Note: Descriptions are shown in the official language in which they were submitted.
CA 02320816 2009-01-26
1
DEVICE RESPONSIVE TO MYOELECTRICAL ACTIVITY
FOR TRIGGERING VENTILATORY SUPPORT
BACKGROUND OF THE INVENTION
1. Field of the invention:
The present invention relates to a method and device for
triggering lung ventilatory support in response to myoelectrical activity of
the diaphragm (or other inspiratory-related muscle), or in response to
myoelectrical activity of the diaphragm (or other inspiratory-related
muscle), inspiratory flow and/or inspiratory pressure in combination.
2. Brief description of the prior art:
Triggering of ventilatory support using airway inspiratory flow
and/or pressure is affected by many factors including:
- inspiratory muscle function, i.e. how activation is
translated into tension, and how tension is translated into
pressure; and
CA 02320816 2008-06-06
2
- respiratory mechanics such as the elastic and
resistive components of the respiratory system.
A drawback of the prior art airway inspiratory flow
and/or pressure based ventilatory support triggering systems is that they
cannot adequately detect inspiratory efforts in, for example, patients
suffering
from severe airflow limitation.
OBJECTS OF THE INVENTION
An object of the present invention is to use
myoelectrical activity of the diaphragm or other respiratory-related muscles
to
trigger ventilatory support and/or to end the ventilatory support, in view of
eliminating inspiratory flow and/or pressure trigger function related problems
due to impedance of the ventilatory support system and the respiratory
system. The present invention will also eliminate the problems related to
leaks in the air flow system (infants).
Another object of the present invention is to provide a
ventilatory support triggering device responsive to a combination of
myoelectrical activity with inspiratory flow and/or pressure to guarantee
adequate triggering of the ventilatory support apparatus in the eventual
presence of delayed onset or absence of myoelectrical activity of the
diaphragm or other respiratory-related muscle. The ventilatory support
triggering device will improve detection of inspiratory efforts without
jeopardizing the patient's ability to use muscles other than the diaphragm to
trigger the ventilatory support system.
CA 02320816 2008-06-06
3
A further object of the present invention is to provide a
ventilatory support triggering device capable of triggering any ventilatory
support system, and of triggering any mode of ventilatory support.
SUMMARY OF THE INVENTION
More specifically, the present invention relates to a
device for triggering ventilatory support from a ventilatory apparatus
connected to a patient's respiratory system to assist respiration of the
patient,
the device comprising:
sensor means for sensing myoelectrical activity of a
respiratory-related muscle of the patient, to thereby detect respiratory
effort
of said patient;
means for producing a myoelectrical signal
representative of the sensed muscle myoelectrical activity; the myoelectrical
signal producing means comprises means for calculating, in response to the
sensed myoelectrical activity, a real-time myoelectrical signal representative
of one of a level and a change in respiratory effort;
means for comparing the real-time myoelectrical signal
to a signal value selected from the group consisting of (a) a given threshold
and (b) a preceding sample of the myoelectrical signal, to produce a
comparison signal; and
means for activating/deactivating ventilatory support in
relation to the comparison signal to synchronise, in real-time,
CA 02320816 2008-06-06
4
activating/deactivating of the ventilatory support to the patient with
respiratory
effort of the patient.
According to one embodiment, the device for triggering
ventilatory support may further comprise means for filtering from the
myoelectrical signal at least one of the following disturbances: motion
artifacts, ECG, electrical interference, and high frequency noise.
According to a second non-restrictive illustrative
embodiment, the comparing means comprises means for comparing at least
one of an amplitude, integral, derivative and combination thereof related to
the real-time myoelectrical signal to the given threshold, and the ventilatory
support activating/deactivating means comprises means for activating
ventilatory support when at least one of said amplitude, integral, derivative
and combination thereof is higher than said given threshold.
According to a third non-restrictive illustrative
embodiment, the comparing means comprises means for detecting an
increment of at least one of an amplitude, integral, derivative and
combination thereof related to the myoelectrical signal, and the ventilatory
support activating/deactivating means comprises means for activating
ventilatory support in response to detection of the increment.
According to a fourth non-restrictive illustrative
embodiment, the increment detecting means comprises:
means for multiplying a current sample of the
myoelectrical signal by a predetermined constant to produce a multiplied
sample;
CA 02320816 2008-06-06
means for comparing the multiplied sample to the
preceding sample of the myoelectrical signal; and
means for detecting the increment when at least one of
5 the amplitude, integral, derivative and combination thereof related to the
multiplied sample is higher than the corresponding amplitude, integral,
derivative and combination thereof related to the preceding sample.
According to a fifth non-restrictive illustrative
embodiment, the comparing means comprises means for comparing at least
one of an amplitude, integral, derivative and combination thereof related to
the myoelectrical signal to the given threshold, and the ventilatory support
activating/deactivating means comprises means for deactivating ventilatory
support when at least one of the amplitude, integral, derivative and
combination thereof is lower than the given threshold.
According to another non-restrictive illustrative
embodiment, the comparing means comprises means for detecting a
decrement of at least one of an amplitude, integral, derivative and
combination thereof related to the myoelectrical signal, and the ventilatory
support activating/deactivating means comprises means for deactivating the
ventilatory support in response to detection of the decrement.
According to a seventh non-restrictive illustrative
embodiment, the decrement detecting means comprises:
means for multiplying a current sample of the
myoelectrical signal by a predetermined constant to produce a multiplied
sample;
means for comparing the multiplied sample to the
preceding sample of the myoelectrical signal; and
CA 02320816 2008-06-06
6
means for detecting the decrement when one of the
amplitude, integral, derivative and combination thereof related to the
multiplied sample is smaller than the respective amplitude, integral,
derivative
and combination thereof related to the preceding sample.
According to a further non-restrictive illustrative
embodiment, the device for triggering ventilatory support further comprises:
means for detecting a level of noise in the
myoelectrical signal; and
means for determining whether the respiratory-related
muscle of the patient is active in relation to the detected level of noise.
According to a ninth non-restrictive illustrative
embodiment, the muscle myoelectrical activity sensing means comprises
means for detecting myoelectrical activity of the respiratory-related muscle
on
two opposite sides of a center of a depolarizing region of the respiratory-
related muscle, and wherein the real-time myoelectrical signal calculating
means comprises:
means for generating two myoelectrical signal
components in response to sensing of the myoelectrical activity of the
respiratory-related muscle on the two opposite sides of the center of the
depolarizing region, respectively, the two myoelectrical signal components
having reversed polarities;
means for subtracting the two myoelectrical signal
components from each other to produce a subtraction signal;
means for adding the two myoelectrical signal
components to each other to produce an addition signal;
means for multiplying the addition signal by a
predetermined constant to produce a multiplied addition signal;
means for comparing the multiplied addition signal to
the subtraction signal; and
CA 02320816 2008-06-06
7
means for accepting the subtraction signal as the real-
time myoelectrical signal when the subtraction signal has one of an
amplitude, integral, derivative and combination thereof higher that a
respective amplitude, integral, derivate and combination thereof related to
the
multiplied addition signal.
According to a tenth non-restrictive illustrative
embodiment, the subtraction signal, the addition signal, the multiplied
addition signal and the myoelectrical signal comprise RMS signals.
According to a still further non-restrictive illustrative
embodiment, the device for triggering ventilatory support further comprises:
a respiratory flow detector for measuring respiratory
flow of the patient, and producing a respiratory flow signal;
a respiratory pressure detector for measuring
respiratory pressure of the patient, and producing a respiratory pressure
signal; and
a logic trigger circuit for triggering ventilatory support in
relation to at least one of the myoelectrical signal, the respiratory flow
signal
and the respiratory pressure signal to assist respiration of the patient in
response to respiratory effort of the patient.
For instance, the diaphragm electromyogram (EMG)
represents the motor unit recruitment and firing rate and hence the
inspiratory effort of the diaphragm which normally is the principal
inspiratory
muscle. Other muscles, for example parasternal intercostal muscles,
sternocleidomatoids, scalenes, alae nasi, etc., associated with inspiratory
efforts can aiso be useful sources for determining the onset of an inspiratory
effort. The inspiratory flow and/or pressure also represents a source of
global inspiratory effort, i.e. the inspiratory effort made by all chest wall
muscles participating in the inspiration. The pressure can be replaced by
CA 02320816 2009-01-26
8
direct measurements of transpulmonary, transabdominal or
transadiaphragmatic pressures. An inspiratory effort can be first detected by
the diaphragm EMG and an instant later as inspiratory flow and/or pressure.
However, limitations of both methods to detect a breathing effort may occur
depending on the condition of the patient. One limitation of using the
diaphragm EMG is that under certain conditions, inspiratory muscles other
than the diaphragm may initiate the inspiration, such that diaphragm EMG
occurs later than inspiratory flow and/or pressure. One limitation of using
airway inspiratory flow and/or pressure measurements is that under certain
conditions, the inspiratory effort is not revealed by such measurements and
consequently the ventilatory support apparatus is not triggered.
The use of EMG to trigger ventilatory support
apparatuses benefits from extremely high quality of the EMG signal. Filtering
and artifacts due to movements of the diaphragm with respect to the muscle
are advantageously minimized. Signal artifacts of non-diaphragmatic origin
are also advantageously eliminated. An example of signal artifacts of non-
diaphragmatic origin is ECG.
The foregoing and other objects, advantages and
features of the present invention will become more apparent upon reading of
the following non restrictive description of an illustrative embodiment
thereof,
given by way of example only with reference to the accompanying drawings.
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
9
BRIEF DESCRIPTION OF THE DRAWINGS
In the appended drawings:
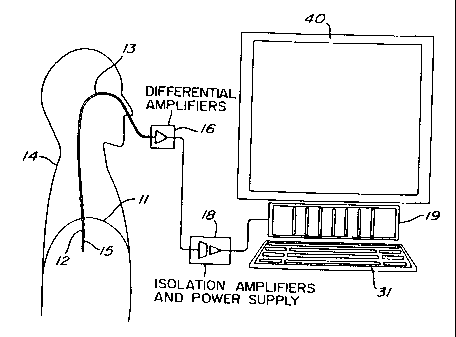
Figure 1 is a schematic representation of a set-up of an
EMG analysis system;
Figure 2 is a section of oesophageal catheter on which
an array of electrodes of the EMG analysis system of Figure 1 is
mounted;
Figure 3 illustrates a section of oesophageal catheter on
which a second embodiment of the array of electrodes is mounted;
Figure 4 is a graph showing a set of EMG signals of the
diaphragm (EMGdi signals) detected by pairs of successive electrodes of
the array of Figure 2;
Figure 5a is a first portion of a flow chart illustrating a
preferred embodiment of the method and device according to the
invention for triggering ventilatory support in response to myoelectrical
activity of a respiration-related muscle, for example the diaphragm;
Figure 5b is a second portion of the flow chart illustrating
a preferred embodiment of the method and device according to the
invention for triggering ventilatory support in response to myoelectrical
activity of the respiration-related muscle, for example the diaphragm;
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
Figure 6a is a graph showing the power density
spectrum of electrode motion artifacts, the power density spectrum of
ECG, and the power density spectrum of EMGdi signals;
Figure 6b is a graph showing an example of transfer
5 function for a filter to be used for filtering out the electrode motion
artifacts, ECG, the 50 or 60 Hz disturbance from electrical mains and high
frequency noise;
Figure 7 is a graph showing the distribution of correlation
10 coefficients calculated for determining the position of the center of the
depolarizing region of the respiration-related muscle, for example the
diaphragm along the array of electrodes of Figure 2;
Figure 8 is a schematic diagram illustrating in the time
domain a double subtraction technique for improving the signal-to-noise
ratio and to reduce an electrode-position-induced filter effect;
Figure 9 is a schematic diagram illustrating in the
frequency domain stabilization by the double subtraction technique of the
center frequency upon displacement of the center of the depolarizing
region of the respiration-related muscle, for example the diaphragm along
the array of electrodes of Figure 2;
Figure 10a is a graph of respiratory and expiratory flow
versus time for quiet breathing of a chronic obstructive pulmonary disease
(COPD) patient qui and Figure 10b is a graph of the RMS value of EMG
versus time for quiet breathing of a COPD patient, the graphs of Figures
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
11
10a and 10b showing the time delay from EMG to airway inspiratory flow;
and
Figure 11a is a graph of esophageal and gastric
pressure versus time for quiet breathing of a chronic obstructive
pulmonary disease (COPD) patient and Figure 11 b is a graph of the RMS
value of EMG versus time for quiet breathing of a COPD patient, the
graphs of Figures 11 a and 11 b showing the relation between EMG and
the esophageal and gastric pressure.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
Although the preferred embodiment of the present
invention will be described in relation to the use of an EMGdi signal
obtained by means of a double subtracted signal and representative of
the myoelectrical activity of the diaphragm, it should be kept in mind that
it is within the scope of the present invention to use another type of
EMGdi signal or to use a signal representative of the myoelectrical activity
of muscles.other than the diaphragm but associated with inspiratory effort
to trigger the ventilatory support apparatus. Examples of other muscles
are parastemal intercostal muscles, sternocleidomatoids, scalenes, alae
nasi, etc. The myoelectrical activity of these muscles can eventually be
detected by means of electrodes directly implanted in the muscle.
Also, although the preferred embodiment of the present
invention will be described in relation to inspiratory support, it should be
kept in mind that the present invention also applies to support of other
respiration-type activity such as expiration support.
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
12
Signal acquisition and processing
The crural diaphragm EMG is recorded from a sheet of
muscle whose fiber direction is mostly perpendicular to an esophageal
bipolar electrode. The region from which the action potentials are elicited,
the electrically active region of the diaphragm (DDR), and the center of
this region, the DDR center, may vary during voluntary contractions, in
terms of their position with respect to an esophageal electrode.
Depending on the position of the bipolar electrode with respect to the
DDR center, the EMGdi signal is filtered to different degrees.
Based on experimental results and anatomical
descriptions of the crural diaphragm, a transfer function for diaphragm
EMG measured with bipolar electrodes was developed:
erpendicular filtering (Ko (w (h-d) /v) -Ko (co (h+d) /v)
~
Ko (wa/v)
where, Ko ()= modified Bessel function, w = angular frequency (i.e. 2rtf
(f being the frequency), h = distance between the signal source and
observation point, d='h inter-electrode distance, v = conduction velocity,
a = muscle fiber diameter.
Based on this transfer function, a new signal analysis
procedure was developed which involves: (a) locating the electrode pair
at the center of the DDR, (b) selecting the signals above and below the
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
13
center of the DDR (reversed in polarity) yielding the highest signal-to-
noise ratio and ( c) subtracting these two signals (double subtraction
technique). The double subtraction technique reduces the influence of
movement of the DDR center relative to the electrode array on the EMG
power spectrum center frequency and root mean square values,
increases the signal to noise ratio by 2 dB, and increases the number of
EMG samples that are accepted by the signal quality indices by 50%. A
more detailed description of the above mentioned double subtraction
technique is given hereinbelow.
For example, to measure EMG activity of the diaphragm
11 (EMGdi) of a human patient 14, an array of electrodes such as 12
(Figures 1 and 2) are mounted on the free end section 15 of an
oesophageal catheter 13, with a constant inter-electrode distance d
(Figure 2). As shown in Figure 1, the catheter 13 is introduced into the
patient's oesophagus through one nostril or the mouth until the array of
electrodes 12 is situated at the level of the gastroesophageal junction.
The diaphragm 11 and/or the oesophagus slightly moves during breathing
of the patient 14 whereby the array of electrodes 12 also slightly moves
about the diaphragm 11. As will be explained in the following
description, automatic compensation for this displacement is provided for.
According to a preferred embodiment, an electrode 12
is mounted on the free end section 15 of the catheter 13 by winding
stainless steel wire (not shown) around that catheter 13. The wound
stainless steel wire presents a rough surface smoothed out by solder,
which in turn is electroplated with nickel, copper and then gold or silver.
Of course, it is within the scope of the present invention to use other
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
14
electrode structures. Also, the electrodes 12 can possibly be applied to
a nasogastric feeding tube (not shown) which is routinely introduced in
intensive-care unit (ICU) patients.
Electric wires (not shown) interconnect each pair of
successive electrodes such as 1-7 (Figure 2) with a respective one of a
group of differential amplifiers 16. Obviously, these electric wires follow
the catheter 13 from the respective electrodes 12 to the corresponding
amplifiers 16, and are preferably integrated to the catheter 13.
Preferably, the electric wires transmitting the EMGdi signals collected by
the various pairs 1-7 of electrodes 12 are shielded to reduce the influence
of external noise, in particular disturbance from the 50 or 60 Hz current
and voltage of the electrical mains.
The group of differential amplifiers 16 amplifies (first
subtraction step of the double subtraction technique) and band-pass
filters each EMGdi signal. This first subtraction step may also be carried
out in the personal computer 19 when the amplifiers 16 are single-ended
or equivalently designed amplifiers (monopolar readings).
In the example illustrated in Figures 1 and 2, the free
end section 15 of the catheter 13 is provided with an array of eight
electrodes 12 defining seven pairs 1, 2, 3, 4, 5, 6 and 7 of successive
electrodes 12 respectively collecting seven different EMGdi signals.
Although it has been found that EMG activity of the diaphragm (EMGdi)
can be measured accurately with an oesophageal catheter 13 provided
on the free end section 15 thereof with an array of eight electrodes 12, a
different number and/or configuration of pairs of electrodes 12 can be
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
contemplated depending on the patient's anatomy and movement of the
diaphragm. Also, the pairs 1-7 do not need to be pairs of successive
electrodes; Figure 3 illustrates an array of nine electrodes to form seven
overlapping pairs of electrodes 1-7.
5 A major problem in recording EMGdi signals is to
maintain the noise level as low and as constant as possible. Since the
electric wires transmitting the EMGdi signals from the electrodes 12 to the
differential amplifiers 16 act as an antenna, it is crucial, as indicated in
the
foregoing description, to shield these electric wires to thereby protect the
10 EMGdi signals from additional artifactual noise. Also, the package
enclosing the differential amplifiers 16 is preferably made as small as
possible (miniaturized) and is positioned in close proximity to the pattent's
nose to decrease as much as possible the distance between the
electrodes 12 and the amplifiers 16.
The amplified EMGdi signals are sampled by a personal
computer 19 through respective isolation amplifiers of a unit 18, to form
signal segments of fixed duration. Unit 18 supplies electric power to the
various electronic components of the differential and isolation amplifiers
while ensuring adequate isolation of the patient's body from such power
supply. The unit 18 also incorporates bandpass filters included in the
respective EMGdi signal channels to eliminate the effects of aliasing. The
successive EMGdi signal segments are then digitally processed into the
personal computer 19 after analog-to-digital conversion thereof. This
analog-to-digital conversion is conveniently carried out by an analog-to-
digital converter implemented in the personal computer 19. The personal
computer 19 includes a monitor 40 and a keyboard 31.
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
16
It is believed to be within the capacity of those of
ordinary skill in the art to construct suitable differential amplifiers 16 and
an adequate isolation amplifiers and power supply unit 18. Accordingly,
the amplifiers 16 and the unit 18 will not be further described in the
present specification.
An example of the seven EMGdi signal components
(hereinafter EMGdi signals) collected by the pairs 1-7 of successive
electrodes 12 (Figures 1 and 2) and supplied to the computer 19 is
illustrated in Figure 4.
The first operation (step 501) performed by the computer
19 is a filtering operation to remove from all the EMGdi signals of Figure
4 electrode motion artifacts, ECG, 50 and 60 Hz interference from the
electrical network, and high frequency noise. The graph of Figure 6a
shows the power density spectrum of the above defined electrode motion
artifacts, the power density spectrum of ECG, and the power density
spectrum of EMGdi signals. Just a word to mention that motion artifacts
are induced by motion of the electrodes 12. More generally, motion
artifacts are defined as a low frequency fluctuation of the EMGdi signals'
DC level induced by mechanical alterations of the electrode metal to
electrolyte interface i.e. changes in electrode contact area and/or
changes in pressure that the tissue exerts on the electrode.
The influence of ECG on the EMGdi signals can be
suppressed or eliminated in different ways. Depending on the working
mode, i.e. on-line or off-line analysis, time domain or frequency domain
processing, different optimal signal conditioning methods can be chosen.
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
17
In time critical applications, an optimized filtering might be a reasonable
choice. Figure 6b presents an optimal filter transfer function to isolate the
diaphragm EMG from a compound signal including ECG and also
disturbed by background noise and electrode motion artifacts. In Figure
6b, the dashed line shows the optimal transfer function, and the solid line
the transfer function implemented by the inventors. Figure 6b is therefore
an example of filter transfer function that can be used in step 501 for
filtering out the electrode motion artifacts, ECG, the 50 or 60 Hz
disturbance from the electrical mains, and the high frequency noise.
Processing of the EMGdi signals by the computer 19 to follow as closely
as possible the optimal transfer function of Figure 6b will conduct
adequately filtering step 501.
An example of integrated EMGdi signal from a COPD
patient in relation to esophageal and gastric pressure is depicted in
Figures 10a and 10b.
Determination of the position of the center of the DDR (step 502)
As the diaphragm is generally perpendicular to the
longitudinal axis of the oesophageal catheter 13 equipped with an array
of electrodes 12, only a portion of the electrodes 12 are situated in the
vicinity of the diaphragm. It is therefore important to determine the
position of the diaphragm with respect to the oesophageal electrode
array.
The portion of the crural diaphragm 11 which forms the
muscular tunnel through which the oesophageal catheter 13 is passed is
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
18
referred to the "diaphragm depolarizing region" (DDR). The thickness of
the DDR is 20-30 mm. It can be assumed that, within the DDR, the
distribution of active muscle fibers has a center from which the majority
of the EMGdi signals originate, i.e. the "diaphragm depolarizing region
center" (DDR center). Therefore, EMGdi signals detected on opposite
sides of the DDR center will be reversed in polarity with no phase shift;
in other words, EMGdi signals obtained along the electrode array are
reversing in polarity at the DDR center.
Moving centrally from the boundaries of the DDR,
EMGdi power spectrums progressively attenuate and enhance in
frequency. Reversal of signal polarity on either side of the electrode pair
4 with the most attenuated power spectrum confirms the position from
which the EMGdi signals originate, the DDR center.
In step 502 of Figure 5a, the position of the center of the
DDR along the array of electrodes 12 is determined. Referring to Figure
5, the first task of the computer 19 is to determine the position of the
center of the DDR along the array of electrodes 12. The center of the
DDR is repeatedly updated, that is re-determined at predetermined time
intervals.
For that purpose, the EMGdi signals are cross-
correlated in pairs in substep 503 to calculate cross-correlation
coefficients r. As well known to those of ordinary skill in the art, cross-
correlation is a statistical determination of the phase relationship between
two signals and essentially calculates the similarity between two signals
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
19
in terms of a correlation coefficient r. A negative correlation coefficient r
indicates that the cross-correlated signals are of opposite polarities.
Figure 7 shows curves of the value of the correlation
coefficient r versus the midpoint between the pairs of electrodes from
which the correlated EMGdi signals originate. In this example, the inter-
electrode distance is 10 mm. Curves are drawn for distances between
the correlated pairs of electrodes 12 of 5 mm (curve 20), 10 mm (curve
21), 15 mm (curve 22) and 20 mm (curve 23). One can appreciate from
Figure 7 that negative correlation coefficients r are obtained when EMGdi
signals from respective electrode pairs situated on opposite sides of the
electrode pair 4 are cross-correlated. It therefore appears that the
change in polarity occurs in the region of electrode pair 4, which is
confirmed by the curves of Figure 4. Accordingly, it can be assumed that
the center of the DDR is situated substantially midway between the
electrodes 12 forming pair 4.
I n substep 504, the correlation coefficients are
systematically compared to determine the center of the DDR. For
example, the center of the DDR can be precisely determined by
interpolation using a square law based fit of the three most negative
correlation coefficients of curve 21 obtained by successive cross-
correlation of the EMGdi signal segments from each electrode pair to the
EMGdi signal segments from the second next electrode pair. Association
of the center of the DDR to a pair of electrodes 12 provides a "reference
position" from which to obtain EMGdi signal segments within the DDR.
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
As mentioned in the foregoing description, the position
of the DDR center along the array of electrodes 12 is continuously
updated, i.e. re-calculated at predetermined time intervals overlapping or
not. In substep 505, update of the position of the DDR center is
controlled by comparing the most negative correlation coefficient rNEG to
5 a constant K3 (substep 506). If rNEe, < K3, it is considered that the EMGdi
signal represents the diaphragm and the position of the center of the DDR
is updated (substep 507); if rNEG> K3, it is considered that the EMGdi
signal does not represent the diaphragm and the position of the center of
the DDR is not updated (substep 508). The control carried out in substep
10 505 is essential in overcoming the artifactual influence on the EMGdi
power spectrum or signal strength measurement.
It has been experimentally demonstrated that EMGdi
signals recorded in the oesophagus of adults are satisfactory as long as
15 they are obtained from electrode pairs (with an inter-electrode distance
situated between 5 and 20 mm) positioned at a distance situated between
5 and 30 mm on the opposite sides of the DDR center (the inter-pair
distance being therefore situated between 5 and 30 mm). With infants,
this may change. Although EMGdi signals obtained from these positions
20 offers a clear improvement in acceptance rate, the signal-to-noise ratio
during quiet breathing still tends to remain unsatisfactorily low.
For example, in Figure 4, the EMGdi signals originating
from the electrode pairs 3 and 5 situated respectively 10 mm below and
10 mm above the DDR are strongly inversely correlated at zero time
delay. In contrast to the inversely correlated EMGdi signals, the noise
components for electrode pairs 3 and 5 are likely to be positively
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
21
correlated. Hence, as illustrated in Figure 8, subtraction of the EMGdi
signals 24 and 25 from electrode pairs 3 and 5 will result into an addition
of the corresponding EMGdi signals (see signal 26) and into a
subtraction, that is an elimination of the common noise components. This
technique is referred to as "the double subtraction technique".
This second subtraction step of the double subtraction
technique can be carried out either in the time domain, or after conversion
of signals 24 and 25 into the frequency domain. Double subtraction
technique can be performed by subtracting other combinations of signals,
or by altering the polarities of electrode pairs. What is important is to
subtract two signals of opposite polarities obtained in the vicinity of the
muscle on opposite sides of the DDR, or if polarity is altered on opposite
sides of the DDR to add signals from opposite sides of the DDR.
Therefore, double-subtracted signal segments 509 are
obtained at the output of step 510 by subtracting the EMGdi signal
segments from the pair of electrodes 12 in optimal location above the
diaphragm from the EMGdi signal segments from the pair of electrodes
12 in optimal location below the diaphragm.
The double subtraction technique compensates for the
changes in signal strength and frequency caused by movement of the
diaphragm 11 (Figure 1) and/or the oesophagus during breathing of the
patient 14 causing movement of the array of electrodes 12 with respect
to the diaphragm 11. Referring to Figure 9, off center of the array of
electrodes 12 (electrode-position-induced filter effect) causes a variation
of center frequency values (see curves 27 and 28) for the EMGdi signals
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
22
from the electrode pairs 3 and 5. The double subtraction technique
eliminates such variation of center frequency values as indicated by curve
29 as well as variation of signal strength. Therefore, the reciprocal
influence of the position of the DDR center on the EMGdi signal
frequency content is eliminated by the double subtraction technique.
It has been found that the double subtraction technique
may improve the signal-to-noise ratio by more than 2 dB and reduce an
electrode-position-induced filter effect. Double subtraction technique is
also responsible for a relative increase in acceptance rate by more than
50%.
Cross-talk signals from adjacent muscles are strongly
correlated at zero time delay and equal in polarity between all pairs of
electrodes 12. Hence, these cross-talk signals appear as a common
mode signal for all electrode pairs and therefore, are eliminated by the
double subtraction technique.
EMG signal strength calculation (step 509)
In step 509, the strength of the EMGdi signal is
calculated. In a first substep 510, a pair of EMGdi signals (see signal 1-7
of Figure 4) obtained from electrode pairs above and below the DDR
center are subtracted from each other and the RMS (Root-Mean-Square)
value of the resulting signal is calculated and referred to as RMSsub
(substep 511). Measures of signal intensity other than the RMS value
can also potentially be used.
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
23
In a substep 512, the above mentioned pair of EMGdi
signals (see signal 1-7 of Figure 4) obtained from electrode pairs above
and below the DDR center are added to each other and the RMS (Root-
Mean-Square) value of the resulting addition signal is calculated and
referred to as RMSadd (substep 513). Measures of signal intensity other
than the RMS value can also potentially be used.
Detection of an increment of the RMS signal amplitude (step 514)
In step 514, a sufficient increment of the RMS signal
amplitude RMSsub is detected. More specifically, in substep 515, the
RMS amplitude RMSsubn of the last EMGdi substraction signal segment
as calculated by substep 511 is compared with the RMSsubn_, of EMGdi
subtraction signal segment last accepted in substep 521. If (RMSsubn x
K,) < RMSsubn-,, no increment is detected and the device will wait until
analysis of the next EMGdi subtraction signal segment is performed. On
the contrary, if (RMSsub, x K,) > RMSsufa,_,, an increment of the RMS
intensity of the EMGdi signal is detected and detection of the common
mode influence (step 518) is activated. Of course, the multiplication
operation (x K,) can be replaced by any other suitable mathematical
operation conducted on either the term RMSsubn or RMSsubn_,.
Detection of common mode influence (step 518)
Step 518 enables detection of signal artifacts of
non-diaphragmatic origin. As indicated in the foregoing description,
EMGdi signals generated by the diaphragm and recorded on either side
of the diaphragm will have reversed polarity and no time delay.
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
24
Accordingly, a subtraction signal representative of the difference between
these two EMGdi signals will have a larger amplitude than an addition
signals representing the sum of such EMGdi signals. In contrast, signals
generated away from and on the same side of the diaphragm will have
the same polarity on all electrode pairs and no time delay. Also signals
from the heart that are not obtained with electrode pairs located too far
apart will have similar shape but with a time delay. Different from signals
with reversed polarity, subtracted signals with same polarity will have
smaller amplitudes than added signals. Hence the ratio or difference
between sum and difference between signals obtained from the same
electrode pairs on either side of the diaphragm can indicate if a signal is
of diaphragm or artifactual origin.
For that purpose, in substep 519, the amplitude
RMSsubn is compared with the amplitude RMSqdd multiplied by a
constant K2. Just a word to recall that the indicia "n" is representative of
the last EMGdi subtraction or addition signal segment. If RMSsub, <
(RMSadd, x KZ), the RMS signal amplitude is rejected (substep 520) and
the two EMGdi signals are considered to have an artifactual origin. If
RMSsubn >(RMSadl xrx ), the RMS signal amplitude is accepted
(substep 521) and the two EMGdi signals are considered to have a
diaphragm origin. Of course, the multiplication operation (x K2) can be
replaced by any other suitable mathematical operation conducted on
either the term RMSsubn or RMSaddn.
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
Replacement of EMGdi signal
The output 522 of the substeps 520 and 521 is
connected to the input 523 of the substep 525. In EMGdi signal
replacement step 524, a substep 525 determines whether the last RMS
5 signal amplitude is accepted. If the last RMS signal amplitude is
accepted, RMSsubn is kept (substep 526). If the last RMS signal
amplitude is not accepted, RMSsubn is replaced by RMSsut~_, or with
another prediction (substep 527).
10 Noise level detection (step 528)
An increase in amplitude of RMSsubn doeg- not
necessarily mean that the diaphragm is the signal source. It is therefore
required to discriminate signals originating from the diaphragm from
15 signals of other origins. In the foregoing description, it has been
described that a technique of sequential cross-correlation of the EMGdi
signals from pairs of electrodes 12 can be used to determine the location
of the diaphragm by the most negative correlation coefficient rNEG. Any
simplified calculation of correlation can be used. The magnitude of the
20 correlation coefficient rNEGis characteristic for each subject but is
typically
negative when the diaphragm is active. If the diaphragm is not active, the
negative correlation coefficient rNEG is very low or the correlation
coefficient is positive. The onset of diaphragm activation can therefore
be detected through the amplitude of the correlation coefficient rNEG.
An alternative to step 528 is to detect the onset of
inspiration through detection of airway inspiratory flow.
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
26
To determine the mean level of noise RMSsubNO,sE (step
528), a mean amplitude of RMSsubn is calculated. For that purpose,
when rNEG> K4, K4 being a constant, this indicates that the diaphragm is
not active (substep 529) and the mean level of RMSsubn, i.e.
RMSsubNO,sE is calculated (substep 530) and supplied to step 532. If r NEG
< K4, step 528 remains in an idle state (step 531).
Steps 532, 533 and 534 is a possible method for
triggering ventilatory support systems from EMGdi signal measurements.
Any increase in EMGdi signal amplitude, it's integrals or derivatives or
combinations thereof, detected via an EMG recording of the diaphragm
or other muscles associated with inspiration above a desired threshold
level and exceeding a desired duration can be used to indicate the onset
of an inspiratory effort. The measurement of inspiratory EMG can be
obtained with any device placed in the vicinity of the inspiratory muscle,
inserted or implanted on the surface of or into the muscle of interest.
Determination of the trigger level to be exceeded in terms of amplitude
and duration can either be performed by manual adjustment supervised
via visual feedback, or automatically by letting the trigger level be relative
to the above described mean noise level. An algorithm can further be
used to trigger the ventilatory support system when the amplitude of a
EMG signal segment of defined duration exceeds the threshold. The
duration that the EMG amplitude remains above the threshold level can
be used to decide the duration of the breath e.g. the ventilatory support
system can start and deliver a full breath independent of the presence of
EMG activity that exceeds the threshold level. The algorithm can also be
adjusted to discontinue the ventilatory support if the EMG amplitude
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
27
drops below the threshold level, or in response to a decrease in amplitude
that exceeds a given magnitude (decrement).
RMS amplitude threshold detection (step 532)
In substep 535, if RMSsubn < K5 the RMS amplitude is
below the threshold and the ventilatory support system is not triggered
(substep 536). Therefore, no ventilatory support is provided to the
patient. K. is a constant equal to RMSsufao,sE x K ,K being another
constant. This will prevent the ventilatory support system from being
triggered in the eventuality that the diaphragm is not active, i.e. in the
case in which rNEC, > K,, (substep 529). Again, the multiplication operation
(x K7) can be replaced by any other suitable mathematical operation
conducted on term RMSsubNO1se=
In substep 535, if RMSsubn > K5 the RMS amplitude is
higher than the threshold and triggering of the ventilatory support system
is requested (substep 537) to provide ventilatory support to the patient.
Otherwise, no ventilatory support is provided (substep 536).
RMS amplitude increment detection (step 533)
In substep 538, RMSsubri., is compared to (RMSsubn x
K6). If (RMSsuq, x K ) < RMSsuo-, , step 533 remains in an idle state
(substep 539) and no ventilatory support to the patient is requested. If
(RMSsubn x K) > RMSsufa-, , this indicates an increment of the RMS
amplitude and triggering of the ventilatory support system is requested
through an increment counting/integrating step 541 to support the patient
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
28
(substep 540). The multiplication operation (x Ks) can be replaced by any
other suitable mathematical operation conducted on either the term
RMSsubn or RMSsubn_,.
The function of the increment counting/integrating step
541 is to determine the time/magnitude response. Step 541 averages the
increment signal to adjust sensitivity.
RMS amplitude decrement detection (step 534)
In substep 543, RMSsubn-, is compared to (RMSsub, x
(1/K6)). If (RMSsub, x(1/Ks)) > RMSsukt,_,, step 534 remains in an idle
state (substep 544) and no ventilatory support to the patient is requested.
If (RMSsub, x(1/Ks)) < RMSsubn-,, this indicates a decrement of the RMS
amplitude and non-triggering of the ventilatory support system is
requested through a decrement counting/integrating step 546 (substep
545). Of course, the multiplication operation (x (1/Ke)) can be replaced
by any other suitable mathematical operation conducted on either the
term RMSsubn and RMSsubn-,.
The function of the decrement counting/integrating step
546 is to determine the time/magnitude response. Step 546 averages the
decrement signal to adjust sensitivity.
Trigger selection step 542
Step 542 is responsive to EMG (signals from substeps
537, 541 and 546), airway inspiratory flow (step 548) and/or pressure
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
29
(step 549) for triggering a ventilatory support system (ventilator) through
an interface 547. The interface 547 may comprise a digital-to-analog
converter and/or other means for analog and digital interface.
More specifically, step 542 is a method for triggering a
ventilatory support system with combined use of EMG, airway inspiratory
flow and/or pressure. The decision for triggering will be made by a logic
circuit on a "first come, first served" basis. For example, if the diaphragm
EMG (or EMG of any other inspiratory related muscle) indicates an
inspiratory effort before airway inspiratory flow and/or pressure indicate
the onset of inspiration, the ventilatory support will be engaged. In the
same fashion, the ventilatory support will be initiated if the inspiratory
effort is detected by a threshold for airway inspiratory flow a-nd/or
pressure being exceeded before the EMG threshold is exceeded.
Any change in airway inspiratory flow and/or pressure,
its integrals or derivatives or combinations thereof, in the inspiratory
direction beyond a desired threshold level and detected via the inspiratory
and/or expiratory lines can be used to indicate the onset of an inspiration.
The graphs of Figures 10a and 10b show, in the case
of quiet breathing of a COPD patient that EMG RMS signal will be
detected approximately 200 ms prior to the onset of airway inspiratory
flow. The graphs of Figures 11 a and 11 b show, still in the case of quiet
breathing of a COPD patient, a similar relation between EMG RMS signal
and the gastric and esophageal pressure. In this particular example,
triggering in response to EMG will enable the lung ventilator to assist the
CA 02320816 2000-08-18
WO 99143374 PCT/CA99/00180
patient directly at the onset of inspiration occuring 200 ms after detection
of EMG RMS amplitude signal.
The method and device according to the invention is
applicable in all patients (adults and infants) on ventilatory support and
5 will enhance the possibilities to obtain spontaneous breathing and
optimize patient ventilator interaction. The method and device apply to
all kinds of ventilatory support systems used in intensive care unit settings
or other wards where assisted ventilation is applied.
10 Finally, it should be kept in mind that:
- the EMG can be measured not only on the diaphragm but on any other
inspiratory related muscle, obtained with the double subtraction technique
or not;
- steps 502 and 518 of Figure 5a are exclusively used with the double
subtraction technique;
- common mode influence detection step 518 is optional;
- step 528 is optional;
- the operation of the device according to the invention can be based
either on the amplitude of the signals or the area under the curve
(integration) of these signals, or any other measure of signal strength.
CA 02320816 2000-08-18
WO 99/43374 PCT/CA99/00180
31
Although the present invention has been described
hereinabove with reference to preferred embodiments thereof, these
embodiments can be modified at will, within the scope of the appended
claims, without departing from the spirit and nature of the subject
invention.