. 22
Eosine YS
D & C RED NO. 27
D & C RED NO. 27 ALUMINUM LAKE
Terabromo Terachloro Fluorescein Lake
D & C RED NO. 27 BARIUM LAKE
D & C RED NO. 27 ZIRCONIUM LAKE
D&CREDN0.28
Phloxine B
D & C RED NO. 30
CA 02311539 2000-OS-19
WO 99/20203 PGT/US97/19445
38
D & C RED NO. 30 ALUMINUM LAKE
D & C RED NO. 30 CALCIUM LAKE
D&CREDN0.31
D & C RED NO. 31 CALCIUM LAKE
D & C RED NO. 33
D & C RED NO. 34
D & C RED NO. 34 CALCIUM LAKE
D&CREDN0.36
D & C RED NO. 36 BARIUM LAKE
D & C RED NO. 36 LAKE
Chlorinated Para Lake. Tange Orange
D & C RED NO. 36 ZIRCONIUM LAKE
D&CREDN0.37
Rhodamine B-Stearate
D & C RED NO. 37 CALCIUM LAKE
Rhodamine B. Stearate Solvent
D &. C RED NO. 39
D & C RED NO. 40
D & C YELLOW NO. 5 ALLUMINUM LAKE
D & C YELLOW NO. 5 ZIRCONIUM LAKE
D & C YELLOW NO. 6 ALUMINUM LAKE
D & C YELLOW NO. 7
D & C YELLOW NO. 8
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
39
Uranine, Sodium Fluorescein, Naphthol Yellow S
D & C YELLOW NO. 10
D & C YELLOW NO. 10 ALUMINUM LAKE
D & C YELLOW NO. 11
EXT. D & C VIOLET NO. 2
EXT. D & C YELLOW NO. 7
EXT. D & C YELLOW NO. 7 ALUMINUM LAKE
FD & C RED NO. 20
FD & C RED NO. 22
FD & C RED NO. 40
FD & C YELLOW NO. 5
FD & C YELLOW NO. 5 ALUMINUM LAKE
FD & C YELLOW NO. 6
FD & C YELLOW NO. 6 ALUMINUM LAKE
SOLVENT RED 48
SOLVENT RED 49:1
SOLVENT RED 72
SOLVENT RED 73
SOLVENT VIOLET 13
SOLVENT YELLOW 13
TARTRAZINE
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
~k~,n From "FEDERAL REGI~TFR~~_
Vol. 43. No. 4 - Frida~r, Jan. 26. 978
Cloflucarban
Para-chloro-mein-xylenof
Povidone-iodine complex
1.5 percent phenol or less aqueous/alcoholic
Triclocarbon
Triclosan
10 Health-care Peronnel Handwash:
Benzalkonium chloride
Benzethonium chloride
Clofluearban
Hexylesorinal
15 Iodine complexed with phophate eater of alkyaryloxy polyethlene glycol
Methyl-benzethonium chloride
Nonyl phenoxypoly (ethyleneoxy) ethanol-iodine
Para-chloro-meta-xylenol
Povidene-iodine complex
20 1.5 percent phenol or less aqueous/alcoholic
Poloxamer-iodine complex
Tricloearban
Undecoylium chloride-iodine complex
Patient preoperative skin, aration
25 Bonzallconium chloride
Benzethonium chloride
Hexylresorcinol
Iodine complexed with phosphate ester of alkylaryloxy
polyethylene glycol
30 Methylbenxethonium chloride
Nonyl phenoxypoly (ethyleneoxy) ethanoilodine
Para-thloro-meta-xylenol
1.5 percent phenol or less aqueouslalcoholic
Poloxamer-iodine complex
35 Povidene-iodine complex
Undecoylium chloride-iodine complex
CA 02311539 2000-OS-19
WO 99/20203 PCT/US97/19445
41
Benzalkonium chloride
Benzathonium chloride
Hexylresorcinoi
Iodine complexed with phosphate ester of alkylaryloxy
polyethylene glycol
Iodine tincture
Methyl-bonzethonium chloride
Nonyl phenoxypoly (ethylencoxy) ethanoliodine
Para-chloro-meta-xylenol
1.5 percent phenol or less aqueous/alcoholic
Poloxamer-iodine complex
Povidene-iodine complex
Triclosan
1 S Triple Dye
Undecoylium chloride-iodine complex
skin wound cleanser
Cloflutarban
Iodine complexed with phosphate ester of alkylaryloxy
polyethylene glycol
Iodine tincture
Nonyl phenoxypoly (ethyleneoxy) ethanoliodine
Para-chloro-meta-xylenol
1.5 percent phenol or less aqueous/alcoholic
Poloxamer-iodine complex
Povidene-iodine complex
Tricloearban
Triclosan
Undecoylium chloride-iodine complex
Skin wound protectant
Benzalkonium chloride
Benzathonium chloride
Hexylresorcinoi
Iodine complexed with phosphate ester of alkylaryloxy
polyethylene glycol
Iodine tincture
Methyl-bonzethonium chloride
CA 02311539 2000-OS-19
WO 99/Z0203 PCTNS97/19445
42
Nonyl phenoxypoly (ethylencoxy) ethanoliodine
Para-chloro-meta-xylenol