Note: Descriptions are shown in the official language in which they were submitted.
CA 02315480 2000-08-11
PROCESS FOR REMOVING METALS FROM A SORBENT
FIELD OF THE INVENTION
The present invention relates generally to removal of metals from metal-
containing
materials and specifically to removal of metals from substrates carrying the
metals, such as
sorbents.
BACKGROUND OF THE INVENTION
This invention relates to the recovery of precious metals from activated
carbon,
specifically to an improved elution process.
Cyanidation is commonly employed for the extraction of gold from its ores. In
this
process, the crushed one is treated with a dilute solution of sodium cyanide
(NaCN), and a
small amount of lime (Ca0) to maintain a pulp pH of z 9. In the presence of
oxygen, gold
dissolves forming gold cyanide complex.
kecovery of the gold is accomplished by adsorbing the gold cyanide complex on
activated carbon. A variety of processes based on this reaction have been
developed.
Effectiveness of these processes is, however, dependent on the development of
an efficient
means of eluting the gold from the gold-loaded carbon.
The most common commercial methods for the elution of gold cyanide from
activated carbon are the Zadra (U.S. Patent No. 2,579,531, issued December 25,
1951) and
Anglo processes.
In the latest Zadra elution process, a hot solution of 1 %weight/volume (w/v)
sodium
hydroxide (NaOH) and 0.2% w/v NaCN are recycled through a gold-loaded
activated carbon
bed for up to 72 hours at 95-100°C. to elute gold cyanide. A modified
Zadra process
operating at 140°C. in a pressurized system reduces elution time to 10-
12 hours.
CA 02315480 2000-08-11
In the Anglo elution process, gold-loaded activated carbon is soaked in a
solution
containing 3-S% w/v NaCN and 1% w/v NaOH followed by elution for 8-12 hours
with
deionized water at 100-120°C. The eluant solution is not recirculated;
therefore, it is a
once-through process.
~ The recycling of the weak gold-loaded eluant from the "tail-end" of an
elution cycle
to the beginning of the next elution cycle is practiced successfully both in
the Zadra and
Anglo processes. However, in the Zadra, high gold value in the recycled eluant
slows down
the elution process.
While both the Zadra and Anglo processes are effective in eluting gold from
activated carbon, these processes suffer from high energy consumption, high
capital costs
for pressurized operations, and a long elution period. Although, conducting
the elution
under pressure or modifying the eluant with organic compounds also improves
the rate of
elution,'these processes are complicated to implement.
There have been other attempts to elute gold from activated carbon involving
the use
of temperatures lower than those used in the Zadra or the Anglo elution
process. D.M. Muir
tried to elute gold by pretreating gold-loaded activated carbon with a
solution of sodium
cyanide and sodium hydroxide, and then eluting the carbon with methanol,
ethanol, or
acetonitrile vapors and condensate at 65-80 ° C. Using this process,
Muir eluted gold cyanide
in 4-6 hours. However, the Muir process requires an expensive sealed system to
minimize
( 1 ) fire hazards of electrowinning due to the flammable organic solvent and,
(2) solvent
losses due to evaporation.
-2-
CA 02315480 2000-08-11
F. Espiell tried to elute gold from activated carbon using mixtures of NaOH
(20 g/L)
and SO% aqueous organic solvents at 30 ° C. Espiell found that this
acetone-water-hydroxide
method was most efficient at a gold desorption with 90% of the gold being
eluted in less
than 40 minutes. However, a loss in gold-binding activity resulted over
several
loading/eluting cycles. This loss resulted from the failure of the acetone
solvent system to
elute the gold most strongly adsorbed to the activated carbon.
Heinen et al., U.S. Patent No. 4,208,378, tried to elute gold at 70-
160°C. with a
solution of abut 20-30% v/v water soluble alcohol and about 80-70% aqueous
solution with
a strong base of sodium or potassium hydroxide.
Parker et al., U.S. Patent No. 4,427,571, tried to elute gold from activated
carbon
using at least 20% v/v polar organic solvents or mixture of polar organic
solvents,
preferably, nitrites containing sodium cyanide or sodium thiocyanate.
~Iarvey et al., U.S. Patent No. 5,769,925, tried to elute gold by adding a
powerful
reducing agent, such as hydrazine monohydrate, to standard eluants, such as
NaOH/NaCN
with or without alcohol.
Belsak et al., U.S. Patent No. 4,968,346, tried to elute gold using an eluant
of about
2-3% v/v alcohol and 97-98% v/v deionized water. This approach involves adding
to the
eluant at least 2.5% w/w of a strong base (sodium or potassium hydroxide) and
at least 0.3%
w/w sodium or potassium cyanide.
Fuller et al., U.S. Patent No. 5,073,354, tried to elute gold using as an
eluant a
compound containing the carboxylate functionality, selected from benzoic or
substituted
benzoic acids and polyacrylic acids of less than about 100,000 M.W.
-3-
CA 02315480 2000-08-11
Fisher, U.S. Patent No. 3,935,006, tried to elute gold using, as eluants,
water-soluble
alcohols or ketones alone or with their aqueous solutions. Adding a strong
base of sodium
or potassium hydroxide facilitate elution.
In the prior art, sodium cyanide and sodium hydroxide are universally used in
the
S elution process. The importance of these two reagents in the elution process
is illustrated
numerous studies on the mechanism of adsorption and elution of gold cyanide
from activated
carbon. Thus, Davidson established that the addition of "spectator cations"
could enhance
appreciably the gold adsorption following the sequence,
Ca2+zMg2+zH+zLi+zNa+zK+. He
proposed a mechanism involving the adsorption of gold as ion pair
Mn+[Au(CN)2']", and the
use of the cations to preserve electroneutrality as counter ions in the
electrical double layer.
According to Van Deventer, the presence of spectator cations (M"~ enhances the
formation of M"+[Au(CN)z J" ion pairs on the carbon, which in turn suppresses
the elution
-- of gold'cyanide. When the concentration of cations in the eluant is high
and cyanide is
absent from the solution or the carbon, very little desorption of gold is
observed. Free
cyanide in the eluant, which causes some competitive adsorption of cyanide
with gold
cyanide, plays a minor role at the elevated temperatures used in the industry.
A more
important effect of cyanide is its reaction with functional groups on the
carbon, the products
of which passivate the surface for adsorption of gold cyanide, with cyanide
enhancing the
elution of gold cyanide. The degree of passivation, which is determined to a
large extent by
the temperature of pretreatment, also affects the elution of cations and the
degradation/adsorption of cyanide itself.
CA 02315480 2000-08-11
As the following table shows, conditions that enhance elution hinder
adsorption.
Conditions Favoring AdsorptionConditions Favoring Elution
Low temperatures High temperatures
Low cyanide concentrations High cyanide concentrations
_ Low Alkalinity High Alkalinity
High ionic strength medium Low ionic strength medium
Presence of Ca'z, Mg+z Absence of Ca+z, Mg+z
Jia observed that ethanol and butanol, adversely affect gold adsorption. He
also
observed that low pH increased adsorption of gold and silver cyanide whereas
organic
solvents and high temperatures decreased gold and silver adsorption.
In summary, prior methods of eluting gold cyanide from activated carbon called
for:
(a) A high temperature/pressure pre-soak of the loaded carbon with
NaCN/NaOH solution followed with hot deionized water;
~---15 ~ Zb) A high temperature/pressure elution with aqueous NaCN/NaOH;
(c) Organic solvents or compounds which contain aqueous NaCN/NaOH; or
(d) Relatively complex unit operations involving distillation with aqueous
NaCN/NaOH, such as the Micron process.
These prior methods had the following disadvantages:
(i) high energy costs because of elution at high temperatures;
(ii) lengthy elution period;
(iii) requirement for high quality water (Anglo process);
(iv) expensive organic solvents or compounds, or both;
(v) fire hazards associated with organic solvents; or
(vi) large volumes of gold-loaded eluant.
Thus, there is a need for a fast, safe, low-temperature, and efficient process
for
eluting gold cyanide from activated carbon for the recovery of metallic gold
from aqueous
solutions containing gold cyanides.
-5-
CA 02315480 2000-08-11
SUMMARY OF THE INVENTION
The present invention provides an effective methodology for removing metals
and
metal complexes, particularly cyano-metal complexes, from substrates, such as
sorbents.
The methodology is useful in extractive metallurgical processes, such as
cyanide extraction
of metals from metal ores followed by adsorption onto a suitable media, the
treatment of
industrial effluents and waste waters, and water purification.
In one embodiment, a method for solubilizing a metal from a substrate (or
sorbent)
carrying the metal is provided that includes the step of contacting the
substrate with a
stripping solution containing a nonpolar or substantially nonpolar molecule or
blinding
agent. The blinding agent displaces the metal from the substrate into the
solution.
The substrate or sorbent can be any material that absorbs, adsorbs, and/or
entraps the
metal or complex containing the metal. In a particularly preferred embodiment,
the sorbent
adsorbs~the metal or metal complex. Preferably, the substratepreferentially
absorbs, adsorbs
and/or entraps nonpolar molecules compared to polar molecules when present in
the same
solution. Preferred substrates include activated carbon, polymers, and resins
with activated
carbon and resins being more preferred.
The process is effective for a variety of metals and metal complexes.
Preferably, the
metal is a member of any one of Groups VIIIA, IB, or IIB, of the Periodic
Table of the
Elements. More preferably, the metal is gold, silver, platinum, copper,
nickel, cobalt,
mercury, and/or mixtures thereof.
The metal can be complexed with a variety of functional groups, including
cyanide,
thiocyanate, thiosulphate, and mixtures thereof. Typically, the metal is
complexed with
cyanide.
-6-
CA 02315480 2000-08-11
The blinding agent is preferably a nonpolar or substantially nonpolar molecule
or
compound that has a molecular size small enough to displace the metal or metal
complex on
the substrate and that is homogeneous or substantially homogeneous in the
solution.
Preferred blinding agents include organic compounds such as carbohydrates and
hydrocarbons (e.g., compounds including only the elements carbon and hydrogen
such as
the aliphatic or straight-chain paraffins (or alkanes having the general
formula C"HZn+2),
olefins (having the general formula CnHz"), alkenes, alkadienes, acetylenes,
acyclic terpenes,
and the cyclic or closed ring alicyclic compounds (e.g., cycloparaffins or
napthenes),
cycloolefins, and cycloacetylenes), aromatic compounds (e.g., benzenes,
napthalenes, and
anthracenes), and cyclic terpenes (both monocyclic or dipentenes and dicyclic
or pinenes)
and inorganic compounds such as ammonia, molecular nitrogen (NZ), and mixtures
thereof.
Particularly preferred blinding agents have one or more saccharose units or
first reaction
products of a saccharose unit, such as monosaccharides, (e.g., simple sugars
such as fructose
(levulose) and its isomer glucose (dextrose) both having the formula C6H,206);
disaccharides
(e.g., sucrose (C,ZH220"), maltose, cellobiose, and lactose); and
polysaccharides (e.g., high
polymeric substances).
The ammonia can be added to the stripping solution in many different ways,
such as
in the form of anhydrous ammonia or as an aqueous solution of ammonia..
The concentration of the blinding agent in the stripping agent can vary.
Preferably,
the blinding agent has a concentration (before the contacting step) ranging
from about 0.1
to about 5 wt.%, more preferably from about 0.2 to about 2 wt.%, and even more
preferably
from about 0.5 to about 1.5 wt.%.
_7_
CA 02315480 2000-08-11
The stripping solution typically includes a solvent for the blinding agent. To
avoid
competing with the blinding agent in displacement of the metal or metal
complex from the
substrate, the solvent preferably is a polar or substantially polar compound.
Preferred
solvents include water, alcohols, and mixtures thereof.
' The pH and temperature in the contacting step are important in achieving a
high rate
of displacement of the metal/metal complex from the substrate. Preferably, the
stripping
solution has a pH in the contacting step of at least about pH 7 and more
preferably ranging
from about pH 10 to about pH 11 and a temperature ranging from about 75 to
about
I 25 ° C, more preferably from about 85 to about 1 I 0 ° C, and
even more preferably from about
95 to about 97°C.
While not wishing to be bound by any theory, it is believed that the blinding
agent
is more attracted to the substrate than the metal/metal complex and causes the
metal/metal
comple3c to be forced into the stripping solution due to its replacement on
the substrate by
the blinding agent. In a gold or silver cyanidation recovery process, for
example, gold or
silver cyanide is adsorbed by activated carbon from a pregnant cyanide
leaching solution.
The loaded activated carbon is then contacted with the stripping solution. The
blinding
agent causes a shift in the equilibrium between adsorbed gold/silver and
dissolved
gold/silver in favor of the dissolution of gold/silver in the stripping
solution. For sugar as
the blinding agent, calcium, the complexing agent in the gold/silver
cyanocomplexes, and
magnesium preferentially react with the sugar to form calcium sucrate or
magnesium
sucrate, respectively, thereby preventing the calcium from again complexing
with the
dissolved gold/silver. As noted, the low concentration of magnesium and/or
calcium ions
favors elution of the metal/metal complex.
_g_
CA 02315480 2000-08-11
The process can have a number of advantages. The process can have low energy
consumption, low capital costs, and relatively short elution periods. The
process is relatively
simple and avoids or eliminates the need for pressurized and/or high
temperature operations.
The process can use blinding agents and solvents that have low flammability,
low toxicity,
and are relatively inexpensive. For example, the eluant can be sugar dissolved
in water. The
process can maintain acceptable levels of metal-binding activity over several
loading/eluting
cycles. The process can use blinding agents that act effectively, even in the
presence of low
quality water as the solvent. The process can be highly efficient. The process
can elute a
high fraction of the metals on the sorbent surface. The eluant can elute
(solubilize) even the
metals most strongly adsorbed on the sorbent.
In one configuration, the elution efficiency of recycled electrowinning
solution is
increased by first eluting the carbon with fresh eluant and then with the
recycled
electrodrinning solution. The process can generate low volumes ofmetal-loaded
eluant. For
example, passing the eluant twice through fresh carbon can reduce the volume
of gold-
loaded eluant by 50% (compared to other processes) without sacrificing elution
efficiency.
The stripping solution can include a mass transfer agent that is different
from the
solvent and the blinding agent. The mass transfer agent is a nonpolar or
substantially
nonpolar molecule. Preferred mass transfer agents include molecular nitrogen,
ammonia,
and mixtures thereof. In one configuration, the mass transfer agent is in the
form of a gas
that is sparged through the stripping solution before and/or during the
contacting or elution
step. The gas, like the blinding agent, displaces the metal/metal complex and
causes
turbulence in the solution adjacent to the substrate surface, thereby
enhancing mass transfer
of the metal/metal complex from the substrate surface into the solution.
-9-
CA 02315480 2000-08-11
The pregnant solution generated by displacement of the metal/metal complex on
the
sorbent will include (a) the blinding agent; (b) the solvent; and (c) the
dissolved metal.
Typically, the concentration of the dissolved metal in the pregnant solution,
after the
contacting step, ranges from about 1 to about 5,000 ppm. More typically from
about 1 to
about 1500 ppm and even more typically from about 5 to about 1000 ppm. The
metal is
typically recovered from the pregnant solution by electrolytic, cementation or
precipitation
techniques.
The residue or substrate, after the contacting step, includes the blinding
agent and the
metal/metal complex. Typically, in the contacting step at least about 90% of
the metaUmetal
complex is displaced into the solution and more typically from about 95 to
about 99.5%.
The residue or substrate is regenerated by contacting the substrate with a
wash
solution having an acidic pH to remove at least most of the blinding agent and
form a
regenerated sorbent. Preferably, the pH of the wash solution is no more than
about pH 3 and
more typically ranges from about pH 0.5 to about pH 2. While not wishing to be
bound by
any theory, at a basic pH (particularly a pH of from about pH 7 to about pH 11
) the
blinding agent is typically more attracted to the sorbent than the metal/metal
complex and
will therefore displace the metaUmetal complex from the sorbent surface. In
the presence
of an acidic pH, the blinding agent is at most only weakly amacted to the
sorbent surface and
will be removed from the sorbent surface.
BRIEF DESCRIPTION OF THE DRAWINGS
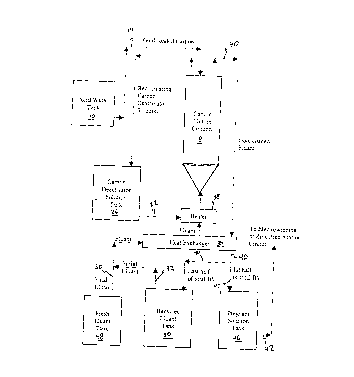
Fig. 1 shows the major components and flow directions of an elution process
according to the present invention;
Fig. 2-A shows the effect of calcium on elution efficiency with or without
sugar;
-10-
CA 02315480 2000-08-11
Fig. 2-B shows the elution recovery curve for sugar, sugar/calcium, and
without
sugar in distilled water and tap water;
Fig. 2-C shows the elution recovery curve of the elution process according to
the
present invention in relation to the Zadra and Anglo processes;
~ Fig. 2-D shows an improvement over the Zadra process brought about by using
sugar/calcium eluant in the first 1.5 BV, 2.25 BV, and then a recycled Zadra
eluant;
Fig. 2-E shows the elution recovery curve of an elution process according to
the
present invention demonstrating the advantage of passing the eluant through
fresh carbon
twice;
Fig. 2-F shows the elution recovery curve of an acid-washed carbon in relation
to a
non-acid-washed carbon;
Fig. 3 shows the maj or components and flow directions of the additional
embodiment
of an elution process according to the present invention.
Fig. 4 depicts an elution process according to another embodiment of the
present
invention;
Fig. 5 depicts an elution process according to another embodiment of the
present
invention; and
Figs. 6 and 7 plot, respectively, eluted carbon assay vs NaCN concentration
and gold
concentration vs time.
-11-
CA 02315480 2000-08-11
DETAILED DESCRIPTION
Although the process embodiments discussed below specifically refer to the
elution
of gold from gold-loaded activated carbon generated from an extractive
metallurgical
process using a cyanide-containing lixiviant, it is to be understood that the
processes are
equally applicable for metals other than gold and for phocesses other than
extractive
metallurgy. For example, the processes can be used on other precious metals
and base
metals, to name but a few, and may be used to elute or remove metals/metal
complexes from
sorbents generated in water treatment and/or purification processes.
The Elution Circuit
A first embodiment of a process involving selected major operations is shown
in Fig.
1. The process assumes that the metal, which in the embodiment is gold, has
been removed
from a cyanide lixiviant onto activated carbon by known techniques. The acid-
washed,
gold-loaded carbon is acid-washed, water-rinsed, and neutralized in the acid
wash tank 10.
The gold-loaded carbon 14 is then transferred to the carbon elution column 18
and preheated
to operating temperatures preferably from about 95-98°C by
recirculating a hot carbon
deactivator solution 22 introduced at the bottom of the column 18. After about
one hour at
the preferred temperature of about 95-98°C, the carbon deactivator
solution 22 is drained
back to the carbon deactivator solution tank 26 and can be reused for the next
batch of gold
loaded carbon. Recycled or fresh eluant (or stripping solution) 30 or 32,
respectively, is
pre-heated in the heat exchanger 34 to a temperature of from about 20 to about
85 °C
and further heated to a temperature of from about 95 to about 100 ° C
with 98 °C being
preferred by a heater 38 before entering the bottom of the carbon elution
column 18. The
process parameters in the column 18 are set forth above and below. The typical
residence
-12-
CA 02315480 2000-08-11
time of the eluant in the column 18 typically ranges from about 30 to about 48
min. The
gold-loaded eluant 40 flows out the top of the tank and is cooled down by the
heat exchanger
34 before it flows into the pregnant solution tank 46 or the recycled eluant
tank 50. From
the pregnant solution tank 46, the gold-loaded eluant 42 goes to the
electrowinning cells or
the'zinc precipitation circuit (not shown).
Operation of the Elution Circuit
The operation of the process will now be described with reference to Fig. 1.
As indicated in Fig. 1, the gold-loaded carbon is prepared for elution by acid
washing in the acid wash tank 10 at about pH 1 with 3-5% v/v mineral acid
solution, such
as nitric acid or hydrochloric acid. This pretreatment is meant to: (a)
dissolve carbonate scale
and reduce levels of silica, magnesium, aluminum, and other metals; (b) reduce
overall
contaminant levels in the pores of the carbon; and (c) remove grit, wood
fiber, plastics and
residual slime by screens integrated into the operation. The acid-washed
carbon is rinsed
with water to remove residual acid solution and neutralized to about pH 10
with minimal
amount of sodium hydroxide.
The acid-washed carbon 14 is transferred from the acid wash tank 10 to the
carbon
elution column 18. The temperature of the carbon is raised to about 95-
98°C by
recirculating a hot carbon deactivator solution (3-5% w/v NaCN) 22 through the
bottom of
the carbon column 18 to fluidize the bed of carbon in the column.
Recirculating the hot
solution 22 through the carbon will: (a) raise the carbon temperature to the
desired elution
temperature; (b) convert the adsorbed gold on the carbon into an elutable form
(i.e.,
from calcium gold cyanide to sodium gold cyanide); and (c) minimize gold
adsorption by
the carbon. This step takes about one hour after the carbon has reached
operating
-13-
CA 02315480 2000-08-11
temperature of about 95-98°C. The solution 22 is then drained back into
the carbon
deactivator solution tank 26. If the presence of hydrogen cyanide in the
carbon deactivator
solution 22 causes concern for safety/environmental reasons, a small amount of
ammonium
hydroxide may be added.
' Hot (95-98°C) eluant 32 from the recycled eluant tank SO and/or fresh
eluant tank
48 at about 1.5 to about 2 bed volumes (BV) per hour is passed through the
carbon in the
carbon column 18 in a fluidized bed configuration to elute the gold from the
carbon. The
fresh (or final) eluant 30 is prepared in fresh eluant tank 48 by adding about
1% w/w of a
blinding agent, preferably sucrose, into commonly available water, such as
process water or
thickener overflow water from other process operations. Fresh eluant 30
preferably contains
about 1 % sugar and the recycled eluant sugar concentration may be increased
to about 2%
by adding sugar into the recycled eluant tank S0. In other words, the recycled
eluant 32
typically has a higher blinding agent concentration than fresh eluant 30.
Assuming that the total volume of eluant used in the elution process is about
10 BV,
1 ~ the first 5 BV preferably comes from the recycled eluant tank 50 and the
last 5 BV from the
fresh eluant tank 48. The first 5 BV of gold-loaded eluant 40 coming out of
the carbon
elution column 18 preferably goes to the pregnant solution tank 46, and the
last S BV of
gold-loaded eluant preferably goes back to the recycled eluant tank 50. This
cycle is
repeated with every batch of carbon. By contacting the eluant twice with fresh
carbon, the
concentration of gold is increased, thereby decreasing the volume of pregnant
solution
subjected to electrowinning.
At this point, the gold can be recovered from the gold-loaded eluant 42 in the
pregnant solution tank 46 by known techniques, such as zinc precipitation or
electrowinning.
-14-
CA 02315480 2000-08-11
The recycled eluant 40 in the recycled eluant tank 50 typically contains from
about
100-200 ppm gold in contrast to a Zadra solution, which is recycled from the
electrowinning
cells that normally has less than about 20 ppm gold. In a Zadra process,
eluant containing
high concentrations of gold significantly slows down the elution process
because equilibrium
conditions prevent the efficient elution of gold from the carbon; that is, the
equilibrium that
exists between gold dissolved in solution and adsorbed on the carbon.
Reeling Circuit for Barren Electrowinning~Solution
An additional embodiment is shown in FIG. 3, where the recycled eluant 60 is
the
spent electrolyte or electrowinning solution 70 from the electrowinning cells.
I 0 FIG. 3 shows the major operations of this embodiment. Figure 3 is the same
as FIG.
1, except that in FIG. 3:
1. At least about 50% by volume of the input to the recycled eluant tank 50
- comes From the electrowinning cells, which is outside of the elution
process, rather than or
in addition to recycled eluant that was not electrowon;
2. At least about 30 % by volume of the fresh eluant 30 is introduced into the
carbon elution column 18 ahead of the recycled eluant 30, rather than in the
reverse
sequence; and
3. All gold-loaded eluants 40 from the carbon elution column 18 are combined
in the pregnant solution tank 46, rather than being split into two streams.
This embodiment relates to the sequence of introducing the eluants (fresh 30
and
recycled 60) into the carbon elution column 18. The fresh eluant 30 is
introduced first, then
the recycled eluant 60 from the electrowinning cells. The gold-loaded eluants
42 are
combined in the pregnant solution tank 46.
-15-
CA 02315480 2000-08-11
Operation of the Recycle Circuit
Referring to Fig. 3 in recovering gold from gold-loaded eluant 40 by
electrowinning,
conductivity reagents, such as sodium hydroxide and sodium cyanide, are added
to improve
the conductivity of the electrowinning solution. The concentration of the
conductivity
reagents) typically ranges from about 0.5 to about 1 % . Recycling the barren
electrowinning solution back to the elution circuit, thus using a recycled
eluant 60, will
reduce the cost of using these reagents.
In an elution/electrowinning circuit with a continuously recirculating of
electrowinning solution, a fraction of the total solution is typically removed
as a "bleed"
solution to prevent the build up of deleterious amounts of salts in the
system. This bleed
solution is typically about 10-30% of the total volume.
To maintain a constant volume of eluant solution, fresh water and reagents are
mixed
- - wali the barren electrowinning solution or recycled eluant 60 that will be
used in the next
elution cycle.
1 S The fresh eluant 30 preferably contains about 1 % sugar. More sugar may be
added
to the recycled eluant to increase its sugar content to 2%. The recycling
process starts after
the carbon in the carbon elution column 18 has been deactivated by the carbon
deactivator
solution 22 and the solution 22 has been drained out of the carbon elution
column 18. The
recycling process involves introducing fresh eluant 30 into the column 18
before the
recycled eluant 60. All gold loaded eluants 40 go to the pregnant solution
tank 46 that feeds
the electrowinning cells. Unlike the prior embodiment, this additional
embodiment brings
the fresh eluant 30 in contact with the fresh carbon typically only once until
it passes through
the electrowinning cells again.
-16-
CA 02315480 2000-08-11
This additional embodiment takes advantage of the high rate of gold elution
using
an eluant containing sugar to improve the overall efficiency. Savings in
reagents are directly
proportional to the fraction of recycled eluant being used.
Regeneration of the Sorbent
' After displacement of the metal/metal complexes by the blinding agent, the
process
further includes a method for regenerating the sorbent by removal of the
blinding agent. In
the method, the stripped sorbent in the carbon elution column or vessel is
contacted with an
acid wash solution. The acid wash solution can include any suitable mineral
acid, such as
nitric acid, hydrochloric acid, sulfuric acid, and/or an organic acid such as
glycolic acid as
described in U.S. Application Serial No. 09/454,584, filed December 6, 1999,
and entitled
"Method for the Regeneration of Activated Carbon" which is incorporated herein
by
reference in its entirety, and preferably has a pH ranging from about pH 0.1
to about pH 5
and mode preferably from about pH 0.5 to about pH 2. The concentration of the
acid in the
acid wash solution preferably arranges from about 0.5 to about 25 vol.% and
more preferably
from about 3 to about 5 vol%.
While not wishing to be bound by any theory, it is believed that the blinding
agent,
in the presence of the acidic pH, is at most only weakly attracted to the
sorbent, thereby
causing a large fraction (typically at least about 90%) of the blinding agent
into the solution
from the sorbent surface. In other words, in the presence of a basic pH, the
equilibrium
between the blinding agent in the solution and the blinding agent on the
surface of the
sorbent strongly favors the attachment of the blinding agent to the sorbent
surface. In
contrast in the presence of an acidic pH, the equilibrium between the
dissolved blinding
-17-
CA 02315480 2000-08-11
agent and the blinding agent on the surface of the sorbent strongly favors the
dissolution of
the blinding agent.
The temperature of the acid wash typically ranges from about 80 to about 95
°C.
After the solvent has been contacted with the acid wash for a time typically
ranging
from about 1 to about 2 hours,~the regenerated sorbent can be rinsed with
water to remove
residual acid solution.
The sorbent can then be reused in a variety of known processes to remove
dissolved
metal/metal complexes from pregnant lixiviant solutions, industrial effluents,
waste waters,
etc., to form metal/metal complex loaded sorbent for use as the feed material
in any of the
elution processes described above.
Other Process Embodiments
A process according to another embodiment is shown in Fig. 4. The process
assumes
- ~~ that the'metal, which in the embodiment is gold, has been removed from a
cyanide lixiviant
onto activated carbon by known techniques. The gold/silver-loaded carbon
(loaded carbon)
1 S is transferred to the Carbon Elution column or Vessel (CEV) 100 and
preheated to operating
temperatures preferably from about 95-98°C by recirculating a hot
pretreatment cyanide
solution 102 introduced at the bottom of the CEV 100 in a fluidized bed
configuration. After
the pretreatment period, the cyanide solution 102 in the CEV is returned to
the Bleed Tank
(BT) 104 by displacing the cyanide solution with a heated barren solution 108
from the
Barren Solution Tank (BST) 112. The carbon is further rinsed with barren
solution 108 and
which into the BT 104.
-18-
CA 02315480 2000-08-11
The barren solution 108 is heated to a temperature of about 95°C to
about 98°C
before entering the CEV 100. The gold/silver-loaded solution (pregnant
solution) 116 flows
out the top of the tank 100 and into the Bleed Tank (BT) 104 or the Pregnant
Solution Tank
(PST) 120. The first 2 to 4 bed volumes (BV) of pregnant solution (which
typically
. 5 represents from about 20 to about 35 vol.% of the total or combined
pregnant solution
volume) go to the BT 104 since it contains the highest concentration of
impurities. Typical
impurities include one or more of sodium cyanide, sodium ions, and base metal
cyano
complexes. The pregnant solution 116 in the BT typically has about 40 wt% or
more
of impurities (more typically about 75 wt% or more) while the pregnant
solution in the PST
120 typically has no more than about 30 wt% impurities (and more typically no
more than
about 20 wt%). The rest of the pregnant solution goes to the PST 120.
Gold/silver in the
BT and the PST solutions are electrowon separately (e.g., with separate
circuits) with the BT
--~ solution 124 going to electrowinning circuit 128 and the PST solution 132
going to
electrowinning circuit 136. The BT barren solution goes to the leach circuit
(not shown),
e.g., Carbon-in Leach (CIL) circuit, and the PST barren solution 144 recycled
back to the
Barren Solution Tank (BST) 112. The process parameters in the CEV 100 are set
forth
above and below. The typical residence time of the eluant in the CEV 100
ranges from 30
minutes to 45 minutes.
The operation of the invention will be described with reference to Fig 4. As
indicated in Fig. 4, the gold-loaded carbon (loaded carbon) is loaded into the
Carbon Elution
Vessel (CEV) 100. The temperature of the carbon is raised to about 95-
98°C by
recirculating a hot cyanide solution 102 between the Bleed Tank (BT) 104 and
the CEV 100.
This step takes about one to four hours depending on the concentration of
cyanide used. The
-19-
CA 02315480 2000-08-11
concentration of cyanide in the pretreatment solution, which typically ranges
from about 3
to about 10 wt%, and the duration of the pretreatment period, which typically
range from
about 1 to about 4 hours play a significant role in the final eluted carbon
assay as shown in
Fig. 6.
~ The pregnant solution 116 and cyanide solution are combined or mixed in the
same
BT tank or vessel as one has a low cyanide concentration and high gold
concentration and
the other has a high cyanide concentration and low gold concentration. The
combined or
mixed solution is subjected to electrowinning for gold recovery. As will be
appreciated, the
presence of the cyanide in the combined solution assists in gold recovery.
The cyanide is rinsed off the carbon preferably with about 2 to 4 bed volume
(BV)
of barren solution 108 with the rinse solution reporting with the used sodium
cyanide in the
BT 104. The BT solution 124 is electrowon to recover the gold, preferably
using a separate
electro~inning cells to that of the pregnant solution 132 from the Pregnant
Solution Tank
(PST) 120.
Hot barren solution from the BST 112 is passed through the carbon elution
vessel
100 to elute the precious metals from the carbon at about two bed volumes (BV)
per hour.
The barren solution when first prepared contains about 0.5-1 wt.% of sucrose
and about 0. I -
0.2 wt.% ammonia in commonly available water, such as mill process water.
After the
initial elution, the barren solution comes from the electrowinning cells after
the precious
metals had been electrowon from the pregnant solution 132 of the PST 120.
-20-
CA 02315480 2000-08-11
Fresh eluant 150 (e.g., water containing about 1 wt.% sucrose) follows the
barren
solution eluant. The amount of fresh eluant 150 used should be enough to
maintain the
volume of barren solution in the BST 112 before each elution cycle (e.g.,
about 4BV if the
bleed used is about 4BV per cycle).
The concentration of sucrose and ammonia are maintained by addition of
sucrose,
and ammonia to the barren solution 108. Ammonia is preferably added in _ the
electrowinning cells to maintain an electrowinning cell pH of at least 10.
Sugar is added to
the fresh eluant (process water)
The concentration of impurities, e.g., nickel, copper, sodium, etc., in the
barren
solution is controlled by the amount of the pregnant solution 116 reporting as
bleed solution
on each elution cycle to the BT 104. Two to four bed volumes of bleed solution
is required
and is always the first couple of bed volumes of barren solution after the
cyanide
pretreatment. The bleed solution 124 in the BT 104 is electrowon in cells 128
with the
.___
barren bleed solution 144 discharged into the leach circuit where the cyanide
carned by the
solution can be used and residual gold/silver can be recovered.
The pregnant solution 132 in the PST 120 is electrowon in cells 136 with the
barren
solution 144 recycling back to the Barren Solution Tank (BST) 112 for reuse.
An additional embodiment is shown in Fig. S, where the pregnant solution is
divided
into two parts: pregnant solution (PS 1 ) 200 and pregnant solution (PS2) 204.
Assuming that
the total volume of eluant (not including the bleed) is about IOBV, the first
SBV goes to the
pregnant solution tank (PST1) 208 and the next SBV goes to the pregnant
solution tank
(PST2) 212. PS 1 200 is electrowon in electrowinning circuit 136 while PS2 204
is not
electrowon but used as the initial eluant after the cyanide pretreatment.
-21-
CA 02315480 2000-08-11
There are two recycled eluants; PS2 216 and barren solution 108 from the
BST112.
PS2 solution 216 did not pass through the electrowinning cell in contrast to
the barren
solution, which is PS1 220 after it passed through the electrowinning cells
136. Typically
the concentration of gold in the barren solution 108 and PS2 216 are, less
than about 2ppm
and greater than about l5ppm respectively.
PS2 216 is used as the initial eluant after the cyanide pretreatment, followed
by the
barren solution 108 thereby decreasing reagent consumption and energy cost,
and reducing
the volume of pregnant solution that goes to the electrowinning cells by
approximately one-
half.
EXPERIMENTAL
Standard conditions used in the examples below are as follows:
1. The samples were derived Carbon-in-Leach (CIL) carbons.
.__ _
2. The carbons were acid-washed with nitric acid solution at pH 1 and
neutralized to about pH 10, unless otherwise noted.
3. Elution Temperature: 95-98°C.
4. Carbons were treated for one hour with hot recirculating 5% NaCN solution
to reduce its activity.
5. Eluant flow: 1.5 bed volumes per hour except in Example 1 (used 2 BV/h).
6. Duration of elution: S hours.
The sugar/calcium eluant in these examples consists of distilled water or tap
water
with lime.
-22-
CA 02315480 2000-08-11
Example 1
FIG. 2-A and Table 1 show how the calcium ions affect the elution recovery
curve
for distilled water with and without sugar.
Table 1
Eluant CarbonAssa %
y Gold 5
(ppm)Head RecoveredAFTER
Tail (HOURS)
1
2
3
4
W D.W.+1% Sugar 5773 118 65.7 88.0 94.3 97.0 98.0
V D.W.+1% Sugar+60 ppm Ca 6850 175 50.7 82.2 91.7 95.8 97.4
T Distilled Water (D.W.) 6444 228 54.8 81.8 91.7 95.1 96.5
S Distilled Water+60 ppm 6863 370 47.5 76.2 86.9 91.9 94.6
Ca
_-- The pr ~sence of calcium ions in the eluant suppresses the elution of gold
cyanide from the
carbon. It is believed that the calcium forms a calcium gold cyanide complex,
which is
strongly held by the carbon. Table 1 shows that, by adding sugar to the
eluant, the formation
of calcium gold cyanide complex is reduced, if not eliminated, thereby
improving the elution
of gold from the carbon.
Example 2
FIG 2-B and Table 2 show an elution method similar to Example 1 at 1.5BV per
hour and
using tap water.
-23-
CA 02315480 2000-08-11
Table 2
Eluant CarbonAssa %
Gold
RecoveredAFTER
y (HOURS)
(ppm)Head 1
2
3
4
5
Tail
P Tap water (lOppmCa)+1% 6525 135 42.1 83.5 93.8 96.6 97.9
Sugar
O Distilled Water+1% Sugar+Ca6109 117 39.4 81.9 93.5 96.9 98.1
(60 ppm)
M Distilled Water+1% Sugar 6054 112 41.7 81.4 93.8 97.2 98.1
L Distilled Water 6361 117 37.4 77.5 92.8 96.9 98.2
Q Tap Water (lOppm Ca) 6259 149 39.1 70.6 87.3 94.8 97.6
Table 2 shows that the elution efficiency can be reduced by 10 ppm calcium
ions in the
eluant. Sugar at 1 percent by weight can improve gold elution recovery, even
in the presence
of higher concentrations of calcium ions.
___ -
Example 3
FIG 2-C and Table 2 show the elution profile of the elution process in
relation to the
Zadra and Anglo processes. The elution process of the present invention has a
higher rate
of elution than the Anglo or Zadra processes, particularly at the early stages
of the elution
cycle.
-24-
CA 02315480 2000-08-11
Table 3
Eluant CarbonAssa %
Gold
RecoveredAFTER
y (HOURS)
(ppm)Head 1 3 4 5
2
Tail
F 1 % Sugar + 70PPM Ca 6692 165 43.6 82.3 93.4 96.3 97.5
G Anglo - Distilled water 6937 143 30.4 76.1 91.3 95.9 97.9
.
I Zadra - Recycled Barren 6540 2540 20.5 35.9 46.6 54.7 61.2
Solution,
1 %NaOH+0.1 %NaCN, 16ppm
AU
Example 4
Fig. 2-D and Table 4 show one way of improving the performance of the Zadra
process. The eluant in the Zadra process is recycled and has a "bleed" to
reduce the build-up
of deleterious concentration of salts in the eluant. Normally, this bleed
represents about 10-
30% of the total volume of eluant. Fresh solution is added to the
recirculating eluant to
replace the bleed solution. Instead of adding the fresh eluant into the
recirculating eluant,
.___
the fresh eluant of sugared water is used as the initial eluant solution. The
higher the
percentage of fresh eluant in the elution, the greater the efficiency of
elution. Gold elution
is favored by low ionic strength eluant, i.e., fresh sugared water eluant. As
will be
appreciated, the recycled electrowinning barren solution has a high ionic
strength due to the
addition of caustic to improve the electrowinning. Therefore, it is
advantageous to the
elution process if fresh eluant is used first and later followed by the
recycled barren
electrowinning barren solution.
In one process configuration, the fresh eluant preferably contains no more
than about
35% by volume, more preferably no more than about 25% by volume, and even more
preferably is substantially free of recycled eluant. Preferably, the fresh
eluant contains no
-25-
CA 02315480 2000-08-11
more than about 1,000 ppm sodium ions and more preferably no more than about
500 ppm
sodium ions. Typically, the recycled eluant contains no more than about 45% by
volume
fresh eluant and more typically no more than about 25% by volume fresh eluant.
Typically,
the recycled eluant contains 5,000 ppm or more sodium ions and more typically
about 7,500
ppm or more sodium ions. During the elution process as a whole the total
volume of eluant
used is typically from about 25 to about 50% by volume fresh eluant.
Table 4
Eluant CarbonAssa % Gold
RecoveredAFTER
y (HOURS)
(ppm)Head 1 2
3
4
5
Tail
F 7.5 BV Sugar/calcium solution6692 165 43.6 82.3 93.4 96.3 97.5
J 2.25BV Sugar/Ca, then 5.25BV6736 779 40.4 75.8 81.4 85.7 88.4
Zadra (5% sugar)
_.
_ D 1.SBV Sugar/Ca, then 6BV 6741 1400 38.4 54.4 66.1 74.0 79.2
_ Zadra
(5% sugar)
I 7.5BV Recycled Zadra solution6560 2540 20.5 36.1 46.7 54.8 61.3
Example 5
FIG. 2-E and Table 5 show the elution efficiency of the sugar/calcium eluant
in three
successive cycles. The elution profiles show that the eluant can be recycled
without negative
effect.
-26-
CA 02315480 2000-08-11
Table 5
Cycl Eluant CarbonAssa % Gold
RecoveredAFTER
a No. y (HOURS)
(ppm)Head 1 2
3
4
5
Tail
F 0 7.5 BV- 1% Sugar+ 6692 165 43.6 82.3 93.4 96.3 97.5
64ppm Ca in Tap Water
Fl 1 3.65 BV - recycled 6644 149 43.0 76.6 89.2 95.3 97.8
Eluant
from Cycle 0, then
3.75
BV - Fresh Eluant
F2 2 3.75 BV - Recycled 6840 236 43.8 72.8 86.4 93.9 96.5
Eluant
from Cycle l, then
3.75
BV - Fresh Eluant
F3 3 3.75 BV - Recycled 6783 122 41.2 75.7 89.6 96.2 98.2
Eluant
from Cycle 2, then
3.75
BV - Fresh Eluant
Example 6
-_ , FIG. 2-F and Table 6 compares the elution efficiency of the acid washed
carbon and
the non-acid washed carbon.
Table 6
Treatment Eluant CarbonAssay %
Gold
RecoveredAFTER
(ppm)Head (HOURS)
Tail 1
2
3
4
5
F Acid-Washed SugarlCa 6692 165 43.6 82.3 93.4 96.3 97.5
H Not-Acid Sugar/Ca 6616 246 36.8 76.6 90.1 94.4 96.3
Washed
K Not-Acid Distilled Water 6748 204 34.6 70:7 86.7 93.4 97.0
Washed
Table 6 shows that the elution of gold is improved by first acid washing the
carbon before
elution to remove impurities. It is believed that by removing the impurities
in the carbon
-27-
CA 02315480 2000-08-11
pores, migration of the solution into and out of the carbon pores is
facilitated, thereby
improving removal of the gold complex from the carbon.
Example 7
Standard conditions used in Examples 7 and 8 below are as follows:
' (a) The samples were dried Carbon-in-Leach (CIL) carbons.
(b) Elution Temperature: 95-98°C.
(c) Carbons were treated for one hour with hot recirculating S% NaCN solution.
A loaded carbon containing about 1500 g/t gold, 285 g/t silver, 146 g/t
copper, and
2058 g/t nickel was subjected to the elution process of Fig. 4 and yielded the
following
results.
Table 7
From Mine Gold, Silver, Copper, Nickel, g/t
A g/t g/t g/t
Loaded Carbon1500 284 146 2058
Eluted Carbon61 66 37 48
The carbon was not acid washed before elution. Due to the presence of sugar
and ammonia
in the eluant, ammonia being a mass transfer agent, the elution of gold,
silver, copper and
nickel was maintained at acceptable levels.
Example 8
A loaded carbon containing 14700/t gold and 156g/t silver from mine "B" was
subjected to the elution process of Fig. S and yielded the following results:
-28-
CA 02315480 2000-08-11
From Mine Gold, g/t Silver, Copper, Nickel, g/t
B g/t g/t
Loaded Carbon14700 156 25 613
Eluted Carbon133 12 13 30
Example 8 shows that results can be obtained that are similar to those of the
prior Example
using the flowsheet of Figure 5.
Example 9
A method for comparing carbon activity was used to compare the following
carbon
regeneration treatments.
~ Spent carbon, with no regeneration treatment;
~ Spent carbon, acid washed at 95°C with nitric acid (HN03);
~ Spent carbon, acid washed at 95°C with hydrochloric acid (HCI);
~ Spent carbon, acid washed at 95°C with glycolic acid (GA);
____
~ Spent carbon, acid washed at 95°C with sulfuric acid (HZSO4); and
~ Spent carbon, with thermal regeneration.
The methodology involves presoaking one gram of carbon sample in "tailings"
slurry
for one hour before adding the carbon to one liter of standard gold cyanide
solution
containing about 10 ppm gold and buffered to pH 10. The purpose of presoaking
the carbon
in the tailings slurry was to neutralize the "pH effect" of the acid treatment
on carbon
activity. The carbon samples were screened to minus 8-mesh and plus 10-mesh.
At 15
minutes time intervals, small aliquots of test solution were removed to
determine the
remaining gold concentration.
-29-
CA 02315480 2000-08-11
Figure 7 shows the carbon activity of carbon that has not been regenerated by
acid
washing to provide a baseline or that has been regenerated by acid washing
using various
acids, namely nitric acid, hydrochloric acid, sulfuric acid, or glycolic acid
or, though not acid
washed, was thermally reactivated or regenerated using conventional
techniques.
The data in Figure 7 shows wo significant improvement in carbon activity was
attributed to the use of nitric and hydrochloric acid wash. Conventional acid
washing allows
for the removal of minerals containing calcium, magnesium and aluminum. The
removal
of these minerals proves helpful in improving thermal regeneration of the
carbon.
On the other hand, the data shows that washing with sulfuric or glycolic acid
solution
significantly improved carbon activity. It is possible that thermal
regeneration can be
significantly reduced or eliminated in gold-adsorption processes such as
carbon-in-leach or
CIL or carbon-in-pulp or CIP.
--- ~ 'Maximum carbon regeneration was achieved by thermal regeneration at a
price of
high carbon losses. It is not always necessary to have a carbon that has very
high activity.
The gold adsorption process will dictate the required carbon activity for
optimum operation.
Conclusions. Ramifications and Scope
It is clear that blinding agents such as sucrose enhance the elution of
metals/metal
complexes such as gold cyanide complexes from sorbents such as activated
carbon. The
eluting process of the present invention extends present knowledge of elution
chemistry.
Furthermore, the process of the present invention has additional advantages
over the prior
art in that:
-30-
CA 02315480 2000-08-11
1. It allows the use of commonly available water and reagents;
2. It allows the recycling of metal-loaded eluant containing high
concentrations
of metal;
3. It provides a fast elution method at 95-98°C and faster still at
higher
temperatures;
4. It provides a metal-loaded eluant one-half the volume required by prior
art;
5. It provides a metal-loaded eluant chemistry suitable for precipitation or
electrowinning;
6. It provides a once-through or recirculation-type elution;
7. It provides a simple means of regenerating the sorbent immediately after
elution using the same vessel; and
8. It provides a simple, economical, and efficient method of eluting
metals/metal
comple~Ces from activated carbon and other sorbents.
The specific data in the examples described above are merely illustrative;
they do not
limit the scope of the invention. Various ramifications are possible within
the scope of the
invention. For example, operating at higher than suggested temperature will
further enhance
the elution of the metal/metal complexes. The metal-loaded sorbent need not be
washed
with acid before elution to benefit from this invention. Sugar is a
carbohydrate containing
carbon, hydrogen and oxygen only. Other carbohydrates include: (a)
monosaccharides
including sugars, such as glucose and fructose, (b) disaccharides including
the sugars
sucrose, maltose, and lactose, and (c) polysaccharides including cellulose,
starch, and
glycogen. Monosaccharides are polyhydroxy aldehydes or ketones, usually
referred to as
aldoses and ketoses, respectively.
-31-
CA 02315480 2000-08-11
Thus, the scope of the invention should be determined by the appended claims
and
their legal equivalents, rather than by the examples given.
-32-