Note: Descriptions are shown in the official language in which they were submitted.
CA 02322931 2000-10-11
MULTI-PROBE CONDUCTIVITY METHOD FOR MONITORING TIME-DEPENDENT
PROCESSES IN FRESH CEMENTITIOUS AND OTHER DENSE SLURRY SYSTEMS
FIELD OF THE INVENTION
The present invention relates to the development of novel devices and methods
for in-situ, non-destructive, continuous and quantitative measurement of
changes
occurring in aqueous-based suspensions, slurries, pastes, sludge and other
colloidal
systems.
BACKGROUND OF THE INVENTION
The basis and significance of the present invention is best described for
systems
in which both physical-type and chemical-type processes are present
simultaneously,
both playing an important role in the evolution of the slurry system. This is
precisely the
situation in fresh cementitious materials, in which, both, physical effects
(i.e., migration
of solid particles (cement, sand, aggregate, pigment, or other) and solution
phase) and
chemical effects (i.e., dissolution or hydrolysis of the reactive minerals,
precipitation
and growth of the hydrate products) can drastically affect the physical
properties of the
hardened material. The invention will therefore be described and illustrated
mainly as it
applies to cementitious systems; similar application to other reactive, or non-
reactive
slurries is obvious to anyone in the field of colloid chemistry or process
engineering.
Physical Effects
In cementitious systems, particularly those which are highly fluid (e.g.,
flowable,
pumpable, or self-levelling concrete), segregation of the various solid
materials may
occur as a result of an upward migration of fines and sedimentation of the
aggregates.
Bleeding may then result from the movement of part of the "free" water in the
fresh
mixtures to the surface, due to the inability of the solid constituents of the
mix to hold
all of the mixing water when they settle downwards (1 ). Migration of
interstitial solution
can lead to surface bleeding and to "channelling", the latter resulting from
preferential
migration paths through the cement paste. Whether these phenomena are
sufficiently
pronounced to be apparent on the external surfaces of the material, or not,
the
CA 02322931 2000-10-11
2
occurrence of segregation and bleeding results in structural heterogeneity
which
reduces the performance characteristics of the material, including surface
finish,
strength, impermeability, and durability. This can also affect the
characteristics of the
interface of the paste with the reinforcing elements, reducing bond strength
(2-4).
In spite of the critical importance of bleeding and segregation effects, there
exists
no direct experimental method to evaluate such effects quantitatively. The
extent of
surface bleeding is usually measured by collecting the excess surface solution
as a
function of time after placement (ASTM-C232, CRD-C9). Bleeding can also be
evaluated
by determining the surface settlement (or subsidence) per unit height of
concrete (5-6).
On the other hand, the degree of segregation occurring in a cementitious
material is
determined by analysis of the distribution of coarse aggregate in the fresh
state or after
the hardening. For example, the testing of the spread of a pile of concrete
subjected to
jolting (ASTM C124) gives an indication of the consistency of the concrete and
its
tendency to undergo segregation during the flow. Similarly, the susceptibility
of fresh
concrete towards coarse aggregate separation can be assessed by observing the
material
scattering following a drop over a cone from two hoppers (7). The observation
of the
distribution of coarse aggregate in a cored sample offers another means of
determining
segregation in hardened concrete (6).
Chemical Effects
In chemically reacting slurries, such as cementitious systems, the chemical
processes occurring in the system, also have important consequences on the
physical
properties of the hardened material. In cement-based materials, the
dissolution/hydration of the reactive components play a crucial role in
determining how
the setting reactions are initiated and how the microstructure evolves to
ensure
development of the early-age strength of the material. These chemical-type
processes,
occurring simultaneously with the physical effects described above, clearly
have a
marked influence on the way in which the stability and the structure of the
slurry evolve
in time, and ultimately on the properties of the hardened material.
CA 02322931 2000-10-11
3
In the case of cement-based materials, the time-dependence of the chemical
processes has been successfully monitored using electrical conductivity (8 to
15). Since
the dissolution/hydration reactions involve ionic solids and soluble
electrolytes, the
chemical processes can be monitored continuously through measurement of the
electrical conductivity of the fresh material. Typically, the electrical
conductivity of a
cement-based slurry (paste) will initially increase as the soluble alkali
salts quickly
dissolve into the mixing water; the conductivity continues to increase as the
aluminate
and silicate phases of the cement react with water producing calcium and
hydroxyl
ions. The paste conductivity will begin to decrease as the hydrate products
(particularly
portlandite) begins to precipitate, and will continue on decreasing as the
microstructure
of the hardening cement matrix develops (10, 13, 14 )
The Need for Monitoring Methods
Its is therefore apparent that, in cementitious systems, there is a need for a
measuring device and method which could provide real-time information on the
evolution of the systems through its initial consolidation and early age
behaviour. Since
this consolidation and early age behaviour depend simultaneously on physical-
type and
chemical-type effects, the required method must be sufficiently incisive to
measure the
evolution of the system properties as affected by both types of effects. To
comply ideally
with the usual constraints of concrete applications, the method must allow non-
destructive, in-situ, real-time measurement; moreover, the method must use
devices
which will not interfere with the processes monitored and will cause minimal
disturbance when they remain in the hardened material. Since no such device or
method currently exist, it is the purpose of the present invention to provide
novel probe
and application conditions to achieve the desired type of measurement and
monitoring
in fresh cement-based material, and in any aqueous slurries and colloidal
system, as
well .
For application in other aqueous-based slurries, the constraints may be less
severe, particularly in systems which exhibit no chemical reactivity between
the various
components and phases present. However, the principle and application of the
probe
and method of the present invention are valid for all such aqueous-based
slurries.
CA 02322931 2000-10-11
4
PRIOR ART
There exist few references regarding the use of electrical conductivity in
studies
on cement-based materials and other aqueous col loidal systems (8 to 15 and 17
to 21 ).
In fresh cementitious systems, typically, the variations in electrical
conductivity have
been used to monitor time-dependent changes in the composition of the solution
phase. During the last decade, there has been a growing interest in the
development
and use of electrical response techniques in cement and concrete research (9).
The
electrical measurement technique has already proven successful in studies of
ion
exchange resins, soils, ion exchange membranes and polyelectrolytes (15, 16).
Previous
work in the area of cement technology has focussed on determining the
relationship
between the evolution in the solution conductivity and the hydration processes
which
lead to the precipitation of portlandite (calcium hydroxide) in the slurry,
the latter effect
associated with the beginning of the setting (stiffening and hardening)
processes in the
cement paste.
In hardened cement materials, conductivity measurements are often used for
evaluating the mobility of ions through the matrix, for example conductivity
of the
hardened concrete due to mobility of ions between two electrodes with a given
potential (ASTM, C 1202). The results are related to the permeability of the
material and
hence to its durability. Recently, it has been reported that rapid estimation
of water-
cementitious ratio and chloride ion diffusivity in hardened and plastic
concrete could be
made by using electrical resistivity measurement, whereby, the concentration
of the
various electrolytes in the interstitial water of the cement paste is
dependent on the
initial water content that affects the conductivity (12).
References are there to the use of electrical conductivity in monitoring or
processing various other types of aqueous slurries and colloidal systems.
Salient features
of some of them are briefly mentioned below.
A new in-situ technique has been developed for hexavalent chromium removal
from sand by imposing of constant electrical potential gradient across the
soil matrix
through graphite cathode and iron anode (17). In another reference, laboratory
experiments and mathematical modelling have been used to study the changes in
the
flows of ions and pore liquid during the process. These flows were directly
related to
CA 02322931 2000-10-11
the removal of charged and uncharged contaminants by electromigration and
electro-
osmosis (18). The use of a low -frequency square wave alternating current was
made in
studying resistivity characteristics of compacted clayas. this method avoids
difficulties
due to electrode polarization and reduces capacitative and lead inductive
effects to
5 minimum (19). Effects of water content, orientation of particles,
electrolyte
concentrations, type of electrolytes, have been studied for the electrial
response
characteristics of soil-water structure. Furthermore, effects of temperatures,
and nature of
surfaces have also been reported regarding soil-liquid system (20).
Electrocoagulation of
bio-organic impurities in waste waters from biochemical processes has been
reported as
an important industrial purification process (21 ).
A brief account of patents of interest to the present invention are given, as
fol lows:
- Patent No:4, 176,038 (Nov. 27, 1979, USA). The patent pertains to, "Water
purification method and apparatus". The process comprises of passing the
liquid between spaced electrode plates in the presence of a fluidized bed
of conductive particles. The liquid suspension is subjected to an
alternating electrical field applied across the electrodes through conductive
particles of the said bed. Under such a system, suspending forces of the
solids are rapidly and efficiently broken. The agglomerated solids may then
be separated from the liquid.
- Patent 5572123 ( Nov. 5, 1996, USA). This patent deals with an apparatus
and a method for on-line inspection of electrically conductive food
products using liquid electrolyte. Changes in electrical conductivity are
measured on-line to correlate the quality of the food product in the
production.
- Patent 548949 ( Feb. 6, 1996, USA). This patent deals with, "High
accuracy calibration-free electrical parameter measurements using
differential measurement with respect to immersion depth". The apparatus
is used to measure electrical parameters of a medium, such as electrical
conductivity and dielectric constant, between a pair of electrodes. By
CA 02322931 2000-10-11
6
obtaining a differential conductance measurement with respect to the
immersion depth, the effects of fringe conductance are eliminated from the
measurement.
- Patent 5458747 (Oct. 17, 1995, USA). This patent deals with, "In-situ bio-
electrokinetic remediation of contaminated soils containing hazardous
mixed wastes". It involves the basic cation and anion movements towards
respective energized electrodes. The pore fluid is moved from the anode
area and collects in the cathode area and may pool at the soil surface. The
technique is dependent on the mobility of the ions and the conductivity of
the medium that plays an important role.
SUMMARY OF THE INVENTION
The present invention relates to the development of novel devices and methods
for in-situ, non-destructive, continuous and quantitative measurement of
changes
occurring in aqueous-based suspensions, slurries, pastes, sludge and other
colloidal
systems. The devices consist of multiple electrode (multi-electrode)
conductivity probes
which can measure, as function of time, variations of electrical conductivity
at different
positions (vertically or horizontally) in the colloidal system. The localised
changes in the
electrical conductivity in the slurry, which result from physical processes or
chemical
reactions occurring in this slurry, yield rate parameters for such processes
as
sedimentation, settling, bleeding, de-watering, etc. When used in reactive
colloidal
systems, such as fresh cement-based materials and hydraulic binders, the
device and
measurement method yield a quantitative measure of bleeding and segregation
effects,
as well as data on the beginning of the hardening reaction and hardening rate
at early
age (e.g., 0-72 hrs).
The principle of the method is as follows: any change in composition (solids
or
solution) occurring in a volume element of an electrically conducting slurry,
will result
in a change of electrical conductivity of this volume element. Hence,
simultaneous
measurement of the electrical conductivity at numerous point locations in a
conducting
CA 02322931 2000-10-11
7
slurry can be used to follow the evolution of physical or chemical processes
in this
slurry.
One important application of the "Multi-probe Conductivity Method" is for
monitoring the evolution, as function of time, of fresh cementitious systems,
grouts,
mortars and concrete. In such systems, the Method allows continuous-real time
monitoring of the following processes:
1. The migration of liquids and solids in the fresh material, leading to
sedimentation, segregation and bleeding
2. Rate of hydration of the reactive materials in the cementious system and
the
onset of the setting (hardening) process
3. The rate of hardening and strength development at early age, typically, 1
to 4
days.
The Method of this invention is non destructive and can be applied "in-situ"
for
monitoring the time-dependent evolution of virtually any aqueous slurry, for
example,
waste water, mine tailings, industrial waste effluents or sludge and
industrial process
slurries.
DETAILED DESCRIPTION OF THE INVENTION
Principles
The method devised for in-situ, non-destructive, continuous and quantitative
measurement of changes occurring in aqueous-based colloidal systems is based
on
changes in the electrical conductivity which occur as a result of composition
variations
in a given volume element of a system. For example, the electrical
conductivity
measured at different depths in an aqueous slurry and as function of time will
reflect
both, the physical and chemical processes occurring in this slurry. Physical
processes,
such as sedimentation or settling, will result in increasing the solids
content of the slurry
(volume or weight fraction) as function of depth; the increased solids content
will be
CA 02322931 2000-10-11
reflected by a proportional decrease in the electrical conductivity. Chemical
processes,
such as dissolution or precipitation of ionic solids, also induce changes in
the electrical
conductivity; if such a process occurs homogeneously throughout the solution
phase, its
time dependence will be reflected in the evolution of the average conductivity
measured over the entire sample. This novel method can be used in any aqueous-
based
colloidal system, typically, suspensions, emulsions, slurries, pastes, sludge,
or other. It
was found particularly useful in monitoring the time evolution of fresh cement-
based
systems, such as grouts, mortars and concrete, wherein both physical-type and
chemical-type processes occur and have an important impact on the final
properties of
the hardened materials. The potential of the novel multi-electrode
conductivity
measurements in fresh cementitious systems is readily visualised from the
following
qualitative description of the phenomena occurring in fresh-cement-based
systems.
The evolution with time of the average conductivity of a fresh portland cement
paste is illustrated schematically in Fig. 1a. The conductivity value measured
in the first
instants following the mixing of the cement and water is low (point A in Fig.
1 a), but
increases rapidly (point B) due to the rapid dissolution of alkali sulphates,
increasing the
concentration of the Na+, K+, and S04-2 ions, and the early surface hydration
of the
most reactive mineral phases in the cement, generating Ca+2 and OH- ions.
During
the dormant period (B to C), the conductivity increases slowly as the
hydration reactions
proceed, further increasing the Ca+2 and OH- solution concentration into
supersaturation (point C). When the conditions for Ca(OH)2 precipitation
become
favourable, a sharp decrease in the Ca+2 and OH- concentration will take
place,
initiating a rapid decrease in conductivity which signals the onset of
setting. As setting
and hardening occur, the conductivity decrease continuously (C-D) due to the
development of the pore structure which greatly limits the mobility of
electrolytes in the
interstitial solution (C-D).
The electrical conductivity measurement (single point or average) performed on
a fresh cement paste as function of time can thus yield key information on the
hydration
reactions and on the setting process. The use of conductivity measurements in
monitoring the evolution of the solution phase and the setting process in
cement-based
CA 02322931 2000-10-11
9
system had already been discussed by Vernet ( 13, 14) and others (8 to 10), as
noted in
the "Prior Art" section.
The novel multi-electrode probe of the present invention allows the
measurement of the electrical conductivity of the fresh cement-based material
at
different positions in the material volume examined. Such measurements
performed
simultaneously (in rapid sequence) as function of time yield results such as
illustrated
schematically in Fig. 1 b (further detailed in the "RESULTS" section). The
latter shows
qualitative trends which may be observed in the conductivity measurements
performed with three pairs of electrodes placed at different depths in the
sample (for
example, top, middle and bottom). In this case, the shape of each conductivity-
vs-time
curve is dependent first, on chemical processes as described above, and
second, on
physical processes, which may also occur simultaneously in the material,
typically
sedimentation, segregation and bleeding. In the hypothetical situation
considered in Fig
1 a, the "Top" curve exhibits high excess conductivity, relative to the other
two curves
(or relative to the average conductivity curve); the reverse is seen with the
bottom
electrode pair, which shows initial decrease in conductivity, and
significantly lower
conductivity compared with the other two pairs of electrodes over the entire
measurement period. These conductivity trends reflects a concomitant migration
of the
conducting solution phase upwards (increasing the conductivity in the upper
portion of
the sample) and a sedimentation of some of the solids (decreasing the
conductivity in
the lower part of the sample volume). Hence, simultaneous measurement of
conductivity at several points in a fresh cement-based material (e.g., at
different heights
in a cylinder), can measure variations in the uniformity of the material
resulting from
bleeding and segregation effects.
The novelty of the present invention lies in its ability to monitor
simultaneously
chemical and physical processes, with a unique probe allowing, quantitative,
in-situ,
non-destructive, continuous measurement and real-time analysis of the data,
which
enable rapid observation of improper system behaviour.
CA 02322931 2000-10-11
Novel Multiple Electrode (multi-electrode) Probes
The configuration and construction of the novel electrical conductivity probes
for
the purpose of the present invention can be performed in a variety of ways.
Two types
of multi-electrode probes are described below as non-limiting examples: a
reusable-type
5 probe for laboratory investigations and a sacrificial probe for laboratory
or field studies
in which the probes remain in the hardened material.
Multiple Electrode Reusable Probe
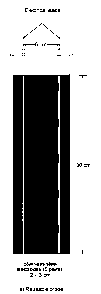
A first conductivity probe was constructed for laboratory investigations on
fresh
10 grout or mortar samples, following the schematic illustration of Fig.2a.
The electrodes
consist of rectangular stainless steel (304) plates measuring 2.0 cm x 3.0 cm,
and 0.15
cm thick; five such plates are mounted on the face of a vertical PVC post (3.0
cm wide x
30 cm high x 1.0 cm thick) equally spaced at 5.0 cm intervals. The electrical
leads to
each electrode are grouped on the back face of the PVC support post and
isolated from
the slurry by a second PVC plate; the latter has a vertical groove which
conveniently
houses the lead wires and is glued onto the PVC post. A second identical
electrode
assembly is mounted facing the first at a distance of 6.0 cm.
The complete probe unit is mounted vertically into a transparent PVC cylinder
having a 10 cm diameter and a height of 35 cm. A level marking at 30 cm from
the
bottom of the cylinder indicates the position of the top surface of the
cementitious
materials to be investigated. The complete conductivity cell thus comprises
five equally-
spaced pairs of electrodes, which are permanently fixed at the following
depths from
the top surface of the cementitious material: 7, 12, 17, 22 and 27 cm. As
noted above,
this type of probe was intended for systematic investigations of bleeding-
segregation
effects in grouts and mortars; hence, it was only suitable for measurements
during the
dormant period.
Multiple electrode sacrificial probes
A second type of probe was designed to perform, again, the measurement of
conductivity as function of time and of depth in the fresh cementitious
samples, but
over extended periods into the setting and hardening phases. This type of
measurement
CA 02322931 2000-10-11
' 11
requires probes, that will remain in the hardened material; these probes were
thus
designed for optimum simplicity of fabrication and use, as well as minimum
cost.
In the probe design reproduced in Fig. 2b, the electrodes consist of stainless
steel
machine screws (0.5 cm dia. x 3 cm length) fixed in a PVC post. The latter is
made by
cutting a PVC pipe (2.5 cm dia.) longitudinally into two half cylinder
channels. Five
electrodes are fixed onto one of these PVC channels, equally spaced at 6.0 cm
intervals;
the lead wires are housed in the channel which, in the end, is filled with an
epoxy
resin. With this type of probe, conductivity measurements are taken between
each pair
of electrodes in succession: 1-2, 2-3, etc.; the readings thus pertain to the
average
conductivity of a 6-cm high narrow column, as opposed to a 3 x 5 cm
rectangular
element in the reusable probe.
Electrical Circuitry for Conductivity Measurement
The electrical conductivity of cementitious system is obtained from
measurements of resistivity between inert metal electrodes using an AC
voltage,
preferably a square wave signal. The electrical circuitry used for these
measurements is
illustrated schematically in Fig. 3. The electrical resistivity between the
various pairs of
electrodes embedded in the cementitious material is measured sequentially
using a low
voltage, 1 kHz square-wave signal; in these conditions, electrode polarization
and
related artifacts are minimized. A computer-controlled switching device
enables
automated measurements on all electrode pairs in a predetermined sequence. A
complete measurement cycle corresponding to the time required to record
consecutive
readings for five pairs of electrodes (r.m.s. current and voltage) requires 20
sec. The
computer management of the system, as well as the data acquisition and
analysis, and
the calculation of stability index values (as described below) allow real-time
evaluation
of changes in the properties of the fresh cementitious material.
EXAMPLES
Typical results obtained using the novel conductivity probe and methods of the
present invention are presented below for three types of fresh cement-based
materials
grout, mortar and concrete. These examples considered are typical, or
representative, of
CA 02322931 2000-10-11
12
a wide range of cement-based systems, which constitute and important class of
colloidal
systems for application of the present invention. The following paragraphs
first describe
the mix compositions of the systems in the selected examples, together with
the
common experimental methods used in the corresponding investigation.
Materials
A Type 10 Canadian portland cement (CSA3-A5-M83, similar to ASTM C 150
Type I cement) was used for all the grouts, mortar and flowable underwater
concrete
(C1 and C2 mixtures). A ternary cement containing approximately 20% Class F
fly ash
and 6% silica fume was used for the self-consolidating concrete (SCC) mixtures
(mixtures C3 and C4).
Continuously graded crushed limestone aggregate with a nominal particle size
of
14 and 10 mm were used for the C1 and C2 mixtures and C3 and C4 mixtures,
respectively. A well-graded siliceous sand with a fineness modulus of 2.5 was
employed for the mortar and concrete mixtures described in this paper, except
for the
M4 mortar where a slightly finer sand was used. The bulk specific gravities of
the
coarse and fine aggregates were 2.68 and 2.64, respectively, and their
absorption values
were 1.3 and 1.5%, respectively.
A sulfonated naphthalene-based HRWR with 42% solid content was used
throughout this study; a dry powdered sodium gluconate was used as a set
retarder. A
powdered welan gum was employed in the SCC mixtures to enhance stability.
Throughout this paper, the concentrations of the HRWR and set retarder are
expressed
in terms of solid content by mass of cementitious materials, while that of the
viscosity
agent (welan gum) by mass of water.
Mixture Preparation
All mixtures were prepared at similar material temperatures of 20 + 3oC. The
grout mixtures were prepared in 3-L batches using a Hobart mixer operating at
a
relatively low speed. The set retarder was consistently pre-blended with the
cement,
then introduced to the water in the mixer. The grout then received 3 minutes
of mixing.
In the case of mortar, a Hobart mixer was also used. The batching sequence
consisted
CA 02322931 2000-10-11
~ 13
of adding the cement to the water with the HRWR diluted in the latter. The
mode of
sand introduction and mixing protocol were consistent with ASTM C 109
recommendations.
The concrete was mixed in an open pan mixer. The sand and coarse aggregate
were homogenized, then the cement, the water, the HRWR, and finally the welan
gum
were introduced. Once all mixture constituents were added, the concrete was
mixed
for 3 minutes. Following a 3-minute rest, the mixing was resumed for 2
additional
minutes
Conductivity Probe Calibration
A simple calibration procedure was developed which allows the calculation of
an effective cell constant for each electrode pair (with either type of
probe). This is
performed by immersing the probe in a standard solution of NaCI or KCI of
known
conductivity and measuring sequentially the electrical resistivity of every
electrode pairs
as configurated for measurements on cementitious systems. The resistivity
values were
obtained by averaging five current/voltage readings under a square-wave at 1.0
kHz
excitation. The readings were taken with increasing voltage in the range of 0
to 10 V.
The constant currendvoltage slope observed ensures that the circuit is mainly
resistive
during the period of observation. This calibration procedure enables
correction for
variations among electrode pairs due to unavoidable geometrical variations in
probe
construction.
Conductivity Measurement Procedures
Reusable probes
Immediately following completion of the mixing process of the grout, mortar,
or
concrete, the fresh material is poured into the test jar (300 mm effective
height), and the
measurement sequence is initiated, taking, as noted above, five consecutive
readings for
each electrode pair, then passing on to the next electrode pair until all five
electrodes
have been scanned. This is repeated at 2-minute intervals, typically up to
four hours,
then the material removed from the cell before setting occurs.
CA 02322931 2000-10-11
~ 14
Sacrificial probes
The measurement procedure followed with the sacrificial probes was identical
to
that described above for the reusable probes. Since sacrificial probes were
designed to
remain permanently in the cementitious material sample, they enabled
measurements
over longer periods, beyond the consolidation and into the hardening stages;
in the
examples given below, the conductivity measurements were recorded for periods
of up
to 72 hours.
Procedures for Measuring Bleeding and Segregation in Concrete
Surface bleeding was determined using standard 150 x 300 mm cylinders. The
external bleeding was determined by measuring the amount of bleed water at the
top of
the column. The cumulative bleeding volume was monitored at fixed intervals of
10
and then of 40 minutes until reaching steady state conditions, corresponding
approximately to the beginning of hardening. Except during the bleeding
measurements, the cylinders were covered to prevent evaporation.
The segregation of the hardened concrete was evaluated by vertically sawing
the
150 x 300 mm cylinders used for the bleeding test to determine the variations
in the
volume fraction of the aggregate as a function of height. The concentration of
coarse
aggregate (> 5 mm) was determined through image analysis of the sawed section
which
yields the surface area of the aggregate on the section, relative to the total
surface of the
section. The segregation coefficient was estimated using a sum of squares
approach by
expressing the deviation of coarse aggregate distribution from a weighted
average value
(6).
Procedures for Data Analysis
The conductivity data collected as function of time and depth in the sample
(from the surface) is examined in three distinct time domains:
- the dormant period: to calculate a "stability index";
- the setting region: to identify an effective setting time
- the hardening period: to determine the rate of hardening
CA 02322931 2000-10-11
Stability Index
The primary purpose of the method presented here is to derive a quantitative
measure of bleeding- segregation effects in a fresh material, using the
conductivity as a
local probe to monitor the development of heterogeneity in the material.
Taking the
5 five conductivity readings at different levels at a given time, the vertical
heterogeneity in
the sample is adequately reflected through the standard deviation of the
conductivity
values (aA). The latter should be normalized to the initial value of the
average
conductivity measured over the five electrodes (Aav) to allow for variations
in
conductivity values between different systems due to differences in their
chemical
10 compositions. An apparent stability index (Is) can then be defined simply
as
Is - ( 1 - aA / Aav )
Eq. 1
A highly stable system will exhibit a unit Is value; a system with a strong
tendency towards bleeding and segregation will show Is < 1 . This definition
of
15 homogeneity is only valid for the plastic stage of the material. Upon
setting and
hardening, large changes in the average conductivity values will induce Is
variations
which are not related to heterogeneity of the material.
Apparent Setting Time
As noted by other workers previously (9 to 14), the onset of paste setting in
a
cement-based material is generally accompanied by a decrease in the
conductivity of
the paste due to the precipitation of portlandite (Ca(OH2)). Since the
conductivity of the
paste usually increases up to the setting point (neglecting segregation
effects), the
maximum value in the conductivity vs. time curve would appear a good indicator
of the
initial set time.
Rate of Hardening
During the hardening period, the strong decrease in the electrical
conductivity of
cementitious systems is largely due to progressive blocking of the ionic
transport
channels as the microstructure of the material develops. The rate of drop in
the
conductivity values between the setting point (measured as maximum
conductivity) and
CA 02322931 2000-10-11
16
a fixed curing period should provide a reasonable estimate of the rate of
strength
development in the material. Therefore, Ot1 values were obtained as [ llset -
Wset +
Ot) ] and compared to the compressive strength developed over the same period
(Ot).
The compressive strength was determined on 50 mm cube samples maintained at
room
temperature and de-moulded immediately before strength testing.
RESULTS
Example 1 : Conductivity Measurements on Grouts with Reusable Multi-electrode
Probes
Seven grout mixtures, labelled G1 to G7 were prepared with various
compositions reported Table 1.
Highly fluid grout with Water/ Cement (W/C) = 1.0
The type of results obtained from multiple electrode (reusable probes)
conductivity measurements is illustrated in Fig. 4 with a highly fluid cement
grout
(W/C = 1 ). The conductivity readings are given from t = 15 minutes, to about
t = 4
hours. The elapsed time here refers to the elapsed period following the
introduction of
water to cement. At t = 0, all electrode pairs must yield the same
conductivity value
since the suspension is still in a homogenous state. The first set of readings
of
conductivity however correspond to 15 minutes after the introduction of the
sample in
the test mould. The time-dependence of the conductivity values at the top (7
cm) and at
the bottom (27 cm) of the 30 cm grout column clearly indicates important
bleeding-
segregation effects during the first two hours. The electrodes located near
the bottom of
the sample show conductivity decreasing with time, which is consistent with a
decrease
of the volume fraction of the conducting solution phase due to sedimentation
of cement
particles. On the other hand, the electrodes near the surface show a rapid
conductivity
increase, corresponding to an increase in the volume fraction of solution in
that region
(i.e., due to bleeding), and to an increasing concentration of electrolytes in
the solution
as hydration reactions proceed. Electrodes located at intermediate heights
reflect the
combined influence of segregation-bleeding and electrolyte solubilization on
the bulk
CA 02322931 2000-10-11
- 17
conductivity throughout the sample. Because of the opposite effects of these
phenomena on bulk conductivity, the readings at intermediate heights (e.g.,
corresponding to the 12 cm and 17 cm electrode pairs) exhibit a maximum as
function
of time (these maxima are not related to the setting phenomenon which occurs
much
later). The position of the maximum on the time scale is related to the
migration rate of
solids and solution in the grout; it thus provides direct indication of the
kinetics of the
bleeding-segregation phenomena as it occurs.
The conductivity data as function of time in Fig. 4 are presented as function
of
the position of the electrodes in the sample, at selected times (15, 75, 135
and 255
minutes) in Fig. 5. At the beginning, the conductivity values measured are
comparable
throughout the sample depth, and this is reflected by a near-vertical A vs.
height curve.
The latter is increasingly curved at longer times reflecting the evolution in
the sample
heterogeneity. This representation illustrates the significance of the
stability index
calculated according to Eq. 1 and reported as function of time in Fig. 6. For
each
selected time, the mean conductivity value is determined; the standard
deviation is then
calculated and normalized to the value at t = 15 minutes, yielding a measure
of the
heterogeneity of the sample. In the present case, the initial stability index
value is
greater than 0.97, but it decreases rapidly with time in the first 90 minutes,
indicating a
highly unstable system.
Reproducibility of method on grouts with W/C=0.75
The repeatability of the method is illustrated in Fig. 7 which shows the
variation
in conductivity with time for the top (7 cm) and bottom (27 cm) pairs of
electrodes, as
observed in three distinct experiments with cement grouts G2, G3 and G4, all
with W/C
of 0.75. The corresponding variations in the calculated stability index (Is)
are illustrated
in Fig. 8. As expected, the Is values for these grouts are higher than that
observed for the
W/C of 1 grout Previous example), but significant bleeding-segregation is
still apparent
during the first hour of consolidation. As may be seen from the consistency in
the
behaviour of the Is values, the repeatability of the measurement is quite
satisfactory.
The coefficient of variations calculated from the conductivity data at each
electrode pair
over the entire duration of the measurement for the three grouts was less than
5%.
CA 02322931 2000-10-11
- 18
Application of method in grouts with varying W/C and with set retarding
admixture
As further test of the method, several other grouts were examined, typically,
as
function of W/C and in the presence of admixtures. The results for three grout
mixtures
G5, G6, and G7 (W/C = 1.0, 0.8, and 0.4) in the presence of a set-retarding
admixture
(0.1 % sodium gluconate) are presented in Fig. 9. The results again show
variations in
conductivity at the top and bottom electrode pairs; the corresponding
stability index for
these systems are illustrated in Fig. 10. In some of these systems, there
appears to be
rapid initial segregation, as indicated by significant differences in the
first conductivity
readings taken 15 minutes after mixing. As observed earlier with other grouts,
the
stability index is rather high initially and decreases with time in a way
related to the
W/C values.
The initial results obtained with the multiple electrode conductivity probe
(reusable type) demonstrate the usefulness of the method for monitoring
variations in
the homogeneity of a cement paste with time.
Example 2. Conductivity Measurements on Mortars with Sacrificial Multi-
electrode
Probes
This Example is given to illustrated the applicability of the novel multi-
electrode
probe and for monitoring cement-based mortars over extended periods typically
24 to
96 hours. In such case, the conductivity-vs-time data can provide information,
initially
on the variations in homogeneity of the system, and later on its behaviour
during the
setting and hardening period.
A total of six mortar mixtures (labelled M1 to M6) were prepared with a ratio
of
cementitious materials to sand of 1:1.5 and W/CM of 0.4 and compositions as
given in
Table 1 Except for the M1 mortar, the mixtures were highly fluid with
approximate
spread diameter values (ASTM C 143) of 31 + 1 cm. The conductivity vs. time
curves
observed with the top and bottom electrode pairs are illustrated in Figs. 11-
14. The
general features of these curves are similar, and their shape is related to
the schematic
illustration presented in Fig. 1a; in accordance with the latter, the mortar
conductivity
may be examined in three distinct periods: consolidation, setting and
hardening.
CA 02322931 2000-10-11
19
Stability of fresh mortars of varying compositions
The conductivity data illustrated in Fig. 11 for a non-superplasticized mortar
with
low fluidity level (spread diameter of 11.5 cm) is typical of a very stable
mortar, with
little difference in the conductivity curves for top vs. bottom electrode
pairs. The
stability index values derived from these measurements in the first 12 hours
of testing is
close to unity, as shown in Fig. 15 for the M1 mix.
Mortars of much greater fluidity (approximate spread of 31 cm) containing 8%
silica fume, no set retarder, and a HRWR yielded conductivity curves which
point to
significant bleeding-segregation, even at very early times, as well as delayed
setting; this
can be seen from the data for mortar M2 in Fig. 12. This behaviour is
exacerbated upon
addition of a set retarding admixture (0.05% and 0.10% sodium gluconate) for
the same
mortar composition, as shown in Fig. 13. The occurrence of bleeding-
segregation
effects is very pronounced in the earliest measurements recorded; the
stability index
values over time for this mortar is also significantly reduced, as shown in
Fig. 15. The
M4 mortar was prepared with a sand that is finer than the sand employed for
the other
mixtures; this explains the higher apparent stability of this mortar relative
to M3.
The conductivity data presented in Fig. 14 illustrates the behaviour of the M5
mortar made with 20% Class F fly ash and 6% silica fume replacement, and a
high level
of retarding admixture (0.10% of sodium gluconate). In spite of an excessive
retardation,
the conductivity curves indicate a reduced level of bleeding-segregation
compared to
the M3 and M4 mixtures reflecting the high stability of the binder
combination.
As with the multi-electrode conductivity measurements on grouts, the
conductivity data obtained on fresh mortars clearly shows the usefulness of
the method
in monitoring variations in the homogeneity of the system, resulting from
bleeding and
segregation effects.
Apparent setting times from multi-electrode conductivity measurements
As expected from prior art on the conductivity of fresh cement-based systems
(8
to 15), the shape of the conductivity-vs.-time curve observed in Figs. 11-14
initially
exhibit increasing conductivity with time during the lag phase; later, a sharp
decrease in
the conductivity occurs due to precipitation of portlandite, and the resulting
maximum
CA 02322931 2000-10-11
signals the beginning of the setting of the cement paste. The actual setting
times (initial)
of the mortar mixes could not be measured through the standard techniques, but
the
time at which the maxima is observed with the different mortars appears
consistent with
the mix design of these mortars. For example, in the case of the M2, M3, and
M4
5 mortars made with the same composition and with increasing dosages of set
retarder (0,
0.05, and 0.10%), the time periods corresponding to the peak before observing
a sharp
reduction in conductivity are 5.5, 24, and 35.5 hours, respectively. These
data confirm
the usefulness of the multi-probe conductivity method for monitoring chemical
reactions occurring in the cement-based system and leading to setting and
hardening of
10 the material.
Hardening rates
The rate of drop in the conductivity of a setting cement-based system should
be
qualitatively related to the rate of strength development (in a way similar to
the heat
evolution which reflects maturity and is related to strength). In attempts to
relate the
15 conductivity variations measured and the strength development in the
mortars, the
following simple procedure was adopted. A mortar hardening rate was calculated
using
the compressive strength, (f'~ ) observed at the end of the sharp conductivity
decline,
and in some cases at intermediate points of the descending curve, taking the
compressive strength as nil at the maximum in the conductivity curves. The
data used
20 for this comparison are collected in Table 2. The slopes Of'~ / ~Aav (where
DAav is the
average conductivity value over the entire sample height) obtained for the
mortars
examined in this part of the study are plotted in Fig. 16. A
strength/conductivity
correlation with a coefficient of approximately 0.9 is observed, which, given
the
extreme range of mortar behaviors, is satisfactory confirmation of the
validity of the
approach.
The multi-electrode conductivity method is thus seen to provide useful
quantitative information on the rate of hardening (rate of strength
development) of the
mortars, an important parameter of these systems in application.
CA 02322931 2000-10-11
21
Example 3 : Measurements on Fresh Concrete with Sacrificial Multi-electrode
probe
The applicability of the multi-electrode conductivity approach to monitor the
stability of plastic concrete was also demonstrated in concrete mixtures
described in
Tables 1 and 3. The mixture compositions were adjusted to allow comparisons at
constant workability between C1 and C2, and C3 and C4 corresponding to highly
flowable concrete for underwater and SCC, respectively. The C1 and C2 mixtures
were
prepared with Type 10 cement and differed by their W/C values (0.41 and 0.55).
Similarly, the C3 and C4 mixtures had similar compositions, except for W/CM of
0.42
and 0.48 and the concentrations of chemical admixtures.
The external bleeding (ASTM C-232) characteristics of these materials were
also
measured and the results are shown in Fig. 17. As expected, the mixtures with
greater
W/C exhibited higher bleeding. Comparing the behaviour of C1 and C2, the rate
of
surface bleeding is found to be highest with the C2 concrete, a
superplasticized
concrete having a rather high W/C of 0.55. With the SCC mixtures C3 and C4,
the
external bleeding is much lower, and it occurs significantly later (i.e., 6 to
10 hours).
The lowest bleeding rate is found with C4, an SCC containing welan gum as a
thickening agent, but a lower W/CM than the C3 concrete.
The segregation of the coarse aggregate is illustrated in Fig. 18 which shows
the
distribution of the coarse aggregate along the height of the cylinders. The
values
indicated on the graphs refer to the segregation coefficients. The relative
segregation
behaviors of these materials follows the same pattern as the bleeding rates:
the greater
the bleeding, the greater the tendency of the coarse aggregate to segregate
towards the
lower part of the fresh concrete sample. The segregation coefficients of the
C1 and C2
mixtures were 4.3 and 6%, respectively, and those for the C3 and C4 mixtures
1.9 and
3.1 %.
The conductivity data measured with these fresh concrete samples yields curves
similar to those observed with mortars and the stability indices obtained from
the
conductivity data are reproduced in Fig. 19. The trend observed in the latter
follows
roughly the trends reported above within the bleeding and segregation data.
However,
CA 02322931 2000-10-11
' 22
the detailed variations in stability index values with time probably reflect
limits of the
equipment used in the present study.
In particular, because of the relatively small size of the electrodes, and the
short
spacing between them, the presence of 10- or 14-mm nominal size aggregate may
have
a considerable influence on the bulk values measured; upon segregation, the
motion of
such large particles in the electrode gap leads to substantial variations in
the volume
fraction of the paste within the measuring volume, thus inducing large
fluctuations in
the measured conductivity. Application of the method to a broad range of
concrete
types will, therefore, need to consider changes in electrode geometry to
alleviate such
difficulties.
Table 1 - Composition of the examined cement-based materials
Mixture W/CM CementitiousChemical Test Consistency
materials admixtures duration
Grout
mixtures
G1 1.0 Non 240 mm h
G2, G3, 0.75 Non 240 mm i
G4
G5 1.0 0.1 % Na 150 mm
bfg
I uconate
G6 0.8 0.1 % Na 150 mm ; '' g,,.
I uconate
G7 0.4 0.1 % Na 150 min
luconate
Mortar xtures
mi
M1 0.40 Non 24 hrs 11.5 cm
spread
diameter
M2 0.40 8% SF 0.6% HRWR 72 hrs 31.5 cm
spread
d iameter
M3 0.40 8% SF 0.6% HRWR 96 hrs 32.5 cm
0.05% Na spread
luconate diameter
M4 0.40 8% SF 0.6% HRWR 92 hrs 30 cm spread
0.1 % Na diameter
I uconate
CA 02322931 2000-10-11
23
Mixture W/CM CementitiousChemical Test Consistency
materials admixtures duration
M5 0.40 6% SF + 0.6% HRWR 96 hrs 31.5 cm
20% fly 0.1 % Na spread
ash
luconate diameter
M6 0.40 30% fly 0.6% HRWR 72 hrs 32 cm spread
ash
0.1 % Na diameter
luconate
Concrete
mixtures
C1 0.41 0.29% HRWR 10 hrs Slump 220
mm
C2 0.55 0.15% HRWR 10 hrs Slump 220
mm
C3 0.42 6% SF + 0.50% HRWR 10 hrs 650 mm
20% fly 0.16% welan slump flow
ash
um
C4 0.48 6% SF + 0.39% 10 hrs 650 mm
20% fly HWRW slump flow
ash
0.14% welan
um
Table 2 - Variations in conductivity vs. strength gain of 1:1.5 mortar
mixtures
Test Binder W/CM Admixtures0t ~f'~ 0A (mS/cm)
No. (hrs)(Mpa)
M1 T a 10 0.40 Non 19 21.4 3.67
M2 8% SF 0.40 0.6% 66 40.5 5.88
HRWR
M3 8% SF 0.40 0.6% 24 32.3 4.35
HRWR 73 45.5 5.01
0.05% Na
I uconate
M5 6% SF 0.40 0.6% 24 12.5 3.17
+
20% FA HRWR 48 26.8 3. 82
0.1 % N
a
I uconate
M6 30% FA 0.40 0.6% 58 34.0 5.17
HRWR
0.1 % Na
I uconate
CA 02322931 2000-10-11
24
Table 3 - Mixture proportioning evaluated concrete
Materials Fluid Self-consolidating
concrete
concrete
C1 C2 C3 C4
Cement 400 400 520 520
(kg/m3) Type 10 Type 10 Ternary Ternary
Cement type cement cement
Water (kg/m3)164 220 218 250
Sand (kg/m3) 705 705 710 700
Coarse 1040 1040 890 875
aggregate (14 mm (14 mm (10 mm (10 mm
(kg/m3) MSA) MSA) MSA) MSA)
HRWR (I/m3) 2.3 1.2 5.2 4.0
Welan gum 0 0 0.35 0.35
(kg/m3)
Water reducer0 0 0.75 0.75
(I/m3)
Slump (mm) 220 220 - -
Slump flow - - 650 650
(mm)
CONCLUSIONS
The results of the measurements reported in the foregoing examples demonstrate
the novelty and usefulness of multi-electrode conductivity measurements in
cementitious materials, specifically:
- The novel multi-electrode probe and measuring method present non
destructive tools to monitor in-situ and continuously the stability (stability
index) of all
types of cementitious systems.
- The AC (square wave at low frequency) electrical conductivity measured at
different levels in a plastic cement-based material can reliably detect
variations in the
homogeneity of the material resulting from bleeding and segregation effects.
- The sensitivity of the method is adequate for monitoring bleeding
segregation, in-situ and in a non disruptive manner, in plastic grout, mortar,
and
concrete.
CA 02322931 2000-10-11
- The conductivity data can be used to define a quantitative measure of the
inhomogeneity in a fresh cement-based material in terms of a "stability index"
(Is) for the
system.
- Using disposable probes, the setting and hardening rate can also be
5 monitored.
- The results also point to a valuable relationship between the strength gain
and the change in the mean conductivity value during the hardening period.
- Within sensitivity limits, mainly set by the geometry of the electrode-probe
assembly, the method provides valuable data on the behaviour of plastic cement-
based
10 materials.
- The method can be exploited either in the development of mixture
formulations with appropriate bleeding characteristics, or for in-situ
monitoring of
concrete consolidation.
- Given that cement-based material are complex colloidal systems in which
15 numerous physical and chemical processes occur simultaneously, the multi-
electrode
conductivity probe and method is most certainly applicable in less reactive
colloidal
systems, typically suspensions, slurries, sludge or pastes.
CA 02322931 2000-10-11
26
REFERENCES
1. Neville, A. M.,"Properties of Concrete," Fourth Ed., Longman Group Ltd.,
1995.
2. Hoshino, M., "Difference of the W/C Ratio, Porosity and Microscopical
Aspect
between the Upper Boundary Paste and the Lower Boundary Paste of the
Aggregate in Concrete," Materials and Structures, V. 21, No. 125, Sept. 1988,
pp.
3 3 6-340.
3. Khayat, K.H., "Use of Viscosity-Modifying Admixture to Reduce Top-Bar
Effect of
Anchored Bars Cast with Fluid Concrete," ACI Materials Journal, V. 95, No. 2,
1998, pp. 158-167.
4. Petrov, N., "Investigation of In-Situ Properties of Self-Consolidating
Concrete:
Influence on Interface with Reinforcement and on Corrosion," Masters Thesis,
UniversitE de Sherbrooke, Qc, 1997, 190 p.
5. Powers, T.C., "The Bleeding of Portland Cement Paste, Mortar, and
Concrete,"
Portland Cement Assoc. Bul. No. 2, Chicago, July 1939.
6. Khayat, K.H., Guizani, Z., "Use of Viscosity-Modifying Admixtures to
Enhance
Stability of Fluid Concrete," ACI Materials Journal, V. 94, No. 4, 1977, pp.
332-
340.
7. Ritchie, A.G.B., "Stability of Fresh Concrete Mixes," Journal of
Construction
Division, ASCE, Proc. V. 92, No. C01, 1966, pp. 17-36.
8. Nonat, A., Mutin, J.C., " From Hydration to Setting," Proceedings of the
International RILEM Workshop on Hydration and Setting Cements, 1991.
9. McCarter, W. J., Brousseu, B., " The A.C. Response of Hydrated Cement
Paste,"
Cement and Concrete Research, V.20, 1990, pp. 891 - 900.
10. McCarter, W. J., Curran, P. N., " The Electrical Response Characteristics
of Setting
Cement Paste," Magazine of Cement Research, V. 30, No. 126, March 1984, pp.
42-49.
CA 02322931 2000-10-11
27
11. McCarter, W. J., " Monitoring the Influence of Water and Ionic Ingress on
cover
zone subjected to Repeated Absorption," ASTM Cement, Concrete, and
Aggregate, V. 18, No., 1, June 1996, pp. 55-63.
12. MacDonald, K. A., Northwood, D. O., " Rapid Estimation of Water-
Cementitious
Ratio and Chloride Ion Diffusion in Hardened and Plastic concrete by
Resistivity
Measurement," Editor, Mohammad Khan, Water-Cement Ratio and Other
Durability parameters, Techniques for Determination, Publication SP-191, ACI
International , year 2000.
13. Vernet, C., " Hydration Kinetics and Mechanical Evolution of Concrete
during the
First Days: Study of the Hardening Mechanism, " Proc. 9th Int. Congress of
Chemistry of Cement, New-Delhi, 1992, pp. 511-517.
14. Vernet, C., Nowaryta, G., " Condctometric Test for Cement-Admixture
Systems,"
Proc. 9th Int. Congress of Chemistry of Cement, New-Delhi, 1992, pp. 627-633.
15. Jolicoeur, C., Simard, M.-A., "Chemical Admixture-Cement Interactions:
Phenomenology and Physico-Chemical Concepts," Cement and Concrete
Composites, V. 20, 1998, pp. 87-101.
16. Taylor, M. A., Arulanandan, K., "Relationships Between Electrical and
Physical
Properties of Cement Pastes," Cement and Concrete Research, V. 4, 1974, pp.
881-897.
17. Haran, B. S., Popov, B. N., Zeng, G., and White, R.E., " Development of a
New
Electrokinetic Technique for Decontamination of Hexavalent Chromium from
Low surface charged soils," Environmental Progress, Vol. 15, No, 3, Fall 1996,
pp. 166-172.
18. Dzenitis, J. M., " Soil chemistry effects and flow prediction in
Electroremediation
of Soil," Environmental Science and Technology, 1997, vol. 31, No. 4, pp. 1191-
1197.
CA 02322931 2000-10-11
' 28
19. McCarter, W. J., " The electrical resistivity characteristics of compacted
clays,"
Geotechnique, Vol. 34, No. 2, 1984, pp. 263-267.
20. Mitchell, J. K., and Arulandan, K., " Electrical Dispersion in Relation to
Soil
Structure," Journal of Soil Mechanics and Foundation Engineering, Proceedings
of
ASCE, Vol. 94, No. SM2, Mar. 1968, pp. 447-471.
21. Matveenko, A. P., Strizhev, E. F., Volkova, A. N., Ivanova, L. V., and
Yakovlev, V.
I., " Electrocoagulation of Bio-organic Impurities in Wastewaters from
Biochemical Processes," Journal. Applied Chemistry, USSR, Vol. 54, 1991, pp.
2282.