Note: Descriptions are shown in the official language in which they were submitted.
CA 02321813 2000-08-24
WO 99/43424 PCT/GB99/00345
-1-
IMPROVEMENTS IN AND RELATING TO GAS GENERATORS
This invention is concerned with improvements in and
relating to gas generators, particularly, but not exclusively,
for the generation of oxygen.
Gas generators are used in a wide variety of applications
to produce or separate gases for breathing, to provide gases
for use in chemical reactions, to separate chemical compounds
into their component parts and for a variety of other purposes .
Gas generators should ideally provide a high level of
purity, at a high gas flow rate, with maximum efficiency and at
as low an operating temperature as possible. Control over the
amount of gas generated is also desirable. Existing technology
faces problems in one or more of these areas and the present
invention aims to provide an improved gas generator.
According to a first aspect of the invention we provide
a gas generator comprising an assembly comprising a layer of a
first material, the first material separating a first space
from a second space, and means for provided a differential
across the layer for at least one species, the layer being
capable of transporting electrons and transporting ions of the
at least one species and resisting the flow of gas from one
space to the other.
The flow of gas ions from one side to the other results
in gas being generated or separated to one space of the
generator.
Preferably the first material is or includes a mixed
conductor, most preferably for both electrons and ions of the
gas to be transported. Preferably the gas to be transported is
oxygen.
Preferably the first material is stable in an oxidising
and reducing environment.
A first material comprising a ceramic oxide is preferred.
Preferably the first material comprises urania. Doped urania,
for instance by one or more rare earth metals, is particularly
preferred. Urania doped with yttria may be provided. The
urania may be provided as a solid solution with a second
CA 02321813 2000-08-24
WO 99/43424 PCT/GB99/00345
-2-
material. The second material may comprise one or more rare
earth metals, such as yttria.
The urania may be depleted urania i.e. the U235 content
may be Less than for naturally occuring urania.
The first material may be provided in a layer less than
200 micrometers and more preferably less than 150 micrometers
thick.
One or more further layers may be provided on the first
layer and/or on each other. One or more of the further layers
may comprise the first material.
A further layer or layers of material may be provided
which have a greater resistance to the flow of gas therethrough
than the first material layer with which it is provided.
Preferably the further layers) acts as a barrier to other
materials, gases, ions in the feed. The further layers) may
be substantially impermeable to the gas or gases and/or other
components present in the first and/or second space. A layer
impermeable to the passage of oxygen and/or nitrogen and/or
carbon dioxide may be provided. Preferably the layer is
impermeable to all three. The layer may be or may further be
impermeable to methane and/or other hydrocarbon type gases.
The further layer may be provided on the first space side
of the first layer and/or on the second space side of the first
layer. The further layer may be exposed to the first space
and/or the second space. The further layer may be provided
separated from the first and/or second space by another layer.
The another layer may comprise a layer of the first material.
A further material may be provided on and/or in
association with and/or in the first material layer to control
the passage of electrons through the first layer and/or to
control the passage of the gas ions through the first layer.
The further material may be provided as a layer on the first
material and/or as a layer separated from the first layer be
another material or layer, such as the further layer.
The further material may be provided in a continuous or
discontinuous manner. The further material may be absent from
some areas or locations relative to the first layer. The level
CA 02321813 2000-08-24
WO 99/43424 PCT/GB99/00345
-3-
of further material present, for instance the proportion of
area for which it is present or absent, may vary with its
location relative to the first layer. The thickness of the
further material may vary with position relative to the first
layer. The nature and/or make up and/or chemical composition
and/or structure of the further material may vary with position
relative to the first layer.
The differential providing means may comprise means for
providing a chemical differential in relation to the species to
be transported. For instance the level of the species may be
increased on the first side of the apparatus relative to the
second side and / or the level may be reduced on the second
side of the apparatus relative to that on the first side of the
apparatus. The species may be substantially absent from the
second side of the apparatus, particularly in the gas feed to
the second side of the apparatus. The differential may take
the form of a concentration gradient across the generator. The
concentration gradient may apply to one or more of the species.
The differential providing means may comprise means for
elevating the pressure of the species to be transported, for
instance above atmospheric, on the first side of the apparatus
and/or means for reducing the pressure of the species to be
transported, for instance below atmospheric, on the second side
of the apparatus.
The differential providing means may alternatively or
additionally comprise means for introducing a gas stream
containing the species to be transported to the first side of
the apparatus and/or means for introducing a gas stream
substantially free of the species to be transported on the
second side of the apparatus.
The gas generator may be formed of a plurality of
assemblies of the type described.
The generator assembly or assemblies may be provided in
substantially planar form, for instance as a planar layer or a
series of planar layers of the various materials. Square,
rectangular or elongate assemblies may be provided.
CA 02321813 2000-08-24
WO 99/43424 PCT/GB99/00345
-4-
In an alternative form the generator assembly or
assemblies may be formed in a tubular and/or cylindrical and/or
hollow elongate form. The assembly or assemblies may be
provided with a through passage, such as a central passage,
enclosed by the first material. The central passage may form
the first side and the space outside the layer the second side
or vice versa. Where more than one layer is provided the
layers may be provided in a concentric manner, for instance to
form layers on a right cylinder. Non- circular or non-regular
cross-sections may be provided.
The through passage may be provided with an inlet end and
an outlet end. The inlet and outlet ends may oppose one
another.
According to a second aspect of the invention we provide
a method for generating gas comprising providing a differential
for at least one species across an assembly comprising a layer
of a first material, the first material separating a first
space from a second space, the layer being capable of
transporting electrons and ions of the at least one species and
resisting the flow of gas from one space to the other.
One or both sides of the generator may be maintained in
contact with a given batch of gas. One side may be depleted
and the other enhanced in one or more gas levels in such a
batch process.
Alternatively one or both sides of the generator may be
contacted with a changing volume of gas. The gas on one or
both sides may periodically or constantly be replaced. In this
continuous system the gas level on one side may be improved,
but the level of gas on one or both sides may remain fairly
constant due to replacement.
Preferably the differential results in a flow of
electrons through the first layer. Preferably the differential
results in a flow of gas ions through the first layer from one
side to another preferentially. The gas ions may flow from the
first side to the second side. Preferably the gas ions reform
gas molecules on reaching the second side. Preferably the gas
CA 02321813 2000-08-24
WO 99/43424 PCT/GB99/00345
_5_
molecules are exhausted to the space surrounding the second
side.
The gas ions may be formed by the disassociation of a gas
molecule in contact with the assembly and/or first layer. The
molecule decomposed may be O2.
The gas generated and/or purified and/or increased in
concentration is preferably oxygen. The gas may be extracted
and/or purified and/or increased in concentration from air.
The method may include the addition of the gas produced,
preferably oxygen, to a gas stream. The addition may occur
remote from or at a surface of the generator. The gas stream
may include or consist of methane. Other gaseous hydrocarbons
may be present. The methane may be generated by the treatment
of coal. The methane may be extracted from an oilwell or other
oil production or processing facility.
The gas stream may flow past the surface of the
generator, for instance from an entrance to an exit, most
preferably in a continuous manner.
Preferably the method includes the reaction of the
oxygen produced with methane to give CO and HZ. The reaction
products may be further processed and/or catalysed to give
higher weight hydrocarbons, such as diesel or petrol.
Other features presented elsewhere in the application,
including the first aspect of the invention, may be provided.
Various embodiments of the invention will now be
described, by way of example only, with reference to the
accompanying drawings in which .
Figure 1 shows a cross-sectioned view of a first
embodiment of the invention; and
Figure 2 shows a partially cross-sectioned perspective
view of a second embodiment of the invention.
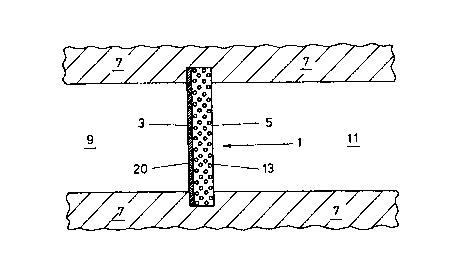
In the first embodiment of the invention illustrated in
Figure 1 the gas generator is formed of an assembly 1 with a
first side 3 and second side 5. The space around the first and
second sides are isolated from one another by the support
structure 7 so as to maintain differences in the make up of the
CA 02321813 2000-08-24
WO 99/43424 PCT/GB99/00345
-6-
volume 9 contacting the first side 3 relative to the volume 11
contacting the second side 5.
The assembly 1 consists of a layer 13 of mixed conductor
material. The layer 13 is formed of a mixture of particles,
the first particles comprising a solid solution of yttria and
uranium dioxide and the second type comprising zirconia.
In use an oxygen containing gas, such as air, is
introduced to the cathode side 3 of the assembly 1. The other
side 5 contains a far lower level of oxygen so giving rise to
a pressure differential, at least with regard to the relative
oxygen levels. The pressure differential driving the system
makes use of the potential arising according to the Nernst
equation
nF PoZ'
_____ In _________
4RT Po2' '
such that an initial differential in the chemical species
balance gives rise to a chemical potential. The difference in
chemical potential gives rise to an electron flow and this in
turn leads to a flow of oxygen ions from side 3 to anode side
5. The process results in the depletion of the oxygen level on
the cathode side 3 of the assembly and its enhancement on the
anode side 5.
As well as arising from a difference in pressure,
differences in the level of one or more chemical species across
the apparatus and / or concentration gradients can be used to
give the variation and give rise to a chemical potential as a
result.
The net result is the production of oxygen at the anode
side 5 which can then be used for the desired purpose at that
side or by its transfer to the location of use. The oxygen
feeding side can be constantly or periodically replaced.
The gas produced, usually oxygen, can be used for a
variety of purposes . The purity of the oxygen produced and the
careful control of the level of oxygen produced make the
CA 02321813 2000-08-24
WO 99/43424 PCT/GB99/00345
_7_
technique particularly suitable for sensitive operations, such
as those involved in semi-conductor manufacture, chemical
vapour deposition and the like. The production of a pure
oxygen output also renders the system useful for injecting
oxygen into a carbon based gas to achieve oxidation. This
technique is applicable to generating CO and Ha from a methane
gas stream, for instance. This reaction is important in
forming intermediaries in the production of petrochemicals from
methane produced from coal and is also believed to offer a
particularly suitable technique for generating useful and more
readily handleable compounds from the methane off gas of oil
extraction facilities.
The generation of oxygen in this way is preferable to the
provision of a cryogenic separator as the size and capital cost
is reduced and the transportability of the system is greater.
The process may be conducted as a batch process or one or
both sides of the assembly may be continually replaced, for
instance to maintain a suitable level of oxygen on the cathode
side from which to extract.
The mixed oxide layer can be produced from an ink style
suspension produced by mixing particles formed of a
yttria/urania solid solution, together with zirconia, cod liver
oil, polyvinyl butyral, polyethylene glycol, dibutyl phthalate
and ethanol. The constituents can be mixed by ball milling
together far several weeks.
A typical mix may comprise .
17.198 50mo1% yttria/UOZ solid solution;
13.658 zirconia;
O.Slg cod liver oil;
4.58 polyvinyl butyral;
1.338 polyethylene glycol;
1.28 dibutyl phthalate;
368 ethanol; with
208 terpineol added after ethanol
evaporation.
CA 02321813 2000-08-24
WO 99/43424 PCT/GB99/00345
_g_
The resulting suspension can be screen printed or
otherwise used to form the layer 3, for instance by spraying.
The layer may then be sintered, at a temperature below 1550°C.
Depending on the layer 3 structure provided it may be
desirable to provide a sealing layer 20 on a side of the layer
3 so as to prevent gas flow, rather than gas ion flow, through
the assembly 1. The layer 3 itself may have too high a
porosity, in certain configurations, to serve this function
alone.
As an alternative to the plate style assembly 1
illustrated in Figure 1, a tubular style generator system 48
may be employed. Such a system, illustrated in Figure 2,
consists of a series tubes 50 formed from a mixed oxide layer
52. The space 54 outside the tube 50 is subjected to a flow of
heated air, as an oxygen source. Each of the tubes 50 is
provided with a flow of methane from inlet end 56 to outlet end
58.
The relative abundance of oxygen outside the tubes 50 and
the relative absence of oxygen inside the tubes 50 gives rise
to a pressure differential and gives rise to the effects
described above as a result. Oxygen ions are generated, flow
through the layer 52 into the tube, the oxygen is the exhausted
into the methane stream inside the tubes 50. The subsequent
reaction generates CO and H2 which can then be used in
subsequent reactions.
As discussed above, the.system 48 may be provided with a
gas impermeable layer or membrane 60 to maintain the pressure
differential.
As the level of oxygen introduced into the tube 50 may
vary between its inlet end 56 and outlet end 58 a further
controlling layer 62 may be provided. In this embodiment the
further layer 62 is provided on the inside surface of the tube .
The control layer 62 is used to influence the amount of oxygen
introduced into the tube 50 and hence gas stream along it
length. This may be to balance the level along the length or
otherwise influence it.
CA 02321813 2000-08-24
WO 99/43424 PCT/GB99/00345
_g_
The layer 62 may control the active area of the tubes
inside surface by forming an ion and gas barrier, which is
perforated to an increasing extent towards the exit 58 to give
an increasing proportion of active area. The control may
alternatively or additionally be effected by varying the
thickness of the layer 62 along the length of the tube 50,
reducing towards the exit 58, as shown.
The materials of the present invention offer a
significant number of advantages over existing gas generators.
In particular the assembly structure used offers a far higher
active area due to the crania layer used. The greater area
leads to higher product flow rates. Additionally the crania
layer has a high catalytic activity which once again increases
the performance of the generator due to improved kinetics.
The materials employed also allow the separator to be
operated at lower temperatures, approximately 800°C, with
benefits in terms of the life of the product and the reduced
cost of the surrounding structure. Cost savings are also
achieved in avoiding the use of platinum group catalysts within
the assembly. The crania also offers significantly improved
resistance to poisoning than many other materials. The layer
is also far more stable in both reducing and oxidising
environments than prior art materials.
The system is particularly useful for the proposed uses
as the pressure differential enables close control of, and
hence an accurate delivery of, the desired amount of gas
through the generator/separator. The gas exhausted is also
particularly pure and suitable for the desired use due to the
highly selective nature of the gas transfer through the layer.