Note: Descriptions are shown in the official language in which they were submitted.
CA 02314387 2000-07-24
METHC>D FOR THE TREATMENT OF A SOIL
CONT AWING SOILBORNE PATHOGENS
FIELD OF THE INVENTION
The present invention relates to the control of soilborne pathogens and to a
method for determining the treatment of a soil containing soilborne pathogens.
BACKGROUND OF THE INVENTION
The treatment of soil in agricultural systems is widely practiced and
essential
since the growing of plant life remains a basic requirement for feeding the
world
population. Over the years, many different types of soil treatments have been
used
and experimented with depending on the desired result. 'Thus, it is common and
widely known to use fertilizers to provide the nutrients for plant growth.
A common problerr~ associated with most crops is the presence of soil
pathogens and weeds within the soil. 'These soil pathogens substantially
reduce the
yields of any given crop and accordingly, many different soil treatments have
been
developed in order to rid the soil of such pathogens.
One of the more common and widely used methods of treating soils to
eliminate pathogens therein is by tlhe use of a methyl bromide. However,
methyl
bromide has been recognized as an ozone depleting chemical and as such,
international agreement has stated that all production in developed countries
must be
phased out by the year 2005.
It has been estimated that the ban on methyl bromide will have a serious
effect
on crop damages and yields. In particular, crops such as potatoes, tomatoes,
peppers,
-1-
CA 02314387 2000-07-24
strawberries, etc are particularly susceptible to soilborne pathogens.
While other products for treating soil have been proposed, they have not
received any wide degree of acceptance. Other methods of controlling soil
pathogens
include crop rotation and field fallowing. However, these approaches lower the
return
on a given parcel of land.
SUMMARY OF THE INVENTION
It is therefore an object of the present invention to provide a method for
controlling soil pathogens.
It is a further object of the present invention to provide a method to
determine
an effective treatment for soil pathogens.
According to one a.5pect of 'the present invention, there is provided a method
of
controlling soilborne pathogens in a soil which comprises the step of adding a
nitrogen containing material and a pH reducing agent to the soil, the pH
reducing
agent being present in an amount sufficient to reduce soil pH below 5.5.
In a further aspect of the present invention, there is provided a method of
determining an effective method for control of soil pathogens in a soil
comprising the
steps of measuring the pH of the soil, measuring the organic carbon content of
the
soil, measuring the buffering capacity of the soil, adding a nitrogen
containing
material and a pH reducing agent to reduce soil pH below 5.5 when the
buffering
capacity is below 2 uL H2S04 g/soil, and adding a nitrogen containing material
at a
pH raising agent to raise the pH above 8.5 when the organic carbon content is
less
than 1.7% by weight.
-2-
CA 02314387 2000-07-24
In a further aspect of the present invention, there is provided a method of
controlling soilborne pathogens in a soil having an organic carbon content
less than
1.7% by weight comprising the step of adding a nitrogen containing material
and a pH
raising agent to raise soil pH above 8.5.
It is being found that the most effective method of controlling soil pathogens
will depend upon key properties of the soil. In particular, it is being found
that
soilborne pathogens can be controlled by exposing the pathogens to either
ammonia
or nitrous acid in a sufficient concentration, one method being selected over
the other
depending upon soil properties.
In particular, it is known that ammonia is effective for the control of
soilborne
pathogens. However, it has been found that the addition of even large amounts
of a
nitrogen containing compound sufficient to generate ammonia will not function
when
the pH of the soil is at below critical level and when the organic carbon
content of the
soil is greater than 1.7% on a weight basis.
Similarly, it has been found that nitrous acid (HN02) and/or nitrite (NOz) is
effective when the pH of the soil is reduced to a level below 5.5 and
preferably
below 5.
The fungus Yerticil!'ium dal~liae Kleb., a wilt pathogen of many crops, is
used
as a model pathogen. In potato (Solanum tuberosum), Verticillium wilt causes
premature senescence and the disease is aptly referred to as "early dying
syndrome".
Infection of potato plants occurs when roots contact microsclerotia (MS) of
Y. dahliae. Microsclerotia over winter in soil and consist of clustered,
melanized,
-3-
CA 02314387 2000-07-24
thick-walled and hyaline, thin-walled hyphal cells. Yerticillium wilt is
difficult to
manage because of the limited success of crop rotations, the slow development
of
resistant cultivars and the absence of chemical control options. Our studies
and those
of others have shown that when one controls this organism in the soil, then
many
other pathogenic agents, nematodes, weeds and pests are similarly controlled.
The following examples and figures examplify different aspects of the
invention, wherein;
Figures la to lk are graphs indicating soil pH and the number of
microsclerotia
germinated as well as NH3 concentration in soil, NOz and N03- content in two
different soils;
Figures 2a through :Ze are graphs showing microsclerotia germinated in a
single soil;
Figures 3a through :3h are graphs showing the effect with and without the
nitrification inhibitor DCD;
Figures 4a through 4d are graphs showing the number of microsclerotia
germinated and HN02 concentration;
Figures Sa through am are graphs similar to those in Figure 3 in a different
type of soil;
Figure 6 is a graph illustrating the number of microsclerotia germinated after
being exposed for two weeks to various concentrations of NH3;
Figure 7 is a graph illustrating the number of microsclerotia germinated for
various time counts after exposure to various concentrations of NH3;
-4-
CA 02314387 2000-07-24
Figure 8 is a graph illustrating the number of microsclerotia germinated at
various times after being exposed to various concentrations of HNOZ and a
citric acid
buffer;
Figure 9 is a graph illustrating the number of microsclerotia germinated after
exposure to various concentrations of 30 mL HNOZ and a citric acid buffer;
Figure 10 is a graph illustrating the peak concentration of NH3 for a soil
amended with 2% MBM;
Figure 11 is a graph. illustrating soil pH in response to H2S04;
Figures 12a through 12k are graphs illustrating the number of microsclerotia
germinated, soil pH, N02 .and NO.,- content and HN02 concentration in a soil
solution
and two different soils;
Figures 14a, 14b, and 14c are graphs illustrating the germination of
microsclerotia and percent colony :forming units of different organisms.
The viability of V. clahliae microsclerotia retrieved from soil was determined
using the following experimental model system. Desired levels of nitrogenous
product comprising meat and bone meal (MBM) were mixed with soil and 20 g of
the
mixture added to 50 mL test tubes. Microsclerotia suspended in crushed silica
sand
was added to a nylon pouch which was then buried in the soil or suspended in
the
head space of each tube. Water was added to each tube to bring soil moisture
to
0.33 bar tension. The tubes were then loosely capped, and placed in the dark
at 24°C.
On each date of analysis, die nylon pouches were retrieved and an Anderson air
sampler used to impact the pouch contents onto a medium (soil-pectate-
tergitol)
-5-
CA 02314387 2000-07-24
selective for growth of V. dahliae. Viability of microsclerotia were
determined two
weeks following plating as the number of microsclerotia germinated from a
total of 50
examined. Resulting death of microsclerotia using this bioassay has been shown
to
correlate to the reduction of wilt disease incidence in greenhouse and field
planted
potatoes.
The levels of NH3, N02 and N03- in soil were determined at each sample date.
Soil (8g) was mixed with cold distilled water (40mL) in sealed plastic bags,
the slurry
mechanically disrupted with a Stomacher laboratory blender, and shaken at
5°C for
one hour. The slurry was once again mechanically disrupted and its pH
determined.
The slurry was centrifuged and supernatant analyzed for total (NH3+NH4+),
(NH02+NOZ ) and N03- using an ion chromatograph. Ammonia and HN02 were
calculated as the fraction of total (NH3+NH4+) or (N02 +HN02) respectively in
solution using the Henderson-Hasselbalch equation knowing soil pH and
incubation
temperature.
Ammonia in excess of 65 mg N kg' soil (20 mMNH3) coincided with a rapid
loss in the viability of microsclerotia (Fig. 1). In two experiments MBM or
soya meal
(SM) were added to various concentrations (0, 0.25, 0.5, 1, and 2%
weight/weight) to
soils from two locations namely, Beauseart and Thorndale. Quite high levels of
ammonia accumulated in the Beauseart soil amended to 2%, but none was detected
in
the 'Thorndale soil. The viability of microsclerotia remained above 60% in
Thorndale
soil compared to less than a 0% in Beauseart soil amended to 2%
(weight/weight).
When 1 % MBM or SM was added to Beauseart soil a gradual decline in
-6-
CA 02314387 2000-07-24
microsclerotia viability to ~0% was seen four weeks after amendment. The
decline in
microsclerotia viability coincided with decreasing soil pH from 8 to 6 and N03-
accumulation in soil. Ammonia accumulation was negligible in Beauseart soil
amended to 1% MBM (weight/weight), suggesting it was not involved in the death
of
microsclerotia at this rate. The results suggest the microsclerotia were
killed by acute
NH3 toxicity in Beauseart soil amended to 2% (weight/weight) with MBM or SM
and
by quite a different mechanism when amended to 1 %.
The Thorndale soil ;amended to 2% MBM or SM failed to accumulate
sufficient NH3 to kill microsclerotia. This provided the opportunity to
confirm NH3 as
responsible for killing of rr~icrosclerotia by inducing high levels of NH3 in
the
Thorndale soil by determining the survival of microsclerotia. This approach
consisted
of adding high rates of MBM to the Thorndale soil. Thus MBM was applied at the
rates of 0,2 and 4% (weight/weight:). The 2% amendment resulted in negligible
NH3
accumulation and survival of microsclerotia greater than 50% by the end of the
study
(Fig. 2). In contrast at 4% MBM, NH3 accumulated to above 150 mM one week
following amendment and continued to the end of the study. This corresponded
to
complete death of microsclerotia.
Accumulation of gr~;ater than 10 mMNH3 was consistently accompanied with
a rapid decline in the viability of microsclerotia in soil. Based on this
observation the
toxicity of NH3 to microsclerotia in solution and atmosphere was tested and
compared
to levels required in soil to kill microsclerotia in soil.
Germination of microsclerotia was prevented by NH3 concentrations larger
CA 02314387 2000-07-24
than 3 mM in agar mediurr~ (Fig. 7). NH3 was generated in SPT medium by
addition
of various concentrations ofNH4C1 to the medium and varying the pH of the
medium
(7, 7.6, 8 and 8.5). Immediately following cooling and hardening of the
medium,
25 microsclerotia per Petri dish were transferred individually with a needle
and
germination recorded two 'weeks later. Microsclerotia that failed to germinate
were
transferred to regular SPT (containing no NH3) and still did not germinate.
Various concentrations of NH3 were generated in glycine or tricine buffer
solution at pH 8.6 with NH4C1 or (NH4)2SO4 added. A lSmL test tube was filled
with
appropriate buffer and NH,~+ solution, microsclerotia added, the tube capped,
then
placed in the dark at 24°C and the tubes inverted daily to suspend the
microsclerotia
in solution. Microsclerotia survival was determined by emptying contents of
the tube
into a Buchner funnel, the microsclerotia being retained on Whatman #42 filter
paper,
rinsed with distilled water, transferred to SPT medium by placing the filter
paper in
contact with the agar medium and removed leaving the microsclerotia adhering
to the
medium.
Microsclerotia germination decreased with concentration and duration of
exposure to NH3 in glycine buffer I;Fig. 8). An exposure of four days to
greater than 5
mMNH3 prevented germination of microsclerotia. Germination of microsclerotia
to
various concentrations of NH3 was not affected by buffer (glycine or tricine),
NH4+
source (NH4C1 or (NH4)zS04) or NaCI concentrations equivalent to the N sources
added.
In previous experiments, NH3 was found to accumulate in the Thorndale but
_g_
CA 02314387 2000-07-24
not Bearseart soil amended to 2% MBM (weight/weight). Therefore a series of
experiments were conducted to determine the soil properties preventing NH3
accumulation and microscl.erotia death in soil.
Several studies have reported that soil organic matter or clay can absorb NH3
to
exchangeable negatively charged sites. Thus various amounts of NH40H were
added
to either soil (varying in levels of NH3 eq. to 0 to 4% MBM weightlweight),
soil
brought to 0.333 bar, incubated for four days with subsequent extraction for
estimation of NH3. Addition of about 1800 mg NH40H-N kg' (eq. to 2 MBM
weight/weight) to the Thorndale or Beauseart soil was sufficient to induce
levels of
NH3 sufficient to kill microsclerotia (data not shown). Therefore, it seems
retention
of NH3 in amended Thorndale soil cannot explain the occurrence of insufficient
NH3
levels to kill microsclerotia.
Rapid nitrification was observed in the Thorndale soil amended to 2% MBM
To test if this rapid nitrification converted NH3 to NOz and N03-, thus
preventing
NH3 accumulation, the nitrification inhibitor dicyandiamide (DCD) was added
with
MBM to the Thorndale soil. Addition of inhibitor prevented the accumulation of
N02 or N03 and the reduction in soil pH following amendment to 2% MBM
However, NH3 levels in soil were not sufficient to kill microsclerotia, thus
nitrification alone cannot be attributed to prevention of NH3 toxicity in the
Thorndale
soil (data not shown).
To determine the factors) that control NH3 accumulation in soil, 2% MBM
was added to twelve soils with a range of soil properties including texture,
organic
-9-
CA 02314387 2000-07-24
carbon, cation exchange capacity, ~IVH3 and acid buffering capacity. The
Beauseart
and Thorndale soils studied previously were included in the twelve soils.
Selected
soil properties including NH3, NHS+, N02 , N03 , pH, soil C:N ratio,
electrical
conductivity, total bacteria, total fungi, ammonifying bacteria, ammonifying
fungi,
proteolytic bacteria and soil respiration which may influence the accumulation
of NH3
in soil were measured over time. Microsclerotia were killed within one week of
addition in four of the twelve soils amended. An accumulation of NH3 (greater
than
65 mg N kg' or 20 mM) was found in each of these soils one week following
amendment. Organic carbon content of soil was the only soil property highly
related
to NH3 accumulation (r=0.,92) with each of the four soils containing less than
1.7%
organic carbon (weight/weight) (dig. 11).
Ammonia failed to accumulate to toxic levels in soils with an organic carbon
content larger than 1.7% amended to 2% MBM. To confirm the role of organic
carbon in soils controlling the level of NH3 accumulation following amendment,
a
recalcitrant source of carbon ( Holland Marsh Muck soil ) was added to
Beauseart and
Habsor (sand) soils and amended with MBM. Amendment of the Beauseart soil to
2% MBM resulted in death of microsclerotia by day 9 with associated high
levels of
NH3 in soil. In contrast addition of Holland Marsh soil to 2 and 4%
(weight/weight)
together with MBM to 2°/. in Beausaert soil resulted in survival of
microsclerotia with
negligible levels of NH3 present in soil (data not shown). Addition of Holland
Marsh
soil to 5% (weight/weight) of the Habsor sand amended to 2% MBM
(weight/weight)
resulted in greater than 80% survival of microsclerotia with an insufficient
amount of
-10-
CA 02314387 2000-07-24
NH3 in soil (less than 1S mg NH3-N kg' or 7 mMNH3) required to kill
microsclerotia
(data not shown).
The lack of NH3 accumulation in the Thorndale soil amended with MBM or
SM was attributed to soil pH remaining below 8.5, being insufficient to
convert NH4+
to NH3. Calcium oxide (Ca0) stabilized sewage sludge (pH 13) was added to soil
to
raise soil pH and induce NH3 toxicity. The sludge raised soil pH above 8.5,
during
the first four days following its addition. Only when the sludge was added
five days
following MBM amendment were microsclerotia killed (Table 1). The NH4+
released
during decomposition and mineralization of MBM at day S was converted to NH3
by
the liming effect of the Ca0 stabilized sludge, thus NH3 toxicity induced.
Table 1. Number of microsclerotia germinated out of 50 counted with 0 or
2% added and 0 or 4% (:a0 stabilized municipal sewage sludge added on day 0
or day 5 after M.M. addition.
Day of MS germination
MBM Sludge Sludge (N=3 standard
(weight/weight) (weight/weight) Addition error of the mean)
0 0 - SO (0,33)
0 4 0 48 (1.15)
2 0 - 4S (0)
2 4 0 44 (1.53)
0 0 - 46 (0.33)
0 4 S 42 (2.19)
2 0 - 47 ( 1.20)
2 4 S 0 (Ol
-11-
CA 02314387 2000-07-24
Base generating agents such as calcium hydroxide, calcium oxide, sodium
hydroxide,
ammonium hydroxide, and potassium hydroxide can be added to various soils to
increase soil pH and encourage the generation of NH3 from N amendments. By
doing
so the required rate of N amendment to disinfect soil of soilborne pathogens
will be
reduced to economical and environmentally suitable levels. Further, the amount
of
the base agents required to bring soil pH to desired levels to induce
generation of NH3
can be determined. Soil properties such as organic matter content and initial
soil pH
are being used to predict the amount of base agent and N amendment required to
disinfest soil of plant pathogens and pests.
The germination of V. dahliae MS having been exposed to varying
concentrations of NH3 was determined. Y. dahliae MS were exposed to NH3 in
solid
agar medium, in buffered solutions, and in the atmosphere above buffered
solutions
containing NH 3. All toxicity studies were done in triplicate and repeated
once.
Agar medium: Various amountes of NH4Cl salt (to 0, 25, 50,100 and 200
mM) was added to soil peclate medium (SPT) and the pH of the medium adjusted
to
7.0, 7.6, 8.0 or 8.5 by addition of 5 MNaOH. For each level of NH4CI and pH,
three
replicate dishes were poured. The agar was allowed to solidify for 2 hours and
immediately thereafter 25 V. dahli'ae MS were individually transferred to each
dish
using a sterile hypodermic. needle. The dishes were wrapped with Parafilm
(American
National Can, Neenah WI) to limit loss of NH3 by volatilization, and incubated
for
two weeks at 24°C in the dark. The viability of V. dahliae MS was
determined as
percent of total MS forming colonies. The concentration of NH3 in medium was
-12-
CA 02314387 2000-07-24
estimated as described below and ranged from 0 to 31 mM. The pH of medium
after
hardening and two weeks after incubation was tested using pH test strips
(ColorpHast
pH 5-10; EM Science, Gibbstown NJ) and was within 0.5 units (limit of
resolution of
the test strips) of that set at the start. The results are shown in Fig. 3(a).
Buffered solutions: Varying amounts of a 2.70 MNH4C1 stock solution was
added (from 0 to 1.0 mL) t:o 40 mI, of 0.05 Mglycine solution adjusted to pH
8.6 with
NaOH (Perrin & Dempsey 1974). The final volume was brought to SOmL with the
same glycine solution. Solutions were then sterilized by filtration through a
0.22 um pore size filter and 15 mL placed into three replicate sterile screw
cap tubes
(total capacity 15.5 mL) About 200 Y. dahliae MS were immediately added to
each
tube and the tube closed and placed at 24°C and in the dark. Tubes were
inverted
every 12 hours to mix and suspend V. dahliae MS in the solution. A maximum
duration of exposure of four days was chosen because Y. dahliae MS germinated
after
five days in solutions of 0 to 0.65 mMNH3. At 8 hours, 1 and 4 days, the
contents of
each tube was passed onto a Buchner funnel containing sterile filter paper
(Whatman
#42). The filter paper retained the Y. dahliae MS and was rinsed with sterile
water
and placed onto the surface of SPT medium such that V. dahliae MS contacted
the
agar surface. The paper was then removed, leaving Y. dahliae MS adhering to
the
surface of the medium. The viability of Y. dahliae MS was determined as the
percentage of MS forming colonies out of 50 counted per replicate dish. The
concentration of NH3 in solutions was estimated as descritted below. The
effect of
type of buffer solution or NH4+ salt on survival of V. dahliae MS was tested
by adding
-13-
CA 02314387 2000-07-24
(NH4)2SO4 to glycine buffer solutions instead of NH4C1, NaCI instead of NH4C1
to
glycine buffered solutions, and NH4C1 added to tricine buffered solutions. The
solution concentration of NH4++NH3 in tubes after 4 days was within 5% of that
measured at the start of the; assay. Solution pH after 4 days did not vary
from the set
pH at the start of the assay by more than 0.1 units. The results are shown in
Fig. 3(b).
Ammonia gas: Fifty mL of a prepared solutions of NH4C1 in glycine solution
described previously were added to sealer jars (each 250 mL). A mesh bag
containing
Y. dahliae MS was then suspended in the atmosphere of the jar using a paper
clip
attached to a septum fitted in the lid of the jar. The jar was sealed, and
placed at 24°C
in the dark for 4 days. The mesh bags were retrieved and the viability of V.
dahliae
MS determined.
The survival of the other test organisms exposed to NH3 was determined in
glycine buffered solutions prepared as described previously. Sclerotia of
S. sclerotiorum ( 15 per treatment), seeds of A. retroflexus, L. sativa and R.
sativus (50
to 100 per treatment) were added to each tube containing a test solution. For
S. scabies and FOL a one mL suspension of spores and chlamydospores
respectively
were added to each tube containing 14 mL of concentrated NH4C1/glycine
solution.
The propagule density in the added suspension was prepared in distilled water
and
adjusted to give about 50 colony forming units (cfu) per 0.1 mL of test
solution at the
start of the experiment. Test solutions containing S. scabies and FOL were
placed on
YME and PDA medium rc;spectively. 'Threee replicate platings were maded for
each
test solution of S. scabies .and FOL and the cfu count were averaged. The
viability of
-14-
CA 02314387 2000-07-24
sclerotia of S. sclerotiorurra was determined as colony formation (non-
carpogenic
germination) on PDA medium and that of seeds on water agar (WA) medium (1.5%
agar) in Petri dishes. Sclerotia and seeds were separated from test solutions
in a
similar manner as that for V. dahlicze using a Buchner funnel and sterile
filter paper.
Sclerotia were cut in two, and a total of 5 placed (cut surface down) onto a
dish
containing medium. Seeds were transferred to WA medium directly from the
filter
paper as done for V. dahliae. Dishes were immediately wrapped using
stretchable
sealer tape. All dishes containing propagules of the test organisms were
placed at
24°C in the dark. Seed survival was based on the development of a 2 mm
radicle.
Seeds of R. sativus that failed to germinate after exposure to NH3, were
checked for
viability by staining with tetxazolium salt (2:3:5-tripenyl-tetrazolium
chloride; BDH,
Poole UK).according the procedure outlined by Moore (1973). The viability of
all
organisms tested was expressed as a percentage of that determined for the 0 mM
test
solution at the start of the experiment. For each test organism, this
experiment was
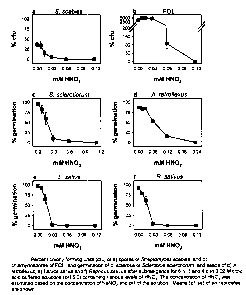
done in triplicate and repeated once. The results are shown in Fig. 3(e).
The germination of V. dahli'ae MS was determined using a setup similar to that
described above for exposure of V. dahliae MS to NH3 in buffered solution.
Exceptions being various amounts of 0.270 MNaN02 stock solution were added
(from 0 to 2.0 mL) to 40 rnL of 0.02 M citric acid solution (set to pH 5.0
with NaOH
(Perrin and Dempsey 1974)) and brought to 50 mL volume with the same citric
acid
solution. All experiments were done in triiplicate and repeated. Solutions
were filter
sterilized and 15 mL placed into sterile screw cap tubes (total capacity 15.5
mL).
-15-
CA 02314387 2000-07-24
Immediately, V. dahliae MS were added to each tube, the tube closed, placed at
24°C
and in the dark and inverted twice daily. At 8 hours, 1 and 4 days, Y. dahliae
MS
viability was determined. The concentration of HN02 in solutions was estimated
as
described below. The effect of NC)2 on survival of V. dahliae MS was tested by
adding various amounts of NaN02 (0 to 53 mMN02 ) to citric acid buffer and pH
set
to 4.0, 5.0 and 6Ø The effect oftype ofN02 salt on survival of V. dahliae MS
was
tested by adding KN02 or NaCI instead of NaN02 to citric acid buffer (set to
pH 5.0).
The concentration of N02 +HNOz and pH of solutions after 4 days was within 5%
and 0.1 units of the solution at day 0, respectively. The results are shown in
Fig.14(a).
Exposure of Y. dahliae MS to HNOZ gas was done using a similar procedure
described above. To sealer jars (2:~0 mL), 50 mL of a prepared solution of
NaN02 in
citric acid solution was added. A ~ dahliae MS bag was suspended in the
atmosphere of the jar. The jar was sealed, and placed at 24°C in the
dark for 4 days.
Thereafter the bags were retrieved and the viability of V. dahliae MS
determined.
This experiment was repeated and done in triplicate. 'The results are shown in
Fig. 14(b).
Determination of the survival of the other test organisms exposed to HNOZ was
done using citric acid buffered solutions as described above and detailed by
Tenuta
and Lazarovits (2000a). Sclerotia of S. sclerotiorum, chlamydospores of FOL,
spores
of S. scabies, seeds of A. retroflexacs, L. sativa and R. sativus were added
to each tube
containing 15 mL of HNO2, in citric acid solution at pH 5Ø Tubes containing
seeds
-16-
CA 02314387 2000-07-24
were opened for 15 s on day 2, as a precaution to prevent generation of
anaerobic
conditions from respiration of seeds. The solution assay was done twice and in
triplicate for each organism tested.
Seeds of R. sativus that failed to germinate after exposure to HN02, were
checked for their viability by staining with tetrazolium salt (2:3:5-tripenyl-
tetrazolium
chloride; BDH, Poole UK) according the procedure outlined by Moore (1973). The
germination or cfu of all organisms tested was expressed as a percentage of
the
germination or cfu of a control solution (0 mM HN02) at the start of the
experiment.
The results are shown in F'ig. 14(c).
Various concentrations of I-INOZ were generated in citric acid buffer solution
at
pH 4.0, 5.0 or 6.0 with NahT02 added. A lSmL test tube was filled with
appropriate
buffer and NaN02 solution, microsclerotia added, the tube capped, placed in
the dark
at 24°C and tubes inverted. daily to suspend microsclerotia in
solution. Microsclerotia
survival was determined as described previously for NH3 in glycine buffer.
Microsclerotia survival decreased with increasing concentration of NaNOz and
decreasing pH at a 24 hour exposure. A calculated concentration of above
0.10 mMHNOz was required to kill all microsclerotia (data not shown). Further,
the
survival of microsclerotia was dependent upon the duration of exposure to
HNOZ,
about 0.025 mMHN02 was sufficient to kill all microsclerotia at a four day
exposure
(Fig. 9). This is within the range of critical HN02 concentration required in
soil to
kill microsclerotia.
In studies described here, microsclerotia died when suspended above soil
-17-
CA 02314387 2000-07-24
amended with MBM, SM, and various fertilizers. Therefore, HN02 was suspected
to
also kill microsclerotia in atmosphere. Various amounts of HN02 in atmosphere
were
subjected to microsclerotia. by suspending microsclerotia in sealer jars
containing
30 mL of citric acid HN02 buffer solution and incubated in the dark at room
temperature for four days. Microsclerotia died when suspended in atmosphere
above
the 0.10 mMHN02 solution (Fig. 10).
This finding is important because it demonstrates that both NH3 and HNOZ can
kill microsclerotia through exposure in soil solution or soil atmosphere.
Since in soil
microsclerotia may reside in both, solution and atmosphere, either compound
has the
potential to kill microsclerotia.
Observations from the experiments described here indicate three factors
determine nitrous acid toxicity in soil. They being: a) amendment rate, b)
rapid
nitrification and c) poor soil acid buffering capacity. Nitrification
determines nitrous
acid toxicity because the intermediate N02 is produced under conditions of
rapid
nitrification and the oxidation NH4 ~ to N02 generates protons which acidifies
soil.
The ability of a soil to buffer against the acidity generated during
nitrification
determines the relative amounts of HNOZ and N02 according to soil pH. A acid
buffering assay was developed in which various amounts of H2S04 were added to
soil, incubated for two hours, distilled water added and the slurry shaken for
one hour,
the slurry then being allowed to settle for one hour with subsequent pH
determination.
Generally, soils group into two categories as may be seen in Fig. 12. Group 1
soils
have the ability to accumulate HNO Z and thus toxicity to microsclerotia.
Soils in this
-18-
CA 02314387 2000-07-24
group require less than 2 u:L HzS0,4 g/soil to lower soil pH to 5. Those in
Group 2 are
soils in which greater than 6uL HZS04 g/soil weight is required to lower soil
pH to 5.
HN02 acid accumulation and toxicity to microsclerotia has not been
demonstrated in
soils belonging to this group. Soils in this group contain CaC03 this being
the source
of their buffering ability.
An example of the importance of nitrification rate in producing HN02 is
evident in a study in which 400 or 800 mg N kg' as (NH4)2504 was added to
Beauseart and Mackenzie soils. The Beauseart soil was air-dried and stored for
1.5
years prior to initiation of the experiment. The Mackenzie soil was recently
collected
and stored at 4°C and at field moisture content. Recently collected
Beauseart soil was
shown previously to generate HNOZ in response to (NH4)ZS04 addition (Fig. 5).
However, the air-dried Beauseart soil failed to accumulate HNOZ and kill
microsclerotia (Fig. 13). In comparison, the Mackenzie soil has rapid
nitrification,
associated reduction in soil pH, accumulation of HN02, and death of
microsclerotia.
The population of autotrophic nitr~iiying bacteria at the start of the
experiment was
higher in the Mackenzie soil ( 1.1 x 105 g' soil) compared to the Beauseart
soil
(5.8 x 103 g' soil) likely accounting for differences in nitrification rate
between soils.
Many acid generating agents such as FeS04, AIS04, S°, S02, HZS04,
ascorbic,
sorbic, citric and acetic acids can be added to various soils to lower soil pH
and
encourage the generation of HN02 :from N amendments. By doing so the required
rate of N amendment to disinfest soil of soilborne pathogens is reduced to
economical
and environmentally suitable levels. The amount of the acid agents required to
bring
-19-
CA 02314387 2000-07-24
soil pH to desired levels to induce generation of HN02 can be determined. Soil
properties such as CaC03 , sand content and initial soil pH are used to
predict the
amount of acid agent and hT amendment required to disinfest soil of plant
pathogens
and pests.
-20-