Note: Descriptions are shown in the official language in which they were submitted.
CA 02313790 2000-06-09
WO 99/34462 PCTIUS98/Z6490
-1-
BATTERY CATHODE
The present invention relates to batteries.
Batteries, such as alkaline batteries, are commonly used as energy
sources. Generally, alkaline batteries have a cathode, an anode, a separator
and an
electrolytic solution. The cathode is typically formed of manganese dioxide,
carbon
particles and a binder. The anode can be formed of a gel including zinc
particles.
The separator is usually disposed between the cathode and the anode. The
electro-
lytic solution, which is dispersed throughout the battery, can be a hydroxide
solution.
The invention relates to batteries, such as alkaline batteries, having
cathodes that include manganese dioxide and relatively small nonsynthetic, non-
expanded graphite particles. These batteries have good performance
characteristics.
For example, the batteries can exhibit high energy output at a high discharge
rate,
such as a discharge rate equal to at least the battery's capacity {in units of
Ampere-
hours) discharged in one hour. The batteries can have various industry
standard
sizes, such as AA, AAA, AAAA, C or D.
"Nonsynthetic graphite particles" refer to graphite particles that are
prepared without using an industrial or laboratory graphitization process.
"Nonexpanded graphite particles" refer to graphite particles that are
prepared without any industrial or laboratory particle expansion process.
In one aspect, the invention features a cathode that includes manganese
dioxide and nonsynthetic, nonexpanded graphite particles having an average
particle
size of less than about 20 microns.
The particle size is measured using a Sympatec HELIOS analyzer. For
a given sample of graphite particles, the average particle size is the
particle size for
which half the volume of the sample has a smaller particle size.
In another aspect, the invention features an electrochemical cell
including a cathode, an anode and a separator disposed between the cathode and
the
anode. The cathode includes manganese dioxide and nonsynthetic, nonexpanded
graphite particles having an average particle size of less than about 20
microns.
In some embodiments, the separator includes a nonwoven, non-
membrane material and a second nonwoven, non-membrane material disposed along
a
surface of the first material. In these embodiments, the separator can be
devoid of a
CA 02313790 2000-06-09
WO 99I344b2 PCTIUS98IZ6490
-2-
membrane layer or an adhesive layer disposed between the nonwoven, non-
membrane materials. A membrane material refers to a material having an average
pore size of less than about 0.5 micron, whereas a non-membrane material
refers to a
material having an average pore size of at least about 5 microns.
S The cathode can have a porosity, of from about 21 % to about 28%.
The porosity of the cathode is the relative volume of the cathode that is not
taken up
by solid material, such as, far example, manganese dioxide, graphite particles
and
binder.
The anode can have a porosity of from about 2 grams of zinc particles
to about 2.45 grams of zinc particles per cubic centimeter of anode volume
that is
taken up by liquid or solid material.
The battery can have a relatively small amount of manganese dioxide
and/or zinc particles compared to the amount of electrolytic solution. For
example,
the weight ratio of manganese dioxide to electrolytic solution can be from
about 2.2
to about 2.9, and the weight ratio of zinc particles to electrolytic solution
can be from
about 0.9 to about 1.25. This is calculated based on the amount of
electrolytic
solution dispersed throughout the cathode, the anode and the separator.
The batteries can be AA or AAA batteries that demonstrate good
results when tested according to the cc photo test, the 1 Watt continuous
test, the half
Watt continuous test, the pulsed test, the half Watt rm test and/or the
quarter Watt rm
test. These tests are described below.
Other features and advantages of the invention will be apparent from
the description of the preferred embodiments thereof and the claims.
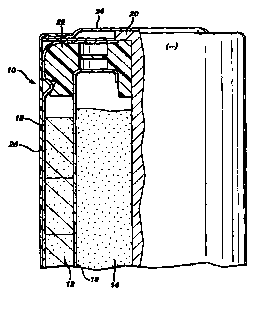
The figure is a cross-sectional view of a battery.
The preferred batteries are alkaline batteries that have a cathode
formed of manganese dioxide, relatively small, nonsynthetic, nonexpanded
graphite
particles and optionally a binder.
Referring to the figure, a battery 10 is shown that has a cathode 12, an
anode 14, a separator 16, an outer wall 18 that contacts the outer diameter of
cathode
12 and insulating Iayer 26. Battery 10 further includes an anode collector 20
that
passed through a meal member 22 and into anode 14. The upper end of anode
collector 20 is connected to a negative end cap 24 which serves as the
negative
CA 02313790 2000-06-09
WO 99/34462 PCT/US98I26490
-3-
external terminal of battery 10. Layer 26 can be formed of an electrically
nonconducting material, such as a heat shrinkable plastic. In addition, an
electrolytic
solution is dispersed throughout battery 10.
If the graphite particles disposed within cathode 12 are too large, the
conductivity of cathode 12 may not be sufficiently low. However, if the
graphite
particles are too small, cathode 12 may be comparatively dense, reducing the
amount
of electrolytic solution in cathode 12 and decreasing the efficiency of
battery 10.
Therefore, the graphite particles in cathode 12 preferably have an average
particle
size of at most 20 microns, more preferably from about 2 microns to about 12
microns and most preferably from about 5 microns to about 9 microns as
measured
using a Sympatec HELIOS analyzer. In some embodiments, the graphite particles
are
nonexpanded, nonsynthetic graphite particles having an average particle size
of about
7 microns as measured by this method. Nonsynthetic, nonexpanded graphite
particles
are available from, for example, Brazilian Nacional de Grafite (Itapecirica,
MG
Brazil).
The amount of graphite particles disposed within cathode 12 should be
enough to improve the overall conductivity of cathode 12 while having minimal
impact on the energy capacity of battery 10. Preferably, cathode 12 is from
about 4
weight percent to about 10 weight percent graphite particles, more preferably
from
about 5 weight percent to about 9 weight percent graphite particles, and most
preferably from about 6 weight percent to about 8 weight percent graphite
particles.
These weight percentage ranges correspond to when the electrolytic solution is
not
dispersed within cathode 12.
Cathode 12 can be a single pellet of material. Alternatively, cathode
12 can be formed of a number of cathode pellets that are stacked on top of
each
other. In either case, the cathode pellets can be made by first mixing the
manganese
dioxide, graphite particles and optionally the binder. For embodiments in
which
more than one pellet is used, the mixhue can be pressed to form the pellets.
The
pellets) are fit within battery 10 using standard processes. For example, in
one
process, a core rod is placed in the central cavity of battery 10, and a punch
is then
used to pressurize the top most pellet. When using this process, the interior
of wall
18 can have one or more vertical ridges that are spaced circumferentially
around wall
CA 02313790 2000-06-09
WO 99/34462 PCTIUS98/26490
-4-
18. These ridges can assist in holding cathode 12 in place within battery 10.
In embodiments in which cathode 12 is formed of a single pellet, the
powder can be placed directly within battery 10. A retaining ring is set in
place, and
an extrusion rod passes through the ring, densifying the powder and forming
cathode
12.
In certain embodiments, a layer of conductive material can be disposed
between wall 18 and cathode 12. This layer may be disposed along the inner
surface
of wall 18, along the outer circumference of cathode 12 or both. Typically,
this
conductive layer is formed of a carbonaceous material. Such materials include
LB1000 (Timcal), Eccocoat 257 (W.R. Grace & Co.), Electrodag 109 (Acheson
Industries, Inc.), Electrodag 112 (Acheson) and EBOUS (Acheson). Methods of
applying the conductive layer are disclosed in, for example, Canadian Patent
No.
1,263,697, which is hereby incorporated by reference.
Using a conductive layer, especially Electrodag 109 or EB005, between
wall 18 and cathode 12 can reduce the pressure used when forming cathode 12
within
battery 10. Thus, the porosity of cathode 12 can be made relatively high
without
causing the pellets) to be crushed or crack when forming cathode 12 within
battery
10. However, if the porosity of cathode 12 is too low, an insufficient amount
of
electrolytic solution can be dispersed within cathode 12, reducing the
efficiency of
battery 10. Thus, in certain embodiments, cathode 12 has a porosity of from
about
21% Co about 28%, more preferably from about 25% to about 27%, and most
preferably about 26%.
Within cathode 12, any of the conventional forms of manganese
dioxide for batteries can be used. Distributors of such manganese dioxide
include
Kerr McGee, Co., Broken Hill Proprietary, Chem Metals, Co., Tosoh, Delta
Manganese, Mitsui Chemicals and JMC.
In certain embodiments, cathode 12 can have from about 8.9 grams of
manganese dioxide to about 9.8 grams of manganese dioxide. In these
embodiments,
cathode 12 preferably includes from about 9.3 grams to about 9.8 grams of
manga-
nese dioxide, more preferably from about 9.4 grams to about 9.65 grams of
manga-
nese dioxide, and most preferably from about 9.45 grams of manganese dioxide
to
about 9.6 grams of manganese dioxide.
CA 02313790 2000-06-09
WO 99134462 PCT/US98lZ6490
-5-
In other embodiments, cathode 12 preferably includes from about 4
grams to about 4.3 grams of manganese dioxide, more preferably from about 4.05
grams to about 4.25 grams of manganese dioxide, and most preferably from about
4.1
grams to about 4.2 grams of manganese dioxide.
In some embodiments, cathode 12 may further include a binder.
Examples of binders for cathode 12 include polyethylene powders,
polyacrlyamides,
Portland cement and fluorocarbon resins, such as PVDF and PTFE. In certain
embodiments, cathode I2 includes a polyethylene binder sold under the
tradename
coathylene HA-1681 {Hoescht). When cathode 12 includes a binder, the binder
preferably makes up less than about 1 weight percent of cathode I2, more
preferably from about 0.1 weight percent to about 0.5 weight percent of
cathode 12,
and most preferably about 0.3 weight percent of cathode 12. These weight
percentages correspond to when the electrolytic solution is not dispersed
within
cathode 12.
Cathode 12 can include other additives. Examples of these additives
are disclosed in U.S. Patent No. 5,342,712, which is hereby incorporated by
reference. In some embodiments, cathode 12 preferably includes from about 0.2
weight percent to about 2 weight percent Ti02, more preferably about 0.8
weight
percent Ti02.
Anode 14 can be formed of any of the standard zinc materials used in
battery anodes. Often, anode 14 is formed of a zinc gel that includes zinc
metal
particles, a gelling agent and minor amounts of additives, such as gassing
inhibitors.
If the porosity of anode 14 is too high, the amount of zinc within
battery 10 is reduced which decreases the energy capacity of battery 10.
However, if
the porosity of anode 14 is too low, an insufficient amount of electrolytic
solution
can be dispersed within anode 14. Therefore, in some embodiments, anode 14
preferably has from about 2 grams to about 2.45 grams of zinc particles per
cubic
centimeter of anode volume, more preferably from about 2.1 grams to about 2.35
grams of zinc particles per cubic centimeter of anode volume, and most
preferably
from about 2.15 grams to about 2.3 grams of zinc particles per cubic
centimeter of
anode volume.
In certain embodiments, anode 14 preferably has from about 3.7 grams
CA 02313790 2000-06-09
WO 99/34462 PCTNS98/26490
-6-
to about 4.25 grams of zinc particles, more preferably from about 3.8 to about
4.15
grams of zinc particles, and most preferably from about 3.9 grams to about
4.05
grams of zinc particles.
In other embodiments, anode 14 preferably has from about 1.5 grams
to about 1.9 grams of zinc particles, more preferably from about 1.55 to about
1.85
grams of zinc particles, and most preferably from about 1.65 grams to about
1.75
grams of zinc particles.
In some embodiments, anode 14 preferably includes from about 64
weight percent to about 76 weight percent zinc particles, more preferably from
about
66 weight percent to about 74 weight percent zinc particles, and most
preferably from
about 68 weight percent to about 72 weight percent zinc particles. These
weight
percentages correspond to when the electrolytic solution is dispersed within
anode 14.
Gelling agents that can be used in anode 14 include polyacrylic acids,
grafted starch materials, polyacrylates, salts of polyacrylic acids,
carboxymethyl-
cellulose or combinations thereof. Examples of such polyacrylic acids are
Carbopol
940 (B.F. Goodrich) and Polygel 4P(3V), and an example of a grafted starch
material
is Waterlock A221 (Grain Processing Corporation, Muscatine, IA). An example of
a
salt of a polyacrylic acid is CL15 (Allied Colloids). In some embodiments,
anode 14
preferably includes from about 0.2 weight percent to about 1 weight percent
total
gelling agent, more preferably from about 0.4 weight percent to about 0.7
weight
percent total gelling agent, and most preferably from about 0.5 weight percent
to
about 0.6 weight percent total gelling agent. These weight percentages
correspond to
when the electrolytic solution is dispersed within anode 14.
Gassing inhibitors can be inorganic materials, such as bismuth, tin, lead
and indium. Alternatively, gassing inhibitors can be organic compounds, such
as
phosphate esters, ionic surfactants or nonionic surfactants. Examples of ionic
surfactants are disclosed in, for example, U.S. Patent No. 4,777,100, which is
hereby
incorporated by reference.
Separator 16 can have any of the conventional designs for battery
separators. In some embodiments, separator 16 is formed of two layers of
nonwoven,
non-membrane material with one layer being disposed along a surface of the
other.
In these embodiments, the separator preferably does not include a layer of
membrane
CA 02313790 2000-06-09
WO 99/34462 PCTIUS98I2b494
-7_
material or a layer of adhesive between the nonwoven, non-membrane layers. To
minimize the volume of separator 16 while providing an efficient battery, each
layer
of nonwoven, non-membrane material can have a basis weight of about 54 grams
per
square meter, a thickness of about 5.4 mils when dry and a thickness of about
10
mils when wet. In one embodiment, the nonwoven, non-membrane material is a
matrix of polyvinyl alcohol (PVA) fibers, cellulose fibers and PVA binder.
Generally, the nonwoven, non-membrane material is devoid of fillers such as,
for
example, inorganic particles.
In other embodiments, separator 16 includes an outer layer of
cellophane with a layer of nonwoven material. Separator 16 also includes an
additional layer of nonwoven material. The cellophane layer can be adjacent
cathode
12 or anode 14. Preferably, the nonwoven layer contains from about 78 weight
percent to about 82 weight percent PVA and from about 18 weight percent to
about
22 weight percent rayon with a trace of surfactant. Such nonwoven materials
are
available from PDM under the tradename PA36.
The electrolytic solution dispersed throughout battery 10 can be any of
the conventional electrolytic solutions used in batteries. Typically, the
electrolytic
solution is an aqueous hydroxide solution. Such aqueous hydroxide solutions
include,
for example, potassium hydroxide solutions and sodium hydroxide solutions. In
some
embodiments, the electrolytic solution is an aqueous solution of potassium
hydroxide
including from about 33 weight percent to about 38 weight percent potassium
hydroxide. .
In certain embodiments, battery 10 preferably includes from about 3.4
grams to about 3.9 grams of electrolytic solution, more preferably from about
3.45
grams to about 3.65 grams of electrolytic solution, and most preferably from
about
3.5 grams to about 3.6 grams of electrolytic solution.
In other embodiments, battery 10 preferably includes from about I.6
grams to about I.9 grams of electrolytic solution, more preferably from about
1.65
grams to about I.85 grams of electrolytic solution, and most preferably from
about
1.7 grams to about 1.8 grams of electrolytic solution.
The weight ratio of manganese dioxide to electrolytic solution can be
from about 2.2 to about 2.9, and the weight ratio of zinc particles to
electrolytic
CA 02313790 2000-06-09
WO 99134462 PCT/US98/Z6490
_g_
solution can be from about 0.9 to about I.25. In some embodiments, the weight
ratio
of manganese dioxide to electrolytic solution is from about 2.5 to about 2.9,
and the
weight ratio of zinc particles to electrolytic solution is from about 1.1 to
about 1.25.
In other embodiments, the weight ratio of manganese dioxide to electrolytic
solution
is from about 2.5 to about 2.65, and the weight ratio of zinc particles to
electrolytic
solution is from about 0.9 to about 1.2. These weight ratios are based on the
amount
of electrolytic solution dispersed throughout the anode, cathode and
separator.
The batteries can be AA or AAA batteries that demonstrate good
results when tested according to the cc photo test, the 1 Watt continuous
test, the half
Watt continuous test, the pulsed test, the half Watt rm test and/or the
quarter Watt rm
test. These tests are described below.
Battery 10 can be a AA battery that exhibits excellent performance
according to the cc photo test (described below). For example, when
discharging to I
Volt according to the cc photo test, the AA battery can give at least about
150 pulses,
at least about 175 pulses, at least about 185 pulses or at least about 200
pulses.
When discharging to 0.8 Volts according to the cc photo test, the AA battery
can
give at least about 350 pulses, at least about 375 pulses, at least about 385
pulses or
at least about 400 pulses.
Battery 10 can be a AA battery that exhibits excellent performance
according to the 1 Watt continuous test (described below). For example, when
dis-
charging to 1 Volt according to the 1 Watt continuous test, the AA battery can
give
at least about 0.6 hours, at least about 0.65 hours, at least about 0.7 hours
or at least
about 0.75 hours. When discharging to 0.8 Volts according to the 1 Watt
continuous
test, the AA battery can give at least about 0.95 hours, at least about 1
hour, at least
about I.OS hours or at least about 1.1 hours.
Battery 10 can be a AA battery that offers excellent performance
according to the pulsed test (described below). For example, when discharging
to 1
Volt according to the pulsed test, the AA battery can give at least about 1.6
hours, at
least about 1.75 hours, at least about 2 hours or at least about 2.15 hours.
When
discharging to 0.8 Volts according to the pulsed test, the AA battery can give
at least
about 2.75 hours, at least about 3 hours, at least about 3.25 hours or at
least about 3.3
hours.
CA 02313790 2000-06-09
WO 99/34462 PCT/US98/Z6490
-9-
Battery 10 can be a AA battery that offers excellent performance
according to the half Watt rm test (described below). For example, when
discharged
to 1.I Volts according to the half Watt rm test, the AA battery can give at
least about
1.5 hours, at least about 2 hours, at least about 2.5 hours or at least about
2.65 hours.
When discharged to 0.9 Volts according to the half Watt rm test, the AA
battery can
give at least 2.9 hours, at Ieast about 3 hours, at least about 3.25 hours or
at least
about 3.4 hours.
Battery 10 can be a AAA battery that offers excellent performance
according to the half Watt continuous test (described below). For example,
when
discharged to I Volt according to the half Watt continuous test, the AAA
battery can
give at least about 0.65 hours, at least about 0.7 hours, at least about 0.75
hours or at
least about 0.8 hours. When discharged to 0.9 Volts according to the half Watt
continuous test, the AAA battery can give at least 0.9 hours, at least about
0.95
hours, at least about 1 hour or at least about 1.05 hours.
Battery 10 can be a AAA battery that offers excellent performance
according to the pulsed test (described below). For example, when discharged
to 1
Volt according to the pulsed test, the AAA battery can give at least about 0.3
S hours,
at least about 0.4 hours, at least about 0.45 hours or at least about 0.5
hours. When
discharged to 0.9 Volts according to the pulsed test, the AAA battery can give
at
least 0.65 hours, at least about 0.7 hours, at least about 0.75 hours or at
Ieast about
0.8 hours.
Battery 10 can be a AAA battery that offers excellent performance
according to the half Watt rm test (described below). For example, when
discharged
to 1.1 Volts according to the half Watt rm test, the AAA battery can give at
least
about 0.4 hours, at Ieast about 0.45 hours, at least about 0.5 hours or at
least about
0.55 hours. When discharged to 0.9 Volts according to the half Watt rm test,
the
AAA battery can give at least 0.9 hours, at least about 0.95 hours, at least
about 1
hour or at least about 1.05 hours.
Battery 10 can be a AAA battery that offers excellent performance
according to the quarter Watt rm test (described below). For example, when
discharged to 1.1 Volts according to the quarter Watt rm test, the AAA battery
can
give at least about 2 hours, at least about 2.1 hours, at least about 2.2
hours or at
CA 02313790 2000-06-09
WO 99/34462 PCT/US98/26490
- 10-
least about 2.3 hours. When discharged to 0.9 Volts according to the quarter
Watt
rm test, the AAA battery can give at least 3.1 hours, at least about 3.25
hours, at
least about 3.4 hours or at least about 3.5 hours.
EXAMPLE I
AA batteries were prepared with the following components. The
cathode included about 9.487 grams of manganese dioxide (Kerr-McGee, Co.),
about
0.806 grams of nonsynthetic, nonexpanded graphite having an average particle
size of
about 7 microns (Brazilian National de Gnrafite) and about 0.3 weight percent
of
coathylene HA-1681. The anode included about 3.976 grams of zinc particles,
about
50 ppm surfactant (RM510, Rhone Poulenc) relative to zinc, and about 0.5
weight
percent total gelling agent (Carbopol 940 and A221 ). The porosity of the
cathode
was about 26%, and the porosity of the anode was about 2.173 grams of zinc per
cubic centimeter of anode. The separator was a two-layer structure with each
layer
formed of a nonwoven material including about 57 weight percent PVA fibers
(about
0.5 denier at 6 millimeters), about 30 weight percent rayon fibers (about 1.5
denier at
6 millimeters) and about 13 weight percent PVA binder. Each layer was about
5.4
mils thick when dry and about 10 mils thick when wet. Each layer had a basis
weight of about 54 grams per square meter. The separator did not include an
adhesive, and the layers were substantially devoid of any filler. The battery
also
included about 3.598 grams of an aqueous potassium hydroxide (about 35.5
weight
percent potassium hydroxide) solution. A thin coating of EB005 (Acheson) was
disposed between the outer wall of the battery and the outer periphery of the
cathode.
The AA batteries were stored at a temperature of from about 20.1
°C to
about 22.1 °C for five days. The AA batteries were then stored
according to the
following procedure.
Each battery is visually examined for leakage or material damage and
identified such that battery identification can be maintained throughout the
test
program. The batteries are oriented on their sides in holding trays such that
the
batteries are not in physical contact with each other. The holding trays are
made to
be resistant to heat and electrolytes. The trays are stored for 1 day at
ambient
conditions, after which the trays are placed into a preheated chamber. The
trays are
spaced so that there is at least about 5 cm (2. inches) of space between the
chamber
CA 02313790 2000-06-09
WO 99134462 PCTNS98/26490
-11-
wall, and the tray above, below, or adjacent to each tray. The following 24
hour test
sequence; shown in Table 1, is repeated for 14 days.
Ta le I
Cycle Number Time (Hrs.) Temperature ( 2C}
1 6.0 Ramp from 28 to 25
2 4.5 Ramp from 25 to 34
3 2.0 Ramp from 34 to 43
4 1.0 Ramp from 43 to 48
5 1.0 Ramp from 48 to 55
i
,
6 1.0 Ramp from 55 to 48
7 1.0 Ramp from 48 to 43
8 3.0 Ramp from 43 to 32
9 4-55 Ramp from 32 to 28
24.0 ( 1 Day)
The trays are removed from the chamber and each battery is visually examined
for
leakage or material damage.
The following tests were subsequently performed on individual AA
batteries. Each test was conducted at a temperature of from about 20.1
°C to about
22.1 °C.
A AA battery was discharged from an open circuit voltage of about 1.6
Volts under constant current conditions of ten seconds per minute for one hour
per
day ("the cc photo test"). The AA battery reached 1 Volt after 203 pulses, and
the
AA battery reached 0.8 Volts after 443 pulses.
A AA battery was continuously discharged from an open circuit
voltage of about 1.6 Volts at 1 Watt ("the 1 Watt continuous test"). The AA
battery
reached 1 Volt after about 0.75 hours, and the AA battery reached 0.8 Volts
after
about 1.00 hours.
A AA battery was continuously discharged from an open circuit
CA 02313790 2000-06-09
WO 99/34462 PGT/US98/26490
- 12-
voltage of about 1.6 Volts at a rate that alternated between I Watt (3 second
pulses)
and 0.1 Watt (7 second pulses) ("the pulsed test"). The AA battery reached 1
Volt
after about 2.16 hours, and the AA battery reached 0.8 Volts after about 3.72
hours.
A AA battery was discharged from an open circuit voltage of about 1.6
Volts at 0.5 Watts for 15 minutes per hour ("the half Watt rm test"). The AA
battery
reached 1.1 Volts after about 1.87 hours, and the AA battery reached 0.9 Volts
after
about 3.34 hours.
EXAMPLE II
A AAA battery was prepared. The cathode 12 included about 4.155
grams of manganese dioxide (Ken McGee, Co.), about 0.353 grams of
nonsynthetic,
nonexpanded graphite having an average particle size of about 7 microns
(Brazilian
Nacional de Grafite) and about 0.3 weight percent of coathylene HA-1681. The
anode 14 included about 1.668 grams of zinc particles and about 0.5 weight
percent
total gelling agent (Carbopol 940 and A221 ). The porosity of the cathode was
about
26%, and the porosity of the anode was about 2.266 grams of zinc per cubic
centi-
meter of anode 14. The separator included two layers of nonwoven material. The
separator was a two-layer structure with each layer formed of a nonwoven
material
including about 57 weight percent PVA binders (about 0.5 denier at 6
millimeters),
about 30 weight percent cellulose fibers (about 1.5 denier at 6 millimeters)
and about
13 weight percent PVA binder. Each layer was about 5.4 millimeters thick when
dry
and about 10 millimeters thick when wet. Each layer had a basis weight of
about 54
grams per square meter. The separator did not include an adhesive, and the
layers
were substantially devoid of any filler. The battery also included about 1.72
grams
of an aqueous potassium hydroxide (about 35.5 weight percent) solution. A thin
coating of EB005 was disposed between the outer wall of the battery and the
outer
periphery of the cathode.
The AAA batteries were stored as described in Example I. Each AAA
battery was discharged from an open circuit voltage of about 1.6 Volts, and
the tests
were conducted within the temperature range described in Example I.
A AAA battery was continuously discharged from an open circuit
voltage of about 1.6 Volts at one half Watt ("the half Watt continuous test").
The
AAA battery reached 1 Volt after about 0.76 hours, and the AAA battery reached
0.8
CA 02313790 2000-06-09
WO 99134462 PCT/US98I26490
-13-
Volts after about 0.96 hours.
With the pulsed test, a AAA battery took about 0.55 hours to reach 1
Volt, and about 0.84 hours to reach 0.8 Volts.
With the half Watt rm test, a AAA battery took about 0.57 hours to
reach 1 Volt, and about 1.08 hours to reach 0.8 Volts.
A AAA battery was discharged from an open circuit voltage of about
1.6 Volts at 0.25 Watts for 15 minutes per hour ("the quarter Watt rm test").
The
AAA battery reached 1.1 Volts after about 2.4 hours, and the AAA battery
reached
0.9 volts after about 3.65 hours.
Example III
AA batteries were prepared with the following components. The
cathode included about 9.11 grams of manganese dioxide (40:60 weight mixture
of
Delta: Tosoh), about 0.810 grams of nonsynthetic, nonexpanded graphite having
an
average particle size of about 7 microns (Brazilian Nacional de Graflte) and
about 0.8
1 S weight percent of titanium dioxide (Kronos). The anode included about 3.89
grams
of zinc particles, about 0.88 weight percent total gelling agent (3 V and CL
15), and
about 50 ppm of surfactant (RM 510, Rhone Poulenc). The porosity of the
cathode
was about 23%, and the porosity of the anode was about 2.173 grams of zinc per
cubic centimeter of anode. The separator included a layer of nonwoven material
{PA36 A, PDM) a layer of PA36C and a layer of cellophane (1 mil. thick). The
cellophane was adjacent to the cathode, and the nonwoven PA36A layer was
adjacent
to the anode. The battery also included about 3.62 grams of aqueous potassium
hydroxide (about 35.5 weight percent potassium hydroxide) solution. A thin
coating
of EB005 (Acheson) was disposed between the outer wall of the battery and the
outer
periphery of the cathode.
The AA batteries were stored at a temperature of from about 20.1
°C to
about 22.1 °C for about five days according to the protocol described
in Example I.
The following tests were subsequently performed on individual AA batteries.
Each
test was conducted at a temperature of from about 20.1 °C to about 22.1
°C.
The AA battery was discharged according to the cc photo test. The
AA battery reached one volt after 180 pulses, and the AA battery reached 0.8
volts
after 347 pulses.
CA 02313790 2000-06-09
WO 99/34462 PCTNS98I26490
- 14-
A AA battery was discharged according to the one Watt continuance
test. The AA battery reached 1 volt after about 0.57 hours, and the AA battery
reached 0.8 volts after about 0.80 hours.
A AA battery was continuously discharged from an open circuit
voltage according to the pulsed test. The AA battery reached 1 volt after
about 1.76
hours, and the AA battery reached 0.8 volts after about 3.11 hours.
A AA battery was discharged according to the half Watt rm test. The
AA battery reached 1.1 volts after about 1.66 hours, and the AA battery
reached 0.9
volts after about 3.05 hours.
Other embodiments are within the claims.