Note: Descriptions are shown in the official language in which they were submitted.
CA 02311738 2000-06-28
Description
FIELD OF INVENTION
This invention relates to a process for retorting oil shale, oil sands, coal
and hydrocarbon containing
soils using a fluidized bed retort using the mixture of steam. off gas and
carbon dioxide as the heat
source and the fluidizing lifr gas.
Page ~
CA 02311738 2000-06-28
Prescott H. Rathborne
BACKGROUND OF THE INVENTION
Economically viable and environmentally acceptable methods for the recovery of
hydrocarbons
from oil shale, oil sands. coal and hydrocarbon containing soils have been
under investigation for
many years. Research efforts have been focused on recovering liquid
hydrocarbons from oil shale,
oil sands and coal for the production of transportation fuels such as
gasoline. diesel and jet fuel.
Direct coal liquefaction was commercially used in Germany to produce aviation
fuel during the
World War II. Indirect coal liquefaction, such as coal gasification followed
by catalytic
hydrogenation of carbon monoxide to hydrocarbons, is already commercialized.
Since late 1960s, production of synthetic crude oii from the oil sands bitumen
became commercial.
In Alberta, Canada, there are two commercial plants producing synthetic crude
oil from Athabasca
oil sands. Caustic hot water extraction process, which is known as the Clark
hot water process, is
used for the extraction of bitumen from the Athabasca tar sands. Extraction
efficiency of the Clark's
hot water process is as high as 90 % to 92 % by weight, for the tar sands
containing about 10 % to
12 % by weight hydrocarbons. After the bitumen extraction process, bitumen is
coked by a thermal
process to produce coker gas oil. The coking process is also called the
primary upgrading, which
yields about 70 % by weight liquid hydrocarbons and 1 ~ % to 20 % by weight
petroleum coke.
During thermal coking process. asphaltenes or coke precursor species present
in bitumen are
converted to coke, liquid and gaseous hydrocarbons. Delayed and fluid cokers
are used for the
thermal coking of bitumen to produce coker gas oil. The coker gas oil is
hydrogenated to produce
synthetic crude oil, which is also known as the secondary upgrading, by using
catalytic
hydrogenation process operating at about 370 °C to 430 °C
temperature and pressures of about 10
MPa to 25 MPa pressure. The primary and secondary upgrading processes convert
bittnnen from
about 8 °API to 10 °API gravity and atomic hydrogen to carbon
ratio of about 1.5 into synthetic
crude oil of about 35 °API gravity and atomic hydrogen to carbon ratio
of about 1.8. Also, a
catalytic hydrocracking process, such as LC-Finer process, can replace the
coking followed by
catalytic hydrotreating processes, or, integrated to the coking followed by
catalytic hydrotreating
processes as it has been used by Suncrude Canada Ltd. since 1988.
Production of synthetic crude oil from oil sands bitumen has two shortcomings,
both of which are
related to the hot water extraction process. The first shortcoming is that it
needs a large volume of
water, in the order of about 9 volumes of water per volume of synthetic crude
oil produced. The
second shortcoming, which remains as an environmental problem, is that hot
water extraction
process produces a tailings effluent stream. Sands particles precipitate
rapidly upon the disposition
of the tailings, while the fine clay particles are carried by water into the
sedimentation lagoons, from
which over the years the mature fine tailings is formed containing about 35 %
by weight of solids.
The solid content of the mature fine tailings is basically fine tails, which
may remain in a fluid state
for centuries because of their very slow consolidation rate.
Hot water bitumen extraction process and conversion of bitumen into coker gas
oil by coking
process can be combined in a single step process, which is the retort process,
if a retort process can
be economically operated in commercial scale. Such a retort process could
eliminate the
requirement of large volume of water for the extraction process and the
formation of mature fine
Page ~
CA 02311738 2000-06-28
Prescott ~I. Rathborne
tailings problem. In other words. a suitable one step retort process could
replace the hot water
extraction and coking processes. Implementation of such a one step retort
process could
significantly reduce the cost of synthetic crude oil production and could
eliminate the environmental
problems associated with the tailings effluent of the hot water extraction
process.
Like oil sands bitumen, oil shale deposits could also be developed for the
commercial production of
synthetic crude oil, economically. Vast deposits of oil shale are found in the
United States, Western
Canada. Australia, Russia, Brazil, Estonia, China and Middle East. Oil shale
is basically a fine-
grained sedimentary rock containing organic matter known as "kerogen" which
has limited
solubility in common solvents and therefore can not be recovered by the
extraction processes.
Upon heating however, kerogen decomposes by pyrolysis, thermal cracking or
distillation to yield
oil, gas, dust and residual carbon. It has been estimated that an equivalent
of 7 trillion barrels of oil
are contained in oil shale deposits in the United Sates with more than half of
those located in the
Green River oil shale deposits of Colorado, Utah and Wyoming. Using existing
retort processes, a
medium grade Colorado oil shale may yield about up to 2~ gallons oil per ton
of ore and a specific
grade of Saskatchewan oil shale may yield about up to 14 gallons of oil per
ton of shale.
Most oil shale ore deposits contain carbonate minerals such as dolomite and
calcite, concentrations
of up to 35 % by weight. Decomposition of these carbonate minerals during the
retort of oil shale
may consume considerable amount of heat, in some cases up to ~ % to 10 % of
the whole thermal
energy injected into the retort, eventually reduces the energy efficiency of
the retort process.
Decomposition of the carbonate minerals can be suppressed by operating the
retort at the lowest
temperature possible, and, allowing the presence of sufficient amount of
carbon dioxide in the
recycle gas. The recycle gas is the mixture of steam, off gas and carbon
dioxide, which is
introduced to the fluidized bed retort as the heat source and the fluidizing
lift gas.
In general, shale oils produced from oil shale ores using the existing retort
processes are of low yield
and of relatively poor quality. Operating conditions such as ore particle
size, hydrodynamic
conditions in the retort and gas phase composition contacting the shale
particles in the retort
determines the oil yield and oil quality. Retort operating conditions control
the mass and heat
transfer between the particles and their surrounding environments, particle
heating rate, contact time
between the ore and the liberated hydrocarbons and suppression of the unwanted
pyrolysis, thermal
cracking or decomposition reactions. All of these are the parameters effecting
the oil yield and oil
quality of the retort process. As a result, retort design and retort operating
conditions are the key
factors for the performance of the retort process.
SUMMARY OF THE INVENTION
A fluidized bed retort process and system is invented for retorting
hydrocarbon containing materials
such as oil shale, tar sands, coal and hydrocarbon containing soils. =The
retort process operates for
the raw ore particles smaller than 7 mm in diameter. A mixture of steam and
off gas is used as the
heat source and the fluidizing lift gas, and recycled in the process. Water is
supplied into the system
to compensate its loses in the system, most preferably in the form of steam
generated from the
quench water.
Page 6
CA 02311738 2000-06-28
Prescott ~I. Rathborne
The retort process described in this invention could also be operated, if
needed, as of a two
stage retort process. The first stake of the retort process, which is a fixed
bed or a fluidized
bed preheating or pretreatment system, operating in any fluidization mode. of
which the
operating temperature is kept in the range of 1 ~0 °C to ?00 °C.
In the first stage of the retort
process. the rav~r ore would lose its moisture water and other recoverable
chemical species
such as ammonia if the raw ore contains ammonium sulfate, and, the recovered
chemical
specie such as ammonia is recovered from the gas effluent stream of the first
stage. After
treating the raw ore in the first stage, ore particles are further heated up
to about 3~0 °C by
using a series of heat exchangers and transferred in to the second retort
stage, which is also
called the retort stage. The retort stage, which is a fluidized bed retort
operating in the
spouted, bubbling, entrained or circulating bed modes. In this stage the
temperature is )<:ept
in the range of 400 °C to 5~0 °C, depending on the overall
process objectives, for the
liberation of the hydrocarbons from the raw ore.
The exit stream of the retort is dedusted using a cyclone. The cyclone
overflow, which is a mixture
of steam, off gas, carbon dioxide and liberated hydrocarbons, cooled for the
condensation of the
liberated hydrocarbons. This process can be made at elevated pressures, up to
4 MPa. to increase
the yield of condensed hydrocarbons. After the cooling, the mixture is fed to
a coalescer and an
electrostatic precipitator for the precipitation of liquid hydrocarbons. The
gaseous hydrocarbons,
which could not be condensed in this process, is called the off gas. The gas
mixture, which is
composed of steam, carbon dioxide and off gas, is heated up to 450 °C
to 600 °C temperature, and,
recycled back to the retort process as the heat source and the fluidizing lift
gas. The recycled
mixture of steam, carbon dioxide and ofF gas contains sufficient amount of
carbon dioxide, which is
generated during the retort of the raw ore. Presence of carbon dioxide in the
recycle gas suppresses
the decomposition of dolomite and calcite content of the raw ore during the
retort process. Also, a
fraction of the mixture of steam, carbon dioxide and off gas is combusted, if
needed, using a
catalytic bed combustor operating at atmospheric or elevated pressures up to 4
MPa for the
generation of thermal energy and using oxygen or air as oxidants. Also, the
carbon deposited in the
spent ore in the cyclone underflow stream is combusted using a fluidized bed
combustor for thermal
energy generation using air as oxidant.
Liquid hydrocarbons, which are liberated from the raw ore in the retort
process, then cooled and
condensed and are upgraded to produce synthetic crude oil or any marketable
hydrocarbon products.
There are many upgrading process options for the upgrading of the
hydrocarbons. Fractionation,
catalytic hydrotreating, catalytic hydrocracking, two step non-catalytic
and/or catalytic hydrotreating
or coking followed by catalytic hydrotreatina processes could be used for this
purpose.
After combusting the coke or residual hydrocarbon deposited on the spent ore,
hot spent ore is
cooled by using a series of heat exchangers or using quench water. The steam
generated from the
quench water is the back-up water for the process, since the steam generated
by injecting the quench
water to cool down the hot spend ore is used in the retort process. After
cooling, the spent ore is
transported back to the mine site, in the form of a paste by the addition of
water, using a pipe-line
transportation system. With the paste technology, land reclamation could take
less than one year,
while it may take possibly centuries in other systems.
Page 7
CA 02311738 2000-06-28
Prescott H. Rathborne
BRIEF DESCRIPTION OF THE DRAfVINGS
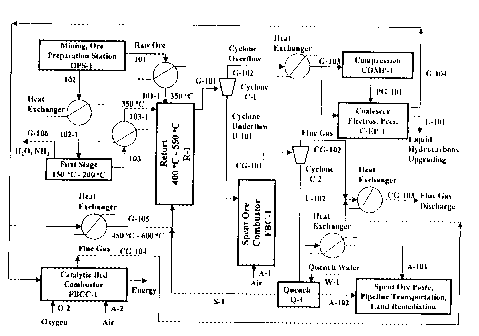
Figure 1 is a schematic process flow diagram of a retortinU system equipped
with a fluidized bed
retort in accordance with principles of the present invention.
DESCRIPTION OF THE PREFERED EMBODIMENT
A novel retorting process system as depicted in Figure 1, equipped with a
fluidized bed reactor is
invented to retort hydrocarbon containing materials such as oil shale, tar
sands, coal and
hydrocarbon containing soils.
Raw ore. which is the term used to define oil shale, tar sands, coal or
hydrocarbon containing soils.
and, blend of any of these. Ore is mined, crushed and screened to smaller than
7 mm in diameter in
the mining and ore preparation station OPS-1 and is fed to a series of heat
exchangers by the ore
feed stream line 101. In these heat exchangers ore particles are heated from
ambient temperature to
about 3~0 °C temperature by recovering the latent heat of the spent ore
discharged from the spent
ore combustor and fed to the retort by the feed line 101-1.
If a two stage retort is needed, the screened ore with smaller than 7 mm in
diameter in the raw ore
feed stream line 102 is fed to a series of heat exchangers and to the first
stage of the retort. This
stage is also defined as the pretreatment stage, operating temperature of
which is kept in the range of
1 ~0 °C to 200 °C. In the first stage of the retort process the
raw ore particles lose the moisture water
and other chemical species which might be liberated as a result of the mild
thermal treatment. As an
example, if the raw ore contains ammonium sulfate, thermal decomposition of
ammonium sulfate to
ammonia and ammonium hydrosulfate, which takes place at about 100 °C,
is achieved in the first
step of the retort. Ammonia in the gas line G-106 is recovered from the gas
effluent of the first step
of the retort, while ammonium hydrosulfate is chemically stable, stays in the
solid phase and carried
over to the fluidized bed retort together with the dried raw ore. After the
pretreatment in the first
stage, ore in line 103 is fed to a series of heat exchangers, and, hot ore at
about 350 °C temperature
in line 103-1 is fed to the retort.
Injection of the heated ore into the retort R-1 is maintained at a solid flux
flow rate of 20 t/m<sup>2</sup>
hr to 500 t/m<sup>2</sup> hr, which is operating in the spouted, bubbling, entrained
or circulating bed
modes. A mixture of steam, off gas and carbon dioxide in line G-105, at about
450 °C to 600 °C
temperature range, is injected into the fluidized bed retort through a
specifically designed nuzzle
orientations at the conical section and at the bottom section of the fluidized
bed retort. The feed gas,
which is a mixture of steam. off gas and carbon dioxide, is the heat source
and the fluidizing lift gas,
which is partially recycled in the process.
In the fluidized bed retort, ore particles of smaller than 7 mm in diameter
are in continuous motion
and contacting to each other by collisions and they are in good contact with
the hot fluidizing gas.
Hydrodynamic conditions in the fluidized bed retort promote mass and heat
transfer between the hot
fluidizing gas and ore particles as well as between the ore particles. These
hydrodynamic conditions
provide fast heating rate for the particles, uniform temperature in the retort
and fast transfer of
Page 8
CA 02311738 2000-06-28
Prescott FI. Rathborne
liberated hydrocarbons from solid ore particles to the gas atmosphere
surrounding the ore particles.
During the retort, part of the elemental oxygen present in the hydrocarbon
structure of the raw ore is
also liberated, most probably as carbon dioxide.
Density of the ore particles in the retort decreases as the retort process
progresses. Density of the
spent ore becomes small enough at an acceptable hydrocarbon liberation level
and spent ore
panicles leave the retort by entraining in the fluidizing gas stream. Also,
diameters of the spent ore
particles get smaller as a result of extensive particle-particle collisions in
the retort. Small size spent
ore particles leaves the retort by entraining in the fluidizing gas also.
The exit stream of the retort in line G-101, which is composed of steam, off
gas, carbon dioxide,
hydrocarbons and spent ore particles, is fed to a cyclone C-1 for dedusting.
Cyclone underflow
stream of U-101, which is the spent ore, is fed to a fluidized bed combustor
FBC-1 for heat
generation by combusting its coke and residual hydrocarbon. Hot air in line A-
1 is injected into the
bottom of the fluidized bed combustor and temperature of the fluidized bed
combustor is maintained
at about 600 °C - 900 °C. Lower operating conditions in the
fluidized bed combustor is desired to
suppress the decomposition of calcium and magnesium sulfates, which are formed
in the combustor
as a result of chemical reactions between the trioxide, and, dolomite and
calcite minerals of the raw
ore. Sulfur trioxide is the oxidation product of the sulfur of the coke and
the residual hydrocarbons,
which is formed in the oxidative atmosphere of the combustion process.
However, operating
temperature of the spent ore combustor is very much depended on the combustion
reactivity of the
coke or residual hydrocarbon on the spent ore pore spaces. Flue gas coming out
from the combustor
in line CG-101 is fed to a cyclone C-2 for dedusting. Cyclone overflow in line
CG-102, which is hot
flue gas is fed to heat exchangers and discharged into the atmosphere as
cooled flue gas in line CG-
103.
Cyclone underflow in line U-102, which is the hot combusted spent ore, is fed
to heat exchangers
and/or cooled by quench water for the recovery of its latent heat. The steam
in line S-1, which is
generated by injecting quench water in line W-1 on to the hot spent ore in the
quench unit Q-1. The
steam in line S-1 is fed to the recycled gas and used for the retort process.
After the quenching,
cooled combusted spent ore in line A-142 is fed to paste making station and
transported to the mine
site in the form of a paste by a pipeline system and discharged for land
reclamation.
Hydrocarbons in the cooled gas in line G-103 are precipitated using a
coalescer and an electrostatic
precipitator C-EP-1, and the recovered liquid hydrocarbons in L-101 is fed to
the upgrading station.
If compression is preferred to increase the efficiency of hydrocarbon
condensation and recovery, the
gas mixture in line G-103 is fed to compression station COMP-1, and compressed
gas in PG-101 is
fed to C-EP-1 for the precipitation of the condensed hydrocarbons. If the
compression of the gas in
line G-103 is desired, the coalescer and electrostatic precipitator C-EP-1 are
also operated under the
pressurized conditions.
A fraction of the cooled recycled gas in line G-104, which is the gas coming
out of the coalescer and
electrostatic precipitator C-EP-1 is heated up to 450 °C to 600
°C temperature range using a series of
heat exchangers. A fraction of the cooled recycled gas in line G-104 is fed to
a fixed bed catalytic
Page 9
CA 02311738 2000-06-28
Prescott H. Rathborne
combustor FBCC-l and its off gas content is combusted, using air in line A-2
or oxygen in line O-2
as oxidant, for heat generation. If the gas in line G-104 is compressed. FBCC-
1 unit is also operated
under the pressurized conditions. The Flue gas effluent of FBCC-1 in line CG-
104 is fed to a series
of heat exchangers to recover its latent heat. and. discharged to the
atmosphere after cooling.
The present invention has the following advantages for the retort of oil
shale. oil sands bitumen, coal
and hydrocarbon and hydrocarbon containing soils:
1. operates by sealing the penetration of the air and/or oxygen into the
retort and hydrocarbon
recovery processes, which provides the best operating conditions for achieve
high hydrocarbon
recovery efficiency and high product quality;
2. provides more trouble free operating conditions;
3. increases raw ore processing capacity;
4. generally uses shelf ready and conventional processes;
5. reduces the capital cost of synthetic crude oil production from non-
conventional
hydrocarbon resources;
6. reduces operating cost of synthetic crude oil production from non-
conventional
hydrocarbon resources; and,
7. eliminates or reduces the major environmental problems associated with the
synthetic crude oil
production from non-conventional hydrocarbon resources.
Although embodiments of this invention have been described and shown, it is to
be
understood that various changes, modifications and substitutions, as well as
rearrangements
of parts and combination of process steps thereof may be made without
departing from the
novel spirit and the scope of this invention.
Page 10
CA 02311738 2000-06-28
References Cited
U.S. Patent
Documents
~041210Aug., 1991 Merrill et
a1.208I407
4404083Sep., 1983Vasalos208/8
441~433Apr., 1985Vasalos202/99
441~4;;Nov., 1983Hoekstra et
a1.208/11
4430195Feb., 198401trogge208/11
4~15679May.,
1985Deering208/11
~009770Apr., 1991 Miller et a1.208i209
4447297May. . 1984Shang et a1.202/99
Page 2