Note: Descriptions are shown in the official language in which they were submitted.
CA 02315490 2000-08-11
BONE PLATE
Backgroand
Currently it is standard practice to reduce bone fractures and in some cases
provide internal
fixation with metal plates arid screws. The common materials currently used
are stainless steel,
chrorae cobalt, titanium and various alloys of such metals. 'The bone plates
arc designed to
support fA-actured bones in place so that they may heal in an appropriate
position. The bone
plates are superior to immobilization of the bone using simple casting
techniques since they
eliminate the inconvenience of long casting periods and thus permits earlier
mobility to the
patient.
Many metallic bone plates were designed over the years for varying purposes.
In general, the
design consists of a bar of the particular metal which is curved on the
surface, and the device is
then placed against the borxe. Series of screws are then introduced into
available screw holes on
the plate to secure the plate to the bone.
It has been suggested that it would be desirable to form such bone plates
;6~om materials which
would be absorbed by the body. Such materials are called bioabsorbable
materials. Examples of
such bioabsorbables are: poly(hydroxy acids), polyorthoesters, polydioxanone,
polyanhydrides,
polyphosphoesters,, and poly(E-caprolactone). Bioabsozbable fracture fixation
devices offer three
major advantages over conventional metallic implants: the need for removal
surgery is obviated,
the degradation products are biocompatible in contrast to harmful metallic
iozts, and the elastic
modulus is closer to that of bone, which could minimize stress concentrations
near the
extremeities of the irnplaztt and reduce, if not eliminate, stress protection
atrophy of the bone.
However, current bioabsorbables are inadequate in strength in comparison to
their metallic
counterparts. Xt is possible to simply increase the bioabsorbable thickness td
meet rraechanical
requirements, but such method is not desirable. In order to compensate for the
lack of strength, a
stronger material can be combined and bonded with the bioabsorbable to for~oa
a complete device.
Sunnnuary of the Invention
'1'l~e present invention provides a bone plate made from two different
materials bonded together:
implant human bone and bioabsorbable polymer. This plate can be used in the
fixation of bones
without fear of the bone plate breaking. The device can he a sandwich design
in which multiple
alternate layers of bones and bioabsorbables are bonded together. Example
combinations of
layering rn:ay be: bone and bioabsorbable, or bone, bioabsozbable and bone.
CA 02315490 2000-08-11
Brief Description of the Drawings
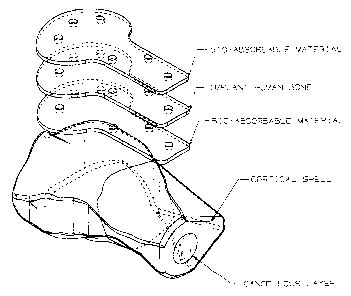
Figure 1 is a perspective exploded view of the present invention comprising
one layer of bone
and two layers of bio-absorbable material;
Figure 2 is a perspective view of the present invention comprising two layers
of bone and one
layer of bio-absorbable material;
Figure 3 is a perspective view of the present invention having an inner
layerof bone and an outer
layer of bio-absorbable material; and
Figure 4 is a perspective view of the present invention having an inner layer
of bio-absorbable
material and an outer layer of bone.
DetaiXed Aescriptioa of the Invention
The bone plate of the present invention is made from a bioabsorbable polymer
(e.g. absorbable
polylactide polymer disclosed in U.S. Pat. Nos. 4,539,981 and 4,550,449).
Zznplant human bones
obtained from parents themselves or the bone bank will be bonded with
bioabsorbable polymers
to produce hone plates and other internal fixation devices. Screws can be made
of either znetal.lic
or bioabsorbable matezials. The construct will maintain its strengti~ for an
appropriate period of
time for the bone, onto which it is placed, to heal. Over tiute, the
bioabsorbable compbncnt will
be absorbed while the implant bone will fuse with the fractured bone. A,s the
bioabsorbable is
absorbed, the bone plate will lose its s~ength. At the same ti~~ e, tl'te
fiactured bone :will ba
healing and eventually be capable of aESUming its normal load.