Note: Descriptions are shown in the official language in which they were submitted.
CA 02321444 2012-01-06
-1-
Description
Utilization of Extracts from Iris Plants, Cimicifuga Racemosa and Tectorigenin
as an
Estrogen-Like Organ-Selective Medicament without Uterotropic Effects
The present invention relates to the use of extracts from Iridaceae
and those from Cimicifuga racemosa as an estrogen-type, organoselective
medicament, and tectorigenin and/or tectorigenin glycosides as a medicament.
17-1 -estradiol, which is formed in the ovaries (whenever estradiol is
mentioned hereinafter, this always refers to physiological 17-f3-estradiol)
[hereinafter also referred to as E2], generally has a proliferation-promoting
effect in the organism. Apart from controlling the female cycle, it i.a. has a
homeostatic influence on the metabolism of the bone and prevents the
formation of atherotic plaques at the endothelium of the vessels.
During menopause, lowering of the estradiol level takes place due to
cessation of the ovarial function. This results in a weakening of
proliferative
processes, and in the hypothalamus results in an intensified activity of the
GnRH impulse generator. (The gonadotropin-releasing hormone impulse
generator is a timer in the hypothalamus, as it were, and times the pulsatile
LH secretion, with steroids influencing amplitude and frequency.) In
climacteric women, the resulting, stimulated LH secretion brings about the
so-called "hot flushes" which are felt to be disturbing.
In the absence of sufficiently high estradiol levels in the blood,
osteoclast activity and thus destruction of the bone mass is predominant,
accompanied by an increased risk of skeleton breakage. At the same time,
there is in the long term a risk of plaque formation in the vascular system
and thus an increased risk of infarctions.
Extracts from Cimicifuga racemosa and from Belamcanda sinensis are
both known from popular medicine to be capable of alleviating peri-
CA 02321444 2012-01-06
-2-
menopausal and post-menopausal disorders. Hitherto this has been
explained through the fact that the extracts of both plant drugs exhibit an
estrogen-type effect with all the positive effects thereof on a multiplicity
of
organs of the human body, particularly the brain, ovaries, bones, vascular
system. Estrogen-type effects on uterus, vagina, breast tissue and liver
would in turn be disadvantagous. What is undesirable, however, is that up to
the present, a medicament from these plant drugs which might be used for
organoselective prophylaxis or therapy in cases of estrogen deficiency, has
not been available in the prior art.
Starting out from this state of the art, it is therefore object of the present
invention to furnish plant medicaments with an estrogen-type effect, the
effect of which is organoselective with no effect or only a slight effect on
the
uterus.
This object is independently achieved through the use of extracts from
Iridaceae, and through the use of extracts from Cimicifuga racemosa. The above
object is moreover achieved with a medicament on the basis of tectorigenin
and/or its glycosides.
Another independent solution is represented by a plant extract
containing tectorigenin and/or tectorigenin glycoside or enriched with
tectorigenin and/or tectorigenin glycoside,
Both in in-vitro and in-vivo experiments it was surprisingly found that
extracts produced both from Iridaceae, particularly Belamcanda sinensis,
and from Cimicifuga racemosa with organic solvents or with supercritical
C02 organoselectively act on the central nervous system, the bone system
and the vascular system, with an effect on the uterus - the so-called
uterotrophic effect - not existing. The extracts used in accordance with the
invention are thus suited for producing a ready-formulated medicament for
the selective treatment and/or prophylaxis of osteoporosis.
They are moreover suited for production of a ready-formulated
'medicament for the selective treatment and/or prophylaxis of cardiovascular
diseases, particularly of atherosclerosis.
CA 02321444 2012-01-06
- 2a -
In one aspect, there is provided use of extracts from Iridaceae for
producing an estrogen-type, organoselective medicament having no uterotrophic
effect or one that is at least negligible, under the proviso that Belamcanda
chinensis extract is not used if the medicament is used for alleviating
peri-menopausal and post-menopausal disorders.
In another aspect, there is provided a use as described herein,
characterised in that the extracts are produced from Belamcanda chinensis.
In another aspect, there is provided use of extracts from Cimicifuga
racemosa for producing an estrogen-type, organoselective medicament having no
uterotrophic effect or one that is at least negligible, under the proviso that
the
medicament is not used for alleviating peri-menopausal and post-menopausal
disorders and dysmenorrhea.
In another aspect, there is provided use of extracts containing tectorigenin
and/or tectorigenin glycoside, with the exception of extracts from Iridaceae,
or
extracts enriched with tectorigenin and/or tectorigenin glycoside far
producing an
estrogen-type, organoselective medicament having no uterotrophic effect or one
that is at least negligible.
In another aspect, there is provided a use as described herein,
characterised in that the extract serves for producing a ready-formulated
medicament for the selective treatment and/or prophylaxis of cardiovascular
diseases, particularly atherosclerosis.
In another aspect, there is provided a use as described herein,
characterised in that the extract serves for producing a ready-formulated
medicament for the selective treatment and/or prophylaxis of osteoporosis.
In another aspect, there is provided a use as described herein,
characterised in that the extract serves for producing a ready-formulated
medicament for the selective treatment and/or prophylaxis of climacteric
disorders, particularly for preventing or alleviating hot flushes.
In another aspect, there is provided a use of tectorigenin and/or its
glycosides for producing an estrogen-type, organoselective medicament having
no uterotrophic effect or one that is at least negligible.
In another aspect, there is provided a use as described herein,
CA 02321444 2012-01-06
- 2b -
characterised in that it is a medicament for the selective treatment and/or
prophylaxis of cardiovascular diseases, particularly atherosclerosis;
osteoporosis; and climacteric disorders, particularly for preventing or
alleviating hot flushes.
In another aspect, there is provided use of an extract from Cimicifuga
racemosa for producing an estrogen-type, organoselective medicament having
substantially no uterotrophic effect, for the treatment or prophylaxis of
atherosclerosis brought about by estrogen deficiency; with the proviso that
the
medicament is not used for alleviating peri-menopausal or post-menopausal
disorders or dysmenorrhea.
In another aspect, there is provided the use as described herein, wherein
the extract serves for producing a ready-formulated medicament for selective
treatment or prophylaxis of atherosclerosis brought about by estrogen
deficiency.
In another aspect, there is provided the use as described herein, wherein,
prior to use, the extract is obtained by extraction with an organic solvent.
In another aspect, there is provided the use as described herein, wherein
the medicament is for oral, intravenous or subcutaneous administration.
In another aspect, there is provided a pharmaceutical composition for the
treatment or prophylaxis of atherosclerosis brought about by estrogen
deficiency,
comprising an extract from Cimicifuga racemosa as an estrogen-type,
organoselective medicament having substantially no uterotrophic effect; and an
excipient, with the proviso that the medicament is not used for alleviating
peri-
menopausal or post-menopausal disorders or dysmenorrhea.
In another aspect, there is provided the composition as described herein,
which is a ready-formulated medicament for selective treatment or prophylaxis
of
atherosclerosis brought about by estrogen deficiency.
In another aspect, there is provided the composition as described herein,
wherein the extract has been obtained by extraction with an organic solvent.
In another aspect, there is provide the composition as described herein,
which is for oral, intravenous or subcutaneous administration,
CA 02321444 2000-08-17
-3-
They are moreover suited for producing a ready-formulated
medicament for the selective treatment and/or prophylaxis of peri-
menopausal and post-menopausal psychovegetative disorders such as, e.g.,
hot flushes.
It was moreover found that the component tectorigenin, which was
isolated from Belamcanda sinensis, essentially exerts the same effects as
the whole extract.
HO , O
CH3O
OH O O
Tectorigenin
This component is also found, besides Belamcanda sinensis, in other
Iridaceae such as, e.g., Iris germanica, I. tectorum, I. illyrica, I.
dichotoma.
Taxonomically speaking, Belamcanda sinensis is classified as follows:
Order Liliales
Family Iridaceae
Genus Belamcanda
Species Belamcanda sinensis (Leman) DC. = Pardanthus
chinensis (L.) Ker-Gawler, also: Ixia chinensis L.
(=Gemmingia chinensis (L.) O. Kuntze)
Preferably rhizomes, stalks, leaves and/or petals of the plants are used
for producing the extracts.
A fundamental phytochemical description of Belamcanda sinensis and
its components was given in the dissertation by Ms. A. Nenninger: (LMU
Munchen, 1997) entitled: "Phytochemische and pharmakologische
Untersuchungen von Belamcanda sinensis, einer Arzneipflanze der TCM
and anderer Irisarten".
CA 02321444 2000-08-17
-4-
With the medicaments of the invention, medicaments from Cimicifuga
racemosa and Belamcanda sinensis and other Iridaceae and tectorigenin-
based medicaments are for the first time available, which act as full estrogen
receptor agonists in bones, in the cardiovascular system and in the brain.
Further advantages and features of the present invention become clear
from the description of experimental data and by referring to the drawings,
showing:
Fig. 1: a comparison of the organic and aqueous phases of
Cimicifuga racemosa. Displacement graph of a
representative estrogen receptor - ligand binding assay. The
concentration of the start solution is 17.66 mg/ml, followed
by dilutions 1:2, 1:4 etc. up to 1:64;
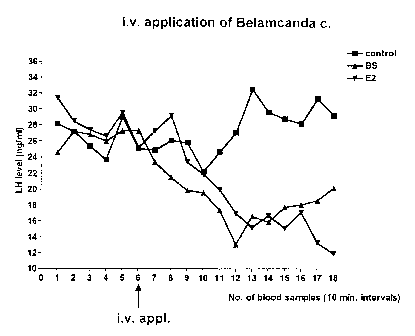
Fig. 2: serum LH prior to, and 2 hours after, intravenous injection of
Belamcanda sinensis extract, E2 and vehicle. The
Belamcanda sinensis extract has a similar capacity of
lowering the elevated Serum LH levels as E2,-
Fig. 3. effects of Cimicifuga racemosa and E2 on uterus weights
(Fig. 3a) and LH levels in the blood (Fig. 3b) in
ovariectomised rats after seven-day subcutaneous
treatment; (mean values + SEM, n = 8, * = p < 0.05 vs.
cremophor as vehicle);
Fig. 3a) uterus weights;
Fig. 3b) LH concentrations in the blood;
Fig 4a) effects of Cimicifuga racemosa and E2 in ovariectomised
rats after seven-day subcutaneous treatment; (mean values
+ SEM, n = 8, * = p < 0.05 vs. cremophor as vehicle) on the
expression of the mRNA for E2-receptor a in the preoptic
region of the hypothalamus;
CA 02321444 2000-08-17
-5-
Fig 4 b) the expression of the mRNA for IGF1 and C3 in the uterus of
ovariectomised rats after 7 days of subcutaneous
administration; and
Fig 4 c) the expression of the mRNA for collagen 1 (Co111) and
osteocalcin in the bone of ovariectomised rats after 7 days of
subcutaneous administration.
Experimental evidence for the estrogenic effect of Cimicifuga racemosa and
Belamcanda sinensis
Selective estrogenic effect was demonstrated in stages in the course of
a series of test systems of various degrees of complexity.
1. in-vitro experimentation
1.1 in-vitro experiments for Cimicifuga racemosa
Recognition of the estrogen-type structure of components by an
antibody directed against 17-f3-estradiol (=E2) was shown in vitro.
The Cimicifuga racemosa extract was evaporated over residue. By
phase distribution between dichloromethane and water, substances having
different polarities were enriched. Binding affinities of the components of
both phases were determined in vitro on estrogen receptors from pig's
uterus. The cytosolic estrogen receptors from the pig uteri were isolated in
accordance with standard procedures and used for the ligand displacement
experiments.
Herein it was found that the estrogen-type structures e.g. from
Cimicifuga racemosa are not hydrophilic in nature but lipophilic inasmuch as
they may be extracted from the extract by means of an organic solvent. The
substances present in the organically extracted phase bind about ten times
more strongly to the antibody than the substances remaining in the aqueous
phase.
The difference between the two phases is even greater in the estradiol
receptor binding assay. The similarity of the binding substance with estradiol
CA 02321444 2000-08-17
-6-
must be high enough to enable a selective - competitive - interaction with the
estradiol receptor to take place in a cell-free preparation. Inside this test
system, the aqueous phase does not possess any activity, whereas the
organic phase binds very strongly to the receptor.
The results are shown in Fig. 1.
1.2 in-vitro Belamcanda sinensis
It is known from other studies that extracts from Belamcanda sinensis
also possess components which are recognised by an antibody against 17-
f3-estradiol and bind to the 17-f3-estradiol receptor (cf. Nenninger
loc.cit.).
Surprisingly, however, the inventors of the present application have found
that these extracts have different estrogenic effects on different organ
systems, particularly that they do not have a uterotrophic effect.
2. in-vivo experiments: Evidence for the estrogenic effect on
ovariectomised rat
Binding to the receptor E2 is very selective; it is, however, not possible
to say whether the subsequent processes within the cell are promoted or
inhibited, i.e. whether the substance is an agonist or an antagonist. This
property can only be determined in suitable cellular systems or in the overall
animal.
The ovariectomised rat is a recognised model for the post-menopausal
woman in whom the endogenous estradiol production has subsided. As a
result of the external supply of 17-(3-estradiol or of substances which have
an estrogen-type effect, there occurs a restauration of estrogen-sensitive
anatomical-morphological parameters, such as increase of the uterus
weights and the occurrence of hornified cells, i.e. plaque epithelium cells at
the vaginal epithelium, or hormonal changes such as lowering of the LH
levels in the blood of the treated animals.
All experiments described hereinbelow were carried out with
ovariectomised Sprague-Dawley rats (=ovx rats) having a weight between
240and280g.
CA 02321444 2000-08-17
-7-
2.1 Single administration of Belamcanda sinensis
The onset of the effect of the estradiol-type effect of Belamcanda
sinensis extract occurs very quickly. Even after a single i.v. administration
of
vehicle, estradiol and Belamcanda sinensis extract to ovx rats, pulsatility
ceases both under E2 and under Belamcanda sinensis. In the medicament
value development, there result significant inhibitions of the serum LH
levels,
both in comparison with the previous values and in comparison with the
cremophor-treated control animals. Cremophor is an emulsifier on the basis
of polyethoxylated castor oil derivatives.
The results are represented in Fig. 2.
In the uterus of the animals six hours after injection of the Belamcanda
sinensis extract, the expression of the uterine VEGF, IGF1 and C3 genes is
not changed in comparison with the controls, whereas the estradiol injection
brings about a clear increase of the gene expression of these three
estrogen-regulated proteins. The constitutively expressed CCO gene was
not significantly influenced by any one of these treatments.
These findings indicate that components of Belamcanda sinensis bring
about an inhibition of the GnRH pulse generator in hypothalamic estrogen-
receptive structures and thus have estrogen-agonistic effects. Hereby the
hypophysary LH secretion is inhibited significantly both by components in
Belamcanda sinensis and by estradiol. In contrast with estradiol, the
components in Belamcanda sinensis do not have a uterotrophic effect.
Estradiol significantly regulates the gene expression of VEGF, IGF1 and C3
upwardly, an effect which is not observed under Belamcanda sinensis.
Execution of the acute experiment on the effect of an i.v. injection of
Belamcanda sinensis extract
24 rats (i.e. 8 animals/group) had a jugular vein catheter implanted
under ether anesthesia on the day preceding the experiment. On the day of
the experiment, 6 blood samples were taken at 10-min intervals. Immediately
following taking of the 6th sample, 62.5 mg of the Belamcanda sinensis
extract or 10 lag 17-f3-estradiol (E2) or the solvent (5 %) cremophor in
isotonic NaCl 1 ml), respectively, were injected intravenously, and blood
CA 02321444 2000-08-17
-8-
samples were taken for another 2 hours in 10-minute intervals. 6 hours after
the intravenous administration, the animals were decapitated, blood was
obtained and the uteri removed, weighed and deep-frozen in liquid nitrogen.
2.2 One-time administration of tectorigenin
Following a single administration of tectorigenin, the time development
of influence on the LH levels in the blood and the estradiol-type
immunoreactivity were determined. The concentration of tectorigenin in the
blood of the animals, determined with the aid of E2-RIA, after 20 min
corresponds to about 100 pg equivalent estradiol.
Tectorigenin triggers a rapid LH reduction. The kinetics of the LH
reduction achieved under tectorigenin up to the time 60 min following i.v.
administration precisely correspond to the one of estradiol, but then do not
result in further reduction but slowly increases again.
Execution: OVX rats had catheters placed in the vena jugularis externa
under ether anesthesia 24 hours before the beginning of the experiment, in
accordance with the method of Harms and Ojeda (Harms PG; Ojeda SR: A
rapid and simple procedure for chronic cannulation of the rat jugular vein. J.
Appl. Physiol. (1974) 36: 391-392). The tube end was positioned in a skin
pocket in the neck. In order not to have to touch the animals for obtaining
the
blood samples, the catheter was prolonged with the aid of a silicone tube.
Catheter and tube were rinsed with Ringer solution containing 50 IU
heparin/ml.
Blood samples of 100 pl each were drawn from the animals at 10-min
intervals, and the withdrawn volume replaced with Ringer/heparin solution.
After the 6th sample, 1.0 ml of the respective test solution was applied
intravenously. As test solutions there were used: 2% cremophor (=vehicle
solution), tectorigenin 7mg/ml vehicle, 17-f3-estradiol 10pg/ml vehicle. Blood
was taken at ten-minute intervals through additional 140 min.
The blood samples thus obtained were filled into a 0.5 ml Eppendorf
reaction vessel containing 10 pl heparin-Losung (5000 IU/ml, Liquemin),
CA 02321444 2000-08-17
-9-
centrifuged for 10 min at 10 000 * g, and the plasma stored at -200C until
performance of the radioimmunoassays.
The RIAs for LH and Prolaktin are based on antisera, reference and
iodisation preparations from NIH (Bethesda, Maryland, USA). The
concentrations of estradiol and of the cross-reactive isoflavones were
measured with the aid of an RIA from DPC, Bad Nauheim.
2.3 Effect of Belamcanda sinensis extract after administration
through 7 days
The effects of repeated administration of estradiol, Belamcanda
sinensis extract and vehicle on overall weight, uterus weight, hormone level
and gene activation of uterus and bone were examined on ovariectomised
rats after daily s.c. application through seven days.
The average body weights of the cremophor- and Belamcanda
sinensis-treated animals do not differ, whereas the E2-treated animals were
significantly lighter. Neither do the uterus weights of the animals treated
with
cremophor and Belamcanda sinensis differ significantly, whereas the E2-
treatment more than tripled the uterus weights.
The serum LH levels in the Belamcanda sinensis-treated animals were
reduced slightly, but significantly in comparison with the cremophor controls;
reduction through estradiol was more marked.
In the uterine mRNA extract, estradiol significantly raised the gene
expression of VEGF to 149% of the control value after a one-week
treatment. Unter Belamcanda sinensis extract, expression was raised slightly
but not significantly. Expression of the non estrogen-regulated constitutive
genes for the cytochrome C oxidase (= CCO) was not influenced.
In extracts of the femur head, the collagen-1 A1, osteocalcin, IGF1 and
TGFf3-mRNA expression was determined. Estradiol as well as Belamcanda
sinensis significantly inhibited the expression of all 4 genes without having
an influence on the constitutive CCO gene.
The different effects of estradiol and Belamcanda become very clear
after the seven-day treatment. Belamcanda sinensis extract has an estradiol-
CA 02321444 2000-08-17
-10-
agonistic influence on the hypophysary LH secretion by inhibiting the GnRH
impulse generator, and on the gene expression of four estrogen-regulated
genes in the bone. In contrast, there is no estrogenic effect on the uterus:
neither the uterus weight nor the estrogen-regulated VEGF gene are
influenced by the Belamcanda sinensis extract. In contrast, estradiol brings
about ballooning of the uterus and an activation of the VEGF gene.
Execution of the subacute test on the effect of daily s.c. injection
through 7 days:
8 animals each per test group (24 altogether) were daily injected
subcutaneously between 8:00 and 9:00 a.m. with 62.5 mg Belamcanda
sinensis extract and 10 lag estradiol or the solvent (cremophor 5%, 1 ml),
respectively. 6 h after the last application, the animals were decapitated and
from every animal the aorta, the uterus and the left femur head were
removed, cleaned, and frozen in liquid nitrogen.
In the blood samples, LH and the estradiol immunoreactivity were
determined.
2.4 Repeated administration of Cimicifuga racemosa
14 days following ovarectomy at the earliest, the animals have the
respective test substance injected subcutaneously in a dose of 62.5 mg
Cimicifuga racemosa/rat or 8 lag estradiol/rat once daily in the morning over
a period of 7 days. Both substances were dissolved in 5% cremophor, the
control animals only received the vehicle.
Following decapitation of the animals, brains, uterus and femur were
prepared for mRNA-recovery. The LH concentration in the blood of the
animals was determined by means of RIA. The expression of the estrogen-
regulated genes in the above identified organs was determined by means of
semi-quantitative RT-PCR.
The uteri of the estradiol-treated animals have more than three times
the weight of those of the animals treated with Cimicifuga racemosa and
vehicle which basically do not differ in their mean values. This means that
the components of Cimicifuga racemosa have no influence on the uterus of
the animals. This is also true for the vagina, where no hornification of the
CA 02321444 2000-08-17
-11-
epithelium tissue occurs in the animals treated with Cimicifuga racemosa
and vehicle, quite contrary to the estradiol-treated animals.
The LH levels of the vehicle-treated animals remain high, however are
lowered significantly both by estradiol and Cimicifuga racemosa.
The results are shown in Figs. 3a) and 3b).
Uterus weights (wet)
Cremophor Cimicifuga E2
[control] racemosa
Number animals 8 8 8
Mean values [mg] 185.6 192.3 702.1
SD 18.81 22.53 194.97
SEM 6.65 7.97 68.92
LH concentrations in the blood
Cremophor Cimicifuga E2
[control] racemosa
Number animals 8 8 8
Mean values [ng/ml] 16.9 12.5 7.83
SID 3.99 3.4 5.57
SEM 1.41 1.2 1.97
As another marker for the estrogene effect, the activation of mRNA of
estrogen-induceable proteines was measured. What was measured here
was tissue from uterus, from bone tissue (femur) and from the preoptic
region of the hypothalamus.
In the hypothalamus, both Cimicifuga racemosa and E2 stimulate the
expression of the mRNA for the estrogen receptor a (Fig 4a). In the bone
tissue, too, Cimicifuga racemosa behaves like an estrogen and reduces, in
analogy with estradiol, the expression of the mRNA for the bone-specific
collagen 1 and for osteocalcin genes (Fig 4b).
CA 02321444 2000-08-17
-12-
In contrast, no effect of Cimicifuga racemosa on estrogen-regulated
genes in the uterus is observed. Only estradiol increases the mRNA for IGF1
and complement factor C3 (Fig 4c).
These findings prove that the components from Cimicifuga racemosa
selectively act on single organs: the extract acts estrogenically in the
hypothalamus (expression of the E2 receptor a, liberation of LH) and on the
bone, proven by the expression of the genes for collagen 1 and osteocalcin.
Other than estradiol, however, Cimicifuga racemosa does not have an effect
on the uterus, as the absence of an effect on the uterus weights and the
expression of the genes for IGF1 and C3 shows.
By the experiments carried out in vitro and in vivo, it could be
demonstrated that Cimicifuga racemosa and Belamcanda sinensis extracts
exert an estrogenic effect. Surprisingly it was found that the extracts from
the
named drugs act organoselectively on central nervous system, bone and
vessels, but not on the uterus, and are thus excellently suited for the
prophylaxis and therapy of estrogen deficiency without having a negative
influence on the endometrium.
Identical effects are achieved by the tectorigenin contained in
Belamcanda.
Thus for the first time medicaments having an estrogen-type effect,
however without a uterotrophic effect, are available.
The like medicaments may be used for the treatment and/or
prophylaxis of cardiovascular diseases, particularly atherosclerosis,
osteoporosis, and of peri- and post-menopausal psychovegetative disorders
such as, e.g., hot flushes.
Among types of application, oral, intravenous and subcutaneous
application are prominent.