Note: Descriptions are shown in the official language in which they were submitted.
CA 02319508 2000-08-O1
WO 99/44660 PCT/US99/02566
FLUID SAMPLING DEVICE WITH RETRACTABLE NEEDLE
FIELD OF INVENTION
The present device relates to the field of medical devices for fluid sampling.
More specifically, the present invention relates to such medical devices
having a
retractable needle, so that the device is rendered safe after use. In
particular, the
present invention relates to a device for drawing blood from a patient,
wherein after
use the needle retracts so that the contaminated needle is enclosed thereby
preventing
inadvertent contact with the contaminated needle.
BACKGROUND
The present invention relates to a type of medical device that is used to take
a
sample of arterial blood. An arterial blood collection is done commonly in
emergency
room settings, as well as hospitals to test for various conditions, such as
blood oxygen
levels and pH. The standard devices currently used are coated with heparin to
prevent
blood clotting and the fit between the plunger piston and the barrel is loose
enough to
allow the arterial blood pressure to move the piston as the device fills with
arterial
blood. These requirements complicate the reaction of the needle.
SUMMARY OF THE INVENTION
In light of the foregoing, the present invention provides an apparatus and
method for collecting fluid samples from a patient. The device comprises a
housing, a
plunger slidably displaceable within the housing and a needle having a
sharpened tip
CA 02319508 2000-08-O1
WO 99/44660 PCT/US99/02566
for piercing a patient. The needle is operable to pierce the skin of a
patient. Fluid
from the patient collects in a fluid chamber within the housing. After the
sample is l
collected, the needle is retracted into the housing so that the sharpened tip
is enclosed.
After the needle is retracted a pair of seals prevent the sample from leaking
from the
fluid chamber. In addition, the seals preferably operate to prevent air from
entering
the fluid chamber after the needle is retracted. The fluid can then be
expelled from
the fluid chamber by displacing the plunger within the housing.
DESCRIPTION OF THE DRAWINGS
The foregoing summary as well as the following detailed description of the
preferred embodiment can be best understood when read in connection with the
following drawings in which:
Fig. 1 is a top view of a fluid sampling medical device having a retractable
needle;
Fig. 2 is a side view of the fluid sampling medical device shown in Fig. 1;
Fig. 3a is a side view of the device shown in Fig. l, illustrating the device
prior to use;
Fig. 3b is a side view of the device shown in Fig. 3a, illustrating the device
after a
quantity of fluid has been withdrawn;
Fig. 3c is a side view of the device shown in Fig. 3a, illustrating the device
with the
needle in a retracted position;
Fig. 3d is a side view of the device shown in Fig. 3a, illustrating the device
after the
fluid sample has been expelled;
Fig. 4 is a side view of a second embodiment of a fluid sampling medical
device
having a retractable needle;
-2-
CA 02319508 2000-08-O1
WO 99/44660 PCT/US99/02566
Fig. Sa is a side view of the device shown in Fig. 4, illustrating the device
prior to use;
Fig. Sb is a side view of the device shown in Fig. Sa, illustrating the device
after a
quantity of fluid has been withdrawn;
S
Fig. Sc is a side view of the device shown in Fig. Sa, illustrating the device
with the
needle in a retracted position;
Fig. Sd is a side view of the device shown in Fig. Sa, illustrating the device
after the
fluid sample has been expelled;
Fig. 6 is a side view of a third embodiment of a fluid sampling medical device
having
a retractable needle;
Fig. 7a is a side view of the device shown in Fig. 6, illustrating the device
prior to use;
Fig. 7b is a side view of the device shown in Fig. 7a, illustrating the device
after a
quantity of fluid has been withdrawn;
Fig. 7c is a side view of the device shown in Fig. 7a, illustrating the device
with the
piston separated from the plunger;
Fig. 7d is a side view of the device shown in Fig. 7a, illustrating the device
with the
needle in a retracted position;
Fig. 8 is a side view of a fourth embodiment of a fluid sampling medical
device
having a retractable needle;
Fig. 9 is an enlarged fragmentary sectional view of the device shown in Fig.
8;
Fig. 10 is a cross-sectional view of the device shown in Fig. 9, taken along
the line 10-
-3-
CA 02319508 2000-08-O1
WO 99/44660 PCT/US99/02566
10;
Fig. 11 is a side view of the device shown in Fig. 8, illustrating the device
with the
needle in a retracted position;
Fig. 12a is an exploded side view of a combination syringe and removable
needle
assembly;
Fig. 12b is a side view of the device shown in Fig. 12a, illustrating the
needle
assembly attached to the syringe;
Fig. 12c is a side view of the device shown in Fig. 12a, illustrating the
needle in a
retracted position;
Fig. 13a is a side view of a second embodiment of removable needle assembly;
and
Fig. 13b is a side view of the needle assembly shown in Fig. 13 a,
illustrating the
needle in a retracted position.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
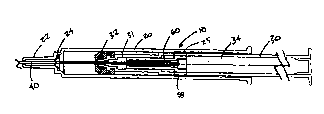
Referring now to the drawings and to Figs. 1-3d specifically, a fluid sampling
device is designated generally 10. The device 10 comprises a barrel 20 and a
needle
40 projecting forwardly from the forward end of the barrel. A plunger 30 is
slidably
displaceable within the barrel 20. Fluid is sampled through the needle. For
instance,
the device may be used to withdraw a quantity of fluid from a patient. The
needle 40
pierces the skin of a patient, and blood from the patient flows into the
barrel 20. After
a sufficient amount of blood has been withdrawn, the needle 40 is retracted
into the
barrel 20 so that the needle is enclosed, preventing inadvertent contact with
the
contaminated sharpened point of the needle.
Referring now to Figs. 1 and 2, the barrel 20 is an elongated generally
-4-
CA 02319508 2000-08-O1
WO 99/44660 PCT/US99/02566
cylindrical hollow housing. The forward end of the barrel 20 forms a reduced
diameter nose piece 22. The nose piece 22 tapers, forming a standard Luer-type
tapered fitting. The nose piece 22 is generally closed, having an aperture for
receiving
the needle 40.
The plunger 30 has a hollow forward stem 31. An elastomeric piston 32 is
attached to the forward end of the plunger stem 31. The piston 32 forms a
fluid-tight
seal with the interior wall of the barrel 20. The stem is integrally formed
with the
rearward portion of the plunger, which is an elongated hollow cylindrical
portion,
which forms a needle chamber 34 for receiving the needle after the needle is
retracted.
The rearward end of the needle chamber is closed to prevent the needle from
being
displaced rearwardly of the needle chamber. An actuator 36 is formed at the
forward
end of the needle chamber 34. The actuator is generally frustoconical, and is
formed
to matingly cooperate with a needle retainer 50 that releasably retains the
needle.
The needle 40 includes a side port 42 formed in the side wall of the needle.
In
addition, the rearward end of the needle is plugged. The needle is disposed so
that the
side port 42 is located forward of the piston. A variable volume is formed in
the
barrel between the piston 32 and the forward end of the barrel 20.
Accordingly, fluid
flowing through the needle is discharged through the side port 42 into the
fluid
chamber between the piston 32 and the nose 22.
The needle 40 is operable between a projecting position in which the
sharpened tip of the needle projects forwardly from the nose 22 of the barrel
20, and a
retracted position in which the needle is enclosed within the barrel. A spring
60
circumscribes the needle 40, biasing the needle rearwardly toward the
retracted
position. The needle retainer 50 releasably retains the needle 40 in the
projecting
position against the bias of the spring 60.. When the actuator 36 engages the
needle
retainer 50, the needle retainer releases the needle 40, allowing the spring
to propel
the needle rearwardly into the needle chamber 34.
The needle retainer 50 is rigidly connected to the barrel 20 so that the
needle
retainer is fixed axially relative to the barrel. The interior wall of the
barrel 20
includes a recess that forms a seat 25 for receiving the needle retainer 50.
As shown
in Fig. 1, the needle retainer 50 comprises a pair of connecting tabs 58 that
form a
-5-
CA 02319508 2000-08-O1
WO 99/44660 PCT/US99/02566
snap fit or friction fit with the seat 25 in the wall ofthe barrel. The
connecting tabs 58 _
project through a pair of slots 38 in the side walls of the plunger 30. The
slots allow
the plunger to be displaced axially relative to the needle retainer.
The needle retainer 50 includes at least one finger or latch 52 for releasably
retaining the needle. In the present instance, the fingers 52 are bonded to
the needle
by UV curable epoxy. Alternatively, a block can be attached to the needle and
the
finger can abut the block to retain the needle against rearward axial
displacement.
The forward end of the fingers form a tapered actuation surface 56 that
cooperates with the tapered actuator 36 on the plunger. When the plunger is
displaced
I 0 rearwardly, the actuator 36 engages the tapered actuation surface 56 of
the needle
retainer, wedging the fingers apart. In this way, the fingers are displaced
radially
outwardly out of engagement with the needle. The spring then propels the
needle
rearwardly into the needle chamber 34.
The needle retainer SO further includes a spring housing 54 projecting
15 forwardly from the fingers 52. The forward end of the spring housing 54
form a
bearing surface against which the forward end of the spring 60 bears. The
rearward
end of the spring is bonded to the needle. Alternatively, if a block is
attached to the
needle, the rearward end of the spring may bear against the block.
As shown in Figs. 1 and 2, in the projecting position, the forward end of the
20 needle projects from the forward end of the barrel 20. The needle also
projects
through the piston 32 and into the needle retainer 50. The piston 32 includes
a
pierceable septum that forms a fluid-tight seal with the exterior surface of
the needle
to prevent fluid from leaking from the barrel into the plunger 30. In
addition, in the
projecting position, the needle pierces a nose seal 24 disposed within the
nose 22 of
25 the barrel. The nose seal forms a fluid-tight seal with the exterior
surface of the
needle to prevent fluid from leaking from the barrel through the nose 22.
The device can be designed to operate in two different manners. In the first
manner, the plunger is withdrawn to form a fluid chamber of a particular
volume. The
needle is then inserted into a patient and blood flows through the needle and
into the
30 barrel, filling the fluid chamber. When designed to be used in this manner,
a
hydrophobic vent is included to prevent the device from becoming airlocked,
which
-6-
CA 02319508 2000-08-O1
WO 99/44660 PCT/US99/02566
would impede the flow of blood into the fluid chamber. The vent is air
permeable, _ _
but is not permeable to blood The vent allows air from the fluid chamber to be
discharged from the fluid chamber as the blood enters the fluid chamber, but
prevents
blood from leaking from the fluid chamber.
Alternatively, the device 10 can be configured to operate so that the blood
pressure displaces the plunger rearwardly as blood enters the fluid chamber.
During
such use, the plunger is displaced forwardly so that the piston is disposed at
the
forward end of the barrel, engaging the forward wall of the barrel. The needle
is then
inserted into the patient and blood flows into the barrel, displacing the
piston 32
rearwardly as blood enters the barrel. When designed to be used in such a
manner, the
device does not need a vent for venting air from the fluid chamber. In
addition, the
piston or the barrel wall is lubricated to reduce the friction between the
piston and the
barrel to facilitate displacing the plunger.
Referring now to Figs 3a-3d, the device operates as follows. In Fig. 3a, the
device is shown prior to use. The needle 40 is inserted into a patient's blood
vessel,
and blood flows into the fluid chamber in the barrel as shown in Fig. 3b.
Referring to
Fig. 3c, the plunger is then displaced axially rearwardly so that the actuator
36
engages the needle retainer 50 displacing the fingers 52 radially outwardly to
release
the needle. The spring 60 then propels the needle rearwardly into the needle
chamber
so that the needle is enclosed with in the barrel. After the needle retracts,
the septum
of the piston that was pierced by the needle reseals to prevent blood from
leaking into
the plunger. In addition, the nose seal 24 reseals to prevent blood from
leaking
through the nose 22. In this way, the sample is sealed within the fluid
chamber
against contact with the air. Referring now to Fig. 3d, after the needle is
retracted, the
sample can discharged from the syringe so that the sample can be tested. The
sample
is discharged by displacing the plunger forwardly. Displacing the piston
forwardly
creates sufficient fluid pressure to expel the fluid through the hole in the
nose seal
membrane that was formed by the needle.
Referring now to Figs. 4-Sd, a second embodiment 110 of a fluid sampling
device is shown. The device 110 includes a barrel 120 and a retractable needle
140
projecting forwardly from the barrel. A plunger 130 is slidably displaceable
within
CA 02319508 2000-08-O1
WO 99/44660 PCT/US99/02566
the barrel. After a fluid sample is collected in the device, the needle is
retracted into
the barrel so that the needle is enclosed to prevent inadvertent contact with
the
contaminated needle. After the needle is retracted, the fluid is sealed within
a fluid
chamber in the barrel. The fluid sample can then be discharged so that the
sample can
be tested.
The plunger 130 includes a tapered hollow stem132. An elastomeric piston
132 is removably attached to the forward end of the stem. The piston forms a
fluid-
tight seal with the interior of the barrel. Preferably, the a hydrophobic plug
136
extends through the piston, providing a vent for gases in the fluid chamber
between
the piston and the forward end of the barrel. An inwardly projecting annular
flange or
stop ring 125 limits the rearward axial displacement of the piston. After the
piston
engages the ring stop 125, continued rearward displacement of the plunger
detaches
the piston from the plunger.
The plunger stem projects forwardly from the rearward portion of the plunger,
which is an elongated generally cylindrical hollow portion, forming a needle
chamber
134. The stem 132 is also hollow, forming a forward chamber 137 for receiving
the
rearward end of the needle when the needle 140 is disposed in the retracted
position.
The forward end of the forward chamber is smaller in diameter than a block 146
attached to the rearward end of the needle. In this way, upon rearward
displacement
of the plunger, the interior wall of the forward chamber 137 engages the block
146 on
the needle urging the needle rearward. This in turn displaces the needle out
of
engagement with the needle retainer 150 so that continued rearward
displacement of
the plunger retracts the needle rearwardly.
The forward end of the barrel 120 forms a reduced diameter nose 122. The
needle projects forwardly from the nose 122 in the projecting position. In
this
position, the needle passes through an opening in the forward end of the
piston. The
forward opening in the piston is smaller in diameter than the needle, so that
the piston
forms a fluid-tight seal around the exterior of the needle.
A needle retainer 150 releasably retains the needle in the projecting
position.
In the present instance, the needle retainer comprises a pair of receptacles
152 that
cooperate with and engage a spherical detent fixed to the needle.
_g_
CA 02319508 2000-08-O1
WO 99/44660 PCT/US99/02566
The device 120 operates as follows. Referring to Fig. Sa, the device 110 is _
_
shown prior to use. The plunger 130 is displaced rearwardly to provide a fluid
chamber for receiving the fluid sample. The needle is then inserted into the
artery of
the patient. Blood flows through the needle into the fluid chamber through a
side port
142 in the needle to collect the sample, as shown in Fig. Sb. Referring to
Fig. Sc, after
the sample is collected, the plunger is displaced rearwardly to detach the
piston from
the plunger. The plunger is further displaced rearwardly to retract the needle
into the
barrel. Referring to Fig. Sd, the sample can then he expelled by driving the
plunger
forward to re-engage the piston and then drive the piston forwardly.
Referring now to Figs. 6-7d, a third embodiment of a fluid sampling medical
device 210 is illustrated. The device includes a barrel 220 and a retractable
needle
240 projecting from the forward end of the barrel. A plunger 230 is slidably
displaceable within the barrel. After a fluid sample is collected from the
patient, the
needle retracts into the barrel to enclose the contaminated needle.
The barrel is generally cylindrical and hollow. The plunger 230 includes an
elastomeric piston 234 that forms a fluid-tight seal with the interior wall of
the barrel.
The plunger 230 is hollow, having a forward chamber 239 housing the spring
before
the needle is retracted, and a rearward needle chamber 237 for receiving the
needle
after the needle is retracted.
A spring 260 circumscribing the needle biases the needle rearwardly towards
the retracted position. The spring is disposed about the needle 240 between a
fixed
spring block 228 and a needle block 244 connected to the rearward end of the
needle.
The spring block 228 is fixedly attached to the barrel 220. Accordingly, slots
233 are
formed in the side of the plunger 230 to provide clearance for the spring
block 228
when the plunger is displaced within the barrel.
A needle retainer 250 releasably retains the needle in the projecting position
against the bias of the spring 260. In the present instance, the needle
retainer is epoxy
252 that bonds the needle to the nose 222.
Referring to Figs 7a-7d, the device operates as follows. In Fig. 7a, the
device
is illustrated prior to use. The plunger 230 is withdrawn to provide a fluid
chamber
between the piston 234 and a resealable seal 224 that is disposed in the nose
of the
-9-
CA 02319508 2000-08-O1
WO 99/44660 PCT/US99/02566
barrel and provides a fluid-tight seal with the exterior of the needle 240.
Referring to z
Fig. 7b, the needle 240 is inserted into a patient's artery, and blood flows
through a
side port 242 in the needle 240 and into the fluid chamber. Once the sample is
collected the needle is withdrawn from the patient. Referring to Fig. 7c, the
plunger
230 is then displaced rearwardly. The rearward displacement brings the piston
234
into engagement with an annular flange projecting inwardly from the interior
wall of
the barrel. Continued rearward displacement of the plunger detaches the piston
234
from the stem 232 of the plunger. In addition, the rearward displacement
brings an
annular flange 238 into engagement with the needle block 244. Referring to
Fig. 7d,
further rearward displacement of the plunger breaks the bond between the nose
222
and the needle, releasing the needle from the needle retainer 250. The needle
then
propels then needle rearwardly into the needle chamber. The nose seal 224
reseals to
prevent the sample from leaking through the nose 222 of the barrel. In
addition, the
forward end of the piston 234 reseals to prevent the sample from leaking into
the
plunger. In this way, the nose seal 224 and the piston 234 seal the sample
within the
fluid chamber to prevent the sample from contacting the air. After the needle
is
retracted, the sample can be expelled from the barrel into equipment for
testing the
sample by driving the plunger forwardly.
Referring now to Figs. 8-11, a fourth embodiment of a fluid sampling device
310 is shown. The device includes a barrel 320, a retractable needle 340 and a
plunger 330 slidably displaceable within the barrel. This third embodiment
allows the
operator to actuate retraction of the needle regardless of the axial position
of the
plunger.
The barrel 320 is generally cylindrical. The forward end of the barrel is
generally closed, forming a reduced diameter opening. A female Luer-type
fitting 322
projects from the forward end of the barrel 320. An elastomeric seal
threadedly
engages the Luer fitting 322. The seal 322 includes a pierceable membrane
through
which the needle 340 projects. The membrane forms a fluid-tight seal with the
exterior of the needle 340.
The plunger 330 includes a piston 332 that forms a fluid-tight seal with the
interior wall of the barrel. In addition, the piston 332 includes a pierceable
membrane
-10-
CA 02319508 2000-08-O1
WO 99/44660 PCT/US99/02566
through with the needle projects. The piston membrane forms a fluid-tight seal
with_
the exterior of the needle. In addition, the piston includes a hydrophobic
plug 336 that
allows gas to vent from the fluid chamber between the piston and the nose seal
224.
Referring to Figs. 10 and 11, the plunger 330 is a generally U-shaped channel,
having
a needle chamber 334 for receiving the retracted needle 340. A longitudinal,
axially
elongated rib 335 projects upwardly into the needle chamber 334.
Referring now to Figs. 8-10, a manually operable needle retainer 350
releasably retains the needle in the projecting position against the bias of
the spring
360 biasing the needle rearwardly toward the retracted position. The needle
retainer
350 comprises an actuating lever 354 and a latch that engages a block 344
attached to
the needle. As shown in Fig. 9, the latch 354 engages the needle block 344 to
releasably retain the needle. By operating the actuator lever352, the latch
354 pivots
radially outwardly out of engagement with the needle block 344. The spring 360
then
propels the needle rearwardly toward the retracted position.
The latch 357 is biased into engagement with the needle block 344. In the
present instance, a spring finger 359 biases the latch into engagement with
the needle
block. The spring finger 359 is integral with the latch and projects
rearwardly from
the latch. The spring finger 359 resiliently flexes and engages the interior
wall of the
barrel 320. When the actuating lever 354 is operated, the latch displaces
radially
outwardly, thereby resiliently deforming the spring finger 359.
Referring to Fig. 10, the needle retainer 350 is attached to the barrel 320 by
mounting brackets 352. The mounting brackets 352 engage a slot 326 formed in
the
top of the barrel. The mounting brackets 352 fix the needle retainer relative
to the
plunger 330. A transverse spring block 351 is connected to the needle retainer
assembly. The spring block forms a forward bearing surface for the spring 360.
The
actuating lever 354 is attached to the spring block 351 by a flexible web or
living
hinge 355. The web 355 forms a pivot point for the actuating lever 354.
The device operates as follows. The plunger 330 is withdrawn to provide a
fluid chamber for receiving a blood sample from a patient. The needle 340 is
inserted
into a patient's artery. Blood flow through a side port 342 in the needle and
into the
fluid chamber. Once a sufficient amount of blood is withdrawn, the needle is
-11-
CA 02319508 2000-08-O1
WO 99/44660 PCT/US99/02566
withdrawn from the patient. The actuating lever is depressed to pivot the
latch 357
thereby releasing the needle from the needle retainer 350. The spring 360 then
propels the needle rearwardly into the needle chamber. After the needle is
retracted,
the nose seal 324 reseals to prevent from blood from leaking from the fluid
chamber.
The fluid sample can then be expelled from the device into separate device to
test the
sample. The sample is expelled by driving the plunger forwardly within the
barrel.
Referring now to Figs 12a-12c, a device for collecting a fluid sample such as
blood is designated generally 410. The device 410 comprises a syringe 420 and
a
removably connectable needle assembly 430. The needle assembly 430 comprises a
retractable insertion needle 460 for piercing a patient's skin. When the
needle
assembly 430 is connected to the syringe 420, the insertion needle 460 is in
fluid
communication with the interior of the syringe. After the fluid sample is
collected in
the syringe 420, the insertion needle 460 can be retracted into the housing of
the
needle assembly 430 to prevent inadvertent contact with the contaminated
insertion
I S needle. The needle assembly 430 can also be removed from the syringe 420
after the
fluid sample is collected. The fluid sample can then be transferred to where
the
sample is to be tested. The sample can then be expelled from the syringe 410
and
tested.
The syringe 420 is similar to a typical syringe, having a barrel 422, a
plunger
424 with a piston 425 slidably displaceable within the barrel and a Luer-type
fitting
428 on the nose of the barrel. The piston 425 forms a fluid-tight seal with
the interior
wall of the barrel 422, and driving the plunger forward expels fluid from the
syringe
420.
The needle assembly 430 is adapted to connect to the Luer fitting 428 of the
syringe so that the needle assembly can be utilized with standard syringes
that are
already in widespread use throughout the medical field. Accordingly, the
housing 440
of the needle assembly 430 includes an opening at the rearward end, forming a
socket
442 for engaging the Luer fitting 428 of the syringe. A seal having a
pierceable
resealable membrane is disposed within the socket 442. The seal 445 is
externally
threaded having threads that cooperate with the Luer fitting 428.
The needle assembly 430 comprises two needles a forward insertion needle
-12-
CA 02319508 2000-08-O1
WO 99/44660 PCT/US99/02566
460 that projects forwardly from the front end of the housing 440, and a fixed
needle
450 disposed within the housing 440. The fixed needle 450 projects into the
socket
442, piercing the Luer seal 445. The fixed needle 450 is attached to a fixed
needle
tube 452 that is fixedly attached to the housing 440. The rearward end of the
fixed
needle tube is generally closed, having a reduced diameter through which the
fixed
needle 450 projects. The fixed needle is fixedly connected to the fixed needle
tube
452 to form a fluid-tight connection between the exterior surface of the fixed
needle
and the generally closed rearward end of the fixed needle tube.
The insertion needle 460 is fixedly connected to a telescoping needle tube 462
that telescopingly engages the interior of the fixed needle tube 452. A needle
seal 456
disposed within the forward end of the fixed needle tube 452 provides a fluid-
tight
seal between the fixed needle tube and the telescoping needle tube. The
insertion
needle projects forwardly from the forward end of the telescoping needle tube
462.
An annular flange 464 projects outwardly from the telescoping needle tube 462.
A
spring circumscribing the telescoping needle tube 462 is disposed between the
flange
464 and the interior of the forward end of the housing. The spring 480 bears
against
the flange 464 biasing the telescoping needle tube 462 and the attached
insertion
needle 460 rearwardly.
A needle retainer 470 releasably retains the insertion needle 460 against the
bias of the spring 480. The needle retainer 470 comprises an actuator button
472 and
a latch 474. The latch 474 has an aperture through which the telescoping
needle tube
462 projects. In the latched position, the latch 474 is disposed so that the
rim of the
aperture engages the flange 464 to retain the telescoping needle tube against
the bias
of the spring. Depressing the actuator button 472 displaces the latch 474
downwardly
so that the latch aperture is aligned with the annular flange 464. The spring
480 then
propels the telescoping needle tube rearwardly into the fixed needle tube, so
that the
insertion needle is enclosed within the housing 440.
Accordingly, the device 410 operates as follows. The plunger 424 is disposed
so that the piston 425 is located at the forward end of the syringe barrel
422. The
needle assembly 430 is connected to the front end of the syringe 420. The rear
fixed
needle 450 projects through the Luer seal 445 and into the barrel. The
insertion
-13-
CA 02319508 2000-08-O1
WO 99/44660 PCTNS99/02566
needle 460 is then inserted into a patient's artery and blood flows from the
patient into
the interior of the syringe. The pressure of the blood flow drives the piston
and
plunger 424 rearwardly as the blood enters the syringe 420. After a sufficient
amount
of blood is collect, the insertion needle is withdrawn from the patient. The
actuator
button 472 is depressed to actuate retraction of the insertion needle. The
insertion
needle then retracts into the housing. The needle assembly 430 is then
detached from
the syringe 420. The Luer seal 445 remains on the Luer-fitting 428 of the
syringe,
sealing the forward end of the syringe to prevent fluid from leaking out of
the nose of
the syringe 420. The piston 425 forms a fluid-tight seal with the barrel to
prevent
fluid from leaking out of the rearward end of the syringe. The sealed fluid
sample can
then be transported to an area for testing the sample and then expelled from
the
syringe by driving the plunger forwardly within the barrel.
Referring now to Figs. 13a and 13b a second embodiment of a needle
assembly that is operable in connection with a syringe is designated generally
510.
The needle assembly includes a housing 520 and a retractable needle 540
projecting
forwardly from the housing. The rearward end of the housing forms a socket 522
for
connecting the needle assembly to a syringe similar to the manner described
above in
connection with the device designated 410 and illustrated in Figs. 12a-12c.
However,
in the present instance, the socket 522 configured as a female tapered Luer-
type fitting
to cooperate with a male Luer-type fitting on a syringe.
The needle assembly 510 includes a generally cylindrical nose piece 530
attached to the forward end of the housing 520. A nose seal 532 forms a fluid-
tight
seal with the exterior of the needle 540. A generally cylindrical needle tube
545 is
disposed within the housing 520 and projects into the rearward end of the nose
piece
545. The forward end wall of the socket 522 has a reduced diameter opening so
that
the needle tube 545 is in fluid communication with the socket. An annular
detent 542
projects inwardly into the needle tube adjacent the forward end of the needle
tube 545.
An elastomeric valve 550 seals the forward end of the needle tube 545. The
valve 550
has an external circumferential groove 552. The annular detent 542 engages the
circumferential groove to releasably retain the valve 550.
The rearward end of the needle 540 projects into the valve 550. The forward
- i 4-
CA 02319508 2000-08-O1
WO 99/44660 PCT/US99/02566
end of the needle 540 projects forwardly from the nose piece. A spring 560
attached_
to the needle biases the spring rearwardly to a retracted position within the
housing
520. A needle retainer 570 releasably retains the needle in the projecting
position
against the bias of the spring.
The needle retainer 570 is configured similarly to the needle retainer
described
above in connection with the previous device 410. The retainer 570 comprises a
button actuator 572 and a latch 574. The latch has an aperture. In the latched
position,
the rim of the latch aperture engages the end of the spring 560. When the
actuator
button 572 is depressed, the latch is displaced downwardly, aligning the latch
aperture
with the spring, thereby allowing the spring to propel the needle rearwardly
as shown
in Fig. 13b.
Accordingly, the device operates as follows. The needle assembly 510 is
attached to a syringe. The needle is then inserted into a patient's artery.
Blood flows
through the needle 540, and through the valve 550 into the needle tube 545.
From the
needle tube the blood flows into the syringe, where the sample collects. After
a
sufficient amount of blood is removed, the needle 540 is removed from the
patient.
The actuator button 572 is depressed to release the needle 540. The spring 560
propels the needle rearwardly. The needle is driven further within the valve,
sealing
the rearward end of the needle. In addition, the spring biases the valve
against the
opening into the socket to seal the socket opening. The needle assembly
thereby
operates as a seal, sealing the forward end of the syringe. The needle
assembly can be
detached if desired. The syringe can then be sealed with a cap and transported
to an
area where the sample is to be tested. The sample can then be expelled from
the
syringe by driving the plunger forward.
While particular embodiments of the invention have been illustrated and
described above, it is not intended to limit the invention to such disclosure.
It will be
recognized that changes and modifications may be made within the scope of the
following claims.
-15-