Note: Descriptions are shown in the official language in which they were submitted.
CA 02314369 2000-06-12
WO 99/30718 PCT/US98I26609
PROSTAGLANDIN E~IFZQ COMBINATION FOR TREATING IMPOTENCE
AND ENHANCING SEXUAL AROUSAL
Area of the Art
This application is a continuation-in-part of, and claims priority based
on U.S. Serial Number 09/038,378 filed March 11, 1998, U.S. Serial Number
09/005,087 filed January 9, 1998, and U.S. Serial Number 08/992,946 filed
December 18, 1997, all of which are continuations-in-part of Serial Number
08/090,483 filed July 12, 1993, now U.S. Patent 5,708,031 issued January
13, 1998 which is a continuation of Serial Number 07/860,107 filed March 30,
1992, which is a continuation of Serial Number 07/725,350 filed July 3, 1991.
The present invention relates to the treatment of impotence in males,
enhancement of sexual arousal in females and devices for delivering
pharmaceutical compositions to treat impotence in males and sexually
stimulate females. In particular, the invention relates to the use of
prostaglandin PGE2 and/or PGF2a in the treatment of sexual disfunction.
In excess of about 10 million men in the United States alone exhibit
sufficient erectile dysfunction that they can be characterized as effectively
impotent. A significant number of men additionally suffer from an inability to
develop an erection which may not meet the clinical definition of impotence
but may not be satisfactory to their desires or those of their partner to
provide
mutually satisfactory sexual intercourse. impotence in the human male can
arise from a variety of psychological and physiological etiologies. For
example, long term diabetes, damage to the spinal cord, multiple sclerosis, or
nerve damage resulting for example from lower abdomen or prostate surgery,
and advancing age can result in impotence. Additionally, there are
psychological causes for impotence. For differing reasons, each of the
foregoing result in an inability to pressurize the corpora cavemosa, which can
result in tum from either an insufficient arterial inflow on the supply side,
or an
insufficient increase in the venous output resistance to blood flow.
A wide variety of mechanical means have been provided, in an effort to
overcome erectile dysfunction. For example, United States Patent No.
CA 02314369 2000-06-12
WO 99/30718 PCTNS98/26609
-2-
4,596,242 to Fischell discloses a surgically implantable hydraulic system,
having a fluid reservoir and pressure generator, a patient manipulable valve,
a pressure reservoir and a distensible member responsive to actuation of the
valve. A variety of other prior art mechanical implants and other devices for
this purpose are described in the Background of the Invention section of the
United States Patent No. 4,596,242.
In addition to the mechanical efforts to overcome erectile dysfunction,
pharmaceutical approaches have been tried as well. For example,
prostaglandin E, has been observed to produce erection in some cases, by
direct percutaneous injection into the penis or as a meltable pellet placed in
the urethra (U.S. Patent Nos. 5,773,020; 5,474,535; 5,242,391). PGE,, in a
drug composition known as "trimix", has also been injected directly into the
corpus cavernosa to treat impotence. One of the additional ingredients in
trimix to phentolamine mesylate.
Most recently Pfizer has made sildenafil citrate available in oral
dosages as a treatment for impotence under the trade name, Viagra.
However, Viagra is counter indicated for use by men with cardiac disease,
death in cardiac patients during or following intercourse has been indicated
as
a possible side effect, and interaction with nitroglycerine may cause
immediate death. In addition, a new warning has been added to labeling for
Viagra warning of a possible occurrence of priapism. Also since sildenafil
citrate is administered orally, the effect of the drug is systemic and not
restricted, as in direct delivery to the penis, to a localized response.
Further, both PGE, and Viagra have been shown to be effective in only
certain causes of impotence. In particular, neither drug appears to be
particularly effective to treat impotence resulting from surgical procedures
on
the prostate.
Still further, the needs for localized drugs to treat female sexual
deficiencies have not been adequately addressed by presently available
products. It is estimated that there are ten million women in the United
States
who suffer from female sexual arousal dysfunction.
CA 02314369 2000-06-12
WO 99/30718 PCT/US98/26609
-3-
Therefore, there remains a need for an improved treatment of erectile
dysfunction in the male and arousal dysfunction in the female. Surgical
implantation andlor repeated injections range from disfavored to medically
disadvantageous, and do not, as a whole, provide a satisfactory solution to
the problem. From a patient usability standpoint, erectile dysfunction would
most advantageously be treated on a self administration basis, without the
need of surgical intervention or repeated injections of a pharmaceutical
agent.
This problem has been addressed by the use of PGE2 as claimed in my U.S.
Patent 5,708,031. However, some patients on using urethral placement of
PGE2 materials have experienced urethral burning and cavernous sinus
aching within the genital area. This unpleasant sensation is also
experienced on use of PGE,. To counteract this side effect an anesthetic
may be added to the prostaglandin formulation. However, this in turn requires
using an increased quantity of the prostaglandin.
Further, the prior gel formulation and the PGE, pellet requires the
delivery of a greater volume of treatment composition than may be desired.
Still further, it would be a significant improvement if a simple and safe
composition is available for application to the female external genitalia
which
would increase sensitivity, enhance lubrication and result in local
vasodilation
and engorgement of external and meatal tissue all of which would enhance
the female sexual arousal and experience.
Therefore, there is a need for a PGE2 formulation which avoids these
negative sensations but does not intertere with the effectiveness of the PGE2
for treatment of impotence.
Summary of the Invention
In accordance with one aspect of the present invention, there is
provided method of treating erectile dysfunction in a male patient, comprising
the step of administering to the patient a unit dose of a formulation
comprising
an erectile dysfunction treating amount of prostaglandin E2 compound, or
pharmaceutically acceptable salts or derivatives thereof. The prostaglandin
CA 02314369 2000-06-12
WO 99/30718 PGT/US98/26609
-4-
E2 compound is preferably formulated together with a pharmaceutically
acceptable delivery medium, which may comprise local anesthetic agents
andlor a lubricant. Preferably, the anesthetic agent comprises lidocaine. The
dose of PGE2 may be in the form of a urethra sized suppository meltable at
body temperature, coated on a removable wand, carried on and/or in a
porous, non-absorbable wand sized for easy placement into and withdrawal
from the urethra, or in physiologically acceptable carrier (saline or water)
placed in the urethra via a small diameter tube. The prostaglandin E2
compound can also be formulated with a small amount of prostaglandin F2«
and/or phentolamine mesylate together with a pharmaceutically acceptable
delivery medium and/or a lubricant.
A unit dose of the formulation in accordance with the present invention
will typically be less than about 5 cc in volume, preferably less than about 3
cc and most preferably below about 1 cc. The amount of active ingredient in
a unit dose will typically be within the range of from about 0.1 mg to about
4.0
mg. More preferably, the amount of prostaglandin E2 in a unit dose will be
within the range of from amount 0.6 mg to about 3.6 mg. This is more than
the amount of PGE, required for a beneficial result. However, tests show that
the results obtained from use of PGE, are much less satisfactory than when
PGE2 is used. Further, if anesthetic, such as lidocaine is added to the
formulation to minimize or eliminate burning, 1.2 mg to about 3.6 mg of PGE2
may be required. It has now been found that adding prostaglandin FZ« in
amounts of about 0.1 micrograms (0.1 ,ug) to about 2.0 ,ug to either
prostaglandin E2 or E, formulations prevents the burning and aching without
noticeably interfering with effectiveness of the prostaglandin. A preferred
amount is 0.25 ~cg of PGF2« per 1 mg of PGE2. It has also been found that
adding phentolamine mesylate to this composition further increases the
effectiveness of the composition (increased rigidity and increased but
controlled longevity).
The administration step of the method in accordance with the present
invention comprises the transurethral administration of the unit dose of
CA 02314369 2000-06-12
WO 99/30718 pCT/US98/26609
-5-
formulation. In an embodiment where the formulation comprises a cream, gel
form or saline solution, the formulation is preferably transurethrally
instilled or
inserted such as by extrusion through syringe or unit dose administration
packet comprising an elongate tubular administration tip.
An alternative delivery procedure comprises the transurethral
placement of a unit dose of the formulation using a coated wand or a
suppository containing a quantity of the formulation suitable for delivery to
the
patient within a preset period of time.
In an embodiment of the present invention, wherein the administrable
form of the formulation comprises a relatively rigid suppository, the
suppository can be manually inserted into the distal opening of the urethra.
A further embodiment provides for urethral insertion of a removable
wand, which may be porous, which carries the unit dose in a form which
transfers over a controlled period of time through the urethral mucosa.
These and further objects and advantages of the present invention will
become apparent from the Drawings and Description which follows,
considered together with the appended Claims.
These and other features, aspects and advantages of the present
invention will become better understood with reference to the following
description, appended claims, and accompanying drawings, where:
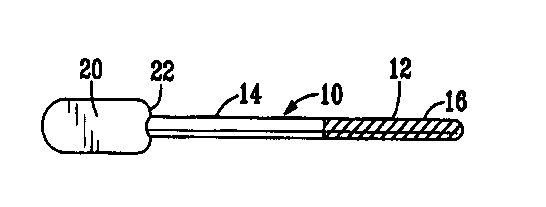
Figure 1 is a perspective side view of a first version of the insertable
drug delivery device.
Figure 2 is a cross sectional view taken along line 2-2 of Figure 1.
Figure 3 is a side view of a second version of the insertable drug
delivery device.
Figure 4 is an end view of the second version of the insertable drug
delivery device.
Figure 5 is a side view of a third version of the insertable drug delivery
device.
CA 02314369 2000-06-12
WO 99/30718 PCT/US98/26609
-6-
Figure 6 is an left end view of the third version of the insertable drug
delivery device.
Figure 7 is an left end view second variation of the third version of the
insertable drug delivery device.
Figure 8 is a cut away side view of the third version of the insertable
drug delivery device of Figure 6 inserted in a carrier.
Figure 9 is a cut away side view of a variation of each of the above
versions inserted in a penis.
Figure 10 is a partial cutaway view of a device for delivery of a liquid
dose of impotence treating drug.
detailed Description of Preferred Embodiments
The prostaglandins are a series of cyclic derivatives of certain
unsaturated fatty acids. They are found in a variety of tissues, including the
prostate gland, the seminal vesicles, the lungs and the brain. These naturally
occurring prostaglandin are derived by cyclization of 20-carbon unsaturated
fatty acids such as arachidonic acid. ~g Lehninger, Albert L., Biochemistry,
2d ed. (1975), p. 300 (hereinafter "Lehninger').
Carbon atoms of the fatty acid backbone are cyclized to form a
characteristic 5-membered ring. The prostaglandin are divided into a number
of groups, including those designated A-F, based on the configuration of the
ring structure. Prostaglandin also differ in stereochemistry and in the number
of side chain double bonds which are conventionally indicated by a subscript
number. Thus, for example, prostaglandin E2 ("PGEz') has the ring
configuration characteristic of the E group and contains two side chain double
bonds. The chemical name for PGEZ is (5Z, 11a, 13E, 15S)-11, 15-
Dihydroxy-9-oxo-prosta-5, 13-lien-1-oic acid and the structural formula of one
form is represented in Formula I, below. The molecular formula is C2oH32~5~
CA 02314369 2000-06-12
WO 99/30718 PCT/US98/26609
-7-
T
The biosynthesis of prostaglandin has been well characterized. Sgt,
g,gs,, Lehninger at p. 687. In a typical biosynthetic pathway, exemplified by
production of PGE2, the essential fatty acid linoleic acid is converted into
the
20-carbon arachidonic acid, which is then acted upon by prostaglandin
synthase, doxygenase enzyme. Oxygen atoms are added at carbon atoms 9
and 15, and the product is cyclized by formation of a bond between carbon
atoms 8 and 12. In the presence of reduced glutathione, this synthesized
product undergoes conversion into prostaglandin PGE2. Other types of
naturally occurring prostaglandins are derived from different polyunsaturated
fatty acids.
In about the 1960's, prostaglandin were isolated from a particular
species of Caribbean coral, which made them more widely available for
research. Catanzarite, Valerian A. and Gary Aisenbrey, Contern ,~or,~p,r
QB/GYN (October 1987), p. 22 (hereinafter "Catanzarite"). A large number of
natural and synthetic analogues of he prostaglandin are now known.
Lehninger at 687.
The various different prostaglandins are known to produce often
unpredictable effects over a very wide range of biological activities of a
hormonal or regulatory nature. Prostaglandin have been reported to both
lower and raise blood pressure, to inhibit gastric secretion, dilate bronchi,
inhibit lipolysis, antagonize vasopressin-induced anti-diarrhesis, constrict
the
pupil, increase and decrease the intraocular pressure and produce
contraction of the uterus. ~, ~,,c ,, Ganong, William F., Review of Medical
Physio~loav~, 7th ed. (1975), p. 226 (hereinafter "Ganong"). The naturally
occurring prostaglandin all appear to be capable of affecting the control of
dH '
OH
CA 02314369 2000-06-12
WO 99/30718 PCTNS98/Z6b09
-$-
vascular and other smooth muscle contractions. In the central nervous
system, prostaglandin are known to modify responses to certain synaptic
transmitters. They have been reported to mimic the actions of some
hormones and to inhibit the actions of certain others. ~g Ganong at 226.
Two of the most extensively studied of the prostagtandin are PGE2,
and PGF2a. Both of these molecules are synthesized within the pregnant and
non-pregnant uterus. lNhile PGE2 and PGFZa are similar in mediating some
effects, they are different with respect to certain others. Both cause uterine
contractions, but they predominate at different sites within the uterus --
PGEZ
in the lower uterine segment, PGF2a is more important in generating uterine
contractions. PGE2 elevates body temperature, whereas PGF2q has no
apparent effect on body temperature. PGE2 is a vasodilator and
bronchodilator, while PGF2a is a bronchoconstrictor and vasoconstrictor. (egg
Catanzarite at 21-22.)
Prostaglandin have been used in gynecology for pregnancy
termination. Preparing the cervix with prostaglandin suppository has been
found to reduce the incidence of cervical laceration and significant bleeding
(Catanzarite at 22). Synthetic analogues of prostaglandin PGE2, such as 16-
16-dimethyl PGE2 and 9-methylene PGE2, have proven useful for the
induction of first trimester abortions. Such procedures typically use vaginal
suppositories containing 20 milligrams PGE2 or 3 milligrams 15-methyl PGF2a,
or by repeated intramyometrial injections of 15-methyl PGF2a, or by infusing a
PGFZa -urea mixture (20 milligrams of PGF2q and 40 milligrams of urea in 100
MI of 5% dextrose in water) into the amniotic sac.
In obstetrics, prostaglandin have been used for cervical ripening, labor
induction and control of post-partum hemorrhage (Catanzarite at 29). For
cervical ripening, PGE2 had been given intravenously, orally and vaginally,
but
the preferred route is intracervically. A PGE2 gel is now commercially
available in Scandinavia, and another PGE2, gel is being investigated in the
United States. The PGE2 gel can also be used for labor induction (3-5 mg of
PGE2, prepared by blending a 20 mg suppository with 60 mL of lubricating
CA 02314369 2000-06-12
WO 99/30718 PCT/I3S98/26609
-9-
jelly and using 9-15 mL of the mixture, is placed in the vagina) (Catanzarite
at
32). Prostaglandin have also been utilized to control post-partum
hemorrhage.
Since circulating prostaglandin can be rapidly metabolized in the lungs,
liver and kidneys, a number of synthetically modified prostaglandins have
been developed that are not metabolized as quickly (egg, g,~, Catanzarite at
32).
Prostaglandin PGE2, also known as the "Prostin E2' brand of
"dinoprostone," is available from Upjohn Company in the form of a vaginal
suppository. Indications and usage reported by Upjohn are (i) termination of
pregnancy from the 12th through the 20th gestational week, (ii) evacuation of
the uterine contents in the management of missed abortion or intrauterine
fetal death up to 28 weeks of gestational age, and (iii) in the management of
non-metastic gestational trophoblastic disease (benign hydatidiform mole).
egg The Upjohn Co., Prostin E2 product description 810 994 009, Oct., 1990.
PGE2 is also available from several sources as a purled, freeze dried
or lypholized product which is readily soluble in saline or distilled water.
Contraindications to the use of prostaglandin PGE2 include
hypersensitivity to dynoprostone, acute pelvic inflammatory disease, or
patients with active cardiac pulmonary renal or hepatic disease. Upjohn
notes that although carcinogenic bioassay studies have not been conducted
in animals for PGEZ (because of the limited indication for use and the short
duration of administration), there was no evidence of mutagenicity in either
the Micronucleus Test or in the Ames Assay. Upjohn also indicates that a
number of adverse reactions may be observed with the use of PGE2 for
abortions. These adverse reactions are related to the contractile effect of
PGE2 on smooth muscle and include vomiting, temperature elevations,
diarrhea, nausea, transient diastolic blood pressure decreases, and a number
of other effects. Upjohn's vaginal suppository contains 20 mg of PGE2 in a
mixture of glycerides of fatty acids.
CA 02314369 2000-06-12
WO 99/30718 PCT/US98/26609
-10-
Upjohn markets a (15S)-15-methyl analogue of prostaglandin PGF2a
under the brand name Hemabate~, also known as "carboprost tromethamine
sterile solution." The structural formula of Hemabate~ is represented in
u~,
OH
Upjohn reports that Hemabate~ is indicated for aborting pregnancy
between the 13th and 20th weeks of gestation, in certain conditions related to
second trimester abortions, and in the treatment of post-partum hemorrhage.
;~ The Upjohn Co., product description 814 350 002, Nov., 1989. For
abortion, the prostaglandin solution is injected using a syringe and
administered deep in the muscle. Intramuscular injection is also used for
treating post-partum uterine bleeding.
Upjohn also markets prostaglandin PGE,, as the "Prostin VR Pediatric"
brand of "alprostadil sterile solution," which is used to temporarily maintain
the patency of the ductus arteriosis until corrective surgery can be performed
in neonates having congenital heart defects and who depend upon the patent
ductus for their survival. For the administration of PGE, in neonates, Upjohn
recommends continuous intravenous infusion into a large vein, or
administration through an umbilical artery catheter placed at the ductal
opening. egg The Upjohn Co., product description 811 987 004, in
Physician's Desk Reference, 45th Edition, p.2250 (1991).
Quite surprisingly, the inventor herein has discovered that transurethral
application of PGE2 can in many cases provide an effective, reversible
treatment of erectile dysfunction in human males. Thus, in accordance with
one embodiment of the present invention, PGE2 or a pharmaceutically
acceptable salt, ester or other derivative thereof is formulated together with
a
carrier medium which may comprise any of a variety of additional excipients
CA 02314369 2000-06-12
WO 99/307$ PCT/US98/Z6609
-11-
or adjuvants into a form suitable for transurethral delivery. One approach is
to apply the composition to a wand, which may be either porous or non-
porous, in sufficient quantities so that the desired dosage is released and
absorbed through the mucosa of the urethra when the coated wand is placed
in the urethra. When an erection of the desired tumescence is obtained the
wand is then removed, terminating delivery of the composition, thus
significantly reducing the possibility of overdosing. While the formulation
may
be provided in the form of a meltable suppository, such a delivery means is
unsuitable for removal to terminate delivery of the composition and can result
in overdosing as well as the delivery of excess PGE2 to the vagina of the
partner.
In a second embodiment a suitable amount of a freeze dried PGE2 is
dissolved in physiological saline or sterile water and placed within the
urethra
using a short, small diameter catheter. The PGEZ absorption is rapid with an
erection, ensuing within 3-5 minutes, persisting for 1-2 hours depending on
dosage. However, alternative replacements for the water carrier include
creams or gels which can flow at or above room temperature, for example at
body temperature.
In accordance with another aspect of the present invention, there is
provided an antidote for reversing the effects of the foregoing PGE2
treatment, comprising administration of an antidotal amount of PGF2a, or
pharmaceutically acceptable salts, esters or derivatives thereof. Preferably,
15-methyl PGF2a is utilized for this purpose. This antidote can be delivered
in
the same manner as discussed above.
Preferably, the PGE2 or PGF2a or combination of PGE2 and PGF2awith
or without other additives, as discussed below, will comprise a cream, gel, or
water based solution although a more solid form such as pellets or a rod-
shaped suppository body may also be used. In the following described
devices or delivery techniques, in each case where PGE2 is referred to, it is
contemplated that the PGF2a may be substituted for delivery purposes.
CA 02314369 2000-06-12
WO 99/30718 PCTNS98/26609
-12-
These devices are also applicable to delivery of PGE, compositions or other
impotence treatment drugs.
Administration of the liquid, cream or gel form may be accomplished by
transurethral delivery using a syringe without a needle, or with a short blunt
cannula attached. The liquid, gel or cream forms are preferably provided in
unit dose amounts for self administration by the patient. For this purpose,
compressible unit dose packages are preferably provided with an elongate
tubular delivery spout, sized for transurethral insertion. Following
transurethral installation of any of the liquid, gei or cream forms, the
distal end
of the urethra is preferably occluded, such as by manual pressure for up to
several minutes, to permit sufficient dwell time for absorption.
A typical male urethra in a flaccid penis is from about 2.5 cm to about 8
cm and the typical diameter of the urethra is from about 1 mm to about 3
mm. Accordingly, the wand with PGE2 and/or PGF2a applied has a diameter
approximating the urethra diameter or slightly larger so that the outer
surface
of the wand is in intimate contact with the mucosal lining of the urethra or
swells when inserted. The typical length of the insertable portion of the wand
is from 20% to 90% of the patients' urethra in the flaccid state, preferred
25%-
40% with some or all of the insertable portion coated with PGE2. The wand
also has an external portion or length for grasping between the users fingers
for placement and removal. This portion may be of a larger diameter or a
flange may be positioned between the insertable portion and the exterior
portion to prevent the wand from being inserted too far into the urethra.
For delivery of the PGE2 dissolved in saline or sterile water, solution, a
catheter of about 1.5 - 3 mm in diameter, possibly with a narrowed insertion
tip or narrowed internal diameter to facilitate the delivery of a spray, is
inserted into the urethra 0.5 to 2 cm and a bolus or spray of about 0.1 cc to
1
cc of a solution containing 0.1 to 4.0 mg PGE2 is placed in the urethra.
A first embodiment of the drug delivery device, shown in Figure 1,
comprises a wand 10 which is sized to be placed within the urethra of the
penis. The wand has an insertable portion 12 which, in use, resides in the
CA 02314369 2000-06-12
WO 99/30718 PC'T/US98I26609
-13-
urethra, and a holding portion 14 for grasping by the user during insertion.
The insertable portion 12 has a partial or complete covering of PGE2 in a
suitable carrier 16.
Figure 2 shows the wand 10 in cross section. The wand is shown as
porous, with pores 18, the PGE2, with or without a carrier, 16 being both on
the surface of the wand 10 and in the pores 18. The invention contemplates
a nonporous wand 10 with the PGE2 and carrier coated only on the surface as
well as an alternate porous version with the PGE2 and carrier being on both
the surface as well as in the pores. The porous version allows delivery of
more drug to the patient. However, because some of the drug is within the
pores, the delivery rate for that portion may be slower than for the PGE2 in
the
surface coating.
A second embodiment of the drug delivery device, shown in Figures 3
and 4, in addition to the features shown in Figures 1 and 2, has a handle 20
for grasping by the user during insertion and removal of the device. The
shoulder 22 prevents the wand from being inserted too deeply.
A third embodiment, shown in Figures 5 and 6, instead of the shoulder
22, has a cap 24 to prevent insertion of the wand 10 to a depth greater than
desired. In Figure 5, the cap is shown to have a curved profile similar to the
rounded shape of the head of the penis. However, any shape is usable as
long as the width of the cap is greater than the diameter of the urethra so as
to prevent insertion of more than the desired length. Figure 6 shows the cap
24 as hemispherical in shape so that it can also serve as a cap to close a
carrying container 26, such as shown in Figure 8. In the particular
embodiment shown in Figures 5, 6 and 8, the cap 24 has threads 28 on its
lower surface which can interact with similarly disposed threads 30 on the
outer upper end of the container 26. In this manner the drug containing wand
10 can be inserted in the container 26 and closed and sealed, such as by a
sonic or heat weld, to keep the PGE2 from contamination or dissipation. It is
also contemplated that the threads 28 can be replaced by other sealing
CA 02314369 2000-06-12
WO 99/30718 PCTNS98/26609
-14-
means such as snap rings or a friction fit, each of which may be further
sealed by an externally applied adhesive tape (not shown).
Figure 7 shows a further variation of the embodiment of Figure 5
wherein the cap 24 is replaced by one or more extensions 32 which radiate
outward from the wand 20 at the juncture of the insertable portion 12 and the
holding portion 16. Figure 7 shows four extensions 32 which can be
extended perpendicular to the wand 10 surface to be curved as in Fig. 5.
Figure 9 is an enlarged cut away view showing a further embodiment
of the PGEZ delivery device incorporating features shown in Fig. 7 placed
within the male urethra. The porous wand 10 has the PGE2 material in a
suitable carrier 16 coated on the surface and in the pores of the wand. The
insertion end of the wand 10 has a rounded tip 42 which has a diameter
which approximates the diameter of the urethra. A length of the inserted
portion 12 is coated with the PGE2 and carrier composition 16, the
composition or the PGE2 alone also being carried in the pores 18 of the wand
10. As the PGE2 coating melts or is absorbed, the diameter of the inserted
coated portion decreases exposing the rear edge 44 of the rounded tip 42.
As a result, when the wand 10 is removed from the urethra, the rear edge 44
acts as a wiper to remove excess PGE2 and carrier from the urethra, thus
substantially stopping the delivery of PGE2 to the tissue of the penis. In
this
manner, the chance of overdose or transfer to the sexual partner during
intercourse is greatly reduced or eliminated.
A delivery device 100 for the PGE2 solution shown in Figure 10
consists of a tube 102 with an integral squeeze bulb 104. The dimensions of
the device are chosen so that a unit dose 106 of PGE2 in a suitable solvent is
held within the length of the tube and adjacent portion of the squeeze bulb
104 and the squeeze bulb 104 can contain a volume of air 108 such that
squeezing of the bulb 104 between two fingers will expel the unit dose. When
some or all of the tube is inserted through the external opening of the
urethra,
squeezing the bulb results in droplet or spray delivery of the tube's contents
along at least a portion of the length of the urethra downstream of the
CA 02314369 2000-06-12
WO 99/30718 PCT/US98/26609
-15-
inserted end of the tube 102. 1n one embodiment of the delivery device 100 .
the tube is from about 1.0 to 3.Omm in length, has an outer diameter of about
2.5mm and an inner diameter of about 1 mm. This inner andlor outer
diameter may be reduced at the insertable end of the tube to ease insertion
and to create a spray of delivered product. The tube is able to contain about
0.01 to 0.03cc of liquid and the bulb is capable of delivering at least 0.1 cc
of
air when compressed. However, the tube inner and outer diameter may be
smaller and a portion or all of the unit dose-may be in the bulb. This allows
retention of about 0.01 cc of a liquid in the lumen of the tube. Further, the
delivery of the liquid composition is not limited to the use of the device of
Figure 10. One skilled in the art is knowledgeable in the selection of a broad
range of catheters for placement within the urethra.
Administration of the PGE2 may be accomplished by the transurethral
placement of the wand to the desired depth. The PGE2 composition applied
to the wand depends on whether a porous or nonporous wand is used. In the
case of a porous wand, a liquid solution of the PGE2, a carrier, and possibly
an anesthetic andlor a transport adjuvant such as a meltable, swellable or
soluble compound, is prepared and the wand is dipped in the solution until
sufficient active material is deposited on and in the pores of the wand. This
may be supplemented by a less fluid composition, with or without PGEZ
applied to the surface of the wand, the surface composition preferably
including a lubricant or having lubricating properties.
While transurethral delivery of PGE2 is a highly effective means of
treating impotence, an undesirable side effect in some individuals is a
sensation of urethral burning or pain and cavernous sinus aching in the
genital area. One approach is to add a lubricant andlor a local anesthetic for
desensitization thus masking these side effects. In one embodiment, the
PGE2, lubricant and anesthetic are all formulated into a convenient cream.
This cream may be prepared, for example, by mixing one Upjohn Prostin E~
PGEZ suppository together with 10 cc of a lidocaine jelly such as Xylocaine~
2% jelly (available from Astra Pharmaceutical Products) and 50 cc. of a
CA 02314369 2000-06-12
WO 99/30718
PCT/US98/26609
-16-
surgical lubricant such as K-Y jelly (available from Johnson & Johnson).
Lidocaine HC1, available in a variety of formulations, comprises acetamide, 2-
(diethyfamino)-N-(2,6-dimethylphenyl) -monohydrochloride.
The amount of lubricant and the amount and concentration of
anesthetic can be varied considerably as will be apparent to one of skill in
the
art. For example, lidocaine jelly can be used having anywhere from about 1
to about 10% and preferably about 2% lidocaine. In general, the anesthetic
level can largely be dictated by patient preference, as determined through
routine experimentation. Although the incidence of adverse effects with
Xylocaine ~ 2% jelly is very low, caution should be exercised when applying
large amounts since the frequency of adverse effects is directly proportional
to the total dosage of the local anaesthetic administered. Sgg Astra
Pharmaceuticals, product description 021838811, June 1986; in Physician's
Desk Reference, 45th Edition (1991), at p. 628.
A variety of other anesthetic agents can also be used with the
formulation of the present invention, as wilt be appreciated by one skilled in
the art. For example, novocaine, procaine, tetracaine or benzocaine may be
selected. Patients allergic to para-aminobenzoic acid derivatives such as
procaine, tetracaine and benzocaine have not appeared to show cross
sensitivity to lidocaine. Lidocaine is also contraindicated in patients with a
history of sensitivity to amide type local anesthetics. Xylocaine~ 2% jelly
also
contains methylparaben, propylparaben and hydroxypropylmethylcellulose, as
well as lidocaine; and, therefor, Xylocainet~ is contraindicated for patients
with
known sensitivities to any of these compounds. ~ Astra Pharmaceuticals,
product description 021838811, June 1986; in Physician's Desk Reference,
45th Edition (1991 ), at p. 628.
As a result of adding the anesthetic to the PGE2, it has been
discovered by the inventor that the effect of the PGE2 treatment is generally
less pronounced. Thus, in a lidocaine-containing formulation, the dosage of
PGE2 must be increased over that in a non-lidocaine-containing formulation,
and more preferably, the PGE2 dosage is preferably doubled in a lidocaine-
CA 02314369 2000-06-12
WO 99/30718 pCTlUS98I26609
17-
containing formulation in order to obtain the same effect on impotence. A
further negative of adding an anesthetic is that it masks the pain rather than
elevating the cause of the pain and may in fact reduce the pleasurable effects
of intercourse by reducing tactile sensitivity. Similar dosage increases are
required with PGE, but since PGE, appears to be less soluble in carriers than
PGE2, adequate dosages of PGE, with anesthetic may not be readily
prepared for delivery.
More or less lubricant may be desired depending upon the delivery
dose and concentration of the anesthetic jelly. In general, the total volume
of
the impotence treating unit dose should be no more than 5 cc, and preferably
from about 1 cc to no more than about 2 cc due to the inherent capacity of
the urethra. Doses of excessive volume can result in painful administration,
and also in retrograde migration of the excess formulation into the prostatic
urethra or bladder.
Preferably, the total amount of PGE2 contained in a unit dose will be
within the range of from about 0.2 mg to about 5.0 mg. Due to differing
etiology of erectile dysfunction, and inherent variations across a population
in
terms of responsiveness to pharmaceutical agents, some routine
experimentation may be desired to determine optimum dosages for a given
patient or class of patients.
In general, however, doses within the range of from about 0.2 to about
5.0 mg, and preferably from about 0.6 to about 3.6 mg PGEZ, have generally
proven sufficient in patients in which a response is likely to occur. Although
it
is not possible to predict with precision what types of patient populations
will
likely respond to the treatments disclosed herein, certain classes of patients
are anticipated to be treatable depending upon the etiology of the condition.
For example, patients in whom erectile dysfunction is associated with
vascular abnormalities such as atherosclerosis which prevents adequate
blood inflow are not likely to respond. Patients in whom the dysfunction is a
result of such conditions as diabetes, denervation, or psychological status
are
expected to be more likely to respond. Where impotence is a result, although
CA 02314369 2000-06-12
WO 99/30718 PGT/US98/26609
-18-
undesired, of a surgical procedure, such as a prostatectomy, the efficacy of
the treatment may depend on the vascular or nerve damage caused by the
surgical procedure. However, clinical trials have shown the PGEZ formulation
is an effective treatment in many patients who show no, or an inadequate
response to PGE, or Viagra~.
In the antidotal or priapism treating PGF2a formulation, the PGF2a will
generally be present in an amount within the range of from about 5 to about
50 ~g per 1 cc of formulation, preferably within the range of from about 8 to
20 ~cg/cc and more preferably about 12 ~cg/cc. As with the PGE2 formulation,
optimum dosage for a given patient can be determined through routine
experimentation.
It has now been discovered that the burning, aching or cramping
sensation can be eliminated without the use of an anaesthetic agent and the
commensurate increase in PGEZ to obtain the same effect. As described
above, PGF2a reverses the effect of PGE2 , i.e. terminates an erection. It has
now been found that mixing small amounts of PGF2a with PGE2 eliminates the
burning or aching experienced by some individuals when PGE2 is used atone.
It has further been discovered that, unlike the addition of an anaesthetic to
PGE2, adding PGF2a in small doses does not reduce the beneficial effects of
PGEZ, alleviates the cause of the pain rather than masking it and does not
reduce the desirable sensory effects of intercourse. Further, additional
amounts of PGE2 are generally not required to obtain the same beneficial
effects.
A suitable PGEZ composition to eliminate the undesirable burning and
aching sensation without noticeably reducing the impotence treatment effect
of the PGEZ includes from about 0.1 Ng to about 2.ONg of PGF2a_ Befow
about 0.1 Ng the burning and aching may persist and above about 2Ng and
erection may occur more slowly and may not be adequate for intercourse. A
preferred composition includes about 0.25Ng of PGF2Q for each milligram of
PGE2.
It has been further discovered that addition of phentolamine mesylate
CA 02314369 2000-06-12
WO 99/30718 PCT/US98/26609
-19-
H
N CH2N ~ CH3~ CH3S03H
N
to PGE2 increases the beneficial characteristics of the PGE2 impotence
treatment; namely rigidity and longevity of the erection. However, the
urethral
burning and cavernous sinus aching still persists. The addition of PGF2a to
the combination, as in the case of addition to PGE2, eliminates this
undesirable side effect without reducing the enhanced benefits of the use of
the phentolamine mesylate. A typical composition to produce an erection
effective for intercourse comprises 1.Omg PGE2 ~ 0.25Ngm PGF2a and
0.25mg phentolamine mesylate in about 0.2cc of a suitable carrier, such as .
sterile water. Smaller or larger doses, volumes or variations on the ratios of
components may be used to adjust to particular patient response and disease
etiology.
Any of several different delivery systems may be utilized in accordance
with the method of the present invention. For example, a fluid, cream, gel
system or solid suppository can be used and the carrier can be absorbed
directly, or allowed to be expelled following sufficient dwell time which may
be
controlled by occluding the distal end of the urethra.
Alternatively, more solid delivery vehicles may be used such as an
ovoid or rod-shaped suppository. Suppositories can be formulated from any
of a variety of materials which exhibit sufficient physical integrity to
permit
transurethral insertion and which will then permit delivery of the medication.
Once installed, the structural component of the suppository may break down
under the influence of body heat. An advantage of applying the meltable
composition to the surface of the wand is that the wand can be removed once
the desired erection is achieved, thus terminating drug delivery, a procedure
not afforded by suppository products. Alternatively; the PGE2 composition
CA 02314369 2000-06-12
WO 99/30718 PCT/US98/26609
-20-
placed in the pores of the wand can be protected until delivery by applying a
sealing material on the surface of the wand, the sealing material melting or
dissolving when placed within the urethra. As a further alternative, materials
can be used which will dissolve in an aqueous environment at a pH within the
range of that typical of the urethra. One suitable composition is a mixture of
glycerides of fatty acids such as that used with the Prostin EZ~ product.
Other suitable compositions include various cellulose based materials such
as hydroxypropylmethylcellulose or collagen compositions which can be
prepared as a fluid water-based material, a viscous solution, a gel or a water
swellable dry coating. One skilled in the art can identify numerous
physiologically acceptable water soluble materials or hydrogels which can be
used for the purposes set forth above.
The above noted burning sensation is also experienced when using
PGE,. It has been found that addition of PGFZa has substantially the same
effect when added to PGE, in a percentage to total dosage equivalent to that
used with PGE2.
As a further alternative, a variety of drug delivery vehicles may be used
which neither dissolve nor break down in the environment of the urethra.
Relatively rigid rod-shaped delivery vehicles may be fashioned from materials
having a micro porous structure for the time release of entrapped
pharmaceutical.
A major advantage of transurethral insertion of a wand is that it can be
inserted for a predetermined period of time and then removed following
delivery of an efficacious amount of drug. The removable time release
delivery structure has the added advantage of providing a range of flexibility
in
the total delivered dose. Thus, the patient, by leaving the implant in place
for
relatively shorter or longer periods of time, can optimize the dose within a
preset maximum range. A particular advantage of the cream, gel, or solution
over prior disclosed pellets of drug containing compositions placed in the
urethra is that the compositions disclosed herein can be readily dispersed
along the length of the urethra allowing delivery through a large area of
tissue
CA 02314369 2000-06-12
WO 99/30718 PCT/US98/Z5609
-21 -
surface, thus reducing or eliminating the undesirable effects of delivering
high
dose concentrations to a very localized area, including slower dispersion,
localized discomfort and less pronounced effectiveness.
Particular embodiments of the present invention will be described in
the Examples which follow.
EXAMPLE I- Preparation of Intraurethral PGE2 Cream
A batch of PGE2 cream was prepared by mixing 40 mg of PGE2
suppository {obtained as the "Prostin E2' suppository from the Upjohn
Company) with 10 cc of 2% Xylocaine jelly and 50 cc of K-Y surgical
lubrication jelly (hydroxyethyl-cellulose, obtained from Johnson & Johnson).
Mixing was accomplished by stirring until the mixture appeared homogenous
upon visual inspection. The result was a PGEZ cream having approximately
1.3 mg of PGEZ per 2 cc of cream. A portion of the cream was reserved for
placement directly into the urethra while a second portion was applied to the
surface of a wand. Approximately 2 cc of cream was applied to a 4 cm of
the insertable length of a porous wand to produce a coated product 5 mm in
diameter. This can be readily inserted and removed from the urethra.
FXAMPLF II - Preparation of I~ptraurethral PGE2~g,[
The homogenecity of a bath of PGE2 is ensured by inclusion of a
methylene blue marker. One 20 mg PGE2 suppository ("Prostin E2" from the
Upjohn Company) is sliced into thin slices and allowed to soften at room
temperature for 15 minutes. A small drop of 1 % methylene blue solution
(American quinine, Shirley, New York) is placed onto each slice to serve as a
marker for homogenicity. The softened slices are thereafter geometrically
mixed with the contents of a 56.7 gram tube of K-Y jelly to yield a
homogenous mixture, as evidenced by blue color uniformity. The theoretical
content of the final product is approximately 0.68 milligrams of PGEZ per 2 cc
of gel. A portion of the cream was reserved for placement directly into the
urethra while a second portion was applied to the surface of a wand. The gel
CA 02314369 2000-06-12
WO 99/30718 PCT/US98/Z6609
-22-
was applied to 4 cm of the insertable length of a porous wand to produce an
insertable product having a 3.0 to 5.0 mm diameter for insertion in the male
urethra.
EXAMPLE I11 _ Preparation of Lipid Based Intraurethral PGE2 Cream
A batch of PGE2 cream in cocoa butter is prepared by placing one 20
mg. PGE2 suppository (Prostin E2 by the Upjohn Company) into a porcelain
evaporating dish and is melted in a 37° C water bath. Shredded cocoa
butter
is added to the melted suppository with stirring to bring the total mass to
approximately 20 grams. As the melting continues, the temperature of the
mixture is kept at or below about 33° C. Higher temperatures are to be
avoided, as they have been reported to cause the crystalline form of the
cocoa butter to change, resulting in aberrations in bioavailability.
Transformations in the crystalline form of the cocoa butter are visually
observed as a change from opalescent to transparent. After complete
melting, the mixture is stirred thoroughly and a first portion poured into
suppository molds. The material is thereafter allowed to cool at room
temperature for about 15 minutes, and thereafter is placed in the refrigerator
to facilitate further solidification. The suppositories may thereafter be
removed from the mold, individually packaged and placed in refrigerated
storage under anhydrous conditions.
A second portion of the mixture was applied to 4 cm of the insertable
length of a porous wand which was about 2 mm in diameter using a tubular
mold. The material is thereafter allowed to cool at room temperature for
about 15 minutes, and thereafter is placed in the refrigerator to facilitate
further solidification in to 5 mm diameter coated wands. The coated wands
are thereafter removed from the mold, individually packaged and placed in
refrigerated storage under anhydrous conditions.
CA 02314369 2000-06-12
WO 99/30718 PCT/US98/26609
-23-
FXAMPI F 1V - Administration of Intraurethral PGEzsream
Two cc of the PGE2 cream from Example 1 was instilled into the
urethral meatus of each of 10 impotent male patients between the ages of 50
and 70, using a syringe. The cream was massaged down the urethra, and
then the distal end of the urethra was occluded for 5 minutes by manual
pressure.
F MP V - Administration of Intraurethral PGE2_
Wands containing about 2cc of a PGE2 composition prepared in
accordance with Examples I - III placed within the urethra of males would be
expected to cause full tumescence suitable for intercourse in a majority of
the
test subjects within about 15 minutes of placement of the wand at which time
the wand can be removed, such removal terminating the delivery from the
wand of the PGE2, except for small amounts which remain on the urethral
mucosa.
~,~MPLE VI - EfficacX of- PGE~ Dream in Treating Human Erectile
Dyrsfunction
The effect of administration of PGEZ cream and the coated wand,
prepared and administered in accordance with the procedures of Examples I
and IV, was observed. After 15 to 30 minutes, treatment response was rated
as no penile tumescence, partial tumescence or full tumescence.
As a result with the cream, two of the ten men treated had no
response, six had partial tumescence, and two had full tumescence. Thus,
80% of the men treated showed at least partial penile tumescence in
response to the intraurethral PGE2 cream. Those treated with the cream
treated wand would be expected to have a similar response.
CA 02314369 2000-06-12
WO 99/30718 PCT/US98IZ6609
-24-
EXAMPLE VII - Efficacy of Lower Concentrations of PGE2 Cream in Treating
PGE2 cream was prepared and administered in accordance with the
procedures of Examples I and IV, except that a 20 mg PGE2 suppository was
used instead of a 40 mg suppository. This cream contained approximately
0.7 mg of PGE2 per 2 cc of cream. Two cc of cream was used to treat each
of ten impotent men between the ages of 50 and 70. After 15 to 30 minutes,
treatment response was rated as no penile tumescence, partial tumescence,
or full tumescence.
As a result, four of the ten men treated had no response, two had
partial tumescence, and four had full tumescence. Thus, even using lower
concentrations of PGE2, 60% of the men treated showed at least partial
penile tumescence in response to the intraurethral PGEZ cream. Those
treated with the cream treated wand would be expected to have a similar
response.
FAX , MPLE VIII - Intraurethral Administration of a PGE~ Hyrdrogel Comy osa
ition
A wand carrying approximately 5 mg of PGE2 in a hydrogel polymer
carrier was placed into the urethral meatus of a 65 year old impotent male
patient.
An effective erection resulted after 15 minutes at which point the wand
was removed. Erection was sufficiently effective for intercourse.
Detumescence commenced at approximately 1 hour after removal. Some
PGE2 remained in the wand after removal, the amount not being ascertained.
As a result, an amount of PGE2 less than 5 mg was delivered.
EXAMPLE IX - Use of PGF2a t~ ~=~unteract FffPCt_ of Administration of PGEZ
Priapism resulting from the use of an excess amount of PGE2 has
been determined to be reversible or treatable through the application of an
effective antidotal amount of a 15 methyl substituted prostaglandin F2a
containing formulation. In addition, it is anticipated that priapism from a
CA 02314369 2000-06-12
WO 99/30718 PCT/US98I26609
-25-
variety of other treatments for impotence (PGE, or Viagra~) will be similarly
treatable.
An antidotal formulation is prepared by mixing approximately 250
micrograms of prostaglandin F2a obtained as Hemabate, marketed by Upjohn,
in approximately 20 cc of K-Y jelly. Mixing is accomplished manually until
visual observation reveals a homogenous composition. A dose of
approximately 1 cc of the foregoing formulation was instilled into the urethra
of an erect penis, to reverse the results of the PGE2 treatment in accordance
with the present invention. Following delivery of the PGF2a there is immediate
relief of pain and within 5 minutes detumescence resulted.
EXAMPLE X - Use of a PGE~,I PGF~a Combination to Treat Impotence
Freeze dried PGEZwas obtained from Chinoin Pharmaceutical and
Chemical Works Co. Ltd., Budapest, Hungary. Three different solutions were
prepared with PGEZ concentration being 0.5, 1.0 or 1.5 mg/ 0.1 cc of
physiological saline. Samples were also prepared with the addition of PGF2a
to the PGE2 solution in the amount of 0.25NgI1.Omg PGE2. The several
different solutions were instilled into the urethra of a normally impotent
male
at a distance of at least about 1 cc from the external opening of the urethra.
An erection suitable for sexual intercourse occurred within about 3 to 5
minutes after placement of the solution and the erection lasted about 1 hour.
In several instances the use of the PGE2 solution resulted in a noticeable
burning or aching feeling within the erect penis. No burning or aching was
experienced in the solutions containing the PGF2a and the erectile effect and
sustainability of the erection appeared to the treated individual to be
undistinguishable from that of a control comprising a similarly prepared
solution without the addition of the PGF2a. The only noticeable difference was
the elimination of the burning sensation along the urethra encountered with
the control solution. The 0.25 pg/1 mg concentration was found to be
effective in most instances and the lesser concentrations were effective in
fewer patients or produced a less satisfying result.
CA 02314369 2000-06-12
WO 99/30718 PCT/US98/26609
-26-
It has been further discovered that PGE2, when applied to the mucous
membrane of the introitus, labia minors, andlor clitoris of female external
genitalia can also cause a physiological response reported to enhance sexual
relations by causing engorgement of the treated tissue with blood in a manner
similar to the creation of an erection in a male.
PGE2 prepared in a cream in the same manner as described above,
when applied to the extemai female genitalia, increases blood flow to the
tissue treated which, in turn, causes a temporary swelling of the external
genitalia to which applied and an enhanced sensitivity to external stimuli. A
typical response is engorgement of the genitalia, redness, swelling and
increased sensitivity typical of a normal response in an aroused female, the
response occurring within about 10-15 minutes following application. A
similar response results from use of PGE2 dissolved in a liquid carrier, such
as water, when applied onto the genitalia or dispensed from a spray
dispenser onto the external genitalia. PGE~IPGF2a can also be used with a
similar result. It is also believed that the addition of phentolamine mesylate
will also have a beneficial effect.
In a study of several women using PGE2 prepared in a liquid
comprising 1.0 mg PGE2 and 0.25 Ng PGF2Q in KY liquid (a water soluble,
physiologically acceptable topical composition commercially available from
Johnson & Johnson as a sexual lubricant) the women reported:
1. an increased pleasurable genital sensation
2. shortened time needed for foreplay to gain desirable stimulation
3. a higher incidence of orgasmic response reached within a
shorter time period of stimulation and/or intercourse, and
4. a tightening of the external genitalia resulting in an increased
stimulation on the erect penis of the sexual partner.
Although this invention has been described in terms of certain
preferred embodiments, other embodiments that are apparent to those of
ordinary skill in the art are also within the scope of the invention. In
particular,
analogs or derivatives of PGE2 and PGFZa which do not affect the basic
CA 02314369 2000-06-12
WO 99/30718 PCTNS98/Z6609
-27-
functionality of those molecules as described herein are also considered
within the scope of the present invention. Accordingly, the scope of the
invention is intended to be defined only by reference to the appended claims.