Note: Descriptions are shown in the official language in which they were submitted.
CA 02318766 2000-07-25
WO 99/46ZZ2 PCT/US99/04514
HIGH GAS YIBLD NON-AZIDS GAS GBNERANTS
BACKGROUND OF THE INVENTION
The present invention relates to nontoxic gas generating
compositions which upon combustion, rapidly generate gases that
are useful for inflating occupant safety restraints in motor
vehicles and specifically, the invention relates to nonazide
gas generants that produce combustion products having not only
acceptable toxicity levels, but that also exhibit a relatively
high gas volume to solid particulate ratio at acceptable flame
temperatures. Additionally, the compositions of the present
invention readily ignite and sustain combustion at burn rates
heretofore thought to be too low for automotive airbag
applications.
The evolution from azide-based gas generants to nonazide
gas generants is well-documented in the prior art. The
advantages of nonazide gas generant compositions in comparison
with azide gas generants have been extensively described in the
patent literature, for example, U.S. Patents No. 4,370,181;
4,909,549; 4,948,439; 5,084,118; 5,139,588 and 5,035,757, the
discussions of which are hereby incorporated by reference.
In addition to a fuel constituent, pyrotechnic nonazide
gas generants contain ingredients such as oxidizers to provide
the required oxygen for rapid combustion and reduce the
quantity of toxic gases generated, a catalyst to promote the
-1-
CA 02318766 2000-07-25
WO 99/46222 PCTNS99/04514
conversion of toxic oxides of carbon and nitrogen to innocuous
gases, and a slag forming constituent to cause the solid and
liquid products formed during and immediately after combustion
to agglomerate into filterable clinker-like particulates.
Other optional additives, such as burning rate enhancers or
ballistic modifiers and ignition aids, are used to control the
ignitability and combustion properties of. the gas generant.
One of the disadvantages of known nonazide gas generant
compositions is the amount and physical nature of the solid
residues formed during combustion. The solids produced as a
result of combustion must be filtered and otherwise kept away
from contact with the occupants of the vehicle. It is
therefore highly desirable to develop compositions that produce
a minimum of solid particulates while still providing adequate
quantities of a nontoxic gas to inflate the safety device at a
high rate.
It is known that the use of ammonium nitrate as an
oxidizer contributes to the gas production with a minimum of
solids. To be useful, however, gas.generants for automotive
applications must be thermally stable when aged for 400 hours
or more at 107°C. The compositions must also retain structural
integrity when cycled between-40°C and 107°C.
Generally, gas generant compositions using ammonium
nitrate are thermally unstable propellants that produce
unacceptably high levels of toxic gases, CO and NOX for
example, depending on the composition of the associated
additives such as plasticizers and binders. Known ammonium
nitrate compositions are also hampered by poor ignitability,
-2-
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
delayed burn rates, and significant performance variability.
Several prior art compositions incorporating ammonium nitrate
utilize well known ignition aids such as BKN03 to solve this
problem. However, the addition of an ignition aid such as
BKN03 is undesirable because it is a highly sensitive and
energetic compound.
Yet another problem that must be addressed is that the
U.S. Department of Transportation (DOT) regulations require
~~cap testing° for gas generants. Because of the sensitivity to
detonation of fuels often used in conjunction with ammonium
nitrate, most propellants incorporating ammonium nitrate do not
pass the cap test unless shaped into large disks, which in turn
reduces design flexibility of the inflator.
Accordingly, many nonazide propellants based on ammonium
nitrate cannot meet requirements for automotive applications.
Two notable exceptions are disclosed in U.S. Patent No.
5,531,941 in which the use of phase-stabilized ammonium
nitrate, triaminoguanidine nitrate, and oxamide is taught, and,
in U.S. Patent No. 5,545,272 .in which the use of phase-
stabilized ammonium nitrate and nitroguanidine is taught.
Despite their usefulness in automotive applications, these
compositions are still problematic because triaminoguanidine
nitrate and nitroguanidine are explosive fuels that complicate
transportation requirements and passing the cap test.
Furthermore, because of poor ignitability and a relatively low
burn rate, the nitroguanidine composition requires a
conventional ignition aid such as BKN03 which is both sensitive
and very energetic..
-3-
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
Certain gas generant compositions comprised of ammonium
nitrate are thermally stable, but have burn rates less than
desirable for use in gas inflators. To be useful for passenger
restraint inflator applications, gas generant compositions
generally require a burn rate of at least .40 ips
(inches/second) at 1000 psi. In general, gas generants with
burn rates of less than 0.40 ips at 1000 psi do not ignite
reliably and often result in "no-fires" in the inflator wherein
only a portion of the gas generant is combusted. Poor
ignitability, even with complete combustion, results in a gas
production rate too slow for automotive airbag applications.
Description of the Prior Art
The gas generant compositions described in Poole et al,
U.S. Patents No. 4,909,549 and 4,948,439, use tetrazole or
triazole compounds in combination with metal oxides and
oxidizer compounds (alkali metal, alkaline earth metal, and
pure ammonium nitrates or perchlorates) resulting in a
relatively unstable generant that decomposes at low
temperatures. Significant toxic emissions and particulate are
formed upon combustion. Both patents teach the use of BKN03 as
an ignition aid.
The gas generant compositions described in Poole, U.S.
Patent No. 5,035,757, result in more easily filterable solid
products but the gas yield is unsatisfactory.
Chang et al, U.S. Patent No. 3,954,528, describes the use
of triaminoguanidine nitrate ("TAGN") and a synthetic polymeric
binder in combination with an oxidizing material. The
oxidizing materials include ammonium nitrate ("AN") although
=4-
CA 02318766 2000-07-25
WO 99/46222 PGT/US99/04514
the use of phase stabilized ammonium nitrate ("PSAN°) is not
suggested. The patent teaches the preparation of propellants
for use in guns or other devices where large amounts of carbon
monoxide and hydrogen are acceptable and desirable.
Grubaugh, U.S. Patent No. 3,044,123, describes a method of
preparing solid propellant pellets containing AN as the major
component. The method requires use of an oxidizable organic
binder (such as cellulose acetate, PVC, PVA, acrylonitrile and
styrene-acrylonitrile), followed by compression molding the
mixture to produce pellets and by heat treating the pellets.
These pellets would certainly be damaged by temperature cycling
because commercial AN is used and the composition claimed would
produce large amounts of carbon monoxide.
Becuwe, U.S. Patent No. 5,034,072, is based on the use of
5-oxo-3-vitro-1,2,4-triazole as a replacement for other
explosive materials (HIS, RDX, TATB, etc.) in propellants and
gun powders. This compound is also called 3-vitro-1,2,4-
triazole-5-one ("NTO"). The claims appear to cover a gun
powder composition which includes NTO, AN and an inert binder,
where the composition is less hygroscopic than a propellant
containing ammonium nitrate. Although called inert, the binder
would enter into the combustion reaction and produce carbon
monoxide making it unsuitable for air bag inflation.
Lund et al, U.S. Patent No. 5,197,758, describes gas
generating compositions comprising a nonazide fuel which is a
transition metal complex of an aminoarazole, and in particular
are copper and zinc complexes of 5-aminotetrazole and 3-amino-
1,2,4-triazole which are useful for inflating air bags in
-5-
CA 02318766 2000-07-25
WO 99/46222 PCT/IJS99/04514
automotive restraint systems, but generate excess solids.
Wardle et al, U.S. Patent No. 4,931,112, describes an
automotive air bag gas generant formulation consisting
essentially of NTO (5-vitro-1,2,4-triazole-3-one) and an
oxidizer wherein said formulation is anhydrous.
Ramnarace, U.S. Patent No. 4,111,728, describes gas
generators for inflating life rafts and similar devices or that
are useful as rocket propellants comprising ammonium nitrate,
a polyester type binder and a fuel selected from oxamide and
guanidine nitrate.
Boyars, U.S. Patent No. 4,124,368, describes a method for
preventing detonation of ammonium nitrate by using potassium
nitrate.
Mishra, U.S. Patent No. 4,552,736, and Mehrotra et al,
U.S. Patent No. 5,098,683, describe the use of potassium
fluoride to eliminate expansion and contraction of ammonium
nitrate in transition phase.
Chi, U.S. Patent No. 5-;074,938, describes the use of phase
stabilized ammonium nitrate as an oxidizer in propellants
containing boron and useful in rocket motors.
Canterberry et al, U. S . Patent No. 4, 925, 503, describes an
explosive composition comprising a high energy material, e.g.,
ammonium nitrate and a polyurethane polyacetal elastomer
binder, the latter component being the focus of the invention.
Hass, U.S. Patent No. 3,071,617, describes long known
considerations as to oxygen balance and exhaust gases.
Stinecipher et al, U.S. Patent No. 4,300,962, describes
explosives comprising ammonium nitrate and an ammonium salt of
-6-
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
a nitroazole.
Prior, U.S. Patent No. 3,719,604, describes gas generating
compositions comprising aminoguanidine salts of azotetrazole or
of ditetrazole.
Poole, U.S. Patent No. 5,139,588, describes nonazide gas
generants useful in automotive restraint devices comprising a
fuel, an oxidizer and additives.
Chang et al, U.S. Patent No. 3,909,322, teaches the use of
nitroaminotetrazole salts with pure ammonium nitrate as gun
propellants and gas generants for use in gas pressure actuated
mechanical devices such as engines, electric crenerators.
motors, turbines, pneumatic tools, and rockets.
Bucerius et al, U.S. Patent No. 5,198,046, teaches the use
of diguanidinium-5,5'-azotetrazolate with KN03 as an oxidizer,
for use in generating environmentally friendly, non-toxic
gases, and providing excellent thermal stability.
Onishi et al, U.S. Patent No. 5,439,251, teaches the use
of a tetrazole amine salt as an air bag gas generating agent
comprising a cationic amine and an anionic tetrazolyl group
having either an alkyl with carbon number 1-3, chlorine,
hydroxyl, carboxyl, methoxy, aceto, nitro, or another
tetrazolyl group substituted via diazo or triazo groups at the
5-position of the tetrazole ring. The focus of the invention
is on improving the physical properties of tetrazoles with
regard to impact and friction sensitivity, and does not teach
the combination of a tetrazole amine salt with any other
chemical.
Lund et al, U.S. Patent No. 5,501,823, teaches the use of
_7_
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
nonazide anhydrous tetrazoles, derivatives, salts, complexes,
and mixtures thereof, for use in air bag inflators.
Highsmith et al, U.S. Patent No. 5,516,377, teaches the
use of a salt of 5-nitraminotetrazole, a conventional ignition
aid such as BKNO3, and pure ammonium nitrate as an oxidizer,
but does not teach the use of phase stabilized ammonium
nitrate.
Therefore, the objects of the invention include providing
high yield (gas/mass > 90%) gas generating compositions that
produce large volumes of non-toxic gases with minimal solid
particulates, that are thermally and volumetrically stable from
-40°C through 110°C, that contain no explosive components, and
that ignite without delay and sustain combustion in a
repeatable manner.
SUMMARY OF T~-IE INVENTION
The aforementioned problems are solved by providing a
nonazide gas generant for a vehicle passenger restraint system
employing ammonium nitrate as an oxidizer and potassium nitrate
as an ammonium nitrate phase stabilizer. The fuel, in
combination with phase stabilized ammonium nitrate, is selected
from the group consisting of amine and other nonmetal salts of
tetrazoles and triazoles having a nitrogen containing cationic
component and an anionic component. The anionic component
comprises a tetrazole or triazole ring, and an R group
substituted on the 5-position of the tetrazole ring, or two R
groups substituted on the 3- and 5-positions of the triazole
ring. The R groups) is selected from hydrogen and any
nitrogen-containing functional groups such as amino, nitro,
-g_
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
nitramino, tetrazolyl and triazolyl groups. The cationic
component is formed from a member of the group including
ammonia, hydrazine; guanidine compounds such as guanidine,
aminoguanidine, diaminoguanidine, triaminoguanidine, and
nitroguanidine; amides including dicyandiamide, urea,
carbohydrazide, oxamide, oxamic hydrazide, Bi-
(carbonamide)amine, azodicarbonamide, and hydrazodicarbonamide;
and substituted azoles including 3-amino-1,2,4-triazole, 3-
amino-5-nitro-1,2,4-triazole, 5-aminotetrazole, 3-nitramino-
1,2,4-triazole, and 5-nitraminotetrazole; and azines such as
melamine.
The gas generants further contain a metallic oxidizer
selected from alkali metal and alkaline earth metal nitrates
and perchlorates. One of ordinary skill will readily
appreciate that other oxidizers such as metallic oxides,
nitrites; chlorates, peroxides, and hydroxides may also be
used. The metallic oxidizer is present at about 0.1-25%, and
more preferably 0.8-15%, by weight of the gas generating
composition.
The gas generants yet further contain an inert component
such as an inert mineral selected from the group containing
silicates, silicon, diatomaceous earth, and oxides such as
silica, alumina, and titania. The silicates include but are
not limited to silicates having layered structures such as talc
and the aluminum silicates of clay and mica; aluminosilicates;
borosilicates; and, other silicates such as sodium silicate and
potassium silicate. The inert component is present at about
0.1-8%, and more preferably at about 0.1-3%, by weight of the
_g_
CA 02318766 2000-07-25
WO 99/46222 PGT/US99/04514
gas generating composition.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
In accordance with the present invention, the preferred
high nitrogen nonazides employed as primary fuels in gas
generant compositions include, in particular, ammonium, amine,
amino, and amide nonmetal salts of tetrazole and triazole
selected from the group including monoguanidinium salt of 5, S' -
Bi-1H-tetrazole (BHT~1GAD), diguanidinium salt of 5,5'-Bi-1H-
tetrazole (BHT~2GAD), monoaminoguanidinium salt of 5,5'-Bi-iH-
tetrazole (BHT~lAGAD), diaminoguanidinium salt of 5,5'-Bi-1H-
tetrazole (BHT~2AGAD), monohydrazinium salt of 5,5'-Bi-1H-
tetrazole (BHT~1HH), dihydrazinium salt of 5,5'-Bi-1H-tetrazole
(BHT~2HH), monoammonium salt of 5,5'-Bi-1H-tetrazole
(BHT~1NH3), diammonium salt of 5,5'-Bi-1H-tetrazole (BHT~2NH3),
mono-3-amino-1,2,4-triazolium salt of 5,5'-Bi-1H-tetrazole
(BHT~lATAZ), di-3-amino-1,2,4-triazolium salt of 5,5'-Bi-1H-
tetrazole (BHT~2ATAZ), diguanidinium salt of 5,5'-Azobis-1H-
tetrazole (ABHT~2GAD), and monoammonium salt of 5-Nitramino-1H-
tetrazole (NAT~1NH3). The primary fuel generally comprises
about 13 to 38%, and more preferably about 23 to 28%, by weight
of the gas generating composition.
R1
N - N N - C
~~ ~~ ' Z ~~ ~~ ' Z
C N C N
/ /
R N R2 N
H g
Formula I Formula II
A generic nonmetal salt of tetrazole as shown in Formula
I includes a cationic component, Z, and an anionic component
-10-
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
comprising a tetrazole ring and an R group substituted on the
5-position of the tetrazole ring. A generic nonmetal salt of
triazole as shown in Formula II includes a cationic component,
Z, and an anionic component comprising a triazole ring and two
R groups substituted on the 3- and 5- positions of the triazole
ring, wherein R, may or may not be structurally synonymous with
R2. An R component is selected from a group including hydrogen
or any nitrogen-containing compound such as an amino, nitro,
nitramino, or a tetrazolyl or triazolyl group from Formula I or
II, respectively, substituted directly or via amine diazo, or
triazo groups. The compound Z forms a cation by displacing a
hydrogen atom at the 1-position of either formula, and is
selected from an amine group including ammonia, hydrazine;
guanidine compounds such as guanidine, aminoguanidine,
diaminoguanidine, triaminoguanidine, and nitroguanidine; amides
including dicyandiamide, urea, carbohydrazide, oxamide, oxamic
hydrazide, Bi-(carbonamide)amine, azodicarbonamide, and
hydrazodicarbonamide; and substituted azoles including 3-amino-
1,2,4-triazole, 3-amino-5-vitro-1,2,4-triazole, 5-
aminotetrazole, 3-nitramino-1,2,4-triazole, and 5-
nitraminotetrazole; and azines such as melamine.
The foregoing nonmetal salts of tetrazole or triazole are
dry-mixed with phase stabilized ammonium nitrate (PSAN). PSAN
is generally employed in a concentration of about 46 to 87%,
and more preferably 56 to 77%, by weight of the total gas
generant composition. The ammonium nitrate is stabilized by
potassium nitrate, as described in Example 16, and as taught in
co-owned U.S. Patent No. 5,531,941, entitled, ~~Process For
-11-
CA 02318766 2000-07-25
WO 99/46222 PCTNS99/04514
Preparing Azide-Free Gas Generant Composition°, and granted on
July 2, 1996, incorporated herein by reference. The PSAN
comprises 85-90% AN and 10-15% KN and is formed by any suitable
means such as co-crystallization of AN and KN, so that the
solid-solid phase changes occurring in pure ammonium nitrate
(AN) between -40°C and 107°C are prevented. Although KN is
preferably used to stabilize pure AN, one skilled in the art
will readily appreciate that other stabilizing agents may be
used in conjunction with AN.
The gas generants further contain a metallic oxidizer
selected from alkali metal and alkaline earth metal nitrates
and perchlorates. One of ordinary skill will readily
appreciate that other oxidizers such as metallic oxides,
nitrites, chlorates, peroxides, and hydroxides may also be
used. The metallic oxidizer is present at about 0.1-25%, and
more preferably 0.8-15%, by weight of the gas generating
composition.
The gas generants yet further contain an inert component
selected from the group containing silicates, silicon,
diatomaceous earth, and oxides such as silica, alumina, and
titania. The silicates include but are not limited to
silicates having layered structures such as talc and the
aluminum silicates of clay and mica; aluminosilicate;
borosilicates; and other silicates such as sodium silicate and
potassium silicate. The inert component is present at about
0.1-8%, and more preferably at about 0.1-3%, by weight of the
gas generating composition.
A preferred embodiment contains 56-77% of PSAN, 23-28% of
-12-
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
diammonium salt of 5,5'-Bi-1H-tetrazole (BHT-2NH3), 0.8-15% of
strontium nitrate, and 0.1-3% of clay.
The combination of the metallic oxidizer and the inert
component results in the formation of a mineral containing the
metal from the metallic oxidizer. For example, the combination
of clay, which is primarily aluminum silicate (A12S14O1o) and
quartz (Si02) with strontium nitrate (Sr(N03)2) results in a
combustion product consisting primarily of strontium silicates
(SrSi04 and Sr3Si05). It is believed that this process aids in
sustaining the gas generant combustion at all pressures and
thus prevents inflator "no-fires~~.
Burn rates of gas generants containing a nonmetal salt as
defined above, PSAN, an alkaline earth metal oxidizer, and an
inert component are low (around .30 ips at 1000 psi), lower
than the industry standard of .40 ips at 1000 psi. Thus, these
compositions quite unexpectedly ignite and sustain combustion
much more readily than other gas generants having burn rates
below .40 ips at 1000 psi, and in some cases, perform better
than gas generants having burn rates greater than .40 ips.
Optional ignition aids, used in conjunction with the
present invention, are selected from nonazide fuels including
triazoles, triazolone, aminotetrazoles, tetrazoles, or
bitetrazoles, or others as described in U.S. Patent No.
5,139,588 to Poole, the teachings of which are herein
incorporated by reference. Conventional ignition aids such as
BKN03 are no longer required because a gas generant containing
a tetrazole or triazole based fuel, phase stabilized ammonium
nitrate, a metallic oxidizer, and an inert component exhibits
-13-
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
improved ignitability of the propellant and also provides a
sustained burn rate with repeatable combustible performance.
The manner and order in which the components of the gas
generating composition of the present invention are combined
and compounded is not critical so long as a uniform mixture is
obtained and the compounding is carried out under conditions
which do not cause decomposition of the components employed.
For example, the materials may be wet blended, or dry blended
and attrited in a ball mill or Red Devil type paint shaker and
then pelletized by compression molding. The materials may also
be ground separately or together in a fluid energy mill, sweco
vibroenergy mill or bantam micropulverizer and then blended or
further blended in a v-blender prior to compaction.
The present invention is illustrated by the following
examples, wherein the components are quantified in weight
percent of the total composition unless otherwise stated.
Values for examples 1-3 and 16-20 were obtained experimentally.
Examples 18-20 provide equivalent chemical percentages as found
in Examples 1-3 and are included for comparative purposes and
to elaborate on the laboratory findings. Values for examples
'4-15 are obtained based on the indicated compositions. The
primary gaseous products are N2, IizO, and CO2, and, the elements
which form solids are generally present in their most common
oxidation state. The oxygen balance is the weight percent of
OZ in the composition which is needed or liberated to form the
stoichiometrically balanced products. Therefore, a negative
oxygen balance represents an oxygen deficient composition
whereas a positive oxygen balance represents an oxygen rich
-14-
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
composition.
When formulating a composition, the ratio of PSAN to fuel
is adjusted such that the oxygen balance is between -4.0% and
+1.0% OZ by weight of composition as described above. More
preferably, the ratio of PSAN to fuel is adjusted such that the
composition oxygen balance is between -2.0% and 0.0% 02 by
weight of composition. It can be appreciated that the relative
amount of PSAN and fuel will depend both on the additive used
to form PSAN as well as the nature of the selected fuel.
In Tables 1 and 2 below, PSAN is phase-stabilized with 15%
KN of the total oxidizer component in all cases except those
marked by an asterisk. In that case, PSAN is phase-stabilized
with 10% KN of the total oxidizer component.
In accordance with the present invention, these
formulations will be both thermally and volumetrically stable
over a temperature range of -40°C to 110°C; produce large
volumes of non-toxic gases; produce minimal solid particulates;
ignite readily and burn in a repeatable manner; contain no
toxic, sensitive, or explosive starting materials; and, be non
toxic, insensitive, and non-explosive in final form.
Table 1
EX CompositionMoles Grams of Oxygen Burn Rate
by Weight of Gas/ Solids/ Balance at 1000
Percent 1008 of 100g of by Weight psi
Generant Generant Percent (in/sec)
1 76.43% PSAN4.00 5.34 0.0% 0.48
23.57%
BHT2NH3
2 75.40% PSAN4.00 5.27 -1.0% 0.47
24.60%
BHT2NH3
3 72.32% PSAN4.00 5.05 -4.0% 0.54
27.68%
BHT2NH3
-15-
CA 02318766 2000-07-25
WO 99/46222 PCTNS99/04514
Table 2
- --
EX Composition Mol Gas/ Grams of Solids/Oxygen
in Weight 100g of 1008 of Balance
Percent Generant Generant in
Weight
Percent
4 73.06% PSAN'4.10 3.40 -4.0%
26.94%
BHT2NH3
76.17% PSAN'4.10 3.55 -1.0%
23.83%
BHT2NH3
5 6 78.25% PSAN'4.10 3.65 +1.0%
21.75%
BHT2NH3
7 73.08% PSAN 3.95 5.11 -4.0%
26.92%
BHT1GAD
8 76.08% PSAN 3.95 5.32 -1.0%
23.92%
BHT1GAD
9 78.08% PSAN 3.95 5.46 +1.0%
21.92%
BHT1GAD
73.53% PSAN 3.95 5.14 -4.0%
26.47%
ABHT2GAD
10 11 76.48% PSAN 3.95 5.34 -1.0%
23.52%
ABHT2GAD
12 78.45% PSAN 3.95 5.48 +1.0%
21.55%
ABHT12GAD
13 46.27% PSAN 3.94 3.23 -4.0%
53.73%
NAT 1NH3
14 52.26% PSAN 3.94 3.65 -1.0%
47.74%
NAT 1NH3
i5 56.25% PsAN 3 , g5 3 . 93 +1
0%
43.75% .
NAT 1NH3
-16-
CA 02318766 2000-07-25
WO 99/46222 PCTNS99/04514
Example 16 - Illustrative
Phase-stabilized ammonium nitrate (PSAN) consisting of 85
wt% ammonium nitrate (AN) and 15 wt% potassium nitrate (KN) was
prepared as follows . 2125g of dried .AN and 3758 of dried KN
were added to a heated jacket double planetary mixer.
Distilled water was added while mixing until all of the AN and
KN had dissolved and the solution temperature was 66-70°C.
Mixing was continued at atmospheric pressure until a dry, white
powder formed. The product was PSAN. The PSAN was removed
from the mixer, spread into a thin layer, and dried at 80°C to
remove any residual moisture.
Example 17 - Illustrative
The PSAN prepared in example 16 was tested as compared to
pure AN to determine if undesirable phase changes normally
occurring in pure AN had been eliminated. Both were tested in
a DSC from 0°C to 200°C. Pure AN showed endotherms at about
57°C and about 133°C, corresponding to solid-solid phase
changes as well as a melting point endotherm at about 170°C.
PSAN showed an endotherm at about 118°C corresponding to a
solid-solid phase transition and an endotherm at about 160°C
corresponding to the melting of PSAN.
Pure AN and the PSAN prepared in example 16 were compacted
into l2mm diameter by l2mm thick slugs and measured for volume
expansion by dilatometry over the temperature range -40°C to
140°C. When heating from -40°C to 140°C the pure AN
experienced a volume contraction beginning at about -34°C, a
volume expansion beginning at about 44°C, and a volume
-17-
CA 02318766 2000-07-25
WO 99/46222 PC'T/US99/04514
contraction beginning at about 90°C and a volume expansion
beginning at about 130°C. The PSAN did not experience any
volume change when heated from -40°C to 107°C. It did
experience.a volume expansion beginning at about 118°C.
Pure AN and the PSAN prepared in example 16 were compacted
into 32mm diameter by lOmm thick slugs, placed in a moisture-
sealed bag with desiccant, and temperature cycled between -40°C
and 107°C. 1 cycle consisted of holding the sample at 107°C
for 1 hour, transitioning from 107°C to -40°C at a constant
rate in about 2 hours, holding at -40°C for 1 hour, and
transitioning from -40°C to 107°C at a constant rate in about
1 hour. After 62 complete cycles, the samples were removed and
observed. The pure AN slug had essentially crumbled to powder
while the PSAN slug remained completely intact with no cracking
or imperfections.
The above example demonstrates that the addition of KN up
to and including 15 wt% of the co-precipitated mixtures of AN
and KN effectively removes the solid-solid phase transitions
present in AN over the automotive application range of -40°C to
107°C.
Example 18
A mixture of PSAN and BHT~2NH3 was prepared having the
following composition in percent by weight: 76.43% PSAN and
23.57% BHT~2NH3. The weighed and dried components were blended
and ground to a fine powder by tumbling with ceramic cylinders
in a ball mill jar. The powder was separated from the grinding
cylinders and granulated to improve the flow characteristics of
-18-
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
the material. The granules were compression molded into
pellets on a high speed rotary press. Pellets formed by this
method were of exceptional quality and strength.
The burn rate of the composition was 0.48 inches per
second at 1000 psi. The burn rate was determined by measuring
the time required to burn a cylindrical pellet of known length
at a constant pressure. The pellets were compression molded in
a l/2~~ diameter die under a 10 ton load, and then coated on the
sides with an epoxy/titanium dioxide inhibitor which prevented
burning along the sides.
The pellets formed on the rotary press were loaded into a
gas generator assembly and found to ignite readily and inflate
an airbag satisfactorily, with minimal solids, airborne
particulates, and toxic gases produced. Approximately 95% by
weight of the gas generant was converted to gas. The ignition
aid used contained no booster such as BKN03, but only high gas
yield nonazide pellets such as those described in U.S. Patent
No. 5,139,588.
As tested with a standard Bureau of Mines Impact
Apparatus, the impact sensitivity of this mixture was greater
than 300 kp~cm. As tested according to U.S. D.O.T. procedures
pellets of diameter 0.184" and thickness of 0.080~~ did not
deflagrate or detonate when initiated with a No. 8 blasting
cap.
Example 19
A mixture of PSAN and BIiT~2NH3 was prepared having the
following composition in percent by weight: 75.40% PSAN and
-19-
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
24.60% BHT~2NH3. The composition was prepared as in Example
18, and again formed pellets of exceptional quality and
strength. The burn rate of the composition was 0.47 inches per
second at 1000 psi.
The pellets formed on the rotary press were loaded into a
gas generator assembly. The pellets were found to ignite
readily and inflate an airbag satisfactorily, with minimal
solids, airborne particulates, and toxic gases produced.
Approximately 95% by weight of the gas generant was converted
to gas.
As tested with a standard Bureau of Mines Impact
Apparatus, the impact sensitivity of this mixture was greater
than 300 kp~cm. As tested according to U.S. Department of
Transportation procedures, pellets of diameter 0.250° and
thickness of 0.125" did not deflagrate or detonate when
initiated with a No. 8 blasting cap.
Example 20
A mixture of PSAN and BHT~2NFi3 was prepared having the
following composition in percent by weight: 72.328 PSAN and
27.68% BHT~2NH3. The composition was prepared as in example
18, except that the weight ratio of grinding media to powder
was tripled. The burn rate of this composition was found to be
0.54 inches per second at 1000 psi. As tested with a standard
Bureau of Mines Impact Apparatus, the impact sensitivity of
this mixture was greater than 300 kp~cm. This example
demonstrates that the burn rate' of the compositions of the
present invention can be increased with more aggressive
-20-
CA 02318766 2000-07-25
WO 99/46222 PGT1US99/04514
grinding. As tested according to U.S:D.O.T. regulations,
pellets having a diameter of .184~~ and thickness of 0.090~~ did
not deflagrate or detonate when initiated with a No. 8 blasting
cap.
In accordance with the present invention, the ammonium
nitrate-based propellants are phase stabilized, sustain
combustion at pressures above ambient, and provide abundant
nontoxic gases while minimizing particulate formation. Because
the nonmetal salts of tetrazole and triazole, in combination
with PSAN, are easily ignitable, conventional ignition aids
such as BKN03 are not required to initiate combustion.
Furthermore, due to reduced sensitivity and in accordance
with U.S.D.O.T. regulations, the compositions readily pass the
cap test at propellant tablet sizes optimally designed for use
within the air bag inflator. As such, a significant advantage
of the present invention is that it contains nonhazardous and
nonexplosive starting materials, all of which can be shipped
with minimal restrictions.
Comparative data of the prior art and that of the present
invention are shown in Table 3 to illustrate the gas generating
benefit of utilizing the tetrazole and triazole amine salts in
conjunction with PSAN.
-21-
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
Table 3 - Comparative Gas Production
U.S. Patent mol gas/ mol gas/ cm3 gas Comparative
No. 100 g prop. 100 cm' generant/ Propellant
gas generant mol gas Volume For
Equal Amount
of Gas
Output
4,931,111 1.46 3.43 29.17 193%
Azide
5,139,588 2.18 4.96 20.16 133%
Nonazide
5,431,103 1.58 5.26 19.03 126%
Nonazide
Present 4.00 6.60 15.15 100%
Invention
As shown in Table 3, and in accordance with the present
invention, PSAN and amine salts of tetrazole or triazole
produce a significantly greater amount of gas per cubic
centimeter of gas generant volume as compared to prior art
compositions. This enables the use of a smaller inflator due
to a smaller volume of gas generant required. Due to greater
gas production, formation of solids are minimized thereby
allowing for smaller and simpler filtration means which also
contributes to the use of a smaller inflator.
In yet another aspect of the invention, it has also been
discovered that certain gas generating compositions containing
PSAN and a nonmetal salt of tetrazole or a nonmetal salt of
triazole may exhibit poor ignitability and incomplete
combustion thereby resulting in an inadequate rate of gas
production and/or in "no-fires" . As shown in Examples 21-27 in
Table 4, by adding a metallic oxidizer and an inert component
in the percentages given above, silicates are formed thereby
improving ignitability and sustaining combustion at all
pressures.
-22-
CA 02318766 2000-07-25
WO 99/4b22Z PCTNS99/04514
Table 4
Exa le 21 22 23 , 24 25 26 27
C oneats
PSAN (lOwt% 75.1 67.2 66.4 73.1 56.3 65.4 74.0
KN)
BHT-2NH3 24.9 19.8 26.1 24.3 26.6 25.8 25.0
Sr(N03)2 7.5 14.5 7.5 0.8
Cha 2.6 2~.6 1.3 0.2
Nitro anidine 13.0
Chas and Solids
1 0 Gas Conversion 97 97 94 94 88 92 96
(wt. %)
60L Tank nd 0.32 0.32 0.24 0.26 0.36 0.35
Solids ( )
100ft3 nd 130 123 110 140 120 174
Particulates
(mg/m3)
Cc~bustioa
Solid Residue nd nd SrC03 K2C03 SrzSi04SrZSi04nd
Inflator yes yes yes no no no no
No-Fires?
Burn at no no no yes yes yes some-
Atmos heric times
P?
Burn at no no some- yes yes
yes some-
100 si? times times
Hura Rates
iK si (in/sec) 0.49 0.44 0.47 0.25 0.28 0.28 0.45
3K si (in/sec) 1.19 0.97 0.84 0.57 0.58 0.66 1.06
5K psi (in/sec)1.37 0.97 1.05 0.80 0.78 0.90 1.27
Low P n (<2.5K)0.89 0.93 1.04 0.75 0.68 0.82 1.00
3 0 Exponent Break 2500 2000 1000, none none none 2000
( si) 3000
Hi h P n (>2.5K0.41 0.16 0.24 0.75 0.68 0.82 0.47
8ffluents~
CO% nd 160 107 98 105 100 92
NH3% nd 141 81 276 117 100 125
NO% nd 58 83 265 83 100 119
NOZ% nd 25 50 1075 30 100 80
nd-indicates that no data is available
The effluents are written as a percentage of values of
Example 26.
-23-
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
Examples 21-27
In Examples 21-27, the phase stabilized ammonium nitrate
(PSAN) contained 10% KN by weight and was prepared by
cocrystallization from a saturated water solution at about
80°C. The diammonium salt of 5,5'-Bi-1H-tetrazole (BHT-2NH3),
strontium nitrate, clay, and nitroguanidine (NQ) were purchased
from an outside supplier.
Each material was dried separately at 105°C. The dried
materials were then mixed together and tumbled with alumina
TO cylinders in a large ball mill jar. After separating the
alumina cylinders, the final product was collected: 15008 of
homogeneous, pulverized powder. The powder was formed into
granules to improve the flow properties, and then compression
molded into pellets (0.184~~ diameter, 0.090~~ thick) on a high
speed tablet press . The tablets were loaded into inf lators and
fired inside a 60L tank and a 100ft3 tank. The 60L tank was
used to determine the pressure over time and to measure the
amount of solids that were expelled from the inflator during
deployment. The 100ft3 tank was used to determine the levels
of certain gases as well as the amount of airborne particulates
produced by the inflator. Table 1 summarizes the results for
each of the compositions.
Examples 21-24 are shown for comparative purposes.
Example 21 contains PSAN and BHT-2NH3. Example 22 contains
PSAN, BHT-2NH3, and NQ. Example 23 contains PSAN, BHT-2NH3,
and strontium nitrate (a metallic oxidizer). Example 24
contains PSAN, BHT-2NH3, and clay (an inert component). In
accordance with the present invention, Examples 25 and 26
-24-
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
contain PSAN, BHT-2NH3, strontium nitrate as a metallic
oxidizer, and clay as an inert component. Finally, Example 27
contains PSAN, BHT-2NH3, strontium nitrate as a metallic
oxidizer, and clay as an inert component, but in amounts other
than as described above. Applicants have discovered that
adding the metallic oxidizer and an inert component to the
compositions of Examples 21 and 22 (and similar compositions as
taught hereinabove), results in sustained combustion and
optimum ignitability. Nevertheless, one of ordinary skill in
the art will readily appreciate that redesigning the inflator
to operate at a higher combustion pressure, for example, would
still make the compositions of Examples 21 and 22 useful in an
automotive airbag application.
As shown in Table 4, Examples 21-27 are typical high yield
gas generants that produce large volumes of gases with minimal
solid particulates. The gas conversion is the percent by
weight of solid gas generant that is converted to gas after
combustion. Although the gas conversion of Examples 25 and 26
is slightly lower than in Bxamples 21-24 and 27, there are no
significant differences in the amount of solids produced by an
inflator in a 60L tank. This demonstrates that the
compositions of Examples 25 and 26 are essentially high yield
gas generants despite a slight decrease in the gas conversion
as compared to Examples 21-24 and 27. All of the Examples
presented in Table 4 are thermally and volumetrically stable
from -40°C to 110°C, and contain no explosive components.
It has been discovered that in certain inflator designs,
the compositions of Examples 21-23 (and similar compositions as
-25-
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
described above) can sometimes experience a ~~no-fire~~ situation
whereby only a portion of the gas generant is combusted. This
is unacceptable for airbag operations demanding a specific rate
of gas production, and therefore requires more complicated
inflators operable at higher pressures. On the other hand, the
compositions of Examples 25-27 when fired consistently result
in complete combustion without delay.
Burn rate data is presented to further describe the
advantages of combining PSAN, a nonmetal salt of tetrazole or
a nonmetal salt of triazole, a metallic oxidizer, and an inert
component . The burn rate model Rb=aP" was assumed to apply,
where Rb = burn rate, a = a constant, P = pressure, and n = the
pressure exponent. Note that the relationship between the burn
rate and pressure, and hence a and n, can change as a function
of pressure. When this occurs, there is a ~~break~~ in the burn
rate vs. pressure curve, indicating a transition to a different
combustion mechanism. Ideally, a gas generant composition
should have a single burning mechanism over the entire inflator
operating pressure. In addition, the gas generant should
ignite easily and sustain combustion over these pressures.
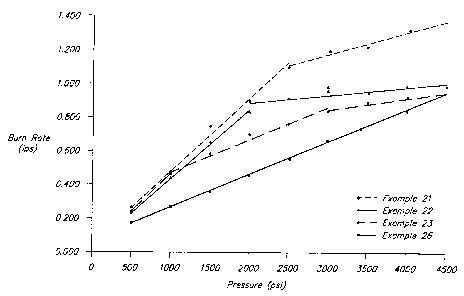
Figure 1 illustrates the ~~break~~ in the pressure exponent of a
gas generant. In Figur 1, the burn rate vs. pressure curves
for Examples 21-23 and 26 are presented. Note that the
composition of Example 26 when combusted shows no ~~breaks~~
thereby indicating a single mechanism of combustion, maintained
and occurring in all of the inflator operating pressures.
At pressures above about 3000 psi, all of the compositions
ignite easily and sustain combustion. As the pressure
-26-
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
decreases below 2000-3000 psi, Examples 21-23 experience a
significant increase in the pressure exponent. This indicates
a transition to a combustion mechanism that is much more
dependent on pressure. At this point, a small decrease in
pressure can dramatically reduce the burning rate of the gas
generant and eventually cause it to extinguish. In fact, it
has been found that certain inflators containing compositions
21-23 sometimes do not function properly because only a small
portion of the gas generant has been consumed. This phenomena
was also observed at very low pressures. When ignited at
atmospheric with a propane torch, compositions 21-23 began to
burn, but always extinguished. Furthermore, these compositions
did not ignite and burn to completion at 100 psi when tested in
a burn rate apparatus.
In contrast, as shown in Figure 1 (note the absence of a
"break" in the curve of composition 26), composition 26 ignites
and burns easily and has the same pressure exponent from 0-4500
psi. When ignited with a propane torch at atmospheric
pressure, composition 26 ignited easily and burned slowly to
completion. At 100 psi in a burn rate apparatus, composition
26 ignited and burned completely. Inflators containing
composition 26 functioned properly on all occasions with easy
ignitability, and complete and steady consumption of the gas
generant. Inflator operating characteristics were relatively
equivalent when composition 25 was used. Note that despite low
levels of a metallic oxidizer and an inert component, and burn
rate properties similar to compositions 21-23, composition 27
functions at the inflator level with complete consumption of
-27-
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
the gas generant.
Composition 24 contains PSAN, the primary fuel
(BHT-2NH3), and an inert component. "No-fires" or combustion
delays were not a problem at the inflator level. However, this
formulation produces high levels of undesirable gases.
Compared to Examples 21-23, and 25-27, composition 24 has a
similar CO level, but much higher levels of ammonia, NO, and
N02, making the composition unsuitable for automotive
applications. This indicates the importance of the metallic
oxidizer in preventing the production of toxic gases.
X-ray dif fraction (XRD) was completed on the solid residue
from compositions 23-26. The major phases are presented in
Table 4. The use of Sr(N03)2 alone in composition 23 results in
the formation of mainly SrC03 with problems of inf lator "no-
fires". The use of clay alone in composition 24 results in the
formation of mainly ICzC03 with problems of high levels of toxic
effluents at the inflator level. The use of both Sr(N03)2 and
clay in compositions 25 and 26 results in the formation of
mainly strontium silicate, Sr2Si04, without occurrence of "no-
fires" or highly toxic effluent levels.
In sum, Examples 21-27 demonstrate that the addition of
both the metallic oxidizer and inert component to PSAN and the
primary fuel is necessary to form a metallic silicate product
during the combustion process. The result is a high-gas yield
generant that is readily ignitable and burns to completion at
all operating pressures, and yet produces minimal solid
particulates and minimal toxic gases.
While the foregoing examples illustrate the use of
-28-
CA 02318766 2000-07-25
WO 99/46222 PCT/US99/04514
preferred fuels and oxidizers it is to be understood that the
practice of the present invention is not limited to the
particular fuels and oxidizers illustrated and similarly does
not exclude the inclusion of other additives as described above
and as defined by the following claims.
-29-