Note: Descriptions are shown in the official language in which they were submitted.
CA 02313696 2000-06-09
WO 00/21633 PCTNS99I24113 _
1
Combined Hydrocycione and Filter System for Treatment of Liquids
Cross Reference to Related Applications
This application is based on a U.S. Provisional Application Serial Number
60/104,175 filed on October 13, 1998 and is a continuation-in-part of U.S.
Patent
Application Serial Number 09/243,553, filed February 2, 1999 entitled "Fluid
Conditioning System and Method", which is a continuation-in-part of U.S.
Application
Serial Number 09/096,254, filed June 11,1998 entitled "Fluid Conditioning
System and
Method", which in turn is based on U.S. Provisional Application Serial No.
60/052,626
filed on July 15, 1997 entitled "Apparatus and Method for Separating
Hydrophobic
Particles from a Solution" and U.S. Provisional Application Serial No.
60/073,971 filed
February 6, 1998 entitled "Flotation Tank Apparatus and Method."
Field of the Invention
This invention relates generally to large scale industrial liquid conditioning
and
filtration systems and more specifically to liquid conditioning components,
methods and
systems including membrane filtration technologies to separate particulates,
gases and
2 0 fluid bound compounds from fluid streams.
Background of the Invention
Filtration technologies that are used to separate particulate matter and gases
from
fluid solutions such as wastewater are often compromised with the buildup of
particulate
2 5 matter on the membranes or filter media which renders the filter useless
and severely
CA 02313696 2000-06-09
WO 00/21633 PCT/US99/24113
2
disrupts the filtering process. For example, traditional static filtration
mechanisms force
fluids through membranes or other filtration media until the membrane is
clogged or has
to be cleaned or replaced. As filters become fouled, their operational
performance
becomes severely reduced. The complexity of particulates, compounds and
microorganisms accumulate along the surface of the filters, retarding the flow
of fluid
through the filters and often irreversibly degrading the performance of the
filter surface.
This requires costly system shutdowns and replacement of expensive filters.
One manner of avoiding the precipitation and compression on filter surfaces of
bulk contaminants such as fine mineral clays, cellulosic fibers,
fats/oils/greases (FOG),
microorganisms and colloidal silica is to change the shape, format and
packaging of filter
membranes. For example, for situations with high contaminant or clogging agent
loading, tubular filters and hollow fiber technologies have been devised.
These filters
coat either tubes or hollow fibers with large bore size passages, permitting
the fluid
streams to pass along the surface of the filters at elevated flow rates
without being
trapped by the support materials and the low tangential flow areas that are
common on
large, flat surface area membrane sheets. Some of these types of tubular and
fiber based
filters are mounted on inert support media such as sintered steel or ceramics
and achieve
reliable performance under aggressive and harsh chemical conditions while
avoiding
heavy buildup of clogging agents. While these filters have proved efficient,
they often
2 0 result in at least a tenfold increase in cost, requiring massive
infrastructure and
operational support in comparison to flat sheet or spiral wound filters that
can package
large filter areas into compact spaces at significantly reduced capital and
operational
expenses. The continued problem of tradeoff of filter surface area to
resistance of fouling
CA 02313696 2000-06-09
WO 00/21633 PCT/US99I24113
3
agents significantly hinders the implementation of filter technologies. Most
applications
where filtration technologies could provide a valuable service can not support
the
expense and complexity of appropriate filtration systems.
Filter systems differ and are selective for defined size classes of
particulates and
dissolved compounds. Surfaces of filters can rej ect compounds based on charge
and their
ability to diffuse through filters. Membrane filters are generally defined in
terms of
microfiltration, ultrafiltration, nanofiltration and reverse osmosis
filtration based on the
size or molecular weight of the compounds that are being filtered, with
micrafiltration
rejecting the largest compounds and reverse osmosis filtering the smallest
compounds.
Membrane filters are rated based on the flux of cleaned water across the
membrane in given defined environmental conditions. The flux rate, defined as
the rate
of flow or transfer of fluid across a given membrane of a given surface area
in a unit of
time, is dependent on such factors as the pressure of the retentate (material
that is rej ected
on the surface of the membrane) on the surface of the membrane, the
temperature of the
fluid, the loading of contaminants and the permeability of the membrane.
Membranes
are deliberately oversized to ensure that there is sufficient capacity for
fluid cleaning and
filtering when the membrane surfaces become fouled by retentate. To ensure and
even
maximize flow rates across the membrane surfaces, systems are operated at
elevated
pressures to overcome the resistance by the fouling agents and to increase
flux rate.
2 0 However, eventually a membrane will become too impacted by retentate and
rendered
useless. Regenerating the membrane for reuse often requires cleaning with
chemical
agents to remove retentate. However, these chemical agents are often corrosive
to the
membrane filter and degrade it after each cleaning.
CA 02313696 2000-06-09
WO OOI21633 PGT/US99l24113
4
To address these fundamental limitations, fluid precleaning or prefiltering
technologies are increasingly being employed to condition the fluid streams
before
filtration through filter systems, in an attempt to reduce the fouling agents
and
contaminants which interfere with optimal performance of filtering membranes.
Fundamental to the concept of precleaning technologies is the removal of
components
from the fluid stream that obstruct fluid flow across filters. Examples of
precleaning
approaches include, clarification technologies and screening and removal of
large
particulates.
Another precleaning device commonly used in treating fluid in non-membrane
I O applications is the use of polymeric coagulants and flocculants. However,
chemical
agents used to trap coagulants such as polymeric coagulants, polymeric
antifoam and
dispersants are generally not compatible with membrane filters. For example,
cationic
polymers attract negatively charged compounds and collect on the filter
surface
disrupting the filtration process. This severely limits the potential utility
of polymeric
coagulants as effective precleaning agents and are therefore generally
avoided. For this
reason, many water treatment systems use filter systems in a series to
compensate for the
lack of efficient precleaning techniques. However, what is needed is a system
whereby
polymeric coagulants and other chemical treatments methods may be used prior
to and
in conjunction with the membrane filtration system.
SUMMARY OF THE INVENTION
The present invention is directed to the use of a conditioning chamber in
combination with various forms of filtration systems designed to remove
particulates,
CA 02313696 2000-06-09
WO OOIZ1633 PCT/US99I24I13 _
including solids, microbes colloids and microscopic gas bubbles in a fluid
stream. The
combination ofpre-treating fluid with the hydrocyclone system prior to
filtering the fluid
stream in filtering systems results in a dramatically more efficient fluid
treatment system
and at a significantly reduced cost.
5 Various embodiments of the invention axe directed to one or more
hydrocyclone
systems in isolation or in combination with separation tanks in the fluid
stream before,
or interspersed between, various filtration systems. Preferred embodiments
include
hydrocyclone systems that reduce the load of filter fouling components from
the fluid
stream at various points before the filtration systems. Such points include
the source of
the fluid load components where these components are generated, collection
points where
various streams combine and fluid systems that directly feed the filtration
system.
After bubble-particle aggregates are formed, they need to be separated from
the
fluid. The separation tank of the present invention incorporates features
which optimize
flotation of bubbles and bubble-particle aggregates from the fluid stream and
the
accumulation of material at the top of the surface where it can be skimmed off
the surface
of the tank. Due to the specific design of the tank for this purpose, this
task is
accomplished in a fraction of the space required for other flotation systems.
This combination of features of increased control of the operational
parameters
and, increased efficiency of the chemical usage in comparison to other
treatment systems,
2 0 permits the repeated treatment of the fluid stream in more compact
equipment than prior
filter pre-treatment technologies. Given the separation of components and
small footprint
of the claimed system of the present invention, several different pre-cleaning
methods are
economical for pre-cleaning of the fluid streams before the filter systems.
CA 02313696 2000-06-09
WO 00/21633 PCT/US99/24113
6
Operational performance from hydrocyclone systems have demonstrated that
these systems remove Total Suspended Solids (TSS), Biochemical Oxygen Demand
(BOD), Volatile Organic Compounds (VOC), metals, microorganisms, Total
Kjehldahl
Nitrogen (TKN) and Total Dissolved Solids (TDS) at unprecedented levels from
fluid
streams. When used in combination with filter systems and prefilter chemical
treatment
by polymers, the versatility and exceptional performance of these systems are
uniquely
suited to pretreat the fluids before they are passed through the various
filtration systems.
Further, due to the optimal performance of the hydrocyclone systems of the
present
invention, prefilter chemical treatment.is permissible and greatly improves
the operation
of the overall system.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a block diagram of the liquid conditioning system according to one
embodiment of the invention;
Figure 2 is a side perspective view of one embodiment of a conditioning
chamber
and separation tank;
Figure 3 is a top plan view of a liquid conditioning chamber;
Figure 4 is a cross-sectional view of a liquid conditioning chamber;
Figure 5 is a cross-sectional view of another embodiment of a liquid
conditioning
2 0 chamber;
Figure 6 is a cross-sectional view of a liquid conditioning chamber;
Figure 7 is a partial cross-sectional view of a collector apparatus;
Figure 8 is a cross-sectional view along lines 10 -10 of Figure 7;
CA 02313696 2000-06-09
WO 00121633 PCTNS99/24113
7
Figure 9 is a perspective view of the collector apparatus of Figure 7;
Figure 10 is apartial vertical cross-sectional view along lines 12-12 ofFigure
9;
Figure 11 is a cross-sectional view of one embodiment of a skimmer apparatus;
Figure 12 is a block diagram of the fluid conditioning system described in
Example 1;
Figure 13 is a block diagram of the fluid conditioning system described in
Example 2;
Figure 14 is a block diagram of the fluid conditioning system described in
Example 3;
Figure 15 is a block diagram of the fluid conditioning system described in
Example 4;
Figure 16 is a block diagram of the fluid conditioning system described in
Example 5;
Figure 17 is a cross-sectional view of one embodiment of a hydrocyclone
system;
and,
Figure 18 is a cross-sectional view of another embodiment of a hydrocyclone
system.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
2 0 The present invention relates to liquid conditioning systems used to pre-
treat fluid
streams before filtration systems to reduce the load of fluid borne components
that
impede the flow of fluid through filters or membranes.
CA 02313696 2000-06-09
WO 00/21633 PCTNS99/24113
8
As previously described, fouling agents and contaminants that reduce the flow
of
fluids through filters reduce the efficiency and raise the overall cost of
fluid treatment
systems. If these agents are removed from the fluid stream, the operational
parameters
of the fluid stream improve. For example, it has been observed in food
processing
streams that contain high levels of Total Suspended Solids (TSS) and
Fats/Oils/Grease
(FOG), that the filters clog and require frequent and intense cleaning. The
FOG also
proves to be damaging to the filtration material directly, irreversibly
damaging the filters
themselves. Removing these agents from the fluid stream can be accomplished to
desired
levels of contaminant parameters by systems as described below.
For example, in a vegetable processing application, where high levels ofTSS
and
FOG are encountered in the fluid stream, a major source of contaminants is
encountered
in the effluent from the cannery. To treat this fluid stream with peak flows
up to 300
gallons per minute (GPM), a three pass chemistry enhanced system is
appropriate. Each
pass constitutes the pumping of the cannery effluent through a hydrocyclone
system
{defined as a cylinder or chamber into which a fluid stream is directed and
swirled on the
inside wall, thereby generating centrifugal forces in the fluid) which is
independently
sparged and from which the contaminants are floated to the surface of a
flotation or
separation tank. Flows of this volume are best accommodated by a 6" Internal
Diameter
(LD.) hydrocyclone; however, it is possible to use smaller or larger
hydrocyclone
2 0 systems. In one such example, before the first pass, the fluid is pumped
from a collection
sump and the pH is adjusted to reduce the surface charges to relative electro-
neutrality
or near zero Zeta-potential (ZP). This commonly requires a pH control loop
which
automatically adjusts the delivery of a strong acid or compressed COZ or Oz to
the fluid
CA 02313696 2000-06-09
WO 00/21633 PC'f/US99I24113 _
9
stream before the hydrocyclone system to bring the solution zeta potential to
zero. The
fluid is pumped from the sump source directly over a coarse screen to remove
large solids
and debris from the stream. From a collection box of the screening device the
fluid is
pumped into the feed pump of the first hydrocyclone system. At the tangential
injection
point where the water is introduced into the hydrocyclone, a high molecular
weight, high
charge density cationic polyacrylamide polymer or other cationic reagent is
injected at
a concentration as required (e.g.,10-15 ppm C-498, Cytec Industries). The
effluent from
the hydrocyclone is delivered into a separation tank that removes the bulk of
the froth and
associated floc particles with it from the water.
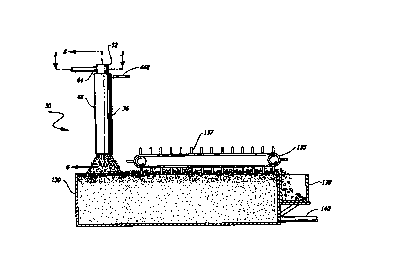
1 o Referring now to Figures 1 and 2, a liquid conditioning system according
to a first
embodiment of the present invention, generally designated 30, includes a
plurality of
modularized components to progressively process an influent carrier liquid
stream 32
originating from a solution source (not shown). The respective modules include
a
conditioning chamber 36 which may be a hydrocyclone system disposed downstream
of
the influent Garner liquid to receive the liquid and create a bubble-rich
environment for
a high incidence of bubble-particle collisions and gas transfer from the
liquid to the
bubbles. However, the conditioning chamber 36 or hydrocyclone system may be
open
to the atmosphere at the top and without a gas sparge system. Further, the
conditioning
chamber 36 or hydrocyclone system may be closed at the top which results in a
closed
2 0 liquid vortex creating a partial vacuum or significantly lower than
atmospheric gas
pressure when liquid is passed through.
The input to the conditioning chamber provides for the application of agents
to
modify the surface chemistry, such as chelating agents, detergents,
surfactants, gases,
CA 02313696 2000-06-09
WO 00/21633 PC"T/US99/Z4113 _
salts, acids and flocculants, at 37, to promote the coagulation and/or modify
the desired
zeta potential of targeted contaminants for efficient collection and removal.
Positioned
proximate the conditioning chamber output is a separation or flotation tank
130. The
unique modularized construction above allows efficient particle and gas
flotation and
5 separation for a wide spectrum of industries and applications while
minimizing the
footprint, and consequently the size, of the overall system. Particles or
particulate matter
are defined as including, but not limited to, solids, microbes, colloids and
microscopic
gas bubbles.
With continued reference to Figure 1, the input to the conditioning
10 chamber or hydrocyclone 36 also allows for delivery, at 37, of surface
chemistry such as
liquid or solid coagulant agents and polymer compounds or other forms
ofapplied energy
(e.g., electromagnetic, sonic, ionic, and the like) injected into the liquid
to break down
and reverse the attraction of the particle to the water and increase particle-
to-particle
attractions or hydrophobic interfaces. One form of energy is disclosed in co-
pending
U.S. Patent Application Serial Number 081979,405 filed November 26,1997 and
entitled
"Multi-Modal Method and Apparatus For Treating a Solution", the disclosure of
which
is expressly incorporated herein by reference. Other potential inputs include
in-line
mixers or static oil interceptors, floc tubes, or chemical injection means.
The general
objective ofthe added surface chemistry is to change the natural particle
attractivity with
2 0 the liquid to a repulsion to the liquid and attractive to air bubbles. It
is highly desirable
to have the particles in the proper state for satisfactory performance of the
present
invention. The particles may then be extracted from the liquid by introducing
large
CA 02313696 2000-06-09
WO 00/21633 PC'f/US99124113
11
quantities of air, or gas bubbles, to which the particles have a greater
likelihood of
attachment.
Referring now to Figures 1-6, gas bubbles such as air, ozone, or chlorine
are injected into the liquid by the conditioning chamber 36 that preferably
comprises an
air-sparged hydrocyclone or referred to just as a hydrocyclone. The
hydrocyclone creates
a predetermined spectrum of bubble sizes from less than one micron to several
hundred
microns in very large quantities. The air-to-water ratio created in the
chamber ranges
from approximately 2:1 to 50:1, with relative velocities of particles and
bubbles of
approximately one meter per second. These high ratios and velocities ensure
that bubbles
and particles collide instantaneously to form an association. This is
especially important
for small colloidal particles. The relatively large ratio of gas/water and
small bubble size
creates orders of magnitude more surface area for gas transfer from the
solution into the
bubbles than in DAFs or other sparged systems.
A fundamental principle of the hydrocyclone is derived from the centrifugal
acceleration of particles, colloidal suspensions, oils and waters in the
spinning fluid
ribbon along the inside wall of the hydrocyclone tube. This causes
classification by
relative densities as well as kinetic coalescence of oil-in-water emulsion,
forming larger
aggregates which are separated by their relative density from water. The other
advantage
is the sparging of gases through the walls of the porous hydrocyclone wall.
This permits
2 0 large volumes of gases, such as air, to be sheared by the spinning fluid
layer into bubbles
of a large size range. The velocity of the fluid ribbon determines the bubble
size inside
the fluid layer and in combination with surfactants the bubble size can be
controlled to
optimally match the size of the contaminants that need to be removed from the
fluid. The
CA 02313696 2000-06-09
wo oonma rcrius99nan3
12
hydrocyclone of the present invention offers several advantages over other
flotation
systems. For example, air to water ratios of 2:1 - 100:1 can be utilized
rather than the
maximal 0.15: I in DAF systems. The bubble size can be optimally tuned to
match the
particle or suspension components that need to be removed from the fluid
stream.
Because centrifugal acceleration can reach about one thousand gravity (Gs) and
the
ribbon is a fraction of an inch deep, coalescence of bubbles and bubble-
particle
aggregates and flotation to the inside surface of the spinning ribbon occurs
in
milliseconds rather than minutes in traditional flotation systems. The
consequence of
these advantages is that the bubble-particle interactions can happen in an
extremely
compact, highly controlled environment.
Due to the kinetic nature of a tangential injection of the fluid at the top of
the
hydrocyclone, this portion of the fluid stream is very effective at mixing and
instantaneously dispersing chemical additives that improve the formation and
stability
of particle aggregations and bubble formation. The intimacy of the mixing
process and
the rapid display of the particulates to the surface active agents,
coagulants, flocculants,
chelatins or pH adjusting agents ensure instantaneous reaction and adjustment
of the
surface chemical forces in the hydrocyclone system. Many applications of the
hydrocyclone system require no chemical enhancements such as the use of
polymers.
However, when chemical enhancements are used, sufficient chemical quantities
to
2 0 achieve optimal flotation are often achieved at a concentration of 10 -
30% of those used
in DAF or chemical precipitation. This results in operational cost savings as
well as
reducing the overall chemical burden on the fluid treatment system.
CA 02313696 2000-06-09
WO OOf21633 PCTIUS99/24113
13
Referring more particularly to Figures 2-4 and 6, the hydrocyclone 36
includes a cylindrical containment vessel having an open ended porous tube 40
(Figures
4 and 6) formed of a gas permeable material. The tube includes an interior
wall 42 (Fig.
4) defining an inner liquid passage with respective inlet and outlet openings
44 and 46
(Fig. 6). An enlarged cylindrical hollow housing 48 is disposed concentrically
around
the first tube to form an annular chamber 50. The chamber includes a gas inlet
44$ (Fig.
6) coupled to a source of regulated pressurized gas such as air or ozone. As
an example,
the porous tube 40 may be of a porosity having pore sizes within the range of
about 20
to 40 microns. The shearing action of the high velocity water passing by the
pores
creates bubbles ranging from sub-micron to several hundred microns in size.
Referring more particularly to Figures 2 through 5, the hydrocyclone 36
further includes a solution input apparatus or accelerator 52 mounted to the
proximal end
of the housing 48 of the hydrocyclone. The input apparatus may take many forms
and
acts to manipulate and tangentially direct the flow of input liquid into a
helical ribbon-
like stream through the liquid passage 42 to eventually exit into the
separation tank 130.
Figure 3 illustrates one form of input apparatus comprising a fixed restrictor
54
configured to generate a predetermined sized ribbon of helically flowing
solution. The
restrictor preferably generates an essentially continuous ribbon of solution
that swirls
around the inner wall of the hydrocyclone. To avoid turbulence that can
disrupt the
2 0 attachment of the particles to the gas-induced bubbles, it is desirable to
avoid ribbon
overlaps 56 (Figure 4, in phantom) or ribbon gaps 58 (Figure S).
As stated previously, other embodiments of the hydrocyclone system 36
may be appropriate. For example in Figure 17, the hydrocyclone 36 may be an
open top,
CA 02313696 2000-06-09
WO 00!21633 PCTNS99IZ4113
14
induced air hydrocyclone in which the hydrocyclone is not gas sparged. In this
hydrocyclone system, the accelerator head 52 is opened to the atmosphere (see
opening
49) where hydrocyclone system operates on the principle that high gravity
loading
centrifucated fluid induces very small bubbles dissolved in the fluid to move
through the
thin layer of fluid and contact the appropriately sized contaminants in the
fluid to form
bubble-particle aggregates. The accelerator head 52 also two has opening 51
and 53 for
inserting chemicals wherein the openings normally remain sealed. While within
the
hydrocyclone 36, the bubble-particle aggregates spiral down the length of the
hydrocyclone terminating in a vortex 55 and exit into the separation tank in a
controlled
manner such as not to disrupt the bubble-particle aggregates. Once in the
separation
tanks, the bubble-particle aggregates float to the surface of the separation
tank to further
aggregate into a large mass aggregation where the aggregation can be removed
with a
skimmer.
Alternatively, the hydrocyclone system 36 may be a closed top, no air
hydrocyclone system, whereby the accelerator head 52 is totally closed to the
atmosphere
as shown in Figure 18. In this embodiment, there is no gas sparging. With the
accelerator head 52 closed to the atmosphere, no air is allowed in the vortex
55 region
(opening 49 is shut by valve 47). This creates a vacuum within the vortex 55
that further
pulls bubble-air aggregates out of the fluid. By removing air as a requirement
in this
2 0 embodiment, power and maintenance requirements are significantly reduced.
The
performance of this hydrocyclone system compares favorably with gas sparged
hydrocyclone systems.
CA 02313696 2000-06-09
WO 00/21633 PGT/US99/24113
With reference to Figures 7-10, the hydrocyclone 36 preferably includes
at its outlet a collector apparatus, generally designated 80, to capture and
controllably
direct substantially particle-free solution. The collector apparatus 80
includes a conical-
shaped splay section 82 coupled axially to the hydrocyclone outlet via a
coupling ring 84
5 and a coupling cylinder 86 that concentrically bind the splay section to the
hydrocyclone.
The splay section is formed with a plurality of radially spaced-apart splay
vectors (not
shown) to urge the separated solution into a modified downwardly directed
flow. The
splay section may also be formed in a straight cylindrical configuration
without any loss
in performance.
10 Further referring to Figure 7, the collector apparatus 80 further includes
a torus-shaped trough 90 (see Figure 8) foamed with an annular slot 102 and
mounted to
the distal end of the splay section 82. The slot includes an engagement edge
or skimmer
101 positioned axially in-line with the expected laminar separation between
particle-rich
froth, and relatively particle-free solution to skim the separated particle-
free solution
15 splaying radially outwardly and downwardly from the conical section. The
trough
includes a unidirectional solution stop 103 (Figure 8} and an outlet formed
into an
outwardly projecting and downwardly directed spout 104 to discharge the
captured
solution as a collected stream. The central portion of the trough defines an
exit passage
106 for discharging the particle-rich froth on the surface of the solution
filled separation
2 0 tank 13 0.
With reference back to Figures 1 and 2, the separation tank 130 is
positioned downstream of the hydrocyclone 36 and is substantially filled with
the output
of the hydrocyclone. The separation tank, as envisioned in one embodiment, may
take
CA 02313696 2000-06-09
WO 00/21633 PCTNS99IZ4113 -
16
the form of a modified dissolved air flotation (DAF) tank (Figure 2), with an
open top to
receive the separated solution and the froth from the hydrocyclone. A froth
skimmer 13 S
having a plurality of paddles 137 (see Fig. 2} is positioned at the surface of
the tank to
push deposited froth or floc from the surface of the solution to a receptacle
area 138. To
exit treated solution from the tank, an effluent outlet 140 is formed near the
bottom
portion of the tank.
in operation, the separation tank 130 is positioned downstream from a
solution source that generates an untreated carrier liquid containing one or
more varieties
of particles or gases. For example, as shown in Figure 12, which illustrates
Example 1,
untreated carrier liquid originates from four separate sources: a cannery
source; a vat
room source; a flume source; and, a pitter source. In this Example, untreated
carrier
liquid (untreated wastewater) is first filtered through a coarse screen to
remove large
solids and then collected in a large reservoir tank. The untreated wastewater
may
optionally be pre-treated at this point by adding surface chemistry, at 37, to
urge the
particles to coalesce. The pH of the water. may also be adjusted at this
point. The water
is then pumped to the hydrocyclone 36.
The hydrocyclone input apparatus 52 (see Figure 2) receives the carrier
liquid stream and restricts the stream to a narrow ribbon, consequently
accelerating the
resulting ribbon flow along the inner passage 42 in Fig. 4 of the housing 48.
The ribbon
2 0 flow is directed tangentially and downwardly to define a helical shape,
and creates a
substantial centrifugal force acting on the solution. As the solution swirls
through the
containment vessel, the sparged gas plenum 448 injects gas bubbles into the
solution
stream. The bubbles collide with particles in the solution and gases dissolved
in the
CA 02313696 2000-06-09
WO 00/21633 PCTIUS99IZ4113
17
water transfer from the higher concentration in the water to the lower
concentration in the
bubbles. This process forms a froth that floats towards the center of the
containment
vessel as a result of the centrifugal force acting on the solution. The action
of the
hydrocyclone on the solution creates a non-turbulent flow between the
relatively particle-
free solution and the particle-rich froth. It has been discovered that by
controlling the
ribbon, a more uniform and turbulent-free ribbon through the hydrocyclone
results.
As the ribbon exits the distal end of the hydrocyclone 36, the swirling
helical action causes the particle-free solution to splay outwardly for
receipt in the
separation tank 130. Simultaneously, the particle-rich froth is deposited on
the surface
of the separation tank solution for subsequent collection by the froth skimmer
135.
In systems utilizing the optional collector apparatus 110 (Fig. 11 ), the
outwardly splaying solution is selectively captured by the trough 115 and
directed
through the spout 117 for delivery into the body of the separation tank
solution. This aids
in reducing the level of turbulence at the surface of the tank which has been
found to
hinder flotation tank performance. The particle-rich froth passes through the
center of
the trough and deposits along surface of the tank. The performance of the
collector
apparatus is substantially improved by employing the optional skimming
apparatus 116
to inject the annular gas stream at a predetermined point between the solution
and froth.
The effluent is then pumped into a volume control tank where the effluent
surface is then
2 0 skimmed.
The effluent from the collection tank is then pumped into a second
hydrocyclone for a second pass. In this pass which is treated at the same (or
slightly
higher) flow rate compared to the first pass, the pH may be adjusted and
cationically
CA 02313696 2000-06-09
WO 00/21633 PGTNS99IZ4113
18
treated effluent is divided into a parallel hydrocyclone systems where a very
high
molecular weight anionic, polyacrylalrlide polymer may be injected (e.g., 5
ppm A-130
HMW; Cytec Industries). The same process as stated previously is repeated. The
effluent from these hydrocyclones is delivered to the attached separation
tanks where the
newly formed floc (a result of the formation of a tight network of residual
cationic and
newly introduced anionic polymer in the second series of hydrocyclones) is
floated out
of water and skimmed off the surface. This effluent is then pumped into a
second storage
tank for volume control. The effluent is then further treated in a series of
filtration
membrane steps which include in sequence bag filtration, ultrafiltration and
reverse
osmosis which will be described in greater detail in the Examples. Other
filtration steps
may be included such as disc filtration, sand filtration, cross membrane
filtration and fine
screen filtration. These filtration steps remove particulates less than 2 mm
in diameter.
The treated effluent may then be further treated in activated carbon filters
and chlorine
dioxide and ozone treatments.
Alternative chemical combinations than the ones stated previously may
be appropriate. For example, fluid streams containing petrochemical products
and metal
contaminants may require alternative coagulants instead ofpH adjustment before
the first
pass through the hydrocyclone system. Inorganic compounds such as aluminum
salts or
organic coagulants such as polyamines may be more appropriate conditioning
agents than
2 0 pH adjustments. These can be injected into the fluid stream, ahead of the
hydrocyclones
or directly into the hydrocyclones. Other agents that can be used to improve
flotation
include detergents or surfactants (e.g., non-ionic nonyl-phenols or anionic
sodium
dodecyl sulfate), that reduce the surface tension of the fluid and thereby
reduce the
CA 02313696 2000-06-09
WO OOI21633 PCTNS99/24113
19
bubble size as the gas is sheared off the wall of the spurge tubes.
Hydrocyclones
containing only surfactants have been very successful at emulsion breaking of
both polar
and non-polar oils, found in the food processing and petrochemical industries
respectively. Other claimed combinations may include metal chelating agents
that are
inj ected into the first treatment pass and then followed by cationic and
anionic polymers
to remove the chelating agents with the associated metals from the stream, in
subsequent
treatment passes. These agents may be used where trace amounts of transition
or heavy
metals are found in large volumes of fluid. Combinations of such chemistries
have been
successful in the removal of cellulosic fibers and mineral clays found in such
diverse
streams as textile processing and paper and pulp manufacturing. These
treatment
combinations, because of their tailored chemistries and repeat treatments,
exceed the
performance of other pretreatments in both economic and operational factors.
Due to high air:water ratios, the small bubble sizes and the dynamic path of
the
gas bubbles through the fluid, gas transfer rates are extremely high and the
hydrocyclone
system of the present invention can be used to remove Volatile Organic
Compounds
(VOC) or light organic compounds. While this may aid in the removal of
components
that interfere with membrane systems, it is also an opportunity to introduce
reactive
gases. Ozone, chlorine or other gases can be introduced in an early pass to
disinfect,
sanitize or deactivate microbial organisms. Since the filtration membranes are
sensitive
2 0 to oxidative compounds and must not be exposed to reactive gases such as
chlorine or
ozone, the initial disinfecting gas may be stripped or removed in subsequent
passes
through hydrocyclones sparged with inert or non-reactive gasses such as
nitrogen or air.
Examples or removal rates of reactive gasses in non-chemical applications with
CA 02313696 2000-06-09
WO 00/21633 PCT/US99/24113 _
hydrocyclones are commonly 30 - 50% per pass. Sequential passes of fluid
through
hydrocyclones have removed VOC and reactive gasses to non-detectable levels.
Other agents that are incompatible with membrane systems can be used in the
hydrocyclones. For the same reason discussed above in the example of the
reactive
5 gasses, agents that interfere with membrane performance are commonly not
used
upstream of membrane filtration systems. The risk of leaks or residual
treatment agents
flowing through the system may not be warranted. However, with the option of
repeat
treatment and the ability to repeat and thereby remove the incompatible agents
from the
stream, the utility of these agents can be exploited. These polishing or self
cleaning
10 treatments can be repeated several times before the stream is exposed to
the membranes.
The removal of such incompatible agents is a novel feature that has not yet
been possible
because of the size and operational limitations of prior pre-treatment
systems.
An alternative to the above treatment is a non-chemical flume or transport
stream
treatment (Example 1). In certain situations, chemical modifications of the
water may
15 be detrimental to the production process and non-chemical treatment may be
more
appropriate. In circumstances where recycled water passes through a partially
or
completely closed system or loop, the entire stream can be passed through the
hydrocyclone systems repeatedly. However, generally, the average removal of
components from the stream by non-chemical means usually is less effective
than in
2 0 chemically enhanced streams, frequent repeat treatment improves the
overall stream
quality and membrane fouling component load. The advantage of this repeat
treatment
is that variability of stream components is reduced while overall loads of TSS
and
associated parameters are commonly reduced as much as 85%. The drain off or
spillage
CA 02313696 2000-06-09
WO 00/21633 PCT/US99/24113 -
21
from this type of stream entering the general effluent stream is much less
contaminated
with the membrane fouling agents. Examples of such transport or flume
treatments
include but are not limited to processing of olives, raisins, grapes, lettuce,
vegetables,
fruits and sugar beats. In some situations, these streams would precede
ultrafiltration to
remove proteinaceous or microbial matter, nanofiltration to remove sugars or
low
molecular weight organics or reverse osmosis to remove minerals.
Often in these situations, due to lack of surface chemical agents that
facilitate the
attachment of the particles to bubbles, mechanical energy and large volumes of
gas are
required to improve the performance of non-chemical systems. For this purpose,
the
air:water ratios can be adjusted to 7:1 - 10:1. This introduces more bubbles
and
opportunities for particles and microbes to attach and be floated out of the
system, even
if they are less tightly associated than in chemically enhanced systems. In
streams that
contain free oils or in oil-in-water emulsions, high G forces or acceleration
can also be
advantageous. When needed, several smaller ID spurge tubes may be run in
parallel. For
example, a 2" ID spurge tube for a given flow rate produces proportionately
higher
acceleration in the spinning fluid ribbon than a 6" tube does.
To increase the treatment passes through the system, internally recirculating
systems may be appropriate. In these situations, the discharge from the
receiving tank
is combined with the untreated effluent corning into the system. This
combination of the
2 0 influent with already treated effluent increases the flow and the volume
of the fluid that
needs to be passed through the hydrocyclones. Due to the acceleration of the
fluid rate
through the hydrocyclone, the resultant fluid volume has to be pumped through
the
hydrocyclone at an accelerated rate to accommodate the extra volume of
recirculating
CA 02313696 2000-06-09
WO OOI21633 PCT/US99/24113
22
fluid. Because of the acceleration of the fluid flow rate through the
hydrocyclone, the
bubble sizes sheared off the wall of the sparge tube is reduced, thereby
improving the
match of the bubble size with that of the particles that need to be removed.
Another
advantage of the adjustment is that the fluid is treated more frequently and
the likelihood
of successful particle flotation is increased even in situations where bubble
particle
aggregates are only temporary associations. Combined with the increased
air:water
ratios, these non-chemical systems can frequently attain removal rates of
stream
components similar to those of chemically enhanced hydrocyclone treatments.
Bulk treatment of all plant or process effluents, in situations where all the
sources
are treated for reuse or discharge, can be warranted. In these situations,
high volume
treatments may be necessary. Hydrocyclone systems or modules are generally run
in
parallel to process large volumes of effluent as shown in the Examples.
However,
hydrocyclone systems may be placed in a serial arrangements. Systems with a
footprint
of 6' x 12' have been used to treat flows in excess of 900 GPM.
Given these advantages in flexibility and modularity combined with the
adjustments to operational parameters such as air-flow-rates and acceleration,
permits the
design of systems that are appropriate for different streams in many
industries and
processes. Because of the modularity, small size and the ability to
selectively introduce
and then remove agents from the fluid streams, unprecedented pretreatments for
2 0 membrane filtration systems are attainable. Some of these systems are
illustrated in the
Examples shown below.
CA 02313696 2000-06-09
WO OOI21633 PGT/US99/24113 _
23
Example I
Non-chemical Treatment
In Olive Processing
Example I, which is illustrated in Figure 12, shows an example of non-chemical
treatment of effluent from several sources in olive processing. Effluent or
waste water
is collected from several sources such as a cannery source 202, a vat room
source 204,
a flume source 206 and a pilfer source 208. Waste water or effluent is then
filtered
through coarse screens 210 to remove large solids and debris from the effluent
sources.
The effluent is then collected in a large storage tank 212 of approximately 1
O6 gallons and
the effluent is at an ambient temperature of approximately 70° -
90°F. The effluent is
then pumped and divided into a parallel row of three hydrocyclone systems 214A
- 214C
in which each hydrocyclone system feeds effluent into an attached separation
tank 30 as
shown in Figure 2 which removed the bulk of the froth and associated bubble-
particle
aggregates by froth skimmer 138. Each hydrocyclone has an inner diameter of 6"
and
a length of 28". The average flow of effluent through each hydrocyclone is
approximately 75 up to 310 GPM. The plenum pressure of the gas inside the
hydrocyclone ranges from 2.5 psi to 6 psi. Thickness of the helical film
ranges from 1/4
inch to 1 inch and the air to water ratio ranges from 2:1 to 10:1.
The effluent was then pumped to a second central volume control tank 216 where
2 0 resulting froth and bubble-particle aggregates are skimmed off of the
surface of the
effluent. The effluent was then pumped to a second trio of parallel
hydrocyclones 218A -
218C of the same type as the first set. In this pass, the flow rate was
approximately the
same compared to the first pass and the effluent was reintroduced into the
parallel
CA 02313696 2000-06-09
WO 00/21633 PCT/US99I24113 _
24
hydrocyclones 218A - 218C. Once again the effluent was sparged and collected
into
three separation tanks where the effluent was skimmed. The effluent was then
pumped
to another central volume control tank 220.
Results of the pre-membrane or polisher portion of the system without the use
of
chemical additives are shown below.
Parameter Range of % Reduction Average
COD 0% - 20% 10%
FOG 0% - 95% 10%
TSS 0% - 76% 10%
Microbes 5% - 67% 32%
The effluent was then pumped though a bag filter system 222 comprised of
static
filtration bags (not cross flow) with 100 micron pore size (10 bags of
approximately 3
feet square for a total of 30 ft2) . The effluent was then pumped to a
ultrafiltration system
224 comprised of 6 banks of 8" type JX constant pressure, variable flow
filters
manufactured by Osmonics. The membrane consists of spiral wound polyvinylidene
diflouride.
After the ultrafiltration step 224, the water was pumped through volume
control
tank 226 to a reverse osmosis filtration step at 228. The reverse osmosis
filter comprised
2 0 of a constant flow, variable pressure trilaminate type AG, Osmonic filter.
The effluent
was then passed through an activated carbon filter 230 and a chlorine dioxide
and ozone
disinfection step 232.
CA 02313696 2000-06-09
WO 00/21633 PCT/US99lZ4113 -
The hydrocyclone system processing resulted in significant improvement to the
effluent stream prior to the bag filter step 222. The results demonstrate that
without the
hydrocyclone system steps of 214A - C and 218A - C, the bag filters were
required to be
changed every 20 minutes to 2 hours. By utilizing hydrocyclone systems 214A -
C and
5 218A - C, the bag filter replacement time increased to 4 - 8 hours.
The chart below demonstrates the increase in runtime (defined as time from the
start of the first bank of filter to startup of last bank. Banks are operated
sequentially to
a minimum flux before switching to the next bank), flux, number of banks used
to treat
the same volume of water and the runtime to shut down.
10 OF BEFORE AFTER
Runtime 2 hours 4 hours
Flux 200,000 gal/day ~ 350,00 gallons/day
15 Number of banks 6 banks 4 - 6 banks
used up treatment of
required volume of
water
2 0 Run time to Shut down 8 - 12 hours I 16 hours
Reverse Osmosis ~ no change
The use of hydrocyclone systems of the present invention for treating the
fluid
25 stream demonstrate a significant increase in runtime of the ultrafilters
with a
compounding increase (200,000 to 350,000 gallons) of fluid processed. Thus,
the run
time increased by a factor of two and the flux nearly doubled. Further, the
number of
banks required to process this increased amount of water was nearly halved.
CA 02313696 2000-06-09
WO 00/21633 PCT/US99I24113
26
Example II
Non Chemical Treatment
In Cheese Processing
Example II, which is illustrated in Figure 13, illustrates an example of non-
chemical treatment of wastewater from cheese processing sources. The object
was
microbe removal from a 350,000 gallon storage tank and to treat up to 400,000
gallons
per day.
The wastewater or effluent from the cheese processing source 240 was pumped
to a collection sump 242 and then pumped to a 350,000 gallon volume control or
equalization tank 244. The effluent was then pumped to three parallel
hydrocyclone
systems 246 with inner diameters of 6" and lengths of 27". Each hydrocyclone
system
was capable of processing 320 gallons per minute and had a stainless steel
porous tube
with 40 um pore size. The plenum pressure of the gas ranged from 6 to 7 psi,
the
air:water ration averaged at 6:1 and the water averaged a temperature of 128
°F.
The effluent was delivered through the hydrocyclone systems 246 and into
separation tanks 30 as illustrated in Figure 2 which removed froth by froth
skimmer 138.
The water was then recirculated to the original 350,000 gallon storage tank to
keep
microbes from growing in the stored water.
The effluent was then pumped and filtered through a nylon fiber screen 248
2 0 manufactured by Laikos. The pH of the effluent was then adjusted by the
additional
NaOH to pH 10 with 30% NaOH. The effluent was then heated to 140° F and
pumped
to oscillating ultra filter membranes for further filtration and then finally
pumped to an
oscillating reverse osmosis membrane systems 252 then to disposal by land
application.
CA 02313696 2000-06-09
WO 00/21633 PCT/US99/24113 _
27
The results demonstrated a dramatic reduction of TSS at the hydrocyclone step
of 54.8%. There was a 16.1 % reduction of COD at the hydrocycione step and a
microbial
reduction of 18 - 24 fold prior to entering into the filtration process. These
results were
achieved by recirculating the tank 244 water 3.8 times over a period of 22.5
hours.
Without treatment, the microbes increased 4 fold (from 14 million to 54
million Standard
Plate Count), significantly impending membrane flux. Microbial byproducts
fouled the
membranes within 8 - 12 hours. With the non-chemical pretreatment these runs
were
extended to 20 hours.
Example III
Chemical Treatment
In Cheese Precessing
Example III, which is illustrated in Fig. 14, shows an example of treatment of
waste water in cheese processing with chemical additives. The objective was
removal
of Total Suspended Solids (TSS) and Chemical Oxygen Demand (COD) compounds in
processing an amount of approximately 1,200,000 gallons of fluid per day.
The waste water from the source 240 was pumped into a collection sump and then
pumped into the 350,000 gal volume control or equalization tank 244. The
effluent was
then pumped to three parallel hydrocyclone systems 246 with inner diameters of
6" and
2 0 lengths of 27". Each hydrocyclone system was capable of processing 320
gallons per
minute. Each hydrocyclone system had a stainless steel porous tube with 40 um
pore
size. The plenum pressure of the gas ranged from 3 to 5 psi, air:water ratio
was
maintained at 4:1 and the water temp averaged at 128 ° F.
CA 02313696 2000-06-09
WO 00/21633 PC'T/US99/24113 _
28
Prior to treatment by the hydrocyclone systems, the pH of the effluent was
adjusted by the addition of sulfuric acid to obtain a pH of 6.2. Also added
prior to
treatment by the hydrocyclone was the addition ofhigh molecular weight
polyacrylamide
aqueous polymers at 10-20 ppm [Cytec 234GD].
The effluent was delivered through the hydrocyclone systems 246 and into
separation tanks 30 as illustrated in Figure 2 which removed froth by froth
skimmer 138.
The water was then pumped to a second 350,000 gallon volume control tank 247
The effluent was then pumped and filtered through a nylon fiber screen 248
manufactured by Laikos. The pH of the effluent was adjusted by the addition of
NaOH
to pH 10. The effluent was then heated and pumped to oscillating ultra filter
membranes
for further filtration and finally pumped to oscillating reverse osmosis
membrane systems
252.
The results show a reduction of 96 to 98% of TSS and a 28% reduction of large
molecular weight COD prior to entry into the filtration steps. This resulted
in a major
increase in efficiency of the overall system by increasing the runtime before
system
cleaning 8 - 16 hours (without treatment) to 16 - 36 hours with treatment.
Example IV
Non Chemical Treatment
2 0 Poultry Effluent
Example IV, shown in Figure 15, demonstrates an example of non-chemical
treatment of effluent from an 80,000 gallon poultry chiller. The objective was
fat/oil/grease [FOG] removal to increase flux rates and to increase run time
before filter
CA 02313696 2000-06-09
WO 00/21633 PCT/US99lZ4113 _
29
failure. The effluent was recirculated through a cooling tower 256 to cool the
effluent
to less than 40° F and then pumped to a first series of two
liydrocyclones 258 in series
each with a porous high density polyethylene tube of a length of 10" and an
internal
diameter of 2". The hydrocyclone systems 258 each have a positive displacement
blower type. The flow rate of the water ranged from S to 12 GPM. The water was
maintained roughly at a temperature of 48 ° F with an air:water ratio
ranging from 4:1 to
11:1. After the effluent was passed through the hydrocyclones, the effluent
was delivered
into the attached separation tanks for removal of surface froth. The effluent
was then
pumped to another hydrocyclone system 260 of the same type previously
described and
the same process was repeated.
The effluent was then pumped to a surge tank 262 where the effluent was heated
to roughly 120° F. The effluent was then pumped to an ultrafiltration
system with
polysulfone membranes manufactured by Koch with pore sizes of 0.02 um. The
effluent
was further pumped to a surge tank 266 and then pumped to a reverse osmosis
system
268 and the effluent was disinfected 270 and then recirculated to the poultry
chiller 254.
The results demonstrated that treatment by the two sets of hydrocyclone
systems
258 and 260 showed a decrease of TSS of 54% [433 ppm to 199 ppm], a decrease
of
CODs of 76% [5525 ppm to 1326 ppm] and a decrease of approximately 85% FOG.
The
downstream filter performance improved and the microfilters showed an ability
to
2 0 process 50 - 60 gallons of effluent in a range of 32 minutes to 50 minutes
down from a
range of 90 minutes to 120 minutes. The overall flux rate of the ultrafilter
showed a 40%
improvement.
CA 02313696 2000-06-09
WO OOI21633 PCTIUS99I24113 _
Example V
Chemical Treatment
Poultry Effluent
Example V, shown in Figure 16, is similar to Example 4, hut uses the addition
of
5 cationic and anionic polyacrylamide polymers in the pre-filtration steps.
Effluent from an 80,000 gallon poultry chiller 254 was circulated through a
cooling tower 256 to cool the effluent to 40° F. The effluent was
pumped to the first
hydrocyclone 258 of the type described in Example IV. However, prior to the
effluent
entering hydrocyclones system 258, a high molecular weight medium charge
density,
10 cationic polyacrylamide polymer was added to the effluent source at a
concentration of
20 ppm. When air sparged operation was used, the air:water ratio ranged from
7:1 to 4:1
with 4:1 being optimal. However, superior results were obtained running no air-
sparge
(hydrocyclone head open to atmosphere) induced air mode and in a partial
vacuum mode
(hydrocyclone head closed to atmospheric and no air sparging). After passing
through
15 the separation tanks 30 for removal of surface froth as previously
described, the effluent
was pumped to a second hydrocyclone 260 identical to the first one. However,
prior to
entry into the second hydrocyclone system, a high molecular weight anionic
polyacrylamide polymer was added [A-130 HMW Cytec Industries at 5 ppm]. After
passage through the hydrocyclone system set 260 the effluent passed through
attached
2 0 separation tanks 30 for the removal of the froth.
The effluent was then pumped to a surge tank 262 from where the effluent was
then pumped to an ultrafiltration system with polysulfone membranes
manufactured by
Koch with a pore size of 0.02 um. The effluent was further pumped to a surge
tank and
CA 02313696 2000-06-09
WO 00121633 PCTNS99I24113 _
31
then further pumped to a reverse osmosis system 268 and finally to a
disinfection step
270 and then recirculated to the poultry chiller 254.
The results demonstrated an 87% COD removal [4238 ppm reduced to 572 ppm]
and a 97% TSS removal [ 1033 ppm reduced to 33 ppm] by the two sets of
hydrocyclone
systems 258 and 260 prior to the effluent entering the ultrafiltration step at
264. The flux
rate increased significantly at the ultrafiltration step in comparison to the
same step in
Example IV. However, flux fell to where it would have been if there had been
no
hydrocyclone system treatment in 25 minutes due to residual polymers
This Example illustrates the importance of choosing the correct polymer-
membrane combination. If the surface chemical properties of the filtration
medium is
incompatible with the polymers (anionic surfaces such as regenerated
cellulose,
sulfonated polysulfone or polysulfone), then even very small amounts of
polymers will
eventually accumulate on the filtration media, degrading their properties.
This also
demonstrates the disproportional advantage of the non-chemical hydrocyclone,
which
significantly enhanced the performance of the filtration media.