Note: Descriptions are shown in the official language in which they were submitted.
CA 02322573 2000-09-07
WO 99/51142 PCTIUS99107565
NON-INVASIVE TISSUE GLUCOSE LEVEL MONITORING
Field of the Invention
This invention relates to instruments and methods for performing non-
invasive measurements of analyte concentrations and for monitoring, analyzing
and
regulating tissue status, such as tissue glucose levels.
Background of the Invention
Diabetes is a chronic life threatening disease for which there is presently no
cure. It is the fourth leading cause of death by disease in the United States
and at least 90
million people worldwide are estimated to be diabetic. Diabetes is a disease
in which the
body does not properly produce or respond to insulin. The high glucose levels
that can
result from this affliction can cause severe damage to vital organs, such as
the heart, eyes
and kidneys.
Type I diabetes (juvenile diabetes or insulin-dependent diabetes mellitus) is
the most severe form of the disease comprising approximately 10% of the
diabetes cases in
the United States. Type I diabetics must receive daily injections of insulin
in order to
sustain life. Type II diabetes, (adult onset diabetes or non-insulin dependent
diabetes
mellitus) comprises the other 90% of the diabetes cases. Type II diabetes is
often
manageable with dietary modifications and physical exercise, but may still
require
treatment with insulin or other medications. Because the management of glucose
to near
- w w ~- w - normal levels can -prevent -the onset and the progression of
complications of diabetes,
persons afflicted with either form of the disease are instructed to monitor
their blood
glucose level in order to assure that the appropriate level is achieved and
maintained.
Traditional methods of monitoring the blood glucose level of an individual
require that blood be withdrawn. This method is painful, inconvenient, costly
and poses
the risk of infection. Another glucose measuring method involves urine
analysis, which,
aside from being inconvenient, may not reflect the current status of the
patient's blood
glucose because glucose appears in the urine only after a significant period
of elevated
levels of blood glucose. An additional inconvenience of these traditional
methods is that
they require testing supplies such as collection receptacles, syringes,
glucose measuring
eTlitcTlTrrrF cT.~'.FT fRTTi.F ~~1
CA 02322573 2000-09-07
WO 99/51142 PCTNS99/07565
2
devices and test kits. Although disposable supplies have been developed, they
are costly
and can require special methods for disposal.
Many attempts have been made to develop a painless, non-invasive external
device to monitor glucose levels. Various approaches. have included
electrochemical and
spectroscopic technologies, such as near-infrared spectroscopy and Raman
Spectroscopy.
Despite extensive efforts, however, none of these methods appears to have
yielded a non-
invasive device or method for the in vivo measurement of glucose that is
sufficiently
accurate, reliable, convenient and cost-ei~ective for routine use.
Summary of the Invention
The invention overcomes problems and disadvantages associated with current
strategies and designs and provides new instruments and methods for
monitoring, analyzing
and regulating in vivo glucose levels or other analyte levels in an
individual.
In one general aspect, the invention features a non-invasive glucose
monitoring instrument useful in vivo. The instrument may comprise a radiation
source
capable of directing radiation to a portion of the exterior or interior
surface of a patient.
That surface may be a mucosal area such as the gums and other mucosal areas,
the
eyeballs and surrounding areas such as the eyelids and, preferably, the skin.
The source
emits radiation at a wavelength that excites a target within the patient such
that the
excited target provides a glucose level indication of the patient. A glucose
level
indication is a quantitative or relative. measurement that correlates with the
blood glucose
___._.~.. ~4.nten t 9r_concendcation of the_patient. The instrurner~-may-
furthercomprise a radiation _ _ _ . __ ._
detector positioned to receive radiation emitted from the excited target, and
a processing
circuit operatively connected to the radiation detector that translates
emitted radiation to a
measurable signal to obtain the glucose level indication. The target is not
glucose itself,
but a molecular component of the patient such as, for example, a component of
skin or
other tissue, that reflects or is sensitive to glucose concentration, such as
tryptophan or
collagen cross-links. Suitable targets are structural components, and
compounds and
molecules that reflect alterations in the environment of matrix components of
the tissue
and are sensitive to or correlate with tissue glucose concentration. The
target provides an
emitted fluorescence signal that is related to the patient's blood glucose
level. The
SUBSTITUTE SKEET (RULE 26)
CA 02322573 2000-09-07
WO 99/51142 PCT/US99/07565
3
radiation detector is responsive to the emission band of the target or species
in the skin.
Preferably the radiation is ultraviolet radiation or light. The emitted
radiation is
preferably fluorescence radiation from the excitation of the non-glucose
target. The
instrument may further include means for measuring scattering re-emitted from
the
irradiated skin. The radiation emitted from the excited target and signal
therefrom
correlates with the blood glucose of the patient.
Another aspect of the invention relates to an instrument for assessing
changes in the superficial structural matrix of the skin or other tissue of a
patient
comprising means for measuring fluorescence, and means for measuring
scattering.
Another aspect of the invention relates to an instrument for assessing
changes in the environment of matrix components of the skin or other tissue of
a patient
comprising means for measuring fluorescence, and means for measuring
scattering.
Preferred embodiments further include means for combining signals from the
means for
measuring fluorescence and the means for measuring scattering.
Another aspect of the invention relates to a non-invasive method of
detecting or assessing a glucose Level compri$ing exciting a target that, in
an excited state,
is indicative of the glucose level of a patient, detecting the amount of
radiation emitted by
the target, and determining the glucose level of the patient from the amount
of radiation
detected. The target is preferably a molecular species in the skin. Preferred
targets are
tryptophan or a matrix target, like PDCCL, which are excited by ultraviolet
radiation and
act as bioamplifiers or bioreporters. Targets may be str<icturar iriatrix or
cellular
components. Suitable targets reflect alterations within the environment of
matrix
components of the skin or other tissue and act as bioamplifiers or
bioreporters when
excited with ultraviolet radiation.
Still another aspect of the invention relates to a non-invasive method of
assessing a change in the superficial structural matrix of a tissue, or a
change in the
environment of matrix components, comprising exposing the tissue to radiation
at a first
wavelength, detecting an amount of fluorescence emitted by exposed tissue,
exposing the
tissue to radiation of a second wavelength, detecting an amount of scattering
re-emitted
SUBSTITUTE SHEET (RULE 26)
CA 02322573 2000-09-07
WO 99/51142 PCT/US99/07565
4
from the exposed tissue, and deriving an indication representative of the
change in the
superficial structural matrix of the tissue, or a change in tissue matrix
components or their
environment, based on of the amount of fluorescence detected and the amount of
scattering detected.
Other objects and advantages of the invention are set forth in part in the
description which follows, and in part, will be obvious from this description,
or may be
learned from the practice of the invention.
Brief Description of the Drawings
Figure 1 A multipurpose skin spectrometer that provides data specifically
relevant to
signals correlating with blood glucose.
Figure 2 Block diagram of one embodiment of a glucose level
monitoring instrument.
Figure 3 Graph of the average fluorescence excitation spectra for
normal and diabetic SKH mice for an emission wavelength of
3 80 nm.
Figure 4 Graph of the average fluorescence excitation spectra for
normal and diabetic SIGH mice for an emission wavelength of
340 nm.
Figure 5 Graph of the average fluorescence excitation spectra for a rat
at an emission wavelength of 380 taken at different blood
glucose levels. __ . . _ .. _ .. _ _ . _ _. _______.... . _._ .. .
Figure 6 Plot of the fluorescence intensity at 346 nm for four different
glucose levels which are taken from Figure 5.
Figure 7 Graph of the average fluorescence excitation spectra for an
emission wavelength of 380 nm for a human male before and
after the ingestion of 100 grams of glucose.
Figure 8 Graph of the average fluorescence excitation spectra for an
emission wavelength of 380 nm for a human male before and
after the ingestion of 100 grams of glucose.
SUBSTTTUTE SHEET (RULE 26)
CA 02322573 2000-09-07
WO 99/51142 PCT/US99107565
Figure 9 Graph of the average fluorescence excitation spectra for an
emission wavelength of 380 nm for a human female before
and after the ingestion of 100 grams of glucose.
Figure l0A A diagram depicting collection of fluorescence spectra with
components
attributable to tryptophan and collagen cross links following irradiation
with LJV light.
Figure l OB A diagram depicting scattering according to a scattering model.
Figure 11 Block diagram of a monitoring instrument that can be used to monitor
tissue glucose levels or evaluate changes in the superficial structural matrix
of a tissue or the environment of matrix components of a tissue.
Detailed Description of Illustrative.Embodiments
As embodied and broadly described herein, the present invention relates to
devices and methods for quantitating, trending and/or reporting an ~analyte,
such as blood
glucose, to devices and methods for monitoring and regulating in vivo glucose
levels, and
to devices and methods for evaluating the superficial structural matrix or
cellular
components of a tissue.
It has been discovered that by measuring fluorescence following irradiation
of a tissue surface of a patient, such as the patient's skin, and by
optionally assessing
scattering, the glucose level of a patient can be evaluated. Evaluation
according to the
invention is based on the surprising discovery that the quantum efficiency of
fluorescence
of a responsive target within the skin is transiently affected by the
irradiation and can be
correlated to the ambient glucose content. Long-term interaction between
diabetes,
collagen and other species has been previously observed (V.M. Monnier et al.,
Diabetes
37:867-872, 1988). However, a reversible component of this interaction that
correlates
with blood glucose levels and possibly depends on the glucose level in the
environment
of collagen and other targets has previously gone unnoticed. More
specifically, although
glucose itself does not fluoresce to any significant degree, when the blood
glucose level
of a patient changes, the quantum efficiency of fluorescence of a target such
as, for
SUBSTITUTE SHEET (RULE 26)
CA 02322573 2000-09-07
WO 99/51142 6 PCT/US99/07565
example, pepsin-digestible collagen cross links (PDCCL), also changes. This
change
may be due, in part, to the direct and indirect effects of the relative
presence of glucose or
other molecules on the environment of target molecules and structures. That
presence
induces a reversible change in the quantum efficiency of fluorescence
production by the
target which can be detected and analyzed. Glucose molecules in the
environment may
be covalently or noncovalently coupled to the target (glycosylated collagen),
or simply
free in the immediate vicinity of the target. Targets may be in the dermal
matrix, in the
epidermal matrix, or in cells or the immediate vicinity of cells associated
with the either
the dermis or the epidermis. In this regard, the invention may also be used to
directly
assess the amount or degree of advanced glycation end products that exist in
an area of
the body such as, for example, in vessels, arteries or organs.
A fluorescent signal originating from dermal collagen cross links has been
identified, which signal slowly increases with aging and is also sensitive to
transient
exposure to ultraviolet radiation. PDCCL fluoresces following excitation at
335-340 nm,
with the emission maximum at 390 nm (N. Kollias et al., Journal of
Investigative
Dermatology, 111:776-81 1998). The fluorescent signal decreases monotonically
with a
single UV exposure, but recovers within hours. With multiple exposures, the
effects
appear cumulative, and recovery takes weeks. However, it has been discovered
that
transient changes in the environment of these collagen cross links causes
significant and
transient alterations in their fluorescence which can be tightly correlated
with blood
glucose determinations.
Targets in the environment of matrix components, such as collagen cross
Iinks serve as bioamplifiers or bioreporters of ambient glucose concentrations
and, thus,
constitute a novel and sensitive means of non-invasively assessing glucose in
real time.
Advantages of this methodology include a large change in signal level for a
relatively
small change in collagen structure or matrix environment. The method is also
unhampered by absorption from competing species in the general area. In
addition, there
are only a few fluorophores which makes signal analysis easier. Further,
detector
sensitivity is generally excellent and instrumentation and optical components,
all of which
CA 02322573 2000-09-07
WO 99/5! 142 7 PCT/US99/07565
are commercially available, are potentially simpler and less expensive than
those used for
infrared measurements. Also, given the robust signals and signal to noise
ratios observed,
there is potentially less of a need to resort to complex algorithmic and
chemometric
analyses.
Accordingly, one aspect of the present invention is related to a non-invasive
in vivo glucose monitoring instrument that determines glucose levels or
changes in
glucose levels by measuring fluorescence of the skin following excitation of
one of these
targets or species. Specifically, fluorescence signals obtained following
irradiation of
skin or other tissue can be correlated with glucose levels, or changes in
glucose levels, by
measuring fluorescence following excitation of targets or species within the
environment
of the matrix components. Preferred targets are structural matrix components
such as
PDCCL. Another preferred target is epidermal tryptophan which, like other
targets, may
be bound to other compounds or structures, and intracellularly or
extracellularly
localized. Other useful matrix targets for excitation include collagenase-
digestible cross
links, elastin cross links, glycosaminoglycans, glycated collagen and
glycosylated
substances in a tissue. These targets may also be referred to as biosensors as
they are
biological substances that detectably change in response to glucose content,
or
bioamplifiers as they may amplify a signal indicative of systemic glucose
levels.
A non-invasive glucose monitoring instrument according to one aspect of
the invention includes a radiation source capable of directing radiation to a
portion of the
surface of the skin (or other tissue} of a patient. The source emits radiation
at a
wavelength that excites a target of species in the tissue that can be
correlated with blood
glucose content, such that the excited target provides a glucose level
indication of the
patient. In a preferred embodiment, the target is a molecule other than
glucose, and most
preferably is a structural matrix component such as, for example, collagen
cross-links.
Alternatively, the target may be tryptophan. When the target being detected is
cross-
linked collagen, the ultraviolet radiation source is preferably operative to
irradiate at
approximately 330-345 nanometers, and the ultraviolet detector is sensitive to
emitted
wavelengths in the range of 370-410 nanometers, more preferably, 380-400
nanorneters
CA 02322573 2000-09-07
WO 99/51142 8 PCT/US99/07565
and, most preferably, 390 manometers. As noted, another useful target whose
change in
emission may be detectable is tryptophan. When the target being detected is
tryptophan,
the ultraviolet radiation source is preferably operative to irradiate at
approximately 285-
305 manometers, more preferably at approximately 295 manometers, and the
ultraviolet
detector is preferably sensitive to emitted wavelengths in the range of 3 I S-
420
manometers, more preferably 340-360 manometers, and most preferably, 345
manometers.
The radiation emitted by the target correlates with the glucose level of the
patient. The
spectral information can be converted into a number correlative to standard
blood glucose
determinations.
The instrument further comprises a radiation detector positioned to receive
radiation emitted from an excited target. The instrument further includes a
processing
circuit operatively connected to the radiation detector and operative to
translate a level of
emitted radiation into a measurable signal that is representative of or may be
correlated
with the blood glucose level. Preferably, the radiation source is ultraviolet
light. In a
preferred embodiment the radiation source may comprise a flexible-fiber optic
arm or
probe that directs said radiation to the target. The probe may comprise a
glass or quartz
fiber and may be flexible and easily manipulated to examine a site anywhere on
the
patient's skin. The portion of skin irradiated may be less than about 1 square
cm, and
more preferably is about 0.2 square cm. Preferably, the portion is a site
which is most
easily measurable on the patient such as on the arm or leg. Differences in
pigmentation
between different areas of the body as well as different patients can be
factored or
eliminated through selection of control input, and overcome.
The instrument may further comprise a display such as, for example, a
visual, auditory or sensory display operatively connected to the processing
circuit and
operative to display the glucose level indication. Optionally, this data may
be analyzed
and transmitted to a pump or other servo mechanism responsive to the
processing circuit.
The pump is incorporated into the system such that the pump administers
insulin or other
medication to the patient at a rate that corresponds to the glucose level
signal.
CA 02322573 2000-09-07
WO 99/51142 PCT/US99/07365
9
Referring to Fig. 1, an embodiment of the glucose monitor of the invention
includes a Xenon arc (Xe-arc) lamp, double excitation and emission
monochromators, a
photomultiplier device, a simple current amplifier and a flexible probe. The
probe may
comprise fiber optic bundles which allow convenient evaluation of living
systems. This
embodiment can take the form of a multipurpose skin spectrometer or it may be
modified
to create a unit optimized to provide data specifically relevant to signals
correlating with
blood glucose. One advantage of utilizing fluorescent excitation spectra
compared to
fluorescence emission spectra is that the former are similar to absorption
spectra, which
aids in the separation and identification of the individual fluorophores in a
complex
spectrum. Although other components can be substituted for the elements in
this
embodiment, a Xe-arc in combination with an excitation monochromator, avoids
the
major constraint of laser sources, namely the limited number of excitation
wavelengths.
Optionally, other types of sources, such as a diode laser, coupled with
enhanced spectral analysis algorithms optimized for the collagen cross links
may be used.
These algorithms may also incorporate variables such as skin type, age,
exposure, etc.,
all of which are analyzed during testing. Hardware modifications and
calibrations may be
incorporated to take into account these and other variables. Specific
algorithms and
software may be embedded into a dedicated processor. For example, one design
may
comprise a night hypo/hyperglycemia monitoring instrument which is programmed
to
alarm by trending analysis parameters that correlate with significant changes
in blood
glucose. Alternatively, monitoring could be performed with a transportable
fiber-based
fluorescence spectrophotometer with double monochromators, both on the
excitation and
emission paths. This allows the evaluation of different subsets of collagen
cross links and
tryptophan signals as well as allowing the estimation of epidermal melanin
pigmentation
or other tissue pigments. Optimized instruments may duplicate and incorporate
the
functionality and data processing requirements incorporated from appropriate
studies.
Another embodiment uses a fiber-based fluorescence spectrometer with two
double monochromators and a high intensity excitation light source (350 W Xe-
arc). The
double monochromator design minimizes stray tight, which tends to be high
because of
CA 02322573 2000-09-07
WO 99151142 PCT/ITS99/07565
the high level of light scattering by the tissues. The probe is preferably a
fiber optic
device that allows collection of data from different skin sites on the body.
Probe design
is optimized to permit ease of use and reproducibility. Optimization of light
sources,
filters and software can be designed to perform three scans that maximize the
collagen
fluorescence signals. One scan is preferably 250-360 nm on the excitation band
and 380
nm on the emission. The second scan is preferably 250-400 nm on the excitation
and 420
nm on the emission. The third scan is preferably 360-480 nm on the excitation
and 500
nm on the emission. This provides information on PDCCL (340/390nm), the
collagenase
digestible collagen crosslinks (370/460nm) and the collagen/elastin crosslinks
(420/500
nm), among other species. The system may also provide data on tryptophan, an
epidermal fluorophore having an excitation wavelength of 290-300 nm and an
emission
wavelength of 340-360 nm, among other species. Devices may be small and
lightweight
desktop units useful in health care provider settings. A remote probe may be
connected
to the system through a flexible fiber optic bundle. Data output may consist
of a
reporting of a quantitative number that correlates with blood glucose
readings, along
with spectral data, which may be displayed on a separate small IIO terminal or
laptop
computer. The software further contains diagnostic overlay capabilities.
Another device allows monitoring of glucose levels, by providing spectral
information reflective of glucose levels, on a continuous or repetitive basis.
In one
embodiment, this would be used throughout the night with a built-in alarm, to
alert the
patient to abnormal decreases or increases in glucose levels. The unit, which
may be the
size of a clock radio, can have a fiber optic cable to connect to the patient,
similar to
existing apnea monitors and pulse oxymeters. Another portable device may be
placed in
contact with the skin for periodic momentary glucose readings. It may have an
LCD
readout for glucose levels, memory to store several hundred glucose readings
and a data
output to download stored data.
An alarm may be operationally coupled to the processing circuit such that
the alarm is activated when the glucose level indication exceeds a first
predetermined
value (such as 200 gm/1), falls below a second predetermined value (such as 70
gm/ml),
CA 02322573 2000-09-07
WO 99/51142 PCT/US99/07565
11
or varies more than 20% from a third predetermined value (such as the
previously
measured Level or a baseline level determined for the patient). Alternately,
the alarm may
be triggered in response to a more complex algorithmic analysis of data or
based on
evaluation by trending analysis over time.
The instrument may further comprise a normalizing detector responsive to
another target in the tissue, such that the processing circuit is responsive
to the
normalizing detector to normalize the glucose level indication. For example, a
current or
latest glucose level signal may be normalized by comparing it to a previously
determined
glucose level signal which has been calibrated by comparing it directly with a
conventionally determined blood glucose level. Alternatively, normalization
may involve
comparison of emissions from the same target but at another wavelength,
comparison of
emissions from a non-target such as glucose or another structural or
circulating
component of the body, or simply taking a reading from another skin site.
Normalization
may also be performed by comparison to similar data from another paint or
points in time
taken from the same individual, or utilizing a stored database or spectral
library.
Normalizing may alternately comprise obtaining a baseline signal before any
prolonged
activity where continual measurements would be difficult such as, for example,
before
driving or sleeping, and watching for changes or trends of changes. The
previously
determined glucose level signal may also be compared with a level assessed
from a
simultaneously drawn blood sample. In addition, scattering evaluation may be
factored
into the normalizing process. , _
The instrument may optionally comprise means for measuring scattering re-
emitted from the tissue. As discussed below, the means for measuring
scattering may
comprise a skin illuminating means that emits radiation at an angle greater
than 60
degrees to said target or it may comprise a skin or tissue illuminating means
which emits
radiation at between about 330 to 420 nm. Re-emitted radiation is then
collected and
analyzed.
The instrument may include a portable housing in which the radiation
source, the radiation detector and the processing circuit are disposed. The
instrument
CA 02322573 2000-09-07
WO 99/51142 PCT/US99/07565
12
may include a battery compartment disposed in the housing and a pair of
battery contacts
operatively connected to the ultraviolet radiation source, the ultraviolet
radiation detector,
and the processor. Data can be electronically recorded, stored or downloaded
for later
review or analysis. The instrument may further comprise attachment means for
attaching
the radiation source, a portion of, or all of the device to the patient. The
portable housing,
the ultraviolet radiation source, the ultraviolet radiation detector, and the
processor may
be designed so that they weigh in combination less than 3 kilograms, more
preferably less
than 1 kilogram, and most preferably, less than 0.5 kilograms. The instrument
may
optionally include an attachment mechanism for attaching the housing to the
patient.
Alternately, the instrument can be miniaturized; in such an embodiment, a
microprocessor
is incorporated onto a dermal patch, which may be operatively connected to
other devices
that provide input directly to a pump or other biodelivery system, such as a
transmucosal
or inhalational system, which may deliver insulin or other appropriate
medication to the
patient.
The instrument may also be constructed using small components composed
of inexpensive, possibly recyclable materials such as plastics and paper, so
that the entire
instrument or a significant portion is disposable. For example, the entire
device can be
incorporated into a patch worn anywhere on the body and secured with adhesive
tape,
hook-and-loop fastener or another suitable means. After expiration or
depletion of an
integral battery, the patch can be safely and easily disposed of and a new
patch secured.
Such instruments weigh less than 1 kg, preferably less than 0.5 kg and more
preferably
less than 0.1 kg.
The processing circuit is preferably operative to translate the level of
detected radiation into a measurable glucose level signal that is indicative
of the glucose
level in the tissue. The signal may be directly evaluated, or it may be
compared to stored
reference profiles, to provide an indication of changes from previous levels
or trends in
the patient's glucose level. Although a preferred embodiment measures
radiation or
fluorescence following irradiation of the skin, the present invention can also
be used to
assess glucose levels by evaluation of other tissues. For instance, glucose
levels may be
CA 02322573 2000-09-07
WO 99/51142 PCT/US99/07565
13
assessed in accordance with the present invention by detecting radiation or
fluorescence
following irradiation of the surface of other tissues, such as mucous
membranes, or
irradiation of the mucosa, submucosa or serosa of any organ.
Another aspect of the invention relates to a non-invasive method of
detecting a glucose concentration or level in vivo comprising the steps of
exciting a target
in the skin or other tissue, preferably using ultraviolet radiation, that is
sensitive to the
patient's tissue glucose content and is indicative of the glucose level of the
patient,
detecting an amount of radiation or fluorescence emitted by the target, and
deriving an
indication representative of or which correlates with a current blood glucose
level based
on the amount of radiation or fluorescence detected. Preferably, the target is
a matrix
target such as collagen cross links. Alternatively, the target may be
tryptophan. The
method may optionally include the step of determining whether to take steps to
adjust the
patient's glucose level in response to the derived glucose level, followed by
the step of
administering insulin or another pharmaceutical composition in response
thereto. The
method may include any one or more of the steps of reporting the information
to the
patient, recommending a dosage, or administering the composition, such as
insulin, to the
patient in response to the indication derived. The step of administering may
be performed
by using a syringe, a pump or another suitable biodelivery system, mechanical
or
chemical, which may be implanted or external to the body. The method may also
include
the step of displaying a glucose level indication related to the indication
derived or
providing a warning to the patient. The method may further include the step of
normalizing the glucose level indication derived in the step of deriving. The
steps of
exciting, detecting, and deriving may be performed continuously or at any
appropriate
interval, for example, by the minute, hourly, daily or every other day for the
same patient
and over a period of days, weeks, months or years.
Optionally, the method may include actuating an alarm in response to the
glucose level when the glucose level exceeds a predetermined first level,
falls below a
predetermined second level or varies more than a set percentage, such as for
example,
10%, 20%, 30%, 50% or 100% or more from a predetermined third level or changes
in
CA 02322573 2000-09-07
WO 99/S1I42 PCT/US99/07565
14
such a way that meets criteria of a specifically designed algorithm. The
method may
further comprise the step of measuring scattering re-emitted from the skin or
irradiated
tissue surface and utilizing the resulting data to initiate or assist in
actuating a process
aimed at adjusting the glucose level.
Instruments according to the invention are advantageous in that they
provide information relative to blood glucose and permit glucose levels to be
evaluated
noninvasively. Such non-invasive instruments allow people with diabetes to
monitor
glucose levels without the pain, inconvenience, and risk of infection
associated with
obtaining a blood sample. By making monitoring safer and more convenient,
people with
diabetes can monitor their glucose levels more frequently and therefore
control levels
more closely. Safer and more convenient glucose level monitoring reduces the
likelihood
of measurements being skipped.
Furthermore, by coupling the instrument according to the invention with a
pump or other device which can deliver insulin or other therapeutic agent to
the patient,
using a transmitter, or other suitable communication device, such that the
pump or device
is responsive to the glucose signal, even finer automatic glucose level
monitoring may be
achievable. For example, the transmitter may remotely transmit the signal to a
pump,
such as a servo pump, having a receiver responsive to the transmitted signal.
The pump is
preferably responsive to information derived from or analysis of the spectral
signal. The
pump may then provide insulin or other appropriate medication to the patient.
Alternately, or in addition, the information may be sent to a remote monitor.
As will be clear to those of skill in the art, the instruments and methods of
the present invention can also be used in forensic applications, to allow the
non-invasive
and non-destructive assessment of forensic tissues. In addition, the
instruments and
methods may be used to detect and diagnose diabetes, monitor the progression
of
diabetes, and detect and monitor other disorders involving hyper or
hypoglycemia or
abnormal blood sugar metabolism. Although the term in vivo is used to refer to
living
material, it is intended herein to encompass forensic applications as well.
CA 02322573 2000-09-07
WO 99/51142 PCTNS99107565
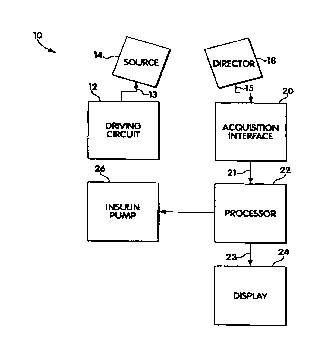
Another embodiment of the present invention is depicted in Figure 2, which
depicts a glucose level monitoring instrument 10 including source driving
circuit 12
having an output provided to an input 13 of a source 14. Source driving
circuit 12
controls the illumination, provided by source 14. Source driving circuit 12
may take a
number of forms, depending on the nature of the source and the acquisition.
Examples
include a regulated power supply or a pulsed modulator.
Source 14 preferably comprises an ultraviolet light source, such as a
continuous mercury lamp, a pulsed or continuous xenon flash lamp, or a
suitable laser.
Useful lasers include, but are not limited to, nitrogen lasers, OPO (tunable
laser) and Nd
YAG pump devices. The output of source 14 may be filtered to restrict
illumination to
within excitation bands of interest. Its intensity (and pulse width if
applicable) is
preferably set at a level that minimizes exposure while optimizing signal-to-
noise
considerations. It is also possible to irradiate the sample with two or more
short (e.g.
fernptosecond) pulses of multiphoton light having a wavelength two or more
times longer
than the wavelength of interest, such that the radiation penetrates to a
different degree or
depth. The source is positioned to illuminate an area of interest on the
patient's skin 16.
Glucose level monitoring instrument 10 also includes a detector 18 that is
sensitive to ultraviolet light emitted by the species that is excited by the
source 14. The
detector has an output 15 operatively connected to an input of an acquisition
interface 20,
which may be an analog-to-digital converter with an analog input operatively
connected
to the detector output. A digital output port 21 of the acquisition interface
is operatively
connected to processor 22.
Processor 22 is operative to convert the digital detector output signal into a
glucose level signal. The processor may perform this conversion by applying
various
signal processing operations to the signal, by comparing signal data with
stored reference
profiles, or by other appropriate methods. It has an output 23 provided to a
display 24,
permitting the glucose level indication to be presented to the user. The
output may be
directly provided to display 24, or sent remotely via a transmitter. Display
24 may be an
alphanumeric display which displays the glucose concentration as a percentage.
CA 02322573 2000-09-07
WO 99151142 PCT/US99/07565
16
The glucose level monitor instrument 10 may also include a medication
delivery device, such as insulin pump 26, which is responsive to the glucose
level signal
or other spectroscopic data or analysis provided by processor 22. A
transmitter may be
used to transmit the glucose level signal of processor 22 to the pump. The
pump is
configured so that it converts the glucose level signal received from
processor 22 into an
insulin dispensing rate. A single bolus of insulin may also be administered
based on the
glucose level signal. The use of an insulin pump allows the glucose level to
be controlled
both continuously and automatically. The medication delivery device can also
deliver
another therapeutic substance, or administer an electrical, chemical, or
mechanical
stimulus. Miniaturized devices may be constructed of disposable materials such
as
plastics and paper to further reduce cost. Instrument 10 may be implemented in
a number
of different ways. It may be implemented at a board level, with the various
elements
described being separate components, mounted on a circuit board. Many of the
elements
may also be integrated into a dedicated special-purpose integrated circuit
allowing a more
compact and inexpensive implementation. Alternatively, the components may be
miniaturized further to create an implantable device or a dermal patch. In
integrating and
miniaturizing the various functions of the instrument, many of them may be
combined.
Important algorithms may be embedded.
Instrument 10 may also include a normalizing section. The normalizing
section is designed to reduce or eliminate the effect of variations, such as
the intensity of
source 14 or day to day variations in the patient's tissue. A normalizing
section may
include a second detector that is responsive to a species in the skin that
fluoresces but
does not respond to glucose concentration. It may also normalize to a signal
collected at
another time, another site, or another wavelength or from a different internal
or external
target. Processor 22 may receive signals from the two detectors and derive a
normalized
glucose level signal. Preferably instrument 10 includes a portable housing
bearing
ultraviolet radiation source 14, ultraviolet radiation detector I $,
acquisition interface 20
and processor 22. Instrument 10 may be powered via battery contacts by a
battery
contained in the battery compartment located within the housing. Preferably,
the entire
CA 02322573 2000-09-07
WO 99/SI 142 PCTlUS99/07565
17
assembly weighs in combination less than 20 kg, preferably less than 10 kg and
more
preferably less than 1 kg. Highly portable embodiments which weigh under one
kg may
be attached to the patient in a monitoring position, such as by an elastic or
hook-and-loop
fastener strap.
In operation, a physician or the patient places source 14 close to an area of
interest on the patient's skin 16. Preferably, this area is one that is not
regularly exposed
to sunlight, such as the inside of the upper arm. The physician or patient may
then start
the instrument's monitoring sequence. The monitoring sequence begins with
driving
circuit 12 producing a driving signal that causes source 14 to irradiate the
area of interest
on the surface of the skin 16 with one or more bands of ultraviolet radiation.
The spectral
content of this radiation is selected to cause one or more targets within the
skin to
fluoresce. These targets may include tryptophan, collagen cross-links or other
suitable
targets. The excitation/emission wave lengths for tryptophan and collagen
cross-links are
295/340-360 nanometers and 335-340/380-400 nanometers, respectively. To
increase the
sensitivity of the measurement, it is also possible to pre-expose the area of
interest with a
higher intensity of radiation, before making measurements. Note also that the
excitation
and emission wavelengths are representative of the molecular species targeted.
Under
circumstances where the target is responsive to multiple different wavelengths
and
provides different information from each, or where targets and non-targets are
responsive
to the same wavelength, more accurate and qualitative values may be obtained
by
identifying and eliminating background and other interfering data.
The target absorbs the radiation from the source and re-emits it back to
detector 18. Detector 18 derives a signal representative of the received
emitted radiation
and provides it to the acquisition interface 20. Acquisition interface 20
translates the
derived signal into a digital value, which it provides to processor 22.
Processor 22
converts the digital value into a display signal, which it provides to display
24. The
display signal may take the form of an alphanumeric representation which
correlates with
the concentration of glucose in the blood, or it may include another kind of
display signal
to be used with another type of display. For example, it is possible to use a
color coding
CA 02322573 2000-09-07
WO 99151142 PCT/US99/07565
18
scheme to indicate levels of glucose, or indicate dosage amounts to the
patient on the
display based on the signal received at the detector. The display need not be
a visual
display; tactile actuators, speakers, or other machine-human interfaces may be
employed.
The glucose level signal produced by the processor may be directly displayed
to indicate
the patient's glucose level. Alternately, the processor may first compare the
data from the
detector with stored reference profiles, such as the patient's prior levels,
to provide
information regarding trends in the patient's glucose level.
Still another aspect of the invention is related to a glucose monitoring
system with alarm features. Parents of children with diabetes are under a
continuous
threat that a severe hyper- or hypoglycemic event may occur without their
knowledge,
such as during the night, with potentially fatal consequences. There are an
increasing
number of individuals with diabetes in need of a device for monitoring their
glucose
levels. Accordingly, this aspect of the invention is related to a monitoring
device with an
alarm that alerts a parent or other interested person in the event of large or
dangerous
changes or trends in the blood glucose levels of a patient. The device reports
systemic
hyperglycemic and/or hypoglycemic events using fluorescent detection of
alterations in
the environment of matrix components that reflect changes in blood glucose.
Alternately,
the device may detect the change in fluorescence from the excitation of
another suitable
species, such as tryptophan. The device may be completely portable,
miniaturized andlor
disposable allowing its use in nearly any environment.
The alarm may be any suitable alarm including, but not limited to, a sound
alarm or a radio transmitter that activates a sound or light emitting unit in
the proximity of
the parents or other interested person. The alarm may be audible, visible,
vibrating or any
other sensory detectible type. For example, in one embodiment, the patient's
glucose
level is measured once or at a plurality of intervals shortly before the
patient goes to sleep
to determine a baseline glucose level. The device is programmed to take
measurements
of the patient's glucose level at periodic intervals during the night, and to
then compare
these results with the baseline. If the glucose level varies more than a
predetermined
percentage from the baseline either simply or utilizing specifically designed
algorithms,
CA 02322573 2000-09-07
WO 99/51142 PCT/US99/07565
19
an alarm sounds. Although any desired percentage variation can be selected, in
a
preferred embodiment, the alarm is activated when the glucose level varies
more than S%,
10%, 20% or more from the previously determined baseline or in accordance with
a
previously defined set of parameters or specifically designed algorithms.
Alternately, or
in addition. the alarm is triggered if the patient's blood glucose level
exceeds a first
predetermined level (i.e. it exceeds 200 gm/ml) or if it falls below a second
predetermined level (i.e. it falls below 70 gm/ml). When the alarm sounds, the
patient
can then be administered insulin (or other suitable medication) if the glucose
level is too
high, or can be given a source of sugar if the glucose Level is too low.
Alternatively, or in
addition, the alarm may be triggered if other analysis or trending patterns
occur.
Optionally, the processor of this device, or any of the monitoring devices
disclosed herein, may include means for storing and displaying a plurality of
sequential
measurements, so that trends which occur during the night or during other time
periods of
interest may be saved and evaluated. The measurements can be taken
continuously or
repetitively at predetermined intervals. For example, a patient can be
periodically
monitored after the administration of one or more of the various forms or
sources of
insulin (i.e. lente, ultralente, semilente, regular, humalog, NPH etc.) or
other glucose
regulatory therapies to determine or help to determine the most suitable
treatment
protocol for the patient. This may be influenced by a comparison to other
readings over
time, a broader data base, a derivation based on the slope of the change of
the signal over
time and where on the scale of patient risk a particular assigned glucose
might fall.
As mentioned above, the fluorescence signals measured from the excitation
of PDCCL and other tissue components are affected by the changes in the
scattering
properties of the superficial structural matrix. As the electrolyte balance in
the micro
environment of collagen cross links changes, changes are induced in
fluorescence. In
addition, the change in electrolytes also produces a change in the local index
of refraction
and thus a change in the scattering properties. The change in scattering
causes a change
in the fluorescence.
CA 02322573 2000-09-07
WO 99/51142 PCT/US99/07565
A diagram depicting fluorescence of species sensitive to glucose
concentration following irradiation of the skin is depicted in Figure 10A.
Incoming
radiation at wavelength ~,i is directed towards the skin. It penetrates the
stratum corneum.
If ~.i is 295 nanometers, fluorescent radiation (~,o) will be emitted at 345
nanometers by
tryptophan in the epidermis of the skin. If ~,i is 335 nm, fluorescent
radiation will be
emitted (~.o) at 390 nm by the collagen cross links in the dermis.
A diagram depicting a scattering according to a scattering model is depicted
in Figure 10B which shows collagen cross links in the superficial dermis
bending
incoming light (~.i) in different directions. The re-emitted light (~,o) is at
the same
wavelength as the incoming light (~,i), but is scattered due to changes in the
local index of
refraction. By independently measuring scattering in the superficial matrix,
the
monitoring of blood glucose levels by measuring fluorescence, as described
above, can be
enhanced. Specifically, the results from the assessment of scattering can be
used to
correct for changes in fluorescence induced by changes in the scattering
properties of the
relevant layers of the dermal matrix.
Accordingly, another aspect of the invention is related to a device that
measures the scattering properties of a target such as superficial collagen
dermal matrix
in the skin, which is affected by changes in the chemical environment which
can be
correlated with blood glucose levels. Although it has been previously been
reported that
the scattering properties of the skin (dermal matrix) change with glucose
concentration
and that these changes are measurable with photon migration techniques in the
near
infrared (NIR), the use of NIR wavelengths provides a sample of the whole
dermis and
subcutis (does not measure one signal specific for glucose, but rather many
signals that
are neither specific for glucose nor reliably linked to glucose levels in a
linear fashion).
In contrast, the present invention assesses the scattering properties of the
superficial
dermis, as opposed to the deeper layers. Such scattering of polarized light by
the
superficial dermal matrix is most noticeable in the range 380-700 nm.
CA 02322573 2000-09-07
WO 99/51142 PCT/US99/07565
21
Assessment of scattering in tissue, such as the superficial dermis, associated
with changes in blood glucose can most preferably be measured by using short
wavelengths (330-420 nm) or launching the illuminating light at large angles
(preferably
>60°). Short wavelengths are preferably used because they penetrate to
a small depth
into the dermis. Alternately, changes in scattering induced by the presence of
glucose
may be measured using light in the visible range of 620-700 nm and looking for
changes
in signal intensity.
One of the benefits of assessing scattering of the superficial dermis, as
opposed to deeper layers of the denmis, is that fluorescent signals from
PDCCL's and
other matrix components originate there and are affected by the changes in the
scattering
properties. Further, the superficial layers of the dermis (in areas of the
body receiving
minimal environmental insults) are well organized and this would be reflected
in
scattering of polarized light. Since glucose has a strong polarization
rotation property,
such changes may be measurable when monitoring at a submillimeter resolution,
but
when monitoring on a gross scale the effects of local organization would be
canceled out.
Increases in fluorescence may be compensated for by decreases in the effective
scattering, making the fluorescence signal difficult to separate from
background noise.
By independent measurement of the scattering with randomly polarized and with
linearly
polarized light, fluorescence detection may be enhanced, allowing it to stand
on its own
merit as a method of indirect measurement of glucose concentration.
Figure 11 depicts an embodiment in which both fluorescence of the
superficial dermis and scattering are evaluated in order to assess glucose
levels.
Although this embodiment is described in connection with monitoring blood
glucose, as
will be clear to those of skill in the art, it can be adapted to assess the
status of other
analytes, or to detect changes in the superficial structural matrix or matrix
components of
a tissue. Instrument 100 comprises a power supply 101 connected via connection
102 to
a light source 104. Light source 104 may be a lamp, an arc lamp, a laser, or
other suitable
illumination device. Power supply 101 receives feedback 103 from data
acquisition
controller 122 to regulate the intensity, synchronization or pulse rate of the
light emitted
CA 02322573 2000-09-07
WO 99/51142 PCTIUS99/07565
22
from light source 104. Light source monitor output 105, which may comprise a
PIN
diode, Avalanche diode, photomultiplier, CCD or other suitable device, couples
the light
source 104 to data acquisition controller 122. Light 106 is directed to
wavelength
selection device 107, where an appropriate wavelength is selected, and
selected light
wavelength output 109 is directed via a fiber, prism or a combination or
directly through
the air, to illuminate skin I 10. Wavelength selection device 107 may comprise
a
monochromator, a filter or a combination of both. If a laser source is used as
light source
104, a filter or other wavelength selection device may not be needed.
Wavelength
selection device 107 is coupled via signal connection 108 to data acquisition
controller
122 to enable selection of the wavelength and to verify the present
wavelength.
Fluorescent signals are emitted and scattered light is re-emitted from skin
110. The fluorescent light and reflective intensity 111 is picked up by
wavelength
selective device 112, which may comprise a monochromator, filter or a
combination.
Wavelength selective device 112 provides a light output 114 to detector 115.
Detector
I 15 may comprise a photomultiplier, diode, avalanche diode, CCD or other
suitable
detection device. The signal from detector 11 S is transmitted to signal
conditioner/processor 120 via signal connector 116. Detector 115 is supplied
power via
power cable connection 117 from power supply 118. Data acquisition controller
122
provides input to power supply 118 via signal connection 119 to allow
selection of
sensitivity or synchronization with the light source. Wavelength selection
device 112 is
coupled via connection 113 to data acquisition controller 122 to select
wavelength and
verify current wavelength. Signal processor/conditioner 120 provides output
via output
connection 121 to data acquisition controller 122. Data acquisition controller
122 is
connected via connection 123 to display 125. Data acquisition controller 122
may also
provide output via connection 124 to an insulin or medication delivery device.
The above described instrument may also be used as a non-invasive device
for assessing changes in the superficial structural matrix or the environment
of matrix
components due to a variety of disease conditions. This embodiment allows the
assessment of changes in the structural matrix non-invasively by measuring the
CA 02322573 2000-09-07
WO 99/51142 PGT/US99/07565
23
combination of fluorescence and scattering, and comparing these results to
measurements
of developed standards, temporal correlates or surrounding normal tissue. This
device
may be used to assess changes in the collagen matrix brought about by diseases
such as
diabetes, scleroderma, scarring, or atrophy induced by the use of steroids. It
is also useful
to assess changes in the matrix due to aging or photoaging and changes induced
by long
exposures to zero gravity environment. This embodiment may be miniaturized,
and may
be used clinically and in research applications to evaluate wound healing,
protein
metabolism, diabetes, collagen diseases and other conditions.
The collagen cross links in the superficial or papillary dermis provide large
fluorescence signals that are indicative of the state of the collagen matrix.
These signals
may be monitored non-invasively without interference with the functions of the
skin.
Specifically, as the matrix is irradiated with UVA, UVB or UVC radiation, the
fluorescence of PDCCL decreases. This fluorescent effect recovers following a
single
exposure; however, the changes induced become permanent after multiple
exposures.
The fluorescence of the skin in the WA (320-400 nm) results mainly from
collagen cross links that lie in the papillary dermis. The fluorescence
signals from these
cross links may be used to evaluate the state of the collagen matrix. In the
skin and other
tissues, as the collagen matrix is degraded due to the expression of matrix
metalloproteinases, such as collagenase, in the stroma of tumors so does the
fluorescence
emission from the collagenase digestible collagen cross links. By assessing
fluorescence,
it has been discovered that degenerative changes in the superficial structural
matrix or of
matrix components may be assessed, such as changes induced by disease or
environmental factors such as diabetes, age, photodamage, topical steroid
application, or
prolonged exposure to zero-gravity. Further, the intensity of scattered light
by the dermis
changes with aging and with changes in the collagen cross links. If the
collagen cross
links in the superficial or papillary derrnis change, then the amount of light
that is
scattered by the dermis and its dependence on wavelength will also change.
These
changes may be monitored by reflectance.
CA 02322573 2000-09-07
WO 99/51142 PCT/US99107565
24
Another aspect of the invention is related to a device that can measure
either fluorescence excited at about 335 nm (pepsin digestible collagen cross
links),
fluorescence excited at about 370 nm (collagenase digestible collagen cross
links), or
both, as well as the reflectance spectrum (450-800 nm), to thereby provide
information on
the state (or changes induced) of the superficial structural matrix or
environment of tissue
matrix components. By combining the assessment of fluorescence and scattering
into one
instrument, a novel device is provided that provides enhanced information on
the state of
the structural matrix or environment of tissue matrix components. Other
wavelengths can
also be used for excitation, such as 295 nm for tryptophan. A preferred
embodiment
incorporates a light source (Hg) and filters to select either 333 nm 365 nm or
visible
broad band. The visible excitation may be provided by a tungsten halogen lamp
of I -2
watts. The light is then conducted to the skin's surface by fibers, reflective
optics or
directly, and the fluorescence from the UVA excitations and the reflectance
from the
visible source are assessed with a photodiode array type of detectors. The
fluorescence
intensities can then be compared to standard signals from collagen samples
(prepared
from gelatin). The reflectance signal is analyzed for scattering and
absorption by iterative
methods at wavelengths of 620-820 nm. Accordingly, another aspect of the
invention is
related to an instrument for assessing changes in a superficial structural
matrix of the skin
or the environment of the matrix components of a patient comprising means for
measuring fluorescence, and means for measuring scattering.
Another aspect of the invention is related to a non-invasive method of
assessing a change in the superficial structural matrix of a tissue or a
change in the
environment of the matrix components of a tissue comprising exposing the
tissue to
radiation at a first wavelength, detecting an amount of fluorescence emitted
by exposed
tissue, exposing the tissue to radiation.of a second wavelength, detecting an
amount of
scattering re-emitted from the exposed tissue, and deriving an indication
representative of
the change in the superficial structural matrix or environment of the matrix
components
of the tissue based on of the amount of fluorescence detected and the amount
of scattering
detected. Preferably, the first wavelength is ultraviolet radiation, or is
between about 320
CA 02322573 2000-09-07
WO 99/51142 PCT/US99/07565
and 420 nm and the second wavelength is between about 330 and 420 nm.
Preferably,
the tissue is skin.
The following examples are offered to illustrate embodiments of the present
invention, but should not be viewed as limiting the scope of the invention.
Examples
Example 1 Glucose Levels of Diabetic versus Non-Diabetic Mice
Experiments were conducted for six shaved hairless (SKH) diabetic mice
made diabetic by the injection of streptozotocin, and six shaved hairless
(SKH) non-
diabetic (normal) mice. Excitation spectra at emission wavelengths of 380 nm
and 340
nm were collected for each of the twelve mice. A Xenon arc source coupled to a
monochromator were fed into a fiber optic probe, which was then used to
illuminate the
backs of all of the mice at an intensity level of approximately 0.1-1.0 mw/cm.
A
spectrometer was used to collect the resulting spectra, which are shown in
Figures 3 and 4
for emission at 380 nm and 340 nm, respectively. The plots indicate a
significantly lower
excitation intensity at 295 nm and a significantly higher excitation intensity
at 340 nm for
the diabetic mice. Urine collected from the animals confirmed that the glucose
levels of
the diabetic mice were higher at 340 nm for the diabetic mice.
Example 2 Glucose Levels of a Non-Diabetic Rat Followin;~ Ketamine
and Insulin Treatments
Referring to Figure 5, experiments were also conducted using a normal rat.
The experimental apparatus used was the same as that used in Example 1.
Fluorescence
excitation spectra were obtained for the rat in the following situations, (A)
at rest
(diamonds), (B) after the administration of Ketamine {squares), (C) after the
administration of insulin (triangles) and (D) after the administration of
additional insulin
(crosses). The glucose levels in situations A-D were determined to be 120,
240, 100, and
40 gm/ml, respectively. The results are believed to be superimposed on a light
leakage
signal that increases steadily with wavelength, although the use of double
monochromators should eliminate this source of background noise. Spectra
collected for
CA 02322573 2000-09-07
WO 99/51142 PCT/US99/07565
26
this rat indicate that blood glucose level has a positive effect on
fluorescence excitation in
the 340 nm range. This is more clearly depicted in Figure 6 in which the
fluorescence
excitation intensity at 346 nm for each of the situations A-D has been
plotted.
Example 3 Glucose Levels of Human Subjects Before and After Glucose In estion
Preliminary experiments were also conducted on humans. Figures 7, 8 and
9 depict fluorescence excitation spectra for three human subjects, two males
and one
female, respectively, before (dashes), 30 minutes after (dotted/dashed line),
and 60
minutes after (solid line) the ingestion of 100 grams of glucose. In each
situation, the
emission monochromator was set to a wavelength of 380 nm. Collagen and
tryptophan
spectra were found to change in ways similar to those for the animal models,
although
there appear to be individual differences. Dashed lines represent measurements
before
glucose intake. Dashed and dotted lines represent changes induced after
glucose intake.
Solid lines represent maximal changes induced by the intake of glucose.
Other embodiments and uses of the invention will be apparent to those
skilled in the art from consideration of the specification and practice of the
invention
disclosed herein. As will be clear to those of skill in the art, the devices
and methods of
the present invention can be easily adapted to reflect or detect the level of
a variety of
substances in tissue, in addition to glucose and the described targets. All
references cited
herein, including all U.S. and foreign patents and patent applications,
including, but not
limited to, United States Provisional Patent Application Serial No.
60/080,794, entitled
Non-Invasive Tissue Glucose Level Monitoring, filed April 6, 1998, are
specifically and
entirely incorporated by reference. The specification and examples should be
considered
exemplary only with the true scope and spirit of the invention indicated by
the following
claims.