Note: Descriptions are shown in the official language in which they were submitted.
CA 02314094 2000-06-09
1
METHOD FOR GASIFYING ~RGANIC SUBSTANCES AND, SUBSTANCE
MIXTURES
The invention relates to a me od for gasifying organic substances and sub-
stance mixtures according to th generic terms of Claim 1.
From US-PS 4,568,362, a method for gasifying organic substances and
substance mixtures is known i which the organic substances are directed into
a pyrolysis reactor in which th organic substances come into contact with a
heat carrier medium which ca see rapid pyrolysis in which the organic sub-
stances are converted into p rolysis products, that is, pyrolysis gases with
substances that can be conde sed and solid residue containing carbon. The
heat energy needed for the p rolysis is generated by firing th~ solid residue
containing carbon. In a second reaction zone, the pyrolysis gases that contain
tar are subjected to cracking r actions and reactions with steam such that a
product gas with a high caloric alue is obtained.
In this method, the pyr lysis as welt as the firing of the solid residue
containing carbon take place i ' a fluidized bed. In the upper part of the
pyroly
sis fiuidiz~d bed r~actor, a re ction zone is provided for the pyrolysis gases
containing tar.
The heat carrier media is discharged together with the solid residue
containing carbon in part thro gh the reactor h~ad of the pyrolysis fluidized
CA 02314094 2000-06-09
2
bed reactor and the remaining portion via a tine that is mounted on the upper
fluidized bed limit, and fed to tl~e fluidized bed firing. There, the solid
residue
containing carbon is fired and t a heat carrier medium heated up. The heated
heat carrier medium and the as e9 ere discharged from the fluidized bed firing
together with the waste gas, a d separated in a gas-solid separator mounted
above the fluidized bed pyroly is reactor, and fed to the reaction zone of the
pyrolysis reactor from which it gain falls into the fluidized bed of the
.pyrolysis
reactor (heat carrier medium cy le).
It is very costly to opera ~ th~ fluidiZed beds and it is hardly possible to
control the reactions of the pyrdlysis gases in the r~action zone.
The object of the inventi n is to .make available a method for generating
a gas with a high caloric value hat is easy to perform. In this pmcess, a
small
condensation portion is preferr d. A further object of the invention is to
mak~
available a simple apparatus fo carrying out the method.
With respect to the meth d, this object is resolved by the combination of
features in Claim 1. According o the invention, the pyrolysis is carried out
in a
fluidizad bed reactor or a rota~ drum, the pyrolysis gases are mixed, if neces-
sary, with a reactant such as seam, and they are fed into an indirect heat, ex-
changer in which the pyrolysis ases react with the reactant. The solid residue
2o containing carbon and the heat exchanger medium are fed to a firing. The
firing
waste gases are fed through t a indirect heat exchanger such that their heat
content is used for the reactio of the pyrolysis gases With the reactant. The
ashes of the solid residue cont fining carbon and the heat carrier medium
taken
from the firing are fed into the ~yrolysis reactor at the entry end for the
organic
substance.
The invention involves t~e basic concept that gasifying methods should
be divided into three method I~teps that can be carried out easily. In a first
method st~p, pyrolysis of the s bstances used takes place rapidly. in the proc-
ess, the goat is to have as littl as possible of the condensable substances in
the pyrolysis gases. The rapid yrolysis is ensured by performing th~ pyrolysis
of the substances used at a to perature of 550-850°C.
CA 02314094 2000-06-09
3
In a second method step, the pyralysis gases are heated and reacted
with steam to adjust the pro uct gas quality. The reaction of the pyrolysis
gases is carried out with steam at a temperature of 900-1000°C.
In a third method step, the solid pyrolysis residue containing carbon is
fired. The heat generated in th process is used for the pyrolysis and the reac-
tion of the pyrolysis gases. Fu hermore, the heat carrier medium is heated up
in the firing and then is conv yed back into the pyrolysis reactor. The, heat
transfer for the reaction of the yrolysis gases with steam taken place in a
heat
exchanger that is heated by th waste gases from the .firing.
The advantage of this ~ivision of the three m~thod steps is that each
method step and the combine ion of the method steps can be arranged ac-
cording to the s~t standard of g s product quality.
The set standard for the as product quality is first of all, a higher caloric
value. Furthermore, the steam ontent is increased by the second method step
so that the gas product is very well suited for use as a synthesis gas, and en-
ergy use in connection with fue cells can also be considered. Naturally, use
to
obtain energy via a gas motor ~r gas turbine is also possible.
The reactant is steam. It is possible to avoid the addition of steam when
sufficient water vapor is conta~ned in the feedstock used, for example, when
the material used is not dried of only to a limited extent. Furthermore, it is
pos~
sible that the pyrolysis gases t at form contain sufficient water vapor when
suf-
ficient steam develops in the p rolysis of the substances used. It is also
possi-
ble to provide the addition of st am in the pyrolysis step.
with the method accor~ing to the invention, basically all organic sub-
stances and substance mixtur~s can be gasified. However, it is preferable to
gasify biomasses.
The substances used m ~ st be pretreated before they are fed to the py-
rolysis. The pretreatment is ge orally limited to drying and if necessary, to
pul-
verization. In the process, no g eat restrictions are set for the lumpiness of
the
substances used because the Ipyrolysis is carried out in a fluidized bed with
a
heat carrier medium.
CA 02314094 2000-06-09
4
To improve the cracking of the noncondensable substances in the py-
rolysis gas, a catalyst can be rovided in the reaction of the pyrolysis gases
with steam. Preferr~d catalysts rB dolomite, calcite, nickel, nick~I oxid~,
nickel
aluminate, or nickel spinal,
When dolomite is used, t is advantag~ous to calcinate the, dolomite at
the reaction temperature of 90 -1000°C, and the resulting
calcium/magnesium
oxide has particularly high catal~tic activity.
The reaction temperatur of 9DD-'1000°C is advantageous for the
reac-
tion of the pyrolysis gas with team, because in this temperature range, the
sulfur sensitivity of the named~catalysts is very much reduced. There is the
possibility of regenerating the c talysts from time to time in. situ by the
addition
of a small amount of air at temc~~ratures abov~ 1000°C.
The catalysts can also b~e used as a heat carrier medium. This manner
of proceeding has the advantage that the catalysts are periodically regener-
1 b ated in the heat carrier cycle.
To prevent the catalyst from being deactivated by dust, if is recom-
mended that the hot pyrolysis g ses be dedusted before addition of the steam.
In cases in which, beca se of the substances used, there is only mini-
mal development of pyrolysis c ke and thus the heat developing in the firing
is
not sufficient for pyrolysis and reaction with steam, a portion of the
pyrolysis
gas can be fired to generate he~t.
The firing of a portion o the pyrolysis gas to generate heat is also re-
quired when the pyrolysis coke is used as a material, for example, for the pro-
duction ofi activated charcoal or grilling charcoal or charcoal briquettes. So
that
the pyralysis cok~ can be traps erred out wall, the grain size of the heat
carrier
medium must be small enough that the heat carrier medium can be separated
from the pyrolysis coke without zany problem.
For th~ device according to the invention, simple and cost-effective
components can be used that dare known as such and easily available. With
these components, the device according to the invention can easily be con-
structed.
CA 02314094 2000-06-09
The pyrolysis takes place in a moving bed reactor using a heat carrier
medium. A shaft kiln is primarily used for this into which the mixture
consisting
of the material to be gasified and tha heat carrier m~dium is loaded from
above. The mixture travets thro~gh the shaft kiln. Rapid pyrolysis occurs due
to
5 the intimate contact of the matehial used with the heat carrier medium.
So that even with heter geneous materials, transport through the shaft
kiln is ensured; built-in structu es or spiral conveyors can be provided
inside
the shaft kiln. The built-in stru ures also have the advantage that the
pyrolysis
gases developing can better a cape upwards through the moving bed. Never-
theless, the equipment expens is increased in this way.
Basically, the' pyrolysis an also be carried out in a rotary drum or a
double-deck oven, although he a as well, the equipment cost would be greater.
The mixture consisting ~f the heat carrier medium and the pyrolysis
residue can be transferred into he firing via commercially available
aggregat~s
such as conveyor worms, s vel grates, rotating grates or cellular wheel
sluices. In combination with a rate firing, however, the use of feeding rams
is
pref~rred. When an underfeeds stoker is used, the use of conveyor worms is
preferred. The firing waste gales are fed through an indirect heat exchang~r
that simultaneously serves as hemical reactor in which the pyrolysis gases
react with st~am. Such heat ~a hangers are known, for example, in refineries
as steam reformers or
Also for the convayance~ of th6 heat carrier medium from the firing into
the shaft kiln, conventional conveyance devices can be used, such as vibrating
conveyors, bucket conveyors, r chain conveyors. The demands on convey-
ance technique also corraspon to the requirements that appear in the steel
industry or in the field of cvkin , so that excessive expense is not required
for
layout of the aggregates.
The heat. carrier medium must have sufficient mechanical, chemical, and
thermal stability in the ,temperature range of 500-1000°C. Fire-
resistant sub-
stances such as sand, silicon,~rit, aluminum silicates, corundum, graywacke,
quartzite, or cvrdierite are us~~1 Th~ use of molded bodies of metallic or non-
CA 02314094 2000-06-09
6
metallic materials or combinati ns of them, such as steel or ceramic balls is
also possible..
With respect to the parti le size, the heat carrier medium must be fine
enough to b~ able to make int mete contact with the material used so that a
good transfer of heat can tak~ place. On' the other hand, the particles of the
heat carrier medium must be bi enough that there is sufficient empty volume
through which the pyrolysis gas s can flow.
These requirements are eat fulfilled when the heat carrier medium has
a grain size of 1-~40 mm. This rain size also has the adwantag~ that th~ h~at
carrier medium can be separat d well from the ash of the pyrolysis residue af
ter the firing.
As mentioned above, a talyst can be provided for the reaction of the
pyrolysis gases with steam. For this purpose, a catalyst bed can be mounted in
the heat exchan~er. Depending on whether the pyrolysis gases are fed through
the pipes of the heat exchange or outside the pipes through heat exchanger,
the catalyst bed is mounted i side or outside of the pipes of the heat ex-
changer. It is also possible to se a catalytically active material for the
heat
exchanger pipes such as corun um with nickel or nickel oxide. It is also possi-
ble to provide a solid bed re ctor with a catalyst bed behind the heat ex-
changer.
If the reaction of the pyr lysis gases with steam is to be supported by a
catalyst, it is recommended tha the hot pyrolysis gases be dedusted with a fil-
ter before contact with the catal st.
The method steps name above as well as those claimed and described
in the embodiment example, w ich are to be used according to the invention,
as well as structural componen s are not subject to any special excsptional re
strictions with respect to their ethod restrictions, their size, shape,
material
selection, and technical conception, so that the selection criteria known in
tt,e
particular area of application in ' ach case can be used without any
limitations.
Further details, features,~and advantages of th~ obj~ct of the invention
result from the following descrl~tion of the related illustration in which, as
an
CA 02314094 2000-06-09
7
example, a preferred ~mbodim nt of th~ gasifying of organic materials is repre-
sented. Shown in the illustratio are:
Figure 1, a diagram of th method according to the invention,
Figure 2, the mass an energy ba4ance of the pyrolysis and reaction
steps,
Figure 3, the mass and nergy
balance
of
the
firing,
and
Figure 4, a, schematic presentation
of
a
device
for
carrying
out
the
method according to the on.
invent
It is evident from Figure 1
that
the
material
tv
be
gasified
1
is
fed
into
pretreatment step 2. Dependig
on
the
material,
this
can
be
a
drying
and/or
pulverization device in a
which t material
is
prepared
for
the
subsequent
py~
rolysis. The pretreated 1
material is
brought
into
pyrolysis
step
3.
The.pyrolysis
step 3 leaves a pyrolysis nd
gas 5 a
pyro(ysis
coke
Sa.
The pyrolysis coke 5a i fired
in
firing
6.
The
heat
from
firing
6
is
di-
rected via a heat couplingyrolysis
7 to step
3
and
via
a
heat
coupling
7a
to
a
reaction zone 4 for pyrolysiss.
The
waste
gases
18
of
firing
6
are
cooled
and diverted in a flue ning
gas cle and
cooling
step
17.
The
waste
heat
ob-
tained writh the flue gas and
cleanin cooling
step
17
can
be
used,
for
example,
for the drying in pretreatmentp
s 2.
Depending on the meth d
conditions,
more
heat
may
develop
in
firing
6
than is needed for heat g
coupli 7
and
7a.
Steam
can
be
generated
with
this
heat. For this, feed waterb~
9 can fed
via
water
treatment
10
and
pump
11
into
heat exchanger 12 which nted
is mo in
firing
6.
The
steam
16
generated
is
fed
into reaction zone 4. The re
press of
the
unneeded
portion
can
be
released
via
turbine 7 3 and further waste
utilized a steam
16a.
The pyrolysis gas 5 is into
fs reaction
zon~
4
with
steam
16.
In
this
re-
action zone, the pyrolysisnd
gas the
crack
products
of
the
condensable
sub-
stances are reacted with to
steam the
desired
gas
product
15.
The
gas
product
15 is then purified in g
a dedusti 8
and
fine
dedustin~
and
quenching
14.
It
is
also possible to feed a 9
portion~ of
the
gas
product
15
into
pyrolysis
3.
The addition of air an~ I_or
oxygen
can
be
provided
in
the
individual
CA 02314094 2000-06-09
8
method steps to influenc~ the method steps of pyrolysis, firing, arsd reaction
with steam.
Figure 2 shows the ma s and energy balance of a pyrolysis st~p 101
and a reaction step 102 in the ~xample of gasifying wood. Wood 104 and heat
carrier medium.104a ar~ introd cad into pyrolysis ~ste~p 101. Furthermor~,
h~at
flow 111 a, that results from the size and consistency of the material filows
con-
sisting of wood 104 and heat c rrier medium 104a, as well as the targeted py-
rolysls temperature, is added. Pyrolysis step 101 leaves a mixture 105 con-
sisting of wood charcoal and h at carrier msdium, and pyrolysis gas 106.
Pyrolysis gas 106 ante reaction step 102. Furthermore, a heat loss
108 occurs. Additionally, the reaction heat of the wood charcoal formation 109
and steam 112 are fed into re ction step 102. In addition, another heat loss
110 occurs. Resulting from the~heat and material streams fed in and diverted
out, is still the heat quantity 11 to be fed in.
In Figure 3, the mass a d energy .balance of the wood charcoal Erring
103 is represented. The mate ial streams, mixture 105, (consisting of wood
charcoal and heat carrier medi m 104a}, water 117, and air 113 enter into the
firing, and also the material streams, waste gas 116, steam 112, and mixture
118 (consisting of heat carrier fnedium 104a and ash), exit. Heat streams that
appear are heat stream 111 t at is fed into reaction step 102, h~at str~am
111 a that is fed into pyrolysis step 101, heat excess 114, and heat loss 115.
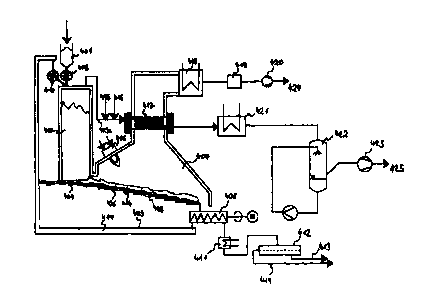
Figure 4 shows a devic for carrying out the method according to the
invention. A material 401 is me~ered via sluice 402 into shaft kiln 403.
Simulta-
neously, heat carrier medium 4~4 is fed into shaft kiln 403 by conveyor 409
via
sluice 410. Mat~rial 401 and the heat carrier medium 414 travel downwards in
shaft kiln 403 and mix, whereb by means of the heat contained in heat carrier
medium 414, material 401 is py olyzed at approximately 600°C.
At the lower end of shaf kiln 403, the mixture consisting of heat carrier
medium 474 and pyrolysis cok 426 forming from material 401 through pyroly..
sis is fed onto grate 405 of br ck-lined firing 407 through feeding 404.
Firing
407 has starting booster 406. n grate 405, pyrvlysis coke 426 burns, giving
CA 02314094 2000-06-09
9
off heat. In this way, heat carrier medium 414 is heated to approximately
1000°C. Heat carrier medium X14 consists of a coarse grained material
such
as sand, grav~I, yr split. During the firing, heat carrier m~dium 414 and
pyroly-
sis coke 426 travel as far as rm 408 at the end of grate 405, by which the
ash of pyrolysis coke 426 and heat carrier medium 414 are discharged. The
majority of this mixture consisting of heat carrier medium 414 and ash is re-
turned to shaft kiln 403 via con eyance 409 and sluice 410, where heat carrier
medium 414 dischar~es the heat absorbed in firing 407 to material 401.
A small portion of the fixture consisting of ash of pyrolysis coke 426
and heat carrier medium 414~s discharged via cooling 411 and sieve 412.
Through sieve 412, the ,ash of yrolysis coke 426 is separated as fine material
413 from the coarser heat carri~r medium 414 and heat carrier medium 414 is
returned to the process. This separation is superfluous when the material to
be
gasified does net contain any a h-forming constituents. ,
The pyrolysis gas formin during the pyrolysis in shaft kiln 403 is with-
drawn from the upper area of s aft kiln 403 via line 403a ahd fed into heat ex-
changer 417. Aside from wate , carbon monoxide, carbon dioxide, hydrogen,
and methane, the pyroiysis gas also contains higher hydrocarbons and tars as
w~II as other organic, especiall~r aromatic compounds as condensable compo-
nents. Heat exchanger 417 is heated to a temperature of approximately
950°C
by the waste gases of firing 40 . At this temperature, th~ pyrolysis gas and
the
condensable substances react with steam that is contained in the pyrolysis
gas. In addition, steam 416 is ed into line 403a for the reactions in heat ex-
changer 417. To further increase the temperature ih heat exchanger 417, air
415 can also be added for a p ~ rtial firing of the pyrolysis gas. To improve
the
cracking of the accompanying ~ars, a catalyst can be provided in the heat ex-
changer.
It is also possible to add the catalyst in the flow stream to the pyrolysis
gas stream and to separate it main after heat exchanger 417 and reuse it.
Heat exchanger 417 leaves a gas product whose portions of carbon
monoxide and hydrogen have been maximized. This gas is fed to heat ex-
CA 02314094 2000-06-09
changer 421 for utilization of w~ste heat and into washer 422 for gas purifica
tion.
Gas product 425 is withd wn via induced draught ventilator 423.
The waste heat from he t exchanger 421 can be used to heat the py-
5 rolysis gas to reaction temperat re for the reaction with steam.
After it has flowed throw h heat exchanger 417, the waste gas of firing
407 is fed through heat exchan er 418 for waste heat utilization. After gas pu-
rification 419, waste gas 424 s discharg~d to the surroundings via induced
draught ventilator 420.
1 O Both firing 407 and also eat exchanger 417 are operated at a pressure
that only slightly deviates from atmospheric pressure and generally is some-
what less than the latter. Indu ed draught ventilator 423 for gas product 425
and 420 for waste gas 424 are regulated and coordinated with one another so
that the pyrolysis gas is fed t rough heat exchanger 417 and is not sucked
through the shaft oven feed int firing 407.
Embodiment example
1000 kg/h wood are ga ified in the device according to Figure 4. The
wood contains 3% ash (free fr m water) and otherwise consists essentially of
50% carbon, 6°~ hydrogen, 42° oxygen, and 1.9% nitrogen,
calculated without
water or ash. The upper caloric value is 17.9 MJlkg in .the anhydrous state.
The
thermal gasification gfficicncy is 4.97 MW. Ths pyrolysis is carried out at
fi00°C and the reaction with st am at 950°C. The workin~
pressure is atmos-
pheric pressure.
Gravel with a grain size om 3 mm to 15 mm is used as heat carrier me-
dium. The gravel is heated fr m 600°C to 950°C. Because of the
required
thermal performance of 380 k , the cycling quantity of the heat carrier medium
is five times that of the wood i put, that is, 5000 kg per hour. The shaft
kiln is
4.5 m high and has a diameter of 1,5 m, corresponding to a fluidized bed vol-
ume of 7.5 m3. The residence ti a in the shaft kiln is two hours.
fn the pyrolysis, the woo is reacted so that 20 wt°i6 of the ~ivood
remains
CA 02314094 2000-06-09
11
as wood charcoal. In the following table, the quantities and compositions of
the
wood and pyrolysis coke (wood charcoal) are listed:
' Material flow Wood Wood charcoal
m [kglh] 1000 200
Hu [MJlkg] dry 17,9 33,5
C [wt%] daf 52,1 82,2
H [wtr6] daf T 4,8 2,6
O [wt~] daf 42,4 5,2
Ash cwt~J d(y __ _ 3 4 I 17,0
The following gas produ t is obtained:
Caloric valueJINm J 10,5
[
H: [upl.-% 51,1
dry]
CO [Vol.-r6 _ ___ _____ _39 ?
dry] __-
CH4 [Vol.-% 0,01
d
COz [Vol.-% 9,2
d
HzO [Vol.-%] 14,8
~~
Chemical. Ipy flow [MW] 3,9
enth
Quantity [Nm 1.338
Ih
The enthalpy flow of the wood charcoal in the firing is 1.86 MW. This is
sufficient to generate a steam flow of 0.45 MW (360 kglh at 950°C and
atrnos-
pheric pressure) as well as tv c ver the heat requirement of the reaction of
the
pyrolysis gas with steam at the level of 0.84 MW. The firing efficiency is
85%.
After accounting fvr tha heat ( ss and Icss through the waste gas flow, only
CA 02314094 2000-06-09
12
0.26 MW remain. With this, 32~ kg/h.supsrheated steam ~rvere generated that
were relaxed via a turbine and sed as heatin~ steam. The cold gas efificiency
IS 7g%.
CA 02314094 2000-06-09
13
List of reference numbers:
1 Mat~rial us~d
2 Pretreatment step
3 Pyrolysis
4 Reaction zone
5 Pyrolysis gas
5a Pyrolysis coke
6 Firing
7 Heat coupling
7a Heat coupling
8 Deducting
9 Feed water
10 Water treatment
11 Pump
12 Heat exchanger
13 Turbine
14 Fine dedusting/quenchin
15 Gas product
16 Steam
16a Waste steam
17 Heat exchang~r/flueleaning
gas
18 Waste gas
19 Gas product
20 Air
101 Pyrolysis step
102 Reaction step
103 Firing
104 Wood
3D 104a Heat carrier msdium
105 Mixture
CA 02314094 2000-06-09
1a
'106 Pyrolysis gas
107 Gas product
108 Lost heat
109 Formation heat
110 Lost heat
111 Heat feed reaction
step
111 a Heat feed pyrolysis
step
112 Superheated steam
113 Air
1 D 114 Excess heat
115 Heat loss
116 Waste gas
117 Water
118 Mixtur~
401 Material
used
402 Sluice
403 Shaft kiln
403a Line
404 Feeding
405 Grate
406 Booster
407 Firing
408 Worm
409 Conveyor
410 Sluice
411 Cooling
412 Sieve
413 Fine material
414 Heat carrier medium
415 Air
416 Steam
CA 02314094 2000-06-09
417 Heat exchanger
41 B Heat exchanger
419 Gas purification
420 Induced draught
ventil
5 421 Heat exchanger
422 Washer
423 Induced draught
ventil
424 Waste gas
425 Gas product
1 O 426 Pyralysis cake