Note: Descriptions are shown in the official language in which they were submitted.
CA 02322199 2000-08-30
WO 99144622 PCTIGB99/00605
- 1 -
INORGANIC NTIR1TE AND ORGANIC ACID IN COMBINATION AS TOPICAL ANTfVIRAL
COMPOSITION
The present invention relates to a complex of nitrogen oxides,
arising from the interaction of nitrite and acid as an
antiviral composition for the treatment of viral diseases of
the skin by topical application thereto. Such nitrogen oxide
include particular NO which is of the importance particularly
if acidified.
In WO 95/22335 we have disclosed a pharmaceutical composition
comprising a pharmaceutically acceptable source of nitrites
and a pharmaceutically acceptable acidifying agent, inter alia
for the direct treatment of disease by topical application.
These compounds have a direct effect on the organism concerned
but the precise mode of action is not known.
US-A-4595591 reveals a composition comprising an aqueous
solution of nitric acid and nitrous acid at a pH below 1
preferably with an organic acid and copper and cadmium ions
for the treatment of superficial lesions of the skin, for
example tumorous growths.
US-A-5648101 provides a vaso-active composition comprising NO
adapted for delivery to a body site inter alia by means of a
cream or ointment. The NO is generated from an admixture of
ferrous sulphate, an organic acid and an inorganic nitrite and
caused to be reactive in the presence of moisture adjacent or
at the site. Acidification is not discussed.
WO 9&/02268 reveals the inhibition of a virus by nitric oxide
(NOZ) but the advantages of reduction of pH at the environment
of use have not been appreciated.
CA 02322199 2000-08-30
WO 99/44622 PGT/GB99/00605
- 2 -
WO 93/25213 reveals a composition comprising nitrous oxide
contained in a dermatological composition comprising as an
essential feature a fatty acid or a lower alkyl ester thereof,
pH values, particulary at the environment of use, are not
mentioned.
The role of NO as a compound which inhibits viral replication
in vitro has been disclosed by J. B. Mannick; 63rd Forum in
Immunology and papers in Intervirology 1995; 38: 206-213,
Trends in Microbiology 1995; 3: 81-82, Science 1993; 261:1445-
1448, and The Journal of Clinical Investigation 1993; 91:
2446-2452. The above papers disclose the effects of NO on
various viruses, for example herpes simplex virus, vaccinia
virus and vesicular stomatitis virus. Exogenous NO donors
such as S-nitroso-N-acetyl penicillamine (SNAP) or SIN-I were
used in vitro to determine the role of NO as an antiviral
compound. Application of exogenous NO to cell-lines infected
with the virus under test resulted in inhibition of the viral
DNA replication. The exact~mechanism of the inhibition seemed
to differ depending on the virus involved. For example in the
case of vaccinia virus it is thought that the NO may inhibit
replication by binding to non-haem iron or thiol groups that
are essential for the catalytic activity of enzymes involved
in vaccinia replication. In this in vitro model the antiviral
effects of NO do not require immune recognition of infected
cells thus providing an early defense against viral pathogens
prior to the development of a specific immune response.
In order for viruses to survive and reproduce they must evade
recognition by the hosts immune responses. The mechanism by
which this is achieved is largely unknown but an effective
immune response eradicates the infection. Viruses are
CA 02322199 2000-08-30
WO 99144622 PGTIGB99/00603
- 3 -
obligate intracellular pathogens. They reproduce using the
host's metabolic machinery.
At present drug treatment of viral diseases is predicated upon
a small number of compounds which block the replication of the
virus. For example Acyclovir, which is effective against
herpes virus, is a deoxyguanosine analogue which competes with
deoxyguanosine triphosphate as a substrate for viral thymidine
kinase and when phosphorylated and incorporated in the viral
DNA causes premature DNA chain termination.
Unfortunately anti-viral drugs are only effective for a
limited number of viral infections and viruses can mutate to
overcome the effectiveness of the drugs. In the case of
molluscum contagiosum 1 and 2, which are related to orthopox
and parapox viruses and share some homology with vaccinia,
other forms of treatment have to be used. Current therapies
comprise physical destruction with manual extrusion, liquid
nitrogen therapy or curettage, all of which are painful and
not very effective and may cause scarring. The pain of these
therapies is particularly pertinent because the majority of
patients are under the age of 3.0 years.
In the case of recalcitrant warts, destructive therapies such
as liquid nitrogen can be used in cases where the conventional
salicylic acid paints have not resulted in the warts
disappearance. One problem with warts is that the viral pool
is in the stem cells which are found at the base of the
epidermis. The aforementioned treatments often remove the
virus particles and thus the infection from the top layer of
the epidermis, but they do not penetrate deep enough to remove
the stem cells and therefore the origins of the infection.
This can result in the re-emergence of the warts.
CA 02322199 2000-08-30
WO 99144622 PGT/GB99100605
- 4 -
An alternative treatment for warts is by use of
dinitrochlorobenzene. Such a treatment is intended to make
the patient allergic to dinitrochlorobenzene, whereupon the
the patient's immune system mounts an immune response to the
dinitrochlorobenzene at the site of the wart and the wart in
some cases disappears, presumably as a result of inununo
potenti~ation. Immuno-potentiation can be an effective
treatment but subjecting the patient to an allergic reaction
caused by dinitrochlorobenzene can be hazardous variable and
difficult to control.
An object of this invention is to provide a treatment for
viral skin infections, such as verrucae, warts and molluscum
contagiosum, which works effectively and is not associated
with the pain involved in the more traditional treatments.
Another object of the invention is to provide a system for
treatment of viral skin diseases which is less susceptible to
mutation of viral DNA.
We have previously suggested the treatment of viral infected
skin with some forms of nitrogen oxides, for example a nitrite
and an acidifying agent which results in the following
reaction:
NOZ' + H+ ~~~ HNOZ . . . . . . . . . . . . . . . . . . . ( 1 )
2HN02 ~ H20 + N203 . . . . . . . . . . . . . . . . ( 2 )
3 0 N203 a NO + NOZ . . . . . . . . . . . . . . . . . . ( 3 )
N203 + C6H806 -- 2N0 + H20 + C6H606 . , . ( 4 )
CA 02322199 2000-08-30
WO 99/44622 PCT/GB99/00605
- 5 -
We have now found that as far as viruses, as opposed to
bacteria for example, are concerned, the above nitrogen oxide
complex comprising for example NO and/or N02 while it may
effect replication to a degree, more importantly modifies the
virally infected cells such that the immune system can
recognize the viral particles. Inter alia, this is supported
by the fact that the complex is markedly less effective in
immunosuppressed hosts. Generally the greater the percent of
nitric oxide (NO) the better the immuno potentiation. If
possible up to 100$ NO can be used.
It is thought, although more work is required, that smaller
molecules, particularly NO and N02 penetrate the skin by direct
diffusion or via the sweat glands or hair follicles through the
epidermis to the sweat cells. It has been found that although
the healthy keratinocytes find the oxides of. nitrogen toxic
they do not die as they are relatively resistant to its
effects. However, the surprising clinical results in our
examples lead us to believe that virally infected cells are
more susceptible to these effects, leading to destruction of
the virally infected cells via a combination of toxicity
leading to programmed cell death and potentiation of the immune
response to the presence of the virus.
According therefore to a first aspect of the invention there
is provided the use in the manufacture of a topical medicament
for the in vi vo potentiation of the immune system during a
viral skin infection resultant from virus replication in the
epidermis, of a topical formulation comprising a source of
nitrogen oxides, and a pharmaceutically acceptable acidifying
agent.
CA 02322199 2000-08-30
WO 99/44622 PCT/GB99/00605
- 6 -
Depending on the type of viral infection the components of the
nitrogen oxide source can work synergistically or alone.
Nitrogen oxides, for example NO and N02, particularly can
diffuse through the epidermis. In the case of warts this
allows them to reach the stem cells which are at the base of
the epidermis and are the cells which contain the pool of
established virus. Once at the infected cells the nitrogen
oxide complex can facilitate programmed cell death, selectively
in infected cells, which may then be taken up by phagocytes and
antigen presenting cells leading to immune recognition of the
previously hidden viral antigens. Once recognized, specific
immunity will lead to destruction of all infected cells through
cellular and humoral responses.
Preferably the viruses replicating in the epidermis which cause
the viral skin infection are selected from molluscum
contagiosum, herpes simplex type 1 and 2, varicella zoster
virus and papilloma virus. Treatment using the acidified
nitrogen oxide source has been shown to be particularly
effective in viral skin infections caused by the aforementioned
viruses.
Conveniently the source of nitrogen oxides contains nitric
oxide and may also contain NO' or N0; nitrosium ions or a
precursor therefor, and is produced when a pharmaceutically
acceptable acidifying agent and a pharmaceutically acceptable
donor of nitrogen oxides, or a precursor therefor are brought
into intimate contact at the site of biological action.
If the pharmaceutically acceptable acidifying agent and the
pharmaceutically acceptable donor of nitrogen oxides, or a
precursor therefor were brought into contact before reaching
the site of biological action the efficacy of the treatment is
CA 02322199 2000-08-30
WO 99/44622 PCT/GB99/00605
diminished as the nitrogen oxides become progressively more
inactive with time.
In a preferred embodiment the pharmaceutically acceptable
acidifying agent or the pharmaceutically acceptable donor of
nitrogen oxides or a precursor therefor is disposed in a
pharmaceutically acceptable carrier or diluent therefor.
Preferably the pharmaceutically acceptable acidifying agent is
an organic acid and is selected from at least one of ascorbic
acid, ascorbyl palmitate, salicylic acid, lactic acid, citric
acid, formic acid, benzoic acid and tartaric acid.
The choice of acidifying agent depends on the type of viral
infection of the skin and the reaction of the infected areas
to treatment. The use of reducing acids such as ascorbic acid
gives a quick burst of NO and N02 with significantly more NO
produced compared to the amount of NOZ produced. The other
organic acids such as salicylic acid give a sustained
concentration of NO and NOZ over a certain time period wherein
the ratio of NO to NOZ is low. The concentration of the
inorganic nitrite, for example sodium nitrite (or other alkali
metal nitrites), as the pharmaceutically acceptable donor of
nitrogen oxides or a precursor therefor depends on the acid
used and the concentration of the acid used. The reducing
acid ascorbic acid is highly reactive so therefore only
between 1-I0~ is required with stoichiometric concentrations
of the pharmaceutically acceptable donor of nitrogen oxides or
a precursor therefor (e. g. sodium or other alkali metal
nitrite). Ascorbyl palmitate is more stable but requires a
higher concentration than ascorbic acid because the palmitate
has a higher molecular weight. A concentration of between 3~
and 25$ of ascorbyl palmitate is thus required. If salicylic
CA 02322199 2000-08-30
WO 99/44622 PCT/GB99/00605
_ g _
acid is used, concentrations of between 0.5~ and 30$ are
appropriate, citric acid requires a yet higher concentration
of up to 45$. (All $ given herein are by weight)
The concentration of sodium nitrite required to react with the
abovementioned concentrations of organic acid is between 0.5~
and 30~, preferably between 5~ and 20$. Other pharmaceutically
acceptable sources of nitrogen oxides or a precursor therefor
require different ranges of concentration.
Preferably the pharmaceutically acceptable acidifying agent and
the pharmaceutically acceptable donor of nitrogen oxides or a
precursor therefor are in stoichiometric concentrations.
I5 If the pharmaceutically acceptable acidifying agent and the
pharmaceutically acceptable donor of nitrogen oxides or a
precursor therefor are in stoichiometric concentrations, after
the reaction is completed there will be no unreacted compounds
left. Accordingly any compounds remaining on the infected area
will not be able to contaminate healthy skin with the active
medicament or anything the treated area touches such as
furniture and clothing.
In a preferred embodiment the medicament is in the form of a
paint, a varnish, an ointment a wax, a salve, or a cream.
These embodiments allow the pharmaceutically acceptable
acidifying agent and a pharmaceutically acceptable donor of
nitrogen oxides, or a precursor therefor to be applied directly
to the infected area. The treatment comprising the topical
application of separate compositions according to this
invention is preferably continued for at least one month, and
more preferably two months.
CA 02322199 2000-08-30
WO 99/44622 PCT/GB99/00605
_ g _
In a further aspect of the present invention there is provided
a two-part delivery system for the topical application of a
medicament for the in vivo treatment of the epidermis the said
system comprising separately;
a first waxy component comprising a pharmaceutically acceptable
acidifying agent;
and a second waxy component comprising a pharmaceutically
acceptable source of nitrogen oxides whereby if topically
applied in vi vo simultaneously, or immediately sequentially,
to the environment of use, active nitrogen oxides ara released
therefrom.
In a further embodiment, the first and second waxy components
comprise a paraffin. The acidifying agent is preferably a
reducing organic acid or salt such as ascorbic acid or ascorbyl
palmitate. The source of nitrogen oxides may be an alkali
metal nitrite such as sodium nitrite.
The use of a reducing acid or salt thereof results in a product
at the environment of use which comprises a major amount of NO
which has significant and therapeutic and immunological
effects.
Thus of the invention provides for the use of a source of
oxides) of nitrogen in the manufacture or prophylaxis of a
viral skin infection by a virus selected from herpes simplex
types 1 and 2, varicella zoster, vaccinia or papilloma, and
particularly from molluscum contagiosum.
In a further aspect of the invention there is provided a
delivery system for the topical application of a medicament for
CA 02322199 2000-08-30
WO 99/44622 PCT/GB99100605
- 10 -
the in vi vo treatment of the epidenais, comprising an adhesive
layer and a support layer impregnated with at least one of the
components of the medicament, characterized in that the
components of the medicament are a pharmaceutically acceptable
acidifying agent and a pharmaceutically acceptable donor of
nitrogen oxides or a precursor therefor, and a means of
combining the pharmaceutically acceptable acidifying agent with
the donor of nitrogen oxides. For example whereby the delivery
system comprises two layers, which when in situ release the
oxides of nitrogen including nitric oxide. The activation can
be by pressure or hydration from the skin.
Preferably the delivery system is adapted for the potentiation
of the immune system during a viral skin infection resultant
from virus replication with the delivery system in place, such
a system may, for example, resemble an adhesive plaster and it
is then simple to apply physical pressure to the exterior of
the plaster.
Conveniently the donor of pharmaceutically acceptable nitrogen
oxides may be aqueous and encapsulated in microspheres or
liposomes disposed in the support, preferably in the form of
a film or a gauze. The film or gauze allows increased
concentrations of the pharmaceutically acceptable acidifying
agent to be used. If a solution of salicylic acid is used then
only a concentration of 20- 26$ by weight is applied, but if
salicylic acid is impregnated in the film or the gauze then a
concentration of 26 to 44~ by weight can be applied.
A further advantage of using an adhesive layer is that it can
be used to occlude the infected area during treatment which
increases the concentration of nitrogen oxides being absorbed
through the epidermis.
CA 02322199 2000-08-30
WO 99/44622 PCT/GB99/00605
- 11 -
Another advantage of using the delivery system as just
described, instead of two creams or ointments, is that the
components of the medicament will only be applied to the
infected site, i.e no spillage will occur. It .is also easier
for the elderly, who may have shaky hands, to apply the
adhesive layer rather than applying a paint. For the treatment
of molluscum contagiosum, which is mainly found in those under
the age of 10 years, the adhesive layer can be a decoratively
patterned in order to appeal to children.
As stated above preferably the integrity of the vehicle is
disrupted by pressure after application of the adhesive layer
and film or gauze to a site of viral infected skin. If the
pharmaceutically acceptable acidifying agent and the
pharmaceutically acceptable nitrogen oxide donors or precursors
therefor are not kept separate until administration at the site
of biological action they will react together thus rendering
the medicament less effective. Accordingly, in this embodiment
it is 'necessary for the pharmaceutically acceptable acidifying
agent and the pharmaceutically acceptable nitrites or
precursors therefor to be retained separately within the film
or gauze layer. The application to the site of biological
action of pressure applied to the adhesive layer, and therefore
the.film or gauze layer; can result in the vehicles, such as
the microspheres or liposomes, breaking and the
pharmaceutically , acceptable acidifying agent and the
pharmaceutically acceptable nitrogen oxide donors or precursors
therefor reacting, thus treating the infected area.
In another aspect the delivery system may be used in
conjunction with a topically applied medicament. The topically
applied medicament being either.a pharmaceutically acceptable
CA 02322199 2000-08-30
WO 99144622 PCT/GB99/00605
- 12 -
acidifying agent or a pharmaceutically acceptable donor of
nitrogen oxides or a precursor therefor.
It is thus possible to provide only one of either the
pharmaceutically acceptable acidifying agent or the
pharmaceutically acceptable nitrogen oxide donors or precursors
therefor impregnated in the film or gauze layer. The other
compound, which is not impregnated in the film or gauze can
then be applied topically to the infected site. The advantage
of this arrangement is that the film or gauze layer can be
larger than the infected site but a reaction between the
pharmaceutically acceptable acidifying agent and the
pharmaceutically acceptable nitrogen oxide donors or precursors
therefor only occurs at the infected site where the medicament
had been topically applied.
It is also possible to vary the treatment regime by changing
the topically applied medicament without changing the compound
in the delivery system. For example if the pharmaceutically
acceptable nitrogen oxide donors or precursors therefor are
impregnated in the film or gauze, then the type of
pharmaceutically acceptable acidifying agent that is topically
applied can be altered and the same adhesive and film or gauze
layers utilized.
The delivery system is an ideal form of treatment for the
verrucae on the feet because the delivery system is hidden from
view.
The invention will now be described, by way of illustration
only, with reference to the following examples and the
accompanying figures.
CA 02322199 2000-08-30
WO 99144622 PGT/GB99/OOb05
- 13 -
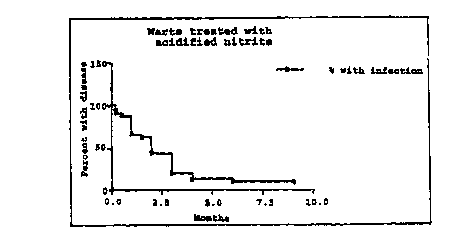
Figure 1 shows a graph of the duration of the warts compared
to the time for wart disappearance with the formulations given
in Example 1, where n=32;
Figure 2 shows the outcome of the treatment of patients with
warts as a function of time, where n=32
Figure 3 shows a Kaplan Meier plot of the outcome of the
treatment of patients with molluscum contagiosum as a function
of time: and
Figure 4 shows a graph of NO and NOZ release from 0.083 g of
10~ Ap wax with 0.014 g of 10~ sodium nitrite wax to give 2lEc
moles of NaN02 and 25~c moles of ascorbyl palmitate. In Figure
4 the curve with "squares" denotes NO values whereas the curve
with "circles" denotes NOZ values.
Example 1
32 subjects with recalcitrant viral warts were treated with
varying formulations of sodium nitrite acidified with the acid
specified. The exact formulations are given in Table 1. All
32 patients had failed to respond to conventional topical wart
applications and at least two treatments with liquid nitrogen.
12 subjects had plantar warts, 12 hand warts, 5 subungal or
peri-ungal and 1 plane of the warts of~the hand, 1 perianal and
1 lip wart.
The warts had a duration with median 24 months this implies
that the patients had a low chance of spontaneous improvement
( see Figure 1 ) .
CA 02322199 2000-08-30
WO 99/44622 PGTIGB99/00605
- 14 -
Table 1
Acid Nitrite No. of patients
treated
Salicylic 5$ cream Sodium nitrite 5~ 5
cream
Ascorbic acid 5$ Sodium nitrite 5$ 7
cream cream
Ascorbic acid 10~ Sodium nitrite 10~ 2
cream cream
Salicylic acid 23~ Sodium nitrite 10~ 9
in alcohol based + copper acetate
wart paint 0.5~
Salicylic acid 23$ Sodium nitrite 10$ 3
in alcohol based cream
wart paint
Salicylic acid 23~ Sodium nitrite 15~ 6
in alcohol based solution
wart paint
The warts were prepared by scraping or abrading the skin to
remove the dead skin then the sodium nitrite containing
formulation was applied before applying the selected acidifying
agent. The warts were treated every night and every three days
the warts were rescrapped or abraded.
Clearance of the warts occurred with a median duration of 2
months regardless of the formulation of the treatment (see
Figures 1 and 2). Copper was included to catalyze the release
of nitrogen oxides from glutathione and proteins that had
become nitrosated to extend the release of nitrogen oxides.
CA 02322199 2000-08-30
WO 99/44622 PCT/GB99I00605
- 15 -
Four treatment failures were seem three of these in patients
who were being treated with immunosuppressive drug therapy for
Lupus erthematosus, kidney transplant and dermatomyositis.
Accordingly there was an 88~ cure rate in all the subjects and
a 96$ cure rate if the innnunosuppressed patients were excluded.
Existing treatments such as using liquid nitrogen or salicylic
acid paints result in 50-80$ clearance.'
Example 2
30 patients with molluscum contagiosum lesions took part in a
double blind trial. They were randomly treated with either 5$
sodium nitrite co-applied with 5~ salicylic acid under
occlusion or 5~ sodium nitrite without acidification. The
I5 mean age of the subjects was 7 years (with one outlier of 47
not included in the mean). The infection had a mean duration
of 8.2313.959 months. No significant difference was found in
the number of lesions per patient or the number of times
treatment was applied in the two groups.
In the case of co-application the sodium nitrite was applied
to the skin with a cotton bud and then a fresh cotton bud was
used to apply the salicylic acid. In the case of the sole
application of sodium nitrite it was applied with a cotton bud.
In both cases, if possible, the area was covered with "cling-
film" or Sellotape~.
As seen in Table 2 in the group treated with the active
treatment 70$ of the patients were cured and 28~ of those in
the control group were cured. The mean time to cure was
1.83~0.91 months.
CA 02322199 2000-08-30
WO 99/44622 PCT/GB99/00605
- 16 -
Table 2
Treatment Cured Not cured
Acid and Nitrite 12 4
Control 4 10
Kaplan Meier plots were performed for active and control
patients (Figure 3) and analyzed by the Logrank test which
showed a significant difference in the survival curves with
cure being greater in the active group (p=0.0183).
Example 3
12 volunteers with no current or recent history of skin disease
and taking no medication randomly applied either low dose (0.5~
nitrite) or high dose (5$ nitrite) of nitrogen oxide complex
to their skin.
Subjects applied 2~ w/w ascorbic acid in aqueous cream to a
control site and an active site. Either the low dose or the
high dose nitrite cream was al,~-.o applied to the active site.
The creams were applied 3 times daily at 8 hourly intervals and
both the control and the active sites were then occluded with
an adhesive polythene/plastic dressing.
The last application of the cream was made 5 hours before the
assessment of the reaction to allow the immediate vasodilatory
effects of the nitrogen oxide complex to subside, so measuring
only residual inflammation.
The thickness of the control and active sites were measured
using a 'Mitotoyu' spring thickness gauge and redness was
measured using reflectance erythema metre. Two 4mm punch
biopsies were taken from the active and control sites; one for
CA 02322199 2000-08-30
WO 99/44622 PCT/GB99/00605
_ 17 -
formalin fixation for histological assessment, mass cell
stains, neutrophil elastase and in situ nick end labeling and
the other for snap freezing and OCT embedding for the other
immunohistochemical stains.
Immunohistochemistry was performed using a streptavidin biotin
method and DAB detection with the antibodies in Table 3 and
using ApopTag Plus in situ nick end labeling detection kit to
identify apoptotic cells.
Staining was quantified by computerized image analysis and data
analyzed by Wilcoxon's test for paired samples and Kruskal-
Wallis' test for non-parametric analysis of variance in the
multiple independent samples analyzed.for effects of dose and
duration (see Tables 4, 5 and 6).
Table 3
Epitope Titre Cells Stained
CDla 0.0451388889 Langerhans cells
CD3 0.0555555556 pan-T cell
CD4 1:150 T-helper cells
CD8 0.0555555556 T-cells suppressor/
cytotoxic
CD54 1:100 ICAM-1
CD6 0.0486111111 Macrophages
CD106 1:100 VCAM-1 .
p53 0.0763888889 Wild type p53
protein
Nitrosotyrosine * 1:100 Nitrosated tyrosine
Neutrophil elastase 1:100 Neutrophils
CA 02322199 2000-08-30
WO 99/44622 PCT/GB99/OU605
- 1B -
Epitope Titre Cells Stained
ApopTag ** Manufacturers Apoptotic cells
instructions
* polyclonal, all other antibodies monoclonal
** based on in situ detection of cleaved DNA with peroxidase
visualization.
The reflectance erythema measurement of the nitrogen oxide
complex treated sites was 32.25 ~ 5.46 (mean ~ sd)
significantly higher than the control sites 18.08 ~ 5.81
(p=0.0022, Wilcoxon's). Skin fold thickness was 5.04 ~ 0.75mm
in the nitrogen oxide complex treated patches which was
significantly greater than that of control skin 3.25 ~ 0.54
(p=0.0022, Wilcoxon's). These measures were not significantly
influenced by dose or duration of exposure, except there was
a trend for greater skin fold thickness in the high dose group
(5.4 mm ~ 0.21 vs 4.7 mm ~ 0.32)(p=0.075).
Histology of all actively treated sites showed a significant
increase in oedema, endothelial swelling, cloudy swelling of
keratinocytes, and a mixed infiltrate of lymphocytes and
neutrophils. These changes were quantified on a 0-4 ordinal
scale and were similar in low dose, high dose, short exposure
and long exposure. The number of mast cells seen in Azure A
stained sections was similar in control and nitrogen oxide
complex treated skin.
A cytotoxic effect was seen in all keratinocytes which was
manifest as cloudy swelling. When extensive this leads to the
formation of bullae high in the epidermis filled with acute
inflammatory cells and cells which have undergone cytotoxic
changes with constriction of the nucleus and cloudy swelling
CA 02322199 2000-08-30
WO 99/44622 PC"T/GB99I00605
- 19 -
of clear cytoplasm around them. Only a minority of these
degenerate cells had undergone apoptosis as judged by staining
with ApopTag. Within the viable epidermis, there was also an
increase in apoptotic cells. This suggests that normal
keratinocytes, not virally infected are relatively resistant
to the well known apoptotic effects of nitric oxide. Apoptotic
cells were also detected in the dermis~, particularly around
adnexal structures. The positive nitrosotyrosine staining
around sebaceous glands suggests that the nitrogen oxide
complex was preferentially absorbed through follicles.
Nitrogen oxide complex treated skin showed significant
increases in immuno-competent cells expressing CD3, CDB, CD68
and neutrophil elastase and in the adhesion molecules which
attract trafficking of the cells to the site, ICAM-1 AND VCAM-
1. The presence of nitrosotyrosine staining in these cells is
indicative of the formation of peroxynitrite (ONOO') and of p53
which indicates that part of the effect of the complex is
mediated through toxicity towards DNA in these cells. In
healthy skin nitrogen oxide complex did cause some apoptosis
but this was surprisingly small at the doses used and we
postulate that the effect is likely to be in infected cells.
The antigen processing cells of the skin (CDla positive) were
seen to lose dendricity and drop from the epidermis so there
were significantly fewer in the treated skin. As these cells
behave in this way when activated and functioning to process
a newly recognized antigen, this would seem to offer further
evidence for an immunopotentiating role for the nitrogen oxide
complex. Ki-67 staining for dividing cells did not differ in
control or active sites. This would suggest that in warts, for
example, the action is not one of reducing cell proliferation.
CA 02322199 2000-08-30
WO 99/44622 PCT/GB99/00605
- 20 -
Kruskal-Wallis test was used to test the effects of time or
duration of nitrogen oxide complex treatment on clinical and
immunohistochemical response. The effect of the dosage on the
skins reaction is given in Table 5. There were fewer CD4
positive cells in the high dose than the low dose group, and
likewise with CD68 positive cells. Although Ki-67 positive
cells were not significantly different between the control site
and the nitrogen oxide complex treated site, there was a
significant increase with high dose compared with low dose.
After 24 and 48 hours exposure to the nitrogen oxide complex
the extent of apoptosis was measured, see Table 6. There was
significantly greater apoptosis after 48 hours than after 24
hours. The CD4 positive cells count rose significantly after
48 hours compared to after 12 hours. The difference for p53
was not quite statistically significant. Similarly, cloudy
swelling tended to be greater in the longer duration treatment
but was not statistically significant.
Table 4
Nitrogen Control Significance
Oxide Wilcoxon's Test
Complex
Mean S.D. Mean S.D.
ApopTag 12.5 10.1 0.41 1.16 0.0033
Ki67 6.82 3.88 6.62 2.854 0.67
CDla* 0.86 0.69 3.43 0.53 0.022
CD3 574.7 396.3 216.1 122.1 0.0186
CD4 608.2 458.2 176.3 149.9 0.0125
CD8 275.7 193.1 122.1 106.1 0.0284
CD68 673.1 542.4 301.4 361.3 0.0044
CA 02322199 2000-08-30
WO 99/44622 PCT/GB99/00605
- 21 -
Nitrogen Control Significance
Oxide Wilcoxon's Test
Complex
Nitrosotyrosine 3.4 0.7 0.9 1.1 0.043
p53 214.4 266.4 22.08 53.8 0.0029
Neutrophils 569.4 385.9 71 113.1 0.043
ICAM-1 705.9 704.5 201.9 160.9 0.0209
VCAM-1 1.5 1.17 0.5 0.9 0.0357
* Where cell counting was difficult e.g. more diffuse
staining/dendritic cells staining was graded subjectively on
a scale of 0-4.
Ki67 was counted in the epidermis and ApopTag positive cells
counted per standard section through a 3mm punch biopsy. All
other counts were done by computerized image analysis on a
fixed standard measuring frame and are expressed as cells per
~2
Table 5
High Low Kruskal-Wallis
Dose Dose Test
(cells/mm2) (cells/mm2)
Mean S.D. Mean S.D.
Ki67 152 35.2 73.6 8 0.01
CD4 280 78.4 936 185.6 0.02
CD68 379.2 65.6 916.8 262.4 0.04
Table 6
12/24* 48 hrs Kruskal-Wallis
hrs Test
Mean S.D. Mean S.D.
ApopTag * 3.5 1.82 14.16 2.56 <0.005
CA 02322199 2000-08-30
WO 99144622 PCT/GB99/00605
- 22 -
CD4 285.9 193.6 824 193.6 0.05
Cloudy swelling 1.7 0.33 2.5 0.224 0.07
**
p53 70.1 47.04 335.2 125.7 0.07
Ki67 was counted in the epidermis and ApopTag positive cells
counted per standard section through a 3mm punch biopsy. All
other counts were done by computerized image analysis on a
fixed standard measuring frame and are expressed as cells per
mm2.
** Where cell counting was difficult e,g. more diffuse
staining/dendritic cells, staining was graded subjectively on
a scale of 0-4.
The nitrogen oxide complexes of the invention may be formed by
a combination of ascorbic acid and nitrite on the skin, which
causes the release of nitrogen oxides, inter alia nitric
oxide, nitrous oxide, nitrogen dioxide and dinitrogen
2G trioxide. The increase in T helper cells and macrophages was
greater in low dose subjects and suggests that at lower doses
nitrogen oxides can be pro-inflammatory but at higher doses
becomes cytotoxic to the immunocvmpetent cells and begins to
exert an inhibitory effect. The nitrogen oxide complex led to
a marked induction of ICAM-1 and a moderate increase in VC.AM-1
expression. The pattern of inflammation was unusual in
showing a marked infiltrate of macrophages after only 24
hours, so showing that activated macrophages use nitrogen
oxides to specifically attract more macrophages to kill a
pathogen.
The promotion of apoptosis and recruitment of all the
immunocompetent cells required for effective recognition of a
CA 02322199 2000-08-30
WO 99/44622 PGT/GB99/00605
- 23 -
pathogen by the immune system of a host, results from
application of a preparation of a combination of nitrite or
precursor of nitrogen oxides and an acidifying agent.
Accordingly, these findings support a potential
immunopotentiating effect of the combination of nitrite or
other precursor of nitrogen oxides such as NO or N02 and a
acidifying agent.
Example 4
A two part component delivery system was made up. Each
component was in the form of a wax stick which can be rubbed
onto an effective area at regular intervals in accordance with
a physician's instructions.
The two components were made up as follows:-
10$ ASCORBYL PALMITATE
Ascorbyl Palmitate 10$
White Soft Paraffin 25
Light Liquid Paraffin 20
Hard Paraffin 20
Arlacel 165 15
Cetosteryl Alcohol, 10
Method
1. Weigh all the components into a vessel.
2. Heat the vessel and stir the mixture until all the
components have melted and the mixture is homogenous.
3. Pour the molten wax into jars and allow to cool to room
temperature.
CA 02322199 2000-08-30
WO 99144622 PCTIGB99/00605
- 24 -
Corlnonents
Phase A
Light Liquid Paraffin 7.5$
White Soft Paraffin 20
Arlacel 582 10
Cetosteryl alcohol 10
Phenoxyethanol 1
Phase B
Sodium Nitrite 10
Purified Water 20
Method
1. Weigh the Phase A components into a vessel, heat to 70°C
and stir until homogenous.
2. Weigh the Phase B components into another vessel heat to
70°C and stir, ensure that all the sodium ni'rite has
dissolved.
3. When both phases have reached 70°C, add phase A to phase
B and homogenize for 5 minutes.
4. . Pour the molten wax into jars and allow to cool to room
temperature.
As is shown from Figure 4, the use of this admixture tends to
release a substantial excess of NO from the two-part delivery
system. This is possibly because NO is a small molecule which
results in a more effective treatment of viral skin diseases.