Note: Descriptions are shown in the official language in which they were submitted.
CA 02314403 2001-05-10
TITLE: HYDROGEN PRODUCTION FROM ALUMINUM,
WATER, AND SODIUM HYDROXIDE AS CATALYST
FIELD OF THE INVENTION
This invention relates to the production of hydrogen gas from
aluminum, water, and sodium hydroxide as catalyst, and to an apparatus
for carrying out the method.
BACKGROUND OF THE INVENTION
Generally speaking, it is known that under certain conditions,
aluminum reacts with water to generate hydrogen and heat. It is also
known, however, that this type of reaction is not sustainable at ambient
temperature. It is believed that a protective oxide layer forms on a metal
surface in contact with water at ambient temperature and hampers the
reaction. Therefore, it has been accepted by those skilled in the art that the
use of aluminum in a reaction with water to generate heat and hydrogen
gas requires that the protective oxide layer is efficiently and continuously
removed, and that the reaction is kept at an elevated temperature.
A number of hydrogen generators have been developed in the past.
The following patent documents constitute a good inventory of the devices
and methods of the prior art in the field of hydrogen gas generation using
the reaction of aluminum or alloys of aluminum with water.
US 909,536 issued on Jan. 12, 1909, and US 934,036 issued on Sept. 14,
1909, both issued to G. F. Brindley et al. These documents disclose
several compositions for generating hydrogen. The compositions comprise
CA 02314403 2001-05-10
any metal which can form an hydroxide when it is brought into contact
with a solution of a suitable hydroxide. For example, aluminum is reacted
with sodium hydroxide to release hydrogen and produce sodium aluminate.
US 2,721,789,issued on Oct. 25, 1955 to Q.C. Gill. This document
discloses the structure of an hydrogen generator for reacting water with a
measured dry charge of aluminum particles and flakes of sodium
hydroxide. The reaction releases hydrogen gas and produces sodium
aluminate.
US 3,554,707 issued on Jan. 12, 1971 to W.A. Holmes et al. This
document discloses a gas generator having bellows to raise or lower the
level of water in response to the pressure inside the generator. As the level
of water drops, the contact surface between the fuel cartridge and the water
is lost and the reaction is terminated.
US 3,957,483 issued on May 18, 1976 to M. Suzuki. This patent discloses
a magnesium composition which produces hydrogen upon contact with
water. The preferred magnesium composition comprises magnesium, and
one or more metals selected from the group consisting of iron, zinc,
chromium, aluminum and manganese.
US 3,975,913 issued on Aug. 24, 1976 to D.C. Erickson. This document
discloses a hydrogen generator wherein molten aluminum is reacted with
water. The generator is kept at a very high temperature to keep the metal
in a molten condition.
US 4,643,166 issued on Feb. 17, 1987, and
US 4,730,601 issued on Mar. 15, 1988 both to H.D. Hubele et al. These
documents disclose the structure of a fuel cell for producing heat energy
2
CA 02314403 2001-05-10
and hydrogen gas. The device has a reaction chamber containing a fuel
composition that is reactive with water. The fuel composition includes a
main fuel part of magnesium and aluminum in a molar ratio of 1:2, and the
second part is composed of lithium hydride, magnesium and aluminum in
equal molar ratio.
US 4,670,018 issued on June 2, 1987, and
US 4,769,044 issued on Sept. 6, 1988, both to J.H. Cornwell. These
documents describe a log made of compressed wood waste and paper. The
log is coated with aluminum particles. Upon burning, the aluminum
particles react with moisture in the log to emit heat due to the generation
of hydrogen gas.
US 4,752,463 issued on June 21, 1988 to K. Nagira et al. This document
discloses an alloy which reacts with water for producing hydrogen gas.
The alloy material comprises essentially aluminum and 5 to 50% tin.
US 5,143,047 issued on Sept. l, 1992 to W.W. Lee. This document
discloses an apparatus and a method for generating steam and hydrogen
gas. In this apparatus, an aluminum or aluminum alloy powder is reacted
with water to generate hydrogen gas. An electric power source is used to
start the reaction. The electric power source is used to explode an
aluminum conductor and to disperse pieces of molten aluminum into a
mixture of water and aluminum powder. A heat exchanger is provided to
extract useful heat.
US 5,867,978 issued on Feb. 9, 1999 to M. Klanchar et al. This document
discloses another hydrogen gas generator using a charge of fuel selected
from the group consisting of lithium, alloys of lithium and aluminum. The
charge of fuel is molten and mixed with water to generate hydrogen gas.
3
CA 02314403 2001-05-10
JP 401,208,301issued to Mito on Aug. 22, 1989. This document discloses
a process for producing hydrogen. Aluminum is reacted with water under
an inactive gas or a vacuum to produce hydrogen gas.
CA 2,225,978 published on June 29, 1999 by J. H. Checketts. This patent
application discloses a hydrogen generation system wherein a coating on
reactive pellets is selectively removed to expose the reactive material to
water for producing hydrogen gas on demand. In one embodiment,
aluminum and sodium hydroxide are reacted with water to release
hydrogen gas and produce sodium aluminate.
Various other processes to produce hydrogen gas have been
described in the art, as reacting water with magnesium, sodium, potassium,
lithium, calcium, iron, zinc or steel.
Although the hydrogen production processes of the prior art deserve
undeniable merits, it is believed that the catalytic reaction of aluminum and
water, using sodium hydroxide as the catalyst, to release hydrogen gas
from water at room temperature has never been anticipated or observed
and disclosed by prior inventors. It is also believed that the prior art is
short of suggestion with regards to a hydrogen production process which
can be improvised in a home workshop using common materials and
equipment, to generate heat and light during a power outage for example.
Concerning hydrogen generators, the prior art discloses a number
of hydrogen generators for use with fuel cells or as heat sources for
thermal engines for examples. These generators are believed to be
complicated and precarious to operate by untrained individuals. These
hydrogen generators are believed to be designed for use by scientists and
other professionals working under laboratory conditions.
4
CA 02314403 2001-05-10
As such , it will be appreciated that there continues to be a need for
a production process and for an apparatus for generating hydrogen gas and
heat using a simple reaction which can be started at room temperature and
carried out safely by ordinary persons not having a formal education in
chemistry and chemical processes.
SUMMARY OF THE INVENTION
Broadly stated, the process for producing hydrogen gas according
to the present invention consists of reacting aluminum with water in the
presence of sodium hydroxide as a catalyst. This process is advantageous
for being carried out at room temperature and for producing large
quantities of heat and hydrogen gas at high purity.
In accordance with another feature of the present invention, there
is provided a process for producing heat, light and hydrogen gas. The
process comprises the steps of providing an expandable receptacle; partly
filling the expandable receptacle with water and introducing an aluminum
element and a catalyst in the water. The process also comprises the steps
of partly sealing the expandable receptacle and reacting the aluminum
element with the water. Then, the expandable receptacle is expanded and
contracted in response to more or less pressure therein, and by the same
action, the fuel element is emerged out or immersed into the water. This
method is advantageous for providing the ability to control the intensity of
the reaction between the water and the aluminum element in response to
the pressure generated inside the expandable receptacle by the reaction.
5
CA 02314403 2001-05-10
In another aspect of the present invention, the aluminum element
comprises a coiled strip of aluminum having several layers set vertically
in the water. As hydrogen gas is generated between the layers of the coiled
strip of aluminum, the hydrogen gas raising to the top of the water causes
a partial vacuum between the layers of the coiled strip, to absorb more
water through the bottom of the coiled strip, thereby promoting an
effective wetting of the aluminum element.
In yet another aspect of the present invention, there is provided an
apparatus for producing heat, light and hydrogen gas. The apparatus
comprises essentially an expandable receptacle having an upper end, a
central portion and a fuel element suspended to the upper end and inside
the central portion. The apparatus also has means for raising and lowering
the fuel element in the central portion in response to more or less pressure
inside the expandable receptacle, respectively.
The apparatus according to the present invention uses the pressure
and temperature of a reaction occurring between a fuel element and the
water contained therein to control the degree of immersion of a fuel
element in the water and consequently to control the intensity and duration
of the reaction between the fuel element and the water.
In yet a further feature of the present invention, the apparatus
comprises a timer mechanism and latch means responsive to the timer
mechanism for timely raising the fuel element out of the water contained
in the receptacle.
The processes and apparatus according to the present invention are
practical and safe for use by the general public to generate heat, light and
6
CA 02314403 2001-05-10
hydrogen gas in power outage situations for example, or in remote
locations where electricity is not available. Furthermore, the method and
apparatus according to the present invention use aluminum waste readily
available in domestic garbage and metal working shops, to promote
recycling and energy conservation.
In accordance with yet another aspect of the present invention, there
is provided a process for producing alumina, comprising the step of
reacting aluminum with water in the presence of a catalyst wherein the
catalyst is sodium hydroxide. This process is advantageous for extracting
available energy from a reaction between aluminum waste and water, and
for simultaneously producing a basic material which can be reused for
manufacturing new aluminum.
Although, the utility of the processes and apparatus of the present
invention can be appreciated by the general public, it is also believed that
the processes and apparatus of the present invention will find
advantageous applications in other more scientific fields, such as the fields
of fuel cells, internal combustion engines, thermal engines, heating systems
and lighting appliances.
Other advantages and novel features of the present invention will
become apparent from the following detailed description of the preferred
embodiment.
BRIEF DESCRIPTION OF THE DRAWINGS
A preferred embodiment of the present invention selected by way
of examples will now be described with reference to the accompanying
drawings, in which:
7
CA 02314403 2001-05-10
FIG. 1 is a side view of the preferred energy production apparatus, also
referred to herein as the hydrogen generator;
FIG. 2 is a cross-section view of the energy production apparatus
illustrating a mode of operation thereof when the fuel cartridge is
entirely immersed in water;
FIG. 3 is another cross-section view of the energy production apparatus
with the fuel cartridge in a raised position when pressure inside the
apparatus force the bellows of the apparatus to expand;
FIG. 4 illustrates yet another cross-section view of the energy production
apparatus with the timer mechanism in an unlatched mode causing
a spring to pull the cartridge out of the water;
FIG. 5 is a schematic diagram of the preferred gas handling manifold and
a burner plate mounted on the energy production apparatus;
FIG. 6 is a side view of the upper fuel support portion of the energy
production apparatus;
FIG. 7 illustrates a side view of a preferred burner plate and an optional
heat storage device for use with the energy production apparatus;
FIG. 8 is a top view of the preferred timer mechanism for use with the
energy production apparatus;
FIG. 9 is a partial cross-section view through the timer mechanism along
line 9-9 in FIG. 8.;
8
CA 02314403 2001-05-10
FIG. 10 illustrates a first arrangement for a fuel cartridge for use with the
energy production apparatus;
FIG. 11 illustrates a second arrangement for a fuel unit for use with the
energy production apparatus;
FIG. 12 illustrates one form for the fuel pellet for use with the energy
production apparatus;
FIG. 13 illustrates a third arrangement for a fuel unit for use with the
energy production apparatus.
FIG.14 illustrates a graph of temperature over time for a typical hydrogen
gas production reaction.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
While this invention is susceptible of embodiments in many
different forms, there is shown in the drawings and will be described in
details herein a specific embodiment of the method and apparatus
according to the present invention, with the understanding that the present
disclosure is to be considered as an example of the principles of the
invention and is not intended to limit the invention to the embodiment
illustrated.
The production of hydrogen gas according to the present invention
is obtained by a reaction of aluminum with water in the presence of sodium
hydroxide (NaOH) as a catalyst. The reaction produces a large amount of
heat and hydrogen gas.
9
CA 02314403 2001-05-10
The catalyst is mixed with tap water in a proportion of about 225 g.
per liter of water. In other words, the sodium hydroxide content of the
catalytic solution is preferably about 18% by weight. The catalyst is not
chemically consumed in the process.
The aluminum used in the reaction comprises aluminum foil,
electrical wire, beverage cans and other similar aluminum waste. The
intensity of the reaction depends upon the surface of contact between the
aluminum and water. Aluminum foil for example reacts faster than a
heavy gauge aluminum wire, and aluminum in a powdered form reacts
instantly to produce hydrogen gas.
A series of eight experiments was carried out to measure the volume
of hydrogen gas produced in a typical reaction. In these experiments,
aluminum foil from Reynolds Aluminum Company of Canada was loosely
crumpled and placed in a one litre plastic bottle containing 500 ml of
catalytic solution. The bottle was quickly capped with a cover fitted with
a tube which led to an inverted volumetric cylinder filled with water. The
bottle was immersed in a water bath to prevent overheating.
The volume of water displaced by the gas produced was measured
and corrected to a gas volume at standard temperature and pressure (STP)
Atmospheric pressure on that day was obtained from a local weather
office. The corrected volume of gas produced was compared to the
theoretical quantity of hydrogen gas, which would be obtained according
to the equation,
2A1 + 3H20 ~ A1 03 +3HZ
catalyst = NaOI->z
CA 02314403 2001-05-10
These experiments were carried out at a room temperature of 21 °C
and an atmospheric pressure of 758 mm of Hg. In all cases the reaction
started in few seconds and continued for few minutes, until depletion of
the aluminum foil. It was noticed that a typical reaction with less than 5
grams of loosely crumpled aluminum foil, is complete in less than 5
minutes. The results of these experiments are shown in Table 1 below.
Table 1: Hydrogen Gas Production from Aluminum Foil
Exp. Al HZ HZ (1) HZ (1) Yield Deviation
(#) (g.) (1) (STP) Theoretical(%) (+/- %)
1 2.08 2.94 2.71 2.59 104 2.6
2 2.03 2.85 2.62 2.53 104 2.6
3 2.21 3.05 2.81 2.75 102 2.5
4 2.16 2.9 2.67 2.69 99 2.6
5 2.2 3.04 2.8 2.74 102 2.5
6 2.21 3.04 2.8 2.76 102 2.5
7 0.73 1.03 0.94 0.91 103 2.4
8 0.83 1.15 1.05 1.03 102 2.2
Ave. 102 2.47
The results from Table 1 show that the reaction is reproducible and
produces stoichiometric quantities of hydrogen gas. The 102% average
yield of hydrogen gas is considered to be within the measurement
uncertainty; however, there are at least two factors which might have
contributed to a slightly higher hydrogen yield. Firstly, the volume of gas
produced was corrected to STP. It is possible that the exhausted fume
hood in which the experiments were carried out could have lowered the
reaction pressure below the atmospheric pressure of 758 mm of Hg. This
would have increased the observed value for the volume of gas produced.
11
CA 02314403 2001-05-10
An exhaust bench typically runs at 1 inch or 2 inches of water pressure.
At a maximum, this could have increased the measured volume by about
0.5%. Secondly, the water used was tap water in all cases, in which
dissolved air may have been present. If any of this air had been released
in the presence of the warm hydrogen gas, this would have increased the
volume of gas measured. This would have affected the results by less than
1 %. Since the results are within the measurement error, and quantification
of these two sources of error would not significantly affect the results, no
further experiment was carried out in this area
The procedure used in the above experiments was repeated, with the
exception that the tube leading from the top of the reaction bottle was
connected to a gas sampling bag. Two samples of gas were obtained and
analysed. The results are presented in Table 2.
Table 2: Gas Analysis
Sample Hydrogen Oxygen & Nitrogen
Concentration
1 92 % balance
2 98 % balance
Table 2 shows that the purity of the hydrogen collected in the
second sample was 98%. This is close to what was theoretically expected.
The lower 92% concentration observed in the first sample was probably
due to the fact the system was not completely purged with hydrogen before
the sample was taken. By the time the second sample was taken, most of
the air had been purged from the tube and the reaction bottle.
The procedure used in the first mentioned experiments was repeated
except that the reaction bottle was placed in a water bath before the
12
CA 02314403 2001-05-10
aluminum was added to the water, and the hydrogen produced was
bubbled through the bath water. The temperature of the bath and the
catalytic solution were measured before and after the reaction, and at about
four minutes after the reaction was completed.
The water equivalent of the plastic containers for absorbing heat
and their specific heat were determined experimentally by adding a known
quantity of hot water to the reaction system at room temperature and then
calculating the heat transfer based on the final temperature.
The quantity of heat produced by the reaction was determined and
compared with the theoretical values. The results are shown in Table 3.
Table 3: Heat of the Reaction
Readings Temp. Temp. Temp. Temp. Time
C C C C
Reactor Bath Reactor Bath
Start Start Finish Finish
1 21.1 20.2 45.5 24.4 5.29
2 21.1 20.2 38.3 25.3 5.33
Table 3: Continued
ReadingsA1 Heats of Heat of Heat Heat Efficiency
(g)
Formation FormationOutput Output (%)
AIZ03 HZO Actual Theoretical
kcal/mole kcal/molekcal kcal
1 9.52 -400.5 -68.3 33.3 34.5 96
2 9.52 -400.5 -68.3 32.5 34.5 94
The results in Table 3 show that the observed heat released in the
production of hydrogen was 96% of the theoretical value. The 94% value
from the second reading can be attributed to the heat lost to the
surroundings during the time that lapsed between the readings.
13
CA 02314403 2001-05-10
The reaction has a net maximum heat production during hydrogen
generation of 195.6 kCal/mole. A further 204.9 kCal/mole will be released
if the hydrogen is burned with oxygen. Stated another way, 51 % of the
reaction energy is used to form hydrogen gas and 49% goes into the
production of heat.
Having explained the preferred method for producing hydrogen gas,
the following disclosure and drawings describe a preferred apparatus for
carrying out the method.
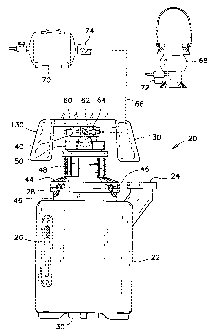
Referring firstly to FIGS. 1 and 2, an energy production apparatus
according to the preferred embodiment of the present invention is
illustrated therein. The energy production apparatus, also referred to herein
as the hydrogen generator 20 is illustrated in these figures in its entirety.
The hydrogen generator 20 uses water and aluminum particles as fuel, and
sodium hydroxide (NaOH) as a catalyst and a surface conditioner to reduce
the formation of oxide layers on the aluminum particles. The sodium
hydroxide may be mixed or otherwise closely associated with the
aluminum particles in a sufficient amount to ensure complete reaction of
the aluminum particles with water in an energy production period. Further
discussion on the incorporation of sodium hydroxide with the aluminum
particles will be provided later, especially when making reference to FIGS.
10-13.
The hydrogen generator 20 comprises firstly a receptacle 22 having
a first closable fill opening 24, a sight glass 26 for monitoring the level of
water therein, and a second closable larger opening 28 in a central upper
region thereof. The receptacle 22 also preferably has a cleanout bung 30
through its bottom surface to facilitate the periodic removal of the reaction
14
CA 02314403 2001-05-10
byproducts such as alumina.
In use, the receptacle 22 is filled with water 32, to a level of
between half and three-quarter of its capacity. A fuel cartridge 34 hanging
from a vertical tube 36 is immersed into the water 32 for causing a
chemical reaction to occur with the water, and for producing heat and
hydrogen gas.
The fuel cartridge 34 is supported in a perforated basket 38 affixed
to the vertical tube 36. The vertical tube 36 is connected to a gas handling
manifold 40 mounted above the receptacle 22, and has a series of holes 42
therein for admitting the hydrogen gas into the gas handling manifold 40.
An annular cap 44 is also provided for mounting over the upper
central opening 28 of the receptable. Several clasps 46 are provided
around the annular cap 44 for securing the annular cap 44 in a sealing
manner to the upper central opening 28. It will be appreciated that the
upper central opening 28 has a dimension to accommodate the insertion of
the fuel cartridge 34 and the basket 38 inside the receptacle 22. It will also
be appreciated that the clasps 46 may be replaced by other closure means
for quickly and easily removing the annular cap 44, for replacing a spent
fuel cartridge for example.
Upon the annular cap 44, there is provided a bellows 48 having an
interior region communicating with the receptacle 22 such that the
expansion and retraction of the bellows are relative to the pressure inside
the receptable. Atop the bellows 48, there is provided a timer mechanism
50, the operation of which will be described later. In the hydrogen
generator according to the preferred embodiment 20, the gas handling
CA 02314403 2001-05-10
manifold 40 is affixed to the upper portion of the vertical tube 36 above
the timer mechanism 50.
The annular cap 44, the bellows 48, the timer mechanism 50 and the
gas handling manifold 40 define with the receptable 22 a closable space
for containing and controlling the hydrogen gas being generated inside the
receptacle 22.
A burner plate 60 is mounted over the gas handling manifold 40.
The gas handling manifold 40 has conduit means communicating with the
burner plate 60. A series of orifices are provided in the burner plate 60 to
allow the burning of hydrogen gas for cooking food for example in a
similar manner as is known of gas stoves. Although the illustrations show
a side view of the burner plate 60 it will be appreciated that the burner
plate 60 is preferably a circular plate similar to those mounted on common
gas stoves.
The gas handling manifold 40 also has a selector valve 62 and a gas
outlet fitting 64 communicating with the selector valve 62. The selector
valve 62 is operable for selectively directing the hydrogen gas to the
burner plate 60 or to the outlet fitting 64.
A flexible hose for example, as represented by dash lines 66, can
be connected to the outlet fitting 64 and to a gas appliance such as a
lantern 68 to conveniently use the hydrogen gas, or to a gas reservoir 70
for accumulating the hydrogen gas for later use. The appliance may have
a water filter 72 thereon if needed or a check valve 74 to prevent any
backflow of gas into the receptacle 22 at the end of an energy production
period.
16
CA 02314403 2001-05-10
With reference to FIGS. 2 and 14, it will be appreciated that a
typical energy production period is known to have a heating phase 'A'
during which the temperature inside the receptacle 22 rises; an active phase
'B' during which the temperature inside the receptacle 22 is preferably
kept at around 85° C, and a cooling phase 'C' during which the reaction
gradually stops. At an operation temperature 'T' during the active phase
'B' of about 85° C, the reaction has been found to be self sustained
and
the hydrogen gas produced contained minimum water vapours.
The heating phase 'A' can be shortened by introducing a fuel pellet
80 inside the receptacle 22, through the fill opening 24. The fuel pellet 80
preferably contains very fine aluminum particles such as saw dust and
filings for examples, compressed with waste paper bits that are
impregnated with sodium hydroxide in a dry form. The small aluminum
particles of the pellet 80 are known to be highly reactive with water to
generate a burst of heat which causes the water temperature to approach
the ideal temperature 'T' quickly, and to accelerate a reaction of the water
with the larger fuel cartridge 34. Another fuel pellet 80 may also be
introduced in the receptacle during the cooling phase 'C' to prolong the
duration of an energy production period.
For example purposes, a fuel cartridge 34 having a volume of about
one liter, that is about 500 ml of aluminum and about 500 ml ofpaper filler
material impregnated with sodium hydroxide in a dry form, immersed in
10 liters of water is believed to be sufficient for producing heat and
maintaining a reaction for about two hours, in which the active phase is
about one hour, and the heating and cooling phases are about one-half hour
each. It is believed that the amount of hydrogen gas produced during the
active phase 'B' is sufficient for cooking food on the burner plate 60.
17
CA 02314403 2001-05-10
Referring now to FIG. 3, the operation of the bellows 48 is
illustrated therein. When the reaction enters its active phase, the heat and
pressure generated inside the receptacle 22 rise. The increase in pressure
inside the receptacle 22 causes the bellows 48 to expand upward as
illustrated in FIG. 3.
Because the basket 38 and the vertical tube 36 are supported to the
gas handling manifold 40, and because the gas handling manifold 40 is
affixed to the movable portion of the bellows 48, the expansion of the
bellows 34 causes the fuel cartridge 34 to be lifted toward an upper region
of the receptacle 22, and by the same doing, causes the water level to fall
in the receptacle 22. The contact surface between water and the fuel
cartridge 34 is thereby greatly reduced. The reaction is slowed down and
the pressure and temperature inside the receptacle 22 are consequently also
reduced. As temperature and pressure inside the receptacle 22 are reduced,
the bellows 48 collapses to re-immerse the fuel cartridge 34 and to resume
the active reaction phase.
Given the structure of the energy production apparatus 20 according
to the preferred embodiment, it is believed possible to calibrate the
characteristics of the bellows 48 for use with a specific size of receptacle
22 and a specific size of fuel cartridge 34, to precisely control the pressure
and temperature of a reaction, such that the apparatus 20 will be practical
and safe for use by the general public.
With reference to FIGS. 4, 8 and 9, the functions of the timer
mechanism 50 of the hydrogen generator 20 will be explained in details.
The timer mechanism 50 is provided for further improving the safety of the
hydrogen generator 20. The timer mechanism 50 is used for lifting the fuel
18
CA 02314403 2001-05-10
cartridge 34 above the water 32 after a set time period, even when the
bellows 48 remains in a collapsed mode. The reaction inside the receptacle
22 can thereby be manually stopped or caused to terminate at a set time
period by adjusting a knob 90 relative to a dial 92.
The preferred timer mechanism 50 comprises a coil spring 94
mounted over the vertical tube 36 and an annular spring-abutment plate 96
affixed to the vertical tube 36 above the spring 94 for retaining the vertical
tube 36 at a fixed position relative to the upper end of the spring 94.
The spring 94 is set in a cylindrical pocket 98 extending downward
through the timer mechanism 50. The depth of the pocket 98 is sufficient
to accommodate the spring 94 in a compressed form when the timer
mechanism is in a latched mode. A seal 100 is affixed to the bottom
portion of the pocket 98 around the vertical tube 36, for allowing a sliding
movement of the vertical tube 36 through the timer mechanism 50, under
the action of the spring 94, and for preventing hydrogen gas from leaking
out of the bellows 48.
One or more latch tabs 102 are movably connected to the timer
mechanism 50 and are linked to the operation of the selector knob 90.
When the burner plate 60 is pushed down to immerse the fuel cartridge 34
in water, the latch tabs 102 engage with the annular spring-abutment plate
96 to keep the spring 94 in a compressed state inside the cylindrical pocket
98.
The linkages, the clockwork and other components mounted inside
the timer mechanism 50 have not been illustrated herein for being common
to those knowledgeable in latches and locks. In the preferred embodiment,
19
CA 02314403 2001-05-10
however, the clockwork is a mechanical device not requiring electric
power. Also in the preferred embodiment, the latched tabs 102 are in a
latching position when the timer knob 90 is set at any time value, and are
in an unlatching position when the knob 90 is set at or reaches zero (0)
time on the dial 92.
Referring now to FIG. 5, the structure of the burner plate 60 and of
the gas handling manifold 40 are explained therein in greater details. The
outline of the gas handling manifold 40 is shown in dash lines to simplify
the illustration. The gas handling manifold 40 comprises a first set of
conduits 110 extending from the vertical tube 36 to the selector valve 62,
to a pressure relief valve 112, and to a flow control valve 114; a second set
of conduits 116 extending from the selector valve 62 to the burner plate
60; and a third conduit 118 extending from the selector valve 62 to the
outlet fitting 64.
The burner plate 60 has a plurality of gas orifices 120 therein, and
each gas orifice is preferably surrounded by one or more air inj ection holes
122 to admit oxygen around the gas orifice 120 during the burning of
hydrogen gas.
In the preferred embodiment, a minimum amount of hydrogen gas
is always directed to the gas orifices 120 to be burnt. The burning of this
minimum amount of gas provides a visual indication of the operation of
the apparatus 20, and prevents any accumulation of hydrogen gas in the
room in which the apparatus is being used. For this purpose, a flow
control valve 114 is provided in the gas handling manifold 40, and has a
fourth conduit 124 bypassing the selector valve 62. Therefore, when the
selector valve 62 is set to direct the hydrogen gas to the outlet fitting 64,
CA 02314403 2001-05-10
a minimum amount of gas is still allowed through the flow control valve
114 and to the gas orifices 120 of the burner plate 60.
The flow control valve 114 is preferably an adjustable type such
that it can be opened fully to bypass both the selector valve 62 and the
pressure relief valve 112, to obtain a larger flame 126 at the center of the
burner plate 60 if needed.
The pressure relief valve 112 is provided to further improve the
safety of the apparatus, as will be understood from the following
description. The pressure relief valve 112 monitors the pressure inside the
vertical tube 36 and releases a pressure over an unsafe level, to a whistle
128 which has an outlet opening positioned near one of the gas orifices
120. The gas flowing from the whistle 128 may thereby be readily ignited
by the flame above that orifice 120, to provide a visual indication of an
abnormal operation of the apparatus. The sound of the whistle 128 is yet
another sign to alert a user of an over pressure inside the receptacle 22, and
to urge that user to set the knob 90 to zero time to cause the timer
mechanism 50 to raise the fuel cartridge 34 out of the water.
As illustrated in FIG. 6, the burner plate 60 preferably has a pair of
handles 130 affixed thereto to manipulate the upper portion of the
apparatus 20 when the clasps 46 are released and the basket 38 is lifted out
of the receptacle 22.
In the preferred apparatus, a gas filter 132 may also be installed
over the gas admitting holes 42, for preventing any accumulation of
reaction byproducts inside the vertical tube 36. In other embodiments, the
vertical tube 36 may be filled with an appropriate granular filtering
21
CA 02314403 2001-05-10
medium for example for preventing reaction byproducts from reaching the
gas handling manifold 40.
As will be appreciated, a pressure gauge 134, a temperature gauge
or both, may also be provided on the annular cap 44 or at another
convenient location allowing a communication with the receptacle 22, for
visually monitoring the development of a reaction occurring inside the
apparatus.
In FIG. 7, the burner plate 60 is shown supporting a heat storage
device 140, for storing heat during the operation of the apparatus 20. The
heat storage device 140 is used for prolonging the beneficial effect of an
energy production period when the apparatus 20 is used to heat a camp in
the wilderness, or a household during a power outage period for example.
The preferred heat storage device 140 comprises a copper plate 142,
supported on legs 144, above the burner plate 60, and a perforated dome-
shape enclosure 146 enclosing one or more rocks 148 laid over the upper
portion of the copper plate 142. The heat storage device 140 is removable
from the burner plate 60 and is preferably used whenever the burner plate
60 is not used for cooking food. Further, the receptacle 22 is preferably
made of steel or similar heat conductive material for radiating heat during
the entire energy production period.
In the preferred embodiment, the inside diameter of the bellows 48
is sufficiently large, 15-25 cm for example, and the spring 94 is calibrated
such that the weight of the heat storage device 140 or the weight of a
common cooking pot (not shown) which may be set on the burner plate 60
does not significantly affect the operation of the bellows 48 or of the timer
mechanism 50.
22
CA 02314403 2001-05-10
Referring now to FIGS. 10-13, several arrangements are proposed
for preparing the fuel elements required for use in the hydrogen generator
20 according to the preferred embodiment. The fuel bundle 34 is
preferably prepared by overlaying a thin strip of aluminum 150 over a
sheet of embossed paper 152 impregnated with sodium hydroxide in a dry
form. The aluminum sheet and the paper layer are coiled together to form
a cylindrical shape. The preferred cartridge 34 is loosely coiled such that
water may be readily absorbed between the layers of the cartridge. The
advantage of a loosely coiled cartridge 34 is that the water is allowed to
seep into the entire cartridge at once to create an intense reaction, and
reduce the duration of the heating phase 'A' of the reaction as illustrated
in FIG. 14.
Another advantage of the cartridge 34 as described above is that
when the layers of the coil are set vertically, the hydrogen gas generated
between the layers rises up and creates a vacuum between the layers at the
lower end of the cartridge 34 to admit more water from the lower end of
the cartridge. This phenomenon is advantageous for wetting the aluminum
strip quickly, entirely and continuously.
The preferred fuel cartridge 34 is packaged in a sealed envelope that
has an indication as to its duration, potential heat energy and volume of
hydrogen gas to be produced by it.
Although the fuel cartridge 34 may be better manufactured with
virgin material, it will be appreciated that there are numerous economical
and environmental advantages in the manufacturing of fuel elements from
waste materials. Accordingly, another preferred fuel unit 160 having a
23
CA 02314403 2001-05-10
loose content in a bag-like envelope is illustrated in FIG. 11. The
envelope 162 is water-permeable, and the loose content comprises
aluminum turning, aluminum saw dust and filings, aluminum shreds and
other aluminum waste particles 164 as normally found in a metal working
shop, or as available from scrap metal vendors.
It is also possible to use aluminum shreds from domestic waste
containers. When the waste aluminum is obtained by shredding food or
drink containers for example, the waste material is preferably pre-treated
to at least partly remove a protective coating on this aluminum material.
The loose content of the fuel unit 160 also comprises waste paper
bits 166 impregnated with sodium hydroxide and dried. The paper bits
166 are made of waste newsprint or similar recyclable paper waste. The
paper bits 166 preferably have sizes and quantities similar to the aluminum
particles, and are mixed with the aluminum particles 164. The presence of
the paper bits 166 prevents the fusion of the aluminum particles 164
together and ensures a continuous absorption of water throughout the loose
content of the fuel unit 160. The fuel unit 160 is also preferably
manufactured and labelled as to indicate its expected energy production
period.
The fuel pellet 80 as illustrated in FIGS. 2 and 12, and as
previously described contains very fine aluminum particles such as saw
dust and filings for example, to provide a better water contact and a more
intense reaction. One or two fuel pellets 80 are preferably packaged in a
sealed envelope and distributed as reaction accelerators with each fuel
cartridge 34, or with each fuel unit 160 sold.
24
CA 02314403 2001-05-10
A third preferred arrangement for a fuel element usable in the
apparatus 20 according to the preferred embodiment is illustrated in FIG.
13. The fuel measure 170 is preferably comprised of a perforated
container 172 filled with aluminum waste 164 and paper bits 166 as
previously described.
When the energy production apparatus according to the preferred
embodiment 20 is used by someone having access to aluminum waste
material, and who does not want to depend on purchased fuel elements, the
fuel measure 170 described herein is recommended and is preferably used
with a nominal quantity of sodium hydroxide 174 set over the fuel measure
170. The sodium hydroxide 174 may be compressed into a tablet form as
illustrated for easy handling and storage. It may be used in a powder form
contained in a water-permeable sachet (not shown), or may be kept in a
sealed container and sprinkled generously over the water 32 before
introducing the fuel measure 170 into the water 32.
As to the manner of manufacture of the preferred apparatus, the
same should be apparent from the above description and accompanying
drawings, and accordingly further discussion relative to this aspect would
be considered repetitious and is not provided.
While one embodiment of a method and of one apparatus have been
described herein above, it will be appreciated by those skilled in the art
that various modifications, alternate materials, compositions and
equivalents may be employed without departing from the true spirit and
scope of the invention. Therefore, the above description and illustrations
should not be construed as limiting the scope of the invention which is
defined by the appended claims.