Note: Descriptions are shown in the official language in which they were submitted.
CA 02323588 2000-09-13
1
FUNGICIDE MIXTURES BASED ON TRIPLE OXIME ETHER
DERIVATIVES AND ADDITIONAL FUNGICIDES
The present invention relates to fungicidal mixtures for
controlling harmful fungi [lacuna)
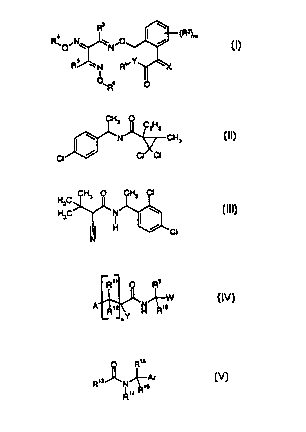
a) phenylacetic acid derivatives of the formula I
3
R
to
R\O~N\ ~N~O ~R2)m
(I)
R5 ~ N R'
I
O~ Rs O
in which the substituents and the index have the following
meaning:
X is NOCH3, CHOCH3, CHCH3;
2 0 Y is O, NR
R1, R independently of one another are each hydrogen and
C1-C4-alkyl;
R2 is cyano, nitro, trifluoromethyl, halogen, C1-C4-alkyl
and C1-C4-alkoxy;
m is 0, 1 or 2, where the radicals RZ may be different if
m is 2;
R3 is hydrogen, cyano, C1-C4-alkyl, C1-C4-haloalkyl,
C3-C6-cycloalkyl;
R4. R6 independently of one another are each hydrogen,
CA 02323588 2000-09-13
la
are C1-C1o-alkyl, C3-C6-cycloalkyl, C2-Clo-alkenyl,
CZ-Clo-alkynyl, C1-Clo-alkylcarbonyl,
C2-Clo-alkenylcarbonyl, C3-Clo-alkynylcarbonyl or
C1-Clo-alkylsulfonyl, where these radicals may be
partially or fully halogenated or may carry one to
0050/48891 CA 02323588 2000-09-13
2
three of the following groups: cyano, nitro, hydroxyl,
mercapto, amino, carboxyl, aminocarbonyl,
aminothiocarbonyl, halogen, C1-C6-alkyl,
C1-C6-haloalkyl, Cz-C6-alkylsulfonyl,
C1-C6-alkylsulfoxyl, C1-C6-alkoxy, C1-C6-haloalkoxy,
C1-C6-alkoxycarbonyl, C1-C6-alkylthio, C1-C6-alkylamino,
di-C1-C6-alkylamino, C1-C6-alkylaminocarbonyl,
di-C1-C6-alkylaminocarbonyl, C1-C6-alkylaminothio-
carbonyl, di-C1-C6-alkylaminothiocarbonyl,
C2-C6-alkenyl, C2-C6-alkenyloxy, C3-C6-cycloalkyl,
C3-C6-cycloalkyloxy, heterocyclyl, heterocyclyloxy,
benzyl, benzyloxy, aryl, aryloxy, arylthio, hetaryl,
hetaryloxy and hetarylthio, where the cyclic groups for
their part may be partially or fully halogenated or may
carry one to three of the following groups: cyano,
nitro, hydroxyl, mercapto, amino, carboxyl,
aminocarbonyl, aminothiocarbonyl, halogen, C1-C6-alkyl,
C1-C6-haloalkyl, C1-C6-alkylsulfonyl,
C1-C6-alkylsulfoxyl, C3-C6-cycloalkyl, C1-C6-alkoxy,
C1-C6-haloalkoxy, C1-C6-alkyloxycarbonyl,
C1-C6-alkylthio, C1-C6-alkylamino, di-C1-C6-alkylamino,
C1-C6-alkylaminocarbonyl, di-C1-C6-alkylaminocarbonyl,
C1-C6-alkylaminothiocarbonyl,
di-C1-C6-alkylaminothiocarbonyl, C2-C6-alkenyl,
C2-C6-alkenyloxy, benzyl, benzyloxy, aryl, aryloxy,
arylthio, hetaryl, hetaryloxy, hetarylthio or
C(=NORM)-An-Re;
are aryl, arylcarbonyl, arylsulfonyl, hetaryl,
het-arylcarbonyl or hetarylsulfonyl, where these
radicals may be partially or fully halogenated or may
carry one to three of the following groups: cyano,
nitro, hydroxyl, mercapto, amino, carboxyl,
aminocarbonyl, aminothiocarbonyl, halogen, C1-C6-alkyl,
C1-C6-haloalkyl, C1-C6-alkylcarbonyl,
C1-C6-alkylsulfonyl, C1-C6-alkylsulfoxyl,
C3-C6-cycloalkyl, C1-C6-alkoxy, C1-C6-haloalkoxy,
C1-C6-alkyloxycarbonyl, C1-C6-alkylthio,
C1-C6-alkylamino, di-C1-C6-alkylamino,
C1-C6-alkylaminocarbonyl, di-C1-C6-alkylaminocarbonyl,
C1-C6-alkylaminothiocarbonyl,
di-C1-C6-alkylaminothiocarbonyl, C2-C6-alkenyl,
C2-C6-alkenyloxy, benzyl, benzyloxy, aryl, aryloxy,
hetaryl, hetaryloxy or C(=NORM)-An-R8;
R5 is hydrogen,
0050/48891 CA 02323588 2000-09-13
3
is C1-C6-alkyl, CZ-C6-alkenyl, CZ-C6-alkynyl, where the
hydrocarbon radicals of these groups may be partially
or fully halogenated or may carry one to three of the
following radicals: cyano, vitro, hydroxyl, mercapto,
amino, carboxyl, aminocarbonyl, aminothiocarbonyl,
halogen, C1-C6-alkylaminocarbonyl,
di-C1-C6-alkylaminocarbonyl, C1-C6-alkylaminothio-
carbonyl, di-C1-C6-alkylaminothiocarbonyl,
C1-C6-alkylsulfonyl, C1-C6-alkylsulfoxyl, C1-C6-alkoxy,
C1-C6-haloalkoxy, C1-C6-alkoxycarbonyl, C1-C6-alkylthio,
C1-C6-alkylamino, di-C1-C6-alkylamino, C2-C6-alkenyloxy,
C3-C6-cycloalkyl, C3-C6-cycloalkyloxy, heterocyclyl,
heterocyclyloxy, aryl, aryloxy, aryl-C1-C4-alkoxy,
arylthio, aryl-C1-C4-alkylthio, hetaryl, hetaryloxy,
hetaryl-C1-C4-alkoxy, hetarylthio,
hetaryl-C1-C4-alkylthio, where the cyclic radicals for
their part may be partially or fully halogenated and/or
may carry one to three of the following groups: cyano,
vitro, hydroxyl, mercapto, amino, carboxyl,
aminocarbonyl, aminothiocarbonyl, C1-C6-alkyl,
C1-C6-haloalkyl, C1-C6-alkylsulfonyl,
C1-C6-alkylsulfoxyl, C3C6-cycloalkyl [sic],
C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6-alkoxycarbonyl,
C1-C6-alkylthio, C1-C6-alkylamino, di-C1-C6-alkylamino,
C1-C6-alkylaminocarbonyl, di-C1-C6-alkylaminocarbonyl,
C1-C6-alkylaminothiocarbonyl,
di-C1-C6-alkylaminothiocarbonyl, C2-C6-alkenyl,
CZ-C6-alkenyloxy, benzyl, benzyloxy, aryl, aryloxy,
arylthio, hetaryl, hetaryloxy, hetarylthio and
C(=NORM)-An-R8;
is C3-C6-cycloalkyl, Cg-C6-cycloalkenyl, heterocyclyl,
aryl, hetaryl, where the cyclic radicals may be
partially or fully halogenated or may carry one to
three of the following groups: cyano, vitro, hydroxyl,
mercapto, amino, carboxyl, aminocarbonyl,
aminothiocarbonyl, halogen, C1-C6-alkyl,
C1-C6-haloalkyl, C1-C6-alkylsulfonyl,
C1-C6-alkylsulfoxyl, C3-C6-cycloalkyl, C1-C6-alkoxy,
C1-C6-haloalkoxy, C1-C6-alkoxycarbonyl, C1-C6-alkylthio,
C1-C6-alkylamino, di-C1-C6-alkylamino,
C1-C6-alkylaminocarbonyl, di-C1-C6-alkylaminocarbonyl,
C1-C6-alkylaminothiocarbonyl, di-C1-C6-alkylamino-
thiocarbonyl, C2-C6-alkenyl, Cz-C6-alkenyloxy, benzyl,
benzyloxy, aryl, aryloxy, hetaryl and hetaryloxy;
0050/48891 CA 02323588 2000-09-13
4
where
A is oxygen, sulfur or nitrogen and where the nitrogen
carries hydrogen or C1-C6-alkyl;
n is 0 or 1;
R~ is hydrogen or C1-C6-alkyl and
R8 is hydrogen or C1-C6-alkyl,
and their salts,
and
b) at least one fungicide selected from the fungicides of the
formulae II to V
CH3 O
CzHs
~N ~CH3 ( II )
CI ~ CI CI
H C CH3 O CH3 CI
3
H3C ~N I \ ( III )
~ ~ H ~ CI
N
R" O R9
I W (IV)
R'~ H~ ~R~o
~ Y
0050/48891 CA 02323588 2000-09-13
O Rts
Rts~N~Ar
5 t5
Rta R
where the substituents have the following meaning:
A is C3-C6-cycloalkyl which may carry one to three
substituents selected from the group consisting of
halogen and C1-C3-alkyl;
R9 is C1-C6-alkyl or C2-C6-alkenyl, where these radicals
may be partially or fully halogenated and/or may
carry one or two of the following groups: C1-C4-
alkoxy, C1-C4-haloalkoxy, C1-CQ-alkylthio, C1-C4-
alkoxycarbonyl, C3-C6-cycloalkyl and phenyl, where
the phenyl may be partially or fully halogenated
and/or may carry one to three of the following
radicals: nitro, cyano, C1-CQ-alkyl, C1-C4-haloalkyl,
C1-C4-alkoxy, C1-C4-haloalkoxy, C1-C4-alkylthio,
C3-Cs-cycloalkyl or heterocyclyl;
Rlo~ R11~ Ri2 are each hydrogen or have one of the meanings of the
radical R9 independently of this meaning;
n is 0 or 1;
Y is cyano or halogen;
w is phenyl, naphthyl or heteroaryl, where these
radicals may carry one to three of the following
groups: nitro, halogen, cyano, C1-C4-alkyl,
C1-C4-haloalkyl, C1-C4-alkoxy, C1-C4-haloalkoxy,
C1-C4-alkylthio, C3-C6-cycloalkyl and
Ci-C4-alkoxycarbonyl,
R13 is C6-C15-bicycloalkyl or C~-C15-bicycloalkenyl, where
these radicals may be completely halogenated and, if they
are not completely halogenated, two carbon atoms of these
radicals together with a C3-CS-alkylidene group may form
a five- to seven-membered saturated carbocyclic ring
and/or may carry one or independently of one another two,
0050/48891 CA 02323588 2000-09-13
6
three, four or five of the following groups: cyano,
Cl-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy, C1-CQ-halo-
alkoxy and aryl, where the aryl may be partially or fully
halogenated and/or may carry one or independently of one
another two or three of the following substituents:
nitro, cyano, Ci-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy,
C1-CQ-haloalkoxy and C1-CQ-alkylthio;
R14, R15, Ris independently of one another are hydrogen,
are C1-Ce-alkyl which may be partially or fully
halogenated and/or may carry one or two of the
following groups: C1-C4-alkoxy, C1-C9-haloalkoxy,
C1-C4-alkylthio, C3-C~-cycloalkyl, C5-C~-cycloalkenyl,
where the cyclic groups for their part may carry one
or independently of one another two or three halogen
atoms, C1-C3-alkyl groups and/or C1-C3-alkoxy groups
and aryl, where the aryl may be partially or fully
halogenated and/or may carry one or independently of
one another two or three of the following
substituents: nitro, cyano, C1-C4-alkyl,
C1-C4-haloalkyl, C1-C9-alkoxy, C1-C4-haloalkoxy ad
C1-Cq-alkylthio or
are C3-C6-cycloalkyl, C3-C6-cycloalkenyl or
heterocyclyl, where these radicals may be partially
or fully halogenated and/or may carry one or
independently of one another two or three of the
following groups: cyano, C1-C6-alkyl,
Ci-Cs-haloalkyl, C2-C6-alkenyl, C1-C6-alkoxy and
C1-C6-alkylthio;
Ar is aryl or heteroaryl, where these radicals may carry
one or independently of one another two or three of
the following groups: halogen, cyano, C1-C4-alkyl,
C1-C4-alkoxyalkyl, C1-C4-haloalkyl, C1-C4-alkoxy,
C1-C4-haloalkoxy, C1-C4-alkylthio, C1-C4-alkoxy-
carbonyl, aryl, aryloxy and heteroaryl, where the
rings in these groups for their part may carry one or
independently of one another two or three of the
following substituents: halogen, cyano, C1-C4-alkyl,
C1-C9-alkoxyalkyl, C1-C4-haloalkyl, C1-C4-alkoxy,
C1-C4-haloalkoxy, C1-C4-alkylthio and
C1-C4-alkoxycarbonyl,
[lacuna]
0050/48891 CA 02323588 2000-09-13
7
It is an object of the present invention to provide fungicidal
mixtures having good fungicidal activity, in particular against
fungal diseases in rice, exceeding the activity of the mixture
components on their own.
We have found that this object is achieved by the mixtures
according to claim 1.
The compounds of the formula I are known per se and are described
in the literature (WO 97/15552).
The fungicides of the formulae II and III are also known and
described in the literature. Moreover, they are commercially
available under the trade names given hereinbelow in brackets:
II: EP 341,475, common name: carpropamide (trade name:WIN~, from
Bayer)
III: CAS RN 139 920-32-4, proposed common name: dichlocymet
(development product of Sumitomo)
The compounds V, and also processes for their preparation, are
described in WO 97/35838.
The compounds IV and processes for their preparation are
explained in a more detailed manner below; the corresponding
compounds are described in the older patent application
PCT/EP 98/01031 or DE 19710618.8.
Owing to their C=C and C=N double bonds, the preparation of the
compounds I may yield E/Z isomer mixtures which can be separated
into the individual compounds in a customary manner, for example
by crystallization or chromatography.
However, if the synthesis yields isomer mixtures, a separation is
generally not necessarily required since in some cases the
individual isomers can be converted into one another during the
preparation for use or upon use (for example under the action of
light, acids or bases). Similar conversions may also occur after
the use, for example in the treatment of plants in the treated
plant or in the harmful fungus or animal pest to be controlled.
0050/48891 CA 02323588 2000-09-13
8
With regard to the C=X double bond, preference is given to the
cis isomers of the compounds I (configuration based on the -OCH3
or the -CH3 group in relation to the -COZR1 group) with respect to
their activity.
With regard to the -C(R3)=NOCHz- double bond, preference is given
to the cis isomers of the compounds I (configuration based on the
radical R3 in relation to the -OCH2- group) with respect to their
activity.
with respect to their biological activity, preference is given to
compounds of the formula I in which m is 0.
Likewise, preference is given to compounds of formula I in which
R1 is methyl.
Besides, preference is given to compounds I in which R3 is
hydrogen, cyano, cyclopropyl, methyl, ethyl, 1-methylethyl or CF3.
Moreover, preference is given to compounds I in which R3 is
methyl.
Besides, preference is given to compounds I in which R3 is cyano.
Furthermore, preference if given to compounds I in which R3 is
cyclopropyl.
Additionally, preference is given to compounds I in which R3 is
CF3 .
Additionally, preference is given to compounds I in which R5 is
hydrogen, cyclopropyl, methyl, ethyl, isopropyl, unsubstituted or
substituted aryl or hetaryl.
Moreover, preference is given to compounds I in which R5 15
methyl.
Additionally, preference is given to compounds I in which RS is
ethyl.
Moreover, preference is given to compounds I in which RS is
isopropyl.
0050/48891 CA 02323588 2000-09-13
9
Moreover, preference is given to compounds I in which R5 is
cyclopropyl.
Moreover, preference is given to compounds I in which R5 is CF3.
Additionally, preference is given to compounds I in which R5 is
unsubstituted or substituted aryl or hetaryl.
Additionally, preference is given to compounds I in which RS is
unsubstituted or substituted pyridyl, pyrimidyl, pyrazinyl,
pyridazinyl or triazinyl.
Additionally, preference is given to compounds I in which R5 is
unsubstituted or substituted furyl, thienyl or pyrrolyl.
Additionally, preference is given to compounds I in which R5 is
unsubstituted or substituted oxazolyl, thiazolyl, isoxazolyl,
isothiazolyl, pyrazolyl or imidazolyl.
Additionally, preference is given to compounds I in which R5 is
unsubstituted or substituted oxdiazolyl [sic], thiadiazolyl or
tri-azolyl.
Moreover, preference is given to compounds I in which R5 is phenyl
which is unsubstituted or carries one or two of the following
groups: nitro, cyano, hydroxyl, amino, aminocarbonyl,
aminothiocarbonyl, halogen, C1-C4-alkyl, C1-C4-haloalkyl,
C1-C4-alkoxy, C1-C4-haloalkoxy, C1-C4-alkylamino,
di-C1-CQ-alkylamino, C1-C4-alkylsulfonyl, C1-C4-alkoxycarbonyl,
C1-C4-alkylaminocarbonyl or di-C1-C4-alkylaminocarbonyl.
Moreover, preference is given to compounds I in which R4 is
hydrogen, C1-C6-alkyl, C2-C6-alkenyl, CZ-C6-alkynyl, allyl,
arylalkyl, hetarylalkyl, aryloxyalkyl, hetaryloxyalkyl, aryl or
hetaryl.
Additionally, preference is given to compounds I in which R4 is
C1-C6_alkyl.
Further preferred compounds I are disclosed in WO 97/15,552.
with respect to the fungicidal activity against harmful fungi,
such as, for example, Pyricularia oryzae, preference is given to
the cycloalkylalkanecarboxamides IV which have the substituents
005048891 CA 02323588 2000-09-13
below, where the preference in each case can be seen on its own
or in combination:
The carbon atom carrying the groups R9 and Rio preferably has the
5 R configuration.
Preference is given to cycloalkylalkanecarboxamides IV in which R9
is methyl and Rlo is either methyl or hydrogen; particular
10 preference is given to compounds IV in which R9 is methyl and Rlo
is hydrogen.
Furthermore, preference is given to cycloalkylalkanecarboxamides
of the formula IV in which W is unsubstituted or substituted
Phenyl which is in particular substituted in the 2-position or in
positions 2 and 4. Very particular preference is given to
substitution in the 4-position of the phenyl ring and here
preferably to substitution by cyano or methoxy, preferably by
methyl and in particular by halogen, where in this case
Preference is in turn given to chlorine.
Additionally, preference is given to cycloalkylalkanecarboxamides
IV where n = 1. The substituents R3 and R4 are preferably
C1-C4-alkyl and in particular methyl. Preference is also given to
the combination in which one of the two substituents is hydrogen
and the other is C1-C4-alkyl and in particular methyl.
Furthermore, preference is given to a-chloro or
a-bromocycloalkylalkanecarboxamides IV (Y = bromine or chlorine).
particular preference is given to
a-cyanocycloalkylalkanecarboxamides IV (Y = cyano).
Finally, preference is given to cycloalkylalkanecarboxamides of
the formula IV in which A is unsubstituted or substituted
cyclopropyl. Particular preference is given to cyclopropyl
carrying one to three substituents selected from the group
consisting of chlorine and C1-C3-alkyl, in particular methyl.
Chlorinated cyclopropyl preferably carries two chlorine atoms,
these being in a geminal position at the cyclapropane ring.
Alkylated or preferably methylated cyclopropyl preferably carries
one of the alkyl(methyl) substituents at the carbon atom of the
site where the cyclopropane ring is linked to the remainder of
the molecule.
0050/48891 CA 02323588 2000-09-13
11
With respect to their use, particular preference is given to the
compounds I which are compiled in the Tables 1 to 13 below.
Table 1
Carboxamides IVa.001 to IVa.108 of the formula IVa
(* = Configuration of the atom labeled "*"; R = R configuration;
S = S configuration; rac. = racemic)
o ~ Z
.CH'
'~ ~ IVa
Z'
N
No. Z1 Z2
IVa.001 H H R
IVa.002 H H S
IVa.003 H H rac.
IVa.004 H C1 R
IVa.005 H C1 S
IVa.006 H C1 rac.
IVa.007 H CH3 R
IVa.008 H CH3 S
IVa.009 H CH3 rac.
IVa.010 H OCH3 R
IVa.011 H OCH3 S
IVa.012 H OCH3 rac.
IVa.013 H F R
IVa.014 H F S
IVa.015 H F rac.
IVa.016 H CN R
IVa.017 H CN S
IVa.018 H CN rac.
IVa.019 C1 H R
IVa.020 C1 H S
IVa.021 C1 H rac.
IVa.022 C1 C1 R
IVa.023 C1 Cl S
0050/48891 CA 02323588 2000-09-13
12
No. Z1 Z2
IVa.024 C1 C1 rac.
IVa.025 Cl CH3 R
IVa.026 Cl CH3 S
IVa.027 C1 CH3 rac.
IVa.028 C1 OCH3 R
IVa.029 C1 OCH3 S
IVa.030 C1 OCH3 rac.
IVa.031 C1 F R
IVa.032 C1 F S
IVa.033 C1 F rac.
IVa.034 C1 CN R
IVa.035 C1 CN S
IVa.036 C1 CN rac.
IVa.037 CH3 H R
IVa.038 CH3 H S
IVa.039 CH3 H rac.
IVa.040 CH3 C1 R
IVa.041 CH3 C1 S
IVa.042 CH3 C1 rac.
IVa.043 CH3 CH3 R
IVa.044 CH3 CH3 S
IVa.045 CH3 CH3 rac.
IVa.046 CH3 OCH3 R
IVa.047 CH3 OCH3 S
IVa.048 CH3 OCH3 rac.
IVa.049 CHg F R
IVa.050 CH3 F S
IVa.051 CH3 F rac.
IVa.052 CH3 CN R
IVa.053 CH3 CN S
IVa.054 CH3 CN rac.
IVa.055 OCH3 H R
IVa.056 OCH3 H S
IVa.057 OCH3 H rac.
IVa.058 OCH3 C1 R
IVa.059 OCH3 C1 S
IVa.060 OCH3 C1 rac.
IVa.061 OCH3 CH3 R
IVa.062 OCH3 CH3 S
0050/48891 CA 02323588 2000-09-13
13
No. Z1 ZZ
IVa.063 OCH3 CH3 rac.
IVa.064 OCH3 OCH3 R
IVa.065 OCH3 OCH3 S
IVa.066 OCH3 OCH3 rac.
IVa.067 OCH3 F R
IVa.068 OCH3 F S
IVa.069 OCH3 F rac.
IVa.070 OCH3 CN R
IVa.071 OCH3 CN S
IVa.072 OCH3 CN rac.
IVa.073 F H R
IVa.074 F H S
IVa.075 F H rac.
IVa.076 F C1 R
IVa.077 F C1 S
IVa.078 F C1 rac.
IVa.079 F CH3 R
IVa.080 F CH3 S
IVa.081 F CH3 rac.
IVa.082 F OCH3 R
IVa.083 F OCH3 S
IVa.084 F OCH3 rac.
IVa.085 F F R
IVa.086 F F S
IVa.087 F F rac.
IVa.088 F CN R
IVa.089 F CN S
IVa.090 F CN rac.
IVa.091 CN H R
IVa.092 CN H S
IVa.093 CN H rac.
IVa.094 CN C1 R
IVa.095 CN C1 S
IVa.096 CN C1 rac.
IVa.097 CN CH3 R
IVa.098 CN CH3 S
IVa.099 CN CH3 rac.
IVa.100 CN OCH3 R
IVa.101 CN OCH3 S
0050/48891 CA 02323588 2000-09-13
14
No. Z1 Zz
IVa.102 CN OCH3 rac.
IVa.103 CN F R
IVa.104 CN F S
IVa.105 CN F rac.
IVa.106 CN CN R
IVa.107 CN CN S
IVa.108 CN CN rac.
Table 2
Carboxamides IVb.001 to IVb.108 of the formula IVb in which the
meanings of the combinations of Z1, Z2 and "*" are given by the
rows of Table 1.
CI Cl
o I Z,
IVb
z'
N
Table 3
Carboxamides IVc.001 to IVc.108 of the formula IVc in which the
meanings of the combinations of Z1, Z2 and "*" are given by the
rows of Table 1.
CI CI
3 5 O ~ Z''
.CH*
IVc
C1
Z
Table 4
Carboxamides IVd.001 to IVd.108 of the formula IVd in which the
meanings of the combinations of Z1, Z2 and "*" are given by the
rows of Table 1.
0050/48891 CA 02323588 2000-09-13
CI CI
o I z-
.a3"
IVd
5
z'
N
Table 5
Carboxamides IVe.001 to IVe.108 of the formula IVe in which the
meanings of the combinations of Z1, ZZ and ~~*" are given by the
rows of Table 1.
CI CI
O ~ z''
.CH"
Ie
Z'
Table 6
Carboxamides IVf.001 to IVf.108 of the formula IVf in which the
meanings of the combinations of Z1, Z2 and ~~*" are given by the
rows of Table 1.
O ~ Z'
.CH"
IVf
Z'
N
Table 7
Carboxamides IVg.001 to IVg.108 of the formula IVg in which the
meanings of the combinations of Z1, Z2 and ~~*" are given by the
rows of Table 1.
0050/48891 CA 02323588 2000-09-13
16
o I z
.ca-t
IVg
~ ~ \ Z'
N
Table 8
Carboxamides IVh.001 to IVh.108 of the formula IVh in which the
meanings of the combinations of Z1, ZZ and "*" are given by the
rows of Table 1.
O ~ zz
.CH*
NH ~ ~ I Vh
Br \ i
Z
Table 9
Carboxamides IVi.001 to IVi.108 of the formula IVi in which the
meanings of the combinations of Z1, Z2 and "*" are given by the
rows of Table 1.
O ~ Zz
,~*
Ivi
\ z'
N
Table 10
Carboxamides IVk.001 to IVk.108 of the formula IVk in which the
meanings of the combinations of Z1, Z2 and "*" are given by the
rows of Table 1.
0050/48891 CA 02323588 2000-09-13
17
C 1 O ~ Z'
CI .CH'
IVk
Z
N
Table 11
Carboxamides IVm.001 to IVm.108 of the formula IVm in which the
meanings of the combinations of Z1, Z2 and "*" are given by the
rows of Table 1.
CI O ~ Za
CI .CSI'
IVm
Z'
N
Table 12
Carboxamides IVn.001 to IVn.108 of the formula IVn in which the
meanings of the combinations of Z1, Z2 and "*" are given by the
rows of Table 1.
C I O ( Z''
CI .CH'
IVn
Z'
N
Table 13
Carboxamides IVo.001 to IVo.108 of the formula IVo in which the
meanings of the combinations of Z1, ZZ and "*" are given by the
rows of Table 1.
0050/48891 CA 02323588 2000-09-13
18
O ~ Z-
~~x
~NE~i ~ I IVo
~ ~ ~ Z'
N
Furthermore, we have found processes by which the carboxamides IV
can be prepared in good yields.
In a preferred process, the carboxamides IV
R" O R'
~
A NH~W I V
R'J Y ~R,
n
are obtained by reacting the carboxylic acid derivatives VI,
R" O
A Z VI
R'z Y
n
with amines of the formula VII
R9
H,N-t-W
VII
R "'
The amide formation is carried out according to the processes
known from the literature. The free carboxylic acids of the
formula VI' where Z is hydroxyl are generally first converted
into an activated carboxylic acid derivative VI where Z is, for
example, chlorine.
The activation of the carboxylic acids VI' can preferably also be
carried out in situ by using the carboxylic acids VI' directly
with addition of, for example, dicyclohexylcarbodiimide, ethyl
chloroformate, diethyl cyanophosphonate, triphenylphosphine/
azodicarboxylic ester, 2-pyridine disulfide/triphenylphosphine,
0050/48891 CA 02323588 2000-09-13
19
carbonyldiimidazole, thionyl chloride, phosphorus trichloride,
phosphorus pentachloride, etc. In general, for example the
carbodiimides are added in equimolar amounts, based on the
carboxylic acids VI'.
Activation of the carboxylic acids via acyl cyanides is carried
out, for example, by reacting the carboxylic acids VI' with
diethyl cyanophosphonate, preferably in an inert solvent such as
tetrahydrofuran, toluene or dichloromethane (cf. Tetrahedron
Lett. 18 (1973), 1595-8).
Activation via anhydrides is carried out, for example, by
reacting the carboxylic acids VI' with chloroformates such as
ethyl chloroformate, generally in the presence of bases and, if
appropriate, in an inert solvent, such as toluene or
tetrahydrofuran (cf. "Houben-Weyl", 4th Ed. (1974), 15/1, pages
28-32).
The amide formation is preferably carried out in the presence of
bases, such as tertiary amines, for example triethylamine or
dimethylcyclohexylamine, alkali metal carbonates, alkali metal
hydroxides, pyridine, etc. The starting materials and the
auxiliary base are advantageously employed in equimolar amounts.
In certain cases, a slight excess of auxiliary base of from
0.1 - 0.5 equivalents may be advantageous.
Suitable solvents are aliphatic hydrocarbons such as hexane and
ligroin, aromatic hydrocarbons such as toluene and xylene,
chlorinated hydrocarbons such as methylene chloride and
1,2-dichloroethane, ethers such as methyl tert-butyl ether and
tetrahydrofuran, polar aprotic solvents, such as acetoni~rile and
dimethylformamide or esters such as ethyl acetate, or mixtures of
these.
The molar ratio of carboxylic acid derivative VI to amine VII is
generally from 0.8 to 1.5 and preferably from 0.9 to 1.1.
After the reaction has gone to completion, work-up is carried out
as usual, for example by introducing the reaction mixture into
water, followed by extraction of the amide.
The amines of the formula VII are known or can be easily obtained
(cf. Organikum (1993) Barth Verlagsgesellschaft mbH Leipzig,
p. 509 ff.; Indian J. Chem. 10 (1972), 366).
0050/48891 CA 02323588 2000-09-13
The preparation of a-cyanocyclopropane acetic acid is described
in Org. Prep. Proced. Int. 5 (1973), 25-29. Scheme 1 shows a
general route for constructing carboxylic acids of the formula
VI' (cf. Collect. Czech. Chem. Commun. 48 (1983), 1597-1601 and
5 J. Polym. Sci., Polym. Chem. Ed. ~ (1976), 2357-9).
Scheme 1
10 R» O R" O
base
A B r -f- ~ OR ~ A OR
R~~ Y R' z Y
n n
20 R" O
A OH
R'z Y
n
Vh
Carboxylic acid derivatives of the formula VIA can furthermore be
prepared in accordance with Scheme 2.
O R" O
A~Z A Z
Y Rt, Y
VIA VIB
45
0050/48891 CA 02323588 2000-09-13
21
Scheme 2
O Br, / P O
A~OH --1 A~OR'
Br
VIII
NaCN
O O
A OR' -----i A OH
NI
VIAA'
The cycloalkylacetic acids of the formula VIII where A is as
defined in claim 1 are known (J. Chem. Technol. Biotechnol.,
Chem. Technol., 33A (1983), 109-15; NL 65 06 881; Chem. Ber. 41
(1908), 2627; Chem. Ber. 35 (1902), 2688).
The cycloalkylacetic acids VIII can be brominated in the a
position in accordance with the protocol in J. Am. Chem. Soc 70
(1948), 3626. Work-up in the presence of a C1-C6-alcohol leads
directly to the corresponding ester. The subsequent bromine/cyano
exchange is carried out as described in Synth. Commun. 23 (1993),
2323-9. The hydrolysis of the esters to give the carboxylic acids
VIAA' is carried out by standard procedures (Organikum 1993 Barth
Verlagsgesellschaft mbH, Leipzig, p. 431ff.).
The carboxylic acid derivatives of the formula VIB can be
obtained, for example, by the route shown in Scheme 3.
40
0050/48891 CA 02323588 2000-09-13
22
Scheme 3
O O
~~ [base)/(H+) O
A-y + ~OR' ---~ A OR'
~Ra Y R.~ Y
15
IX X
R3MgHa1 MI)
R3 O R' O
A OR' ~ A OH
Ra Y Rs Y
VIB'
The starting materials, acyl- or formylcycloalkanes of the
formula IX, are generally accessible (cf. inter alia J. Chem.
Soc., Perkin Trans. I, 6 (1994), 739 -52). These are reacted in a
Knoevenagel reaction with C1-C6-alkyl a-halo- or a-cyanoacetates
to give the Michael systems X (cf. Chem. Heterocycl. Compd. 24
(1988), 860-4).
The condensation is generally carried out in a solvent that is
immiscible with water, such as hexane, toluene or xylene, with
removal of the water that is formed during the reaction. To this
end, the reaction mixture is heated at the boil and under reflux
for several hours.
Bases, such as, for example, piperidine, pyridine, ammonia or
(3-alanine, in the presence of an acid, such as, for example,
glacial acetic acid, serve as catalysts.
An alkyl Grignard reagent of the formula XI where R3 is as defined
in claim 1 and Hal is chlorine, bromine or iodine is subsequently
added to a Michael system of the formula X to give saturated
systems of the type VIB.
The reaction is carried out in solvents which are inert under the
reaction conditions. Particular preference is given to ethers
such as tetrahydrofuran, diethyl ether, dimethoxyethane or methyl
tert-butyl ether. The reaction temperature is generally set to
from -10 to 80°C and preferably to from 10 to 60°C.
0050/48891 CA 02323588 2000-09-13
23
The Grignard reagent XI is generally employed in equimolar
amounts, based on the Michael system X. In some cases, it proves
advantageous to employ an excess of from 0.2 to 0.5 molar
equivalents of the Grignard reagent.
10
In general, the addition is carried out, catalyzed by copper, by
addition of 1 - 10 mol% of, for example, copper(I) iodide. This
results in a higher selectivity for 1,2-addition versus
1,4-addition.
The free carboxylic acids VIB' are finally prepared by alkaline
hydrolysis of the corresponding esters (Organikum 1993 Barth
Verlagsgesellschaft mbH, Leipzig, p. 431ff.).
An elegant route to
2-cyano-3-(2,2-dichlorocyclopropyl)-3-methylbutanoic acid is
shown in Scheme 4.
Scheme 4
o ~ o
_ / \
O
O
CHC13/MOH
C1
C1
N
The preparation of 2-cyano-3,3-dimethylpent-4-enoic acid from the
3-methylbut-2-enyl ester of cyanoacetic acid is described in DE
26 49 711 and Res. Discl. 249 (1985), 55. By addition of
dichlorocarbene, which is obtainable from chloroform and alkali
metal hydroxides by standard methods, 2-cyano-3-(2,2-dichloro-
cyclopropyl)-3-methylbutanoic acid can be obtained directly. To
increase the yield it is advantageous to protect the carboxylic
acid function prior to the cyclopropanation step (for example by
conversion into the tert-butyl ester).
0050/48891 CA 02323588 2000-09-13
24
By the abovementioned processes, carboxylic acid derivatives VI
are obtainable which are suitable, for example, for preparing the
carboxamides IV according to the invention.
The particularly preferred embodiments of the carboxylic acid
derivatives VI with respect to the substituents R11, Riz, A and Y
correspond to those of the carboxamides IV.
Z is a nucleophilically replaceable radical, such as hydroxyl,
C1-C4-alkoxy, halogen, for example bromine or chlorine, hetaryl,
for example imidazolyl or pyridyl, carboxylate, for example
acetate or trifluoroacetate, etc.
particular preference is given to carboxylic acid derivatives of
the formula VI in which n is 1 and/or A is unsubstituted or
substituted cyclopropyl. In the case that n is 0, preference is
given to carboxylic acid derivatives of the formula VIA in which
A is cyclopropyl which may carry one to three substituents, such
as, for example, chlorine and/or C1-C3-alkyl. Chlorinated
cyclopropyl preferably carries two chlorine atoms in a geminal
position at the cyclopropane ring.
The compounds V can be prepared starting from the corresponding
carboxylic acids XIIa
R13-COON (XIIa)
by reaction with amines XIII (the literature references
"Houben-Weyl" are based on : Houben-Weyl, Methoden der
Organischen Chemie, 4th Edition, Thieme Verlag, Stuttgart).
The carboxylic acids XIIa are known from EP-A 653 418.
The amines XIII are also generally known or obtainable by known
methods (cf. WO-A 95/23 784).
The reaction is preferably carried out by initially converting
the carboxylic acids XIIa into carboxyl-activated derivatives
XII, especially into acyl halides - for example the chlorides -,
acyl cyanides or anhydrides (cf. Tetrahedron Letters, Volume 18,
page 1595 to page 1598 (1973) or "Houben-Weyl", Volume 15/1, page
0050/48891 CA 02323588 2000-09-13
28 to page 32). These derivatives XII are then reacted with the
amines XIII in the presence of bases.
Suitable for preparing the acyl cyanides is, for example, the
5 reaction of the carboxylic acids XIIa with diethyl
cyanophosphonate, especially in an inert solvent such as
tetrahydrofuran, toluene or dichloromethane.
10 To prepare the carboxyl-activated anhydrides, preference is given
to reacting the carboxylic acids XIIa with chloroformates such as
isobutyl chloroformate in the presence of bases and, if
appropriate, in an inert solvent such as toluene or
tetrahydrofuran.
The reaction of the amines XIII with the carboxyl-activated
carboxylic acids III [sic] is preferably carried out in a solvent
such as dichloromethane, tetrahydrofuran or toluene.
The amines XIII themselves may also act as the bases, and they
are usually recovered from the crude product.
In a preferred embodiment of this process step, the carboxylic
acid XII [sic], the amine XIII, the reagent suitable for
preparing the carboxyl-activated derivative of the
carbamoylcarboxylic acid XII and the base are reacted in a
one-pot process, if appropriate in an inert solvent.
The reaction mixture obtained in this manner is worked-up in a
customary manner to give the compounds V, for example by mixing
with water, separation of the phases and, if appropriate,
chromatographic purification of the crude products. Some of the
end products are obtained in the form of colorless or slightly
brownish, viscous oils, which can be freed of volatile components
under reduced pressure and at moderately elevated temperature. If
the end products are obtained as solids, purification can also be
carried out, for example, by recrystallization or digestion.
Depending on the nature of the substituents, the compounds of the
formula V may be present as geometrical and/or optical isomers or
isomer mixtures. In particular, the carbon atom in the compounds
V which carries the groups R3 and R4 may have R or S configuration
according to IUPAC nomenclature. Both the pure isomers described
here and the mixtures of the isomers have fungicidal activity.
0050/48891 CA 02323588 2000-09-13
26
In the compounds V, with respect to the radical R13, the remaining
part of the molecule may be arranged exo or endo. Both isomers
and their mixtures are in each case fungicidally active.
With respect to their biological action against harmful fungi,
preference is given to compounds V in which the carbon atom
carrying groups R15 and R16 has R configuration.
M°reover, preference is given to compounds V in which R15 is
hydrogen and R16 is C1-C4-alkyl, especially methyl.
Furthermore, preference is given to compounds V in which Ar is
optionally substituted phenyl which is substituted in particular
in the 2-position or in the 2- and 4-position and especially in
the 4-position. Preferred sole substituents in the 4-position are
cyano, preferably methyl and in particular halogen, especially
chlorine.
Additionally, preference is given to compounds V in which R13 is
unsubstituted or substituted bicycloalkyl having 6 to 10 carbon
atoms.
Moreover, preference is given to compounds V in which R13 is
unsubstituted or substituted bicycloalkenyl having 7 to 10 carbon
atoms.
Particular preference with respect to their use is given to the
compounds compiled in Tables 1 to 29 of WO-A 97/35838.
In the definition of the compounds I, IV and V given at the
outset, collective terms were used which generally represent the
following groups:
Halogen: fluorine, chlorine, bromine and iodine;
Alkyl: straight-chain or branched alkyl groups having 1 to 4, 6
or 10 carbon atoms, for example C1-C6-alkyl such as methyl, ethyl,
propyl, 1-methylethyl, butyl, 1-methylpropyl, 2-methylpropyl,
1,1-dimethylethyl, pentyl, 1-methylbutyl, 2-methylbutyl,
3-methylbutyl, 2,2-dimethylpropyl, 1-ethylpropyl, hexyl, 1,1-
dimethylpropyl, 1,2-dimethylpropyl, 1-methylpentyl, 2-methyl-
pentyl, 3-methylpentyl, 4-methylpentyl, 1,1-dimethylbutyl,
1~2_dimethylbutyl, 1,3-dimethylbutyl, 2,2-dimethylbutyl, 2,3-
dimethylbutyl, 3,3-dimethylbutyl, 1-ethylbutyl, 2-ethylbutyl,
0050/48891 CA 02323588 2000-09-13
27
1,1,2-trimethylpropyl, 1,2,2-trimethylpropyl,
1-ethyl-1-methylpropyl and 1-ethyl-2-methylpropyl;
Haloalkyl: straight-chain or branched alkyl groups having 1 to 6
carbon atoms, it being possible for some or all of the hydrogen
atoms in these groups to be replaced by halogen atoms as
mentioned above, for example C1-C2-haloalkyl, such as
chloromethyl, dichloromethyl, trichloromethyl, fluoromethyl,
difluoromethyl, trifluoromethyl, chlorofluoromethyl,
dichlorofluoromethyl, chlorodifluoromethyl, 1-fluoroethyl,
2-fluoroethyl, 2,2-difluoroethyl, 2,2,2-trifluoroethyl,
2-chloro-2-fluoroethyl, 2-chloro-2,2-difluoroethyl,
2,2-dichloro-2-fluoroethyl, 2,2,2-trichloroethyl and
pentafluoroethyl;
C1-C4-Alkoxy and the alkoxy moieties of C1-C4-alkoxycarbonyl:
methoxy, ethoxy, propoxy, 1-methylethoxy, butoxy,
1-methylpropoxy, 2-methylpropoxy and 1,1-dimethylethoxy;
C1-CQ-Haloalkoxy: a C1-C4-alkoxy radical as mentioned above which
is partially or fully substituted by fluorine, chlorine, bromine
and/or iodine, i.e., for example, fluoromethoxy, difluoromethoxy,
trifluoromethoxy, chlorodifluoromethoxy, bromodifluoromethoxy,
2-fluoroethoxy, 2-chloroethoxy, 2-bromomethoxy, 2-iodoethoxy,
2,2-difluoroethoxy, 2,2,2-trifluoroethoxy,
2-chloro-2-fluoroethoxy, 2-chloro-2,2-difluoroethoxy,
2,2-dichloro-2-fluoroethoxy, 2,2,2-trichloroethoxy,
pentafluoroethoxy, 2-fluoropropoxy, 3-fluoropropoxy,
2-chloropropoxy, 3-chloropropoxy, 2-bromopropoxy, 3-bromopropoxy,
2,2-difluoropropoxy, 2,3-difluoropropoxy, 2,3-dichloropropoxy,
3,3,3-trifluoropropoxy, 3,3,3-trichloropropoxy,
2,2,3,3,3-pentafluoropropoxy, heptafluoropropoxy,
1-(fluoromethyl)-2-fluoroethoxy, 1-(chloromethyl)-2-chloroethoxy,
1-(bromomethyl)-2-bromoethoxy, 4-fluorobutoxy, 4-chlorobutoxy,
4-bromobutoxy and nonafluorobutoxy;
Cycloalkyl: monocyclic alkyl groups having 3 to 6 carbon ring
members, for example cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl;
Alkenyl: straight-chain or branched alkenyl groups having 2 to 6
or 10 carbon atoms and a double bond in any position, for example
CZ-C6-alkenyl, such as ethenyl, 1-propenyl, 2-propenyl,
1_methylethenyl, 1-butenyl, 2-butenyl, 3-butenyl,
1-methyl-1-propenyl, 2-methyl-1-propenyl, 1-methyl-2-propenyl,
2-methyl-2-propenyl, 1-pentenyl, 2-pentenyl, 3-pentenyl,
0050/48891 CA 02323588 2000-09-13
28
4-pentenyl, 1-methyl-1-butenyl, 2-methyl-1-butenyl,
3-methyl-1-butenyl, 1-methyl-2-butenyl, 2-methyl-2-butenyl,
3-methyl-2-butenyl, 1-methyl-3-butenyl, 2-methyl-3-butenyl,
3-methyl-3-butenyl, 1,1-dimethyl-2-propenyl,
1,2-dimethyl-1-propenyl, 1,2-dimethyl-2-propenyl,
1-ethyl-1-propenyl, 1-ethyl-2-propenyl, 1-hexenyl, 2-hexenyl,
3-hexenyl, 4-hexenyl, 5-hexenyl, 1-methyl-1-pentenyl,
2-methyl-1-pentenyl, 3-methyl-1-pentenyl, 4-methyl-1-pentenyl,
1-methyl-2-pentenyl, 2-methyl-2-pentenyl, 3-methyl-2-pentenyl,
4-methyl-2-pentenyl, 1-methyl-3-pentenyl, 2-methyl-3-pentenyl,
3-methyl-3-pentenyl, 4-methyl-3-pentenyl, 1-methyl-4-pentenyl,
2-methyl-4-pentenyl, 3-methyl-4-pentenyl, 4-methyl-4-pentenyl,
1,1-dimethyl-2-butenyl, 1,1-di-methyl-3-butenyl,
1,2-dimethyl-1-butenyl, 1,2-dimethyl-2-butenyl,
1,2-dimethyl-3-butenyl, 1,3-dimethyl-1-butenyl,
1,3-dimethyl-2-butenyl, 1,3-dimethyl-3-butenyl,
2,2-dimethyl-3-butenyl, 2,3-dimethyl-1-butenyl,
2,3-dimethyl-2-butenyl, 2,3-dimethyl-3-butenyl,
3,3-dimethyl-1-butenyl, 3,3-dimethyl-2-butenyl,
1-ethyl-1-butenyl, 1-ethyl-2-butenyl, 1-ethyl-3-butenyl,
2-ethyl-1-butenyl, 2-ethyl-2-butenyl, 2-ethyl-3-butenyl,
1,1,2-trimethyl-2-propenyl, 1-ethyl-1-methyl-2-propenyl,
1-ethyl-2-methyl-1-propenyl and 1-ethyl-2-methyl-2-propenyl;
plkynyl: straight-chain or branched alkynyl groups having 2 to 10
carbon atoms and a triple bond in any position, for example
CZ-C6-alkynyl, such as ethynyl, 2-propynyl, 2-butynyl, 3-butynyl,
1-methyl-2-propynyl, 2-pentynyl, 3-pentynyl, 4-pentynyl,
1-methyl-2-butynyl, 1-methyl-3-butynyl, 2-methyl-3-butynyl,
1,1-dimethyl-2-propynyl, 1-ethyl-2-propynyl, 2-hexynyl,
3-hexynyl, 4-hexynyl, 5-hexynyl, 1-methyl-2-pentynyl,
1-methyl-3-pentynyl, 1-methyl-4-pentynyl, 2-methyl-3-pentynyl,
2-methyl-4-pentynyl, 3-methyl-4-pentynyl, 4-methyl-2-pentynyl,
1,1-dimethyl-2-butynyl, 1,1-dimethyl-3-butynyl,
1,2-dimethyl-3-butynyl, 2,2-dimethyl-3-butynyl,
1-ethyl-2-butynyl, 1-ethyl-3-butynyl, 2-ethyl-3-butynyl and
1-ethyl-1-methyl-2-propynyl;
Heterocyclyl or heterocyclyloxy, heterocyclylthio and
heterocyclylamino: three- to six-membered saturated or partially
unsaturated mono- or polycyclic heterocycles which contain one to
three hereroatoms [sic] selected from a group consisting of
oxygen, nitrogen and sulfur and which are attached to the
skeleton directly or (heterocyclyloxy) via an oxygen atom or
(heterocyclylthio) via a sulfur atom or (heterocyclylamino) via a
nitrogen atom, such as, for example, 2-tetrahydrofuranyl,
oxiranyl, 3-tetrahydrofuranyl, 2-tetrahydrothienyl,
0050/48891 CA 02323588 2000-09-13
29
3-tetrahydrothienyl, 2-pyrrolidinyl, 3-pyrrolidinyl,
3-isoxazoldinyl, 4-isoxazolidinyl, 5-isoxazolidinyl,
3-isothiazolidinyl, 4-isothiazolidinyl, 5-isothiazolidinyl,
3-pyrazolidinyl, 4-pyrazolidinyl, 5-pyrazolidinyl,
2-oxazolidinyl, 4-oxazolidinyl, 5-oxazolidinyl, 2-thiazolidinyl,
4-thiazolidinyl, 5-thiazolidinyl, 2-imidazolidinyl,
4-imidazolidinyl, 1,2,4-oxadiazolidin-3-yl,
1,2,4-oxadiazolidin-5-yl, 1,2,4-thiadiazolidin-3-yl,
1,2,4-thiadiazolidin-5-yl, 1,2,4-triazol-idin-3-yl,
1,3,4-oxadiazolidin-2-yl, 1,3,4-thiadiazolidin-2-yl,
1,3,4-triazolidin-2-yl, 2,3-dihydrofur-2-yl, 2,3-dihydrofur-3-yl,
2,3-dihydrofur-4-yl, 2,3-dihydrofur-5-yl, 2,5-dihydrofur-2-yl,
2,5-dihydrofur-3-yl, 2,3-dihydrothien-2-yl,
2,3-dihydrothien-3-yl, 2,3-dihydrothien-4-yl,
2,3-dihydrothien-5-yl, 2,5-dihydrothien-2-yl,
2,5-dihydrothien-3-yl, 2,3-dihydropyrrol-2-yl,
2,3-dihydropyrrol-3-yl, 2,3-dihydropyrrol-4-yl, 2,3-dihydro-
pyrrol-5-yl, 2,5-dihydropyrrol-2-yl, 2,5-dihydropyrrol-3-yl,
2,3-dihydroisoxazol-3-yl, 2,3-dihydroisoxazol-4-yl,
2,3-dihydroisoxazol-5-yl, 4,5-dihydroisoxazol-3-yl,
4,5-dihydroisoxazol-4-yl, 4,5-dihydroisoxazol-5-yl,
2,5-dihydroisothiazol-3-yl, 2,5-dihydroisothiazol-4-yl,
2,5-dihydroisothiazol-5-yl, 2,3-dihydroisopyrazol-3-yl,
2,3-dihydroisopyrazol-4-yl, 2,3-dihydroisopyrazol-5-yl,
4,5-dihydroisopyrazol-3-yl, 4,5-dihydroisopyrazol-4-yl,
4,5-dihydroisopyrazol-5-yl, 2,5-dihydroisopyrazol-3-yl,
2,5-dihydroisopyrazol-4-yl, 2,5-dihydroisopyrazol-5-yl,
2,3-dihydrooxazol-3-yl, 2,3-dihydrooxazol-4-yl,
2,3-dihydrooxazol-5-yl, 4,5-dihydrooxazol-3-yl,
4,5-dihydrooxazol-4-yl, 4,5-dihydrooxazol-5-yl,
2,5-dihydrooxazol-3-yl, 2,5-dihydrooxazol-4-yl,
2,5-dihydrooxazol-5-yl, 2,3-dihydrothiazol-2-yl,
2,3-dihydrothiazol-4-yl, 2,3-dihydrothiazol-5-yl,
4,5-dihydrothiazol-2-yl, 4,5-dihydrothiazol-4-yl,
4,5-dihydrothiazol-5-yl, 2,5-dihydrothiazol-2-yl,
2,5-dihydrothiazol-4-yl, 2,5-dihydrothiazol-5-yl,
2,3-dihydroimidazol-2-yl, 2,3-dihydroimidazol-4-yl,
2,3-dihydroimidazol-5-yl, 4,5-dihydroimidazol-2-yl,
4,5-dihydroimidazol-4-yl, 4,5-dihydro-imidazol-5-yl,
2,5-dihydroimidazol-2-yl, 2,5-dihydroimidazol-
4-yl, 2,5-dihydroimidazol-5-yl, 2-morpholinyl, 3-morpholinyl,
2-piperidinyl, 3-piperidinyl, 4-piperidinyl,
3-tetrahydropyridazinyl, 4-tetrahydropyridazinyl,
2-tetrahydropyrimidinyl, 4-tetrahydropyrimidinyl,
5-tetrahydropyrimidinyl, 2-tetrahydropyrazinyl,
1,3,5-tetrahydrotriazin-2-yl, 1,2,4-tetrahydrotriazin-3-yl,
1,3-dihydrooxazin-2-yl, 1,3-dithian-2-yl, 2-tetrahydropyranyl,
0050/48891 CA 02323588 2000-09-13
1,3-dioxolan-2-yl, 3,4,5,6-tetrahydropyridin-2-yl,
4H-1,3-thiazin-2-yl, 4H-3,1-benzothiazin-2-yl,
1,1-dioxo-2,3,4,5-tetrahydrothien-2-yl, 2H-1,4-benzothiazin-3-yl,
2H-1,4-benzoxazin-3-yl, 1,3-dihydrooxazin-2-yl, 1,3-dithian-2-yl;
5
Aryl or aryloxy, arylthio, arylcarbonyl and arylsulfonyl:
aromatic mono- or polycyclic hydrogen radicals which are attached
to the skeleton directly or (aryloxy) via an oxygen atom (-0-) or
(arylthio) a sulfur atom (-S-), (arylcarbonyl) via a carbonyl
10 group (-CO-) or (arylsulfonyl) via a sulfonyl group (-S02-), for
example phenyl, naphthyl and phenanthrenyl or phenyloxy,
naphthyloxy and phenanthrenyloxy and the corresponding carbonyl
and sulfonyl radicals;
15 Hetaryl or hetaryloxy, hetarylthio, hetarylcarbonyl and
hetarylsulfonyl: aromatic mono- or polycyclic radicals which,
beside carbon ring members, can additionally contain one to four
nitrogen atoms or one to three nitrogen atoms and one oxygen or
20 °ne sulfur atom or one oxygen or one sulfur atom and which are
attached to the skeleton directly or (hetaryloxy) via an oxygen
atom (-O-) or {hetarylthio) a sulfur atom (-S-),
(hetarylcarbonyl) via a carbonyl group (-CO-) or
{hetarylsulfonyl) via a sulfonyl group (-S02-), for example
- 5-membered heteroaryl, containing one to three nitrogen
atoms: 5-membered heteroaryl groups which, beside carbon
atoms, can contain one to three nitrogen atoms as ring
members, for example 2-pyrrolyl, 3-pyrrolyl, 3-pyrazolyl,
4-pyrazolyl, 5-pyrazolyl, 2-imidazolyl, 4-imidazolyl,
1,2,4-triazol-3-yl and 1,3,4-triazol-2-yl;
- 5-membered heteroaryl, containing one to four nitrogen atoms
or one to three nitrogen atoms and one sulfur or oxygen atom
or one oxygen or one sulfur atom: 5-membered heteroaryl
groups which, beside carbon atoms, can contain one to four
nitrogen atoms or one to three nitrogen atoms and one sulfur
or oxygen atom or one oxygen or sulfur atom as ring members,
for example 2-furyl, 3-furyl, 2-thienyl, 3-thienyl,
2-pyrrolyl, 3-pyrrolyl, 3-isoxazolyl, 4-isoxazolyl,
5-isoxazolyl, 3-isothiazolyl, 4-isothiazolyl, 5-isothiazolyl,
3-pyrazolyl, 4-pyrazolyl, 5-pyrazolyl, 2-oxazolyl,
4-oxazolyl, 5-oxazolyl, 2-thiazolyl, 4-thiazolyl,
5-thiazolyl, 2-imidazolyl, 4-imidazolyl,
1,2,4-oxadiazol-3-yl, 1,2,4-oxadiazol-5-yl,
1,2,4-thiadiazol-3-yl, 1,2,4-thiadiazol-5-yl,
0050/48891 CA 02323588 2000-09-13
31
1,2,4-triazol-3-yl, 1,3,4-oxadiazol-2-yl,
1,3,4-thiadiazol-2-yl, 1,3,4-triazol-2-yl;
- benzo-fused 5-membered heteroaryl, containing one to three
nitrogen atoms or one nitrogen atom and/or one oxygen or
sulfur atom: 5-membered heteroaryl groups which, beside
carbon atoms, can contain one to four nitrogen atoms or one
to three nitrogen atoms and one sulfur or oxygen atom or one
oxygen or one sulfur atom as ring members, and in which two
adjacent carbon ring members or one nitrogen and one adjacent
carbon ring member may be bridged by a buta-1,3-dien-1,4-diyl
group;
5-membered heteroaryl bonded via nitrogen and containing one
to four nitrogen atoms, or benzo-fused 5-membered heteroaryl,
bonded via nitrogen and containing one to three nitrogen
atoms: 5-membered heteroaryl groups which, beside carbon
atoms, can contain one to four nitrogen atoms and one to
three nitrogen atoms, respectively, as ring members, and in
which two adjacent carbon ring members or one nitrogen and
one adjacent carbon ring member can be bridged by a
buta-1,3-dien-1,4-diyl group, these rings being attached to
the skeleton via one of the nitrogen ring members;
- 6-membered heteroaryl containing one to three and one to four
nitrogen atoms, respectively: 6-membered heteroaryl groups
which, beside carbon atoms, can contain one to four nitrogen
atoms as ring members, for example 2-pyridinyl, 3-pyridinyl,
4-pyridinyl, 3-pyridazinyl, 4-pyridazinyl, 2-pyrimidinyl,
4-pyrimidinyl, 5-pyrimidinyl, 2-pyrazinyl,
1,3,5-triazin-2-yl, 1,2,4-triazin-3-yl and
1,2,4,5-tetrazin-3-yl;
- benzo-fused 6-membered heteroaryl containing one to four
nitrogen atoms: 6-membered heteroaryl groups in which two
adjacent carbon ring members can be bridged by a buta-
1,3-dien-1,4-diyl group, for example quinoline, isoquinoline,
quinazoline and quinoxaline,
and the corresponding oxy, thio, carbonyl or sulfonyl groups.
Hetarylamino: aromatic mono- or polycyclic radicals which, beside
carbon ring members, can additionally contain one to four
nitrogen atoms or one to three nitrogen atoms and one oxygen or
005048891 CA 02323588 2000-09-13
32
one sulfur atom and which are attached to the skeleton via a
nitrogen atom.
Bicycloalkyl: bicyclic alkyl groups having 6 to 15 carbon ring
members, for example bicyclo[2.1.1]hex-5-yl, bicyclo[2.2.1]-
hept-2-yl, bicyclo[2.2.2]oct-2-yl, bicyclo[3.2.1]oct-6-yl,
bicyclo[3.2.2]non-6-yl, bicyclo[4.2.2]dec-7-yl, bicyclo[3.1.0]-
hex-1-yl, bicyclo[4.1.0]hept-1-yl, bicyclo[4.3.0]non-1-yl,
bicyclo[4.4.0]dec-1-yl, particularly preferably 5-methylbicyclo-
[2.1.1)hex-5-yl, 2-methylbicyclo[2.2.1]hept-2-yl, 2-methyl-
bicyclo[2.2.2)oct-2-yl, 6-methylbicyclo[3.2.1]oct-6-yl,
6-methylbicyclo[3.2.2]non-6-yl, 7-methylbicyclo[4.2.2]dec-7-yl,
1-methylbicyclo[3.1.0]hex-1-yl, 1-methylbicyclo[4.1.0]hept-1-yl,
1-methylbicyclo[4.3.0]non-1-yl, 1-methylbicyclo[4.4.0]dec-1-yl,
2-methylbicyclo[3.1.0]hex-1-yl, 2-methylbicyclo[4.1.0]hept-1-yl,
2-methylbicyclo[4.3.0]non-1-yl, 2-methylbicyclo[4.4.0]dec-1-yl,
adamantyl;
Bicycloalkenyl: bicyclic alkenyl groups having 7 to 15 carbon
ring members, for example bicyclo[2.2.1]kept-2-en-5-yl,
bicyclo[2.2.2]oct-2-en-5-yl, bicyclo[4.2.2]dec-7-en-2-yl,
bicyclo-[4.3.0]non-7-en-1-yl, bicyclo[4.4.0]dec-3-en-1-yl,
bicyclo-[4.1.0]hept-3-en-1-yl, 5-methylbicyclo[2.2.1]hept-
2-en-5-yl, 5-methylbicyclo[2.2.2]oct-2-en-5-yl, 2-methylbicyclo-
[4.2.2]dec-7-en-2-yl, 2-methylbicyclo[4.3.0]non-7-en-1-yl,
2-methyl-bicyclo[4.4.0)dec-3-en-1-yl,
2-methylbicyclo[4.1.0]hept-3-en-1-yl;
Alkylidene: straight-chained or branched alkylidene groups having
3 to 5 carbon atoms, for example 1,3-propylidene, 1,4-butylidene,
1-methyl-1,3-propylidene, 2-methyl-1,3-propylidene,
2,2-dimethyl-1,3-propylidene, 1,5-pentylidene,
I-methyl-1,4-butylidene;
Cycloalkenyl: monocyclic alkyl groups having 5 to 7 carbon ring
members and containing one or more double bonds, for example
C5-C~-cycloalkenyl, such as cyclopentenyl, cyclohexenyl and
cycloheptenyl;
Heterocyclyl: three to six-membered saturated or partially
unsaturated mono- or polycyclic heterocycles which contain one to
three hereroatoms [sic] selected from a group consisting of
°xygen, nitrogen and sulfur and which are attached to the
skeleton, for example 2-tetrahydrofuranyl, oxiranyl,
3-tetrahydrofuranyl,
2-tetrahydrothienyl, 3-tetrahydrothienyl, 2-pyrrolidinyl,
0050/48891 CA 02323588 2000-09-13
33
3-pyrrolidinyl, 3-isoxazolidinyl, 4-isoxazolidinyl,
5-isoxazolidinyl, 3-isothiazolidinyl, 4-isothiazolidinyl,
5-isothiazolidinyl, 3-pyrazolidinyl, 4-pyrazolidinyl,
5-pyrazolidinyl, 2-oxazolidinyl, 4-oxazolidinyl, 5-oxazolidinyl,
2-thiazolidinyl, 4-thiazolidinyl, 5-thiazolidinyl,
2-imidazolidinyl, 4-imidazolidinyl, 1,2,4-oxadiazolidin-3-yl,
1,2,4-oxadiazolidin-5-yl, 1,2,4-thiadiazolidin-3-yl,
1,2,4-thiadiazolidin-5-yl, 1,2,4-triazolidin-3-yl,
1,3,4-oxadiazolidin-2-yl, 1,3,4-thiadiazolidin-2-yl,
1,3,4-triazolidin-2-yl, 2,3-dihydrofur-2-yl, 2,3-dihydrofur-3-yl,
2,3-dihydro-fur-4-yl, 2,3-dihydro-fur-5-yl, 2,5-dihydro-fur-2-yl,
2,5-dihydro-fur-3-yl, 2,3-dihydrothien-2-yl,
2,3-dihydrothien-3-yl, 2,3-dihydrothien-4-yl,
2,3-dihydrothien-5-yl, 2,5-dihydrothien-2-yl,
2,5-dihydrothien-3-yl, 2,3-dihydropyrrol-2-yl,
2,3-dihydropyrrol-3-yl, 2,3-dihydropyrrol-4-yl,
2,3-dihydropyrrol-5-yl, 2,5-dihydropyrrol-2-yl, 2,5-dihydro-
pyrrol-3-yl, 2,3-dihydroisoxazol-3-yl, 2,3-dihydroisoxazol-4-yl,
2,3-dihydroisoxazol-5-yl, 4,5-dihydroisoxazol-3-yl,
4,5-dihydro-isoxazol-4-yl, 4,5-dihydroisoxazol-5-yl,
2,5-dihydroisothiazol-3-yl, 2,5-dihydroisothiazol-4-yl,
2,5-dihydroisothiazol-5-yl, 2,3-dihydroisopyrazol-3-yl,
2,3-dihydroisopyrazol-4-yl, 2,3-dihydroisopyrazol-5-yl,
4,5-dihydroisopyrazol-3-yl, 4,5-dihydroisopyrazol-4-yl,
4,5-dihydroisopyrazol-5-yl, 2,5-dihydroisopyrazol-3-yl,
2,5-dihydroisopyrazol-4-yl, 2,5-dihydroisopyrazol-5-yl,
2,3-dihydrooxazol-3-yl, 2,3-dihydrooxazol-4-yl,
2,3-dihydrooxazol-5-yl, 4,5-dihydrooxazol-3-yl,
4,5-dihydrooxazol-4-yl, 4,5-dihydrooxazol-5-yl,
2,5-dihydrooxazol-3-yl, 2,5-dihydrooxazol-4-yl,
2,5-dihydrooxazol-5-yl, 2,3-dihydrothiazol-2-yl,
2,3-dihydrothiazol-4-yl, 2,3-dihydrothiazol-5-yl,
4,5-dihydrothiazol-2-yl, 4,5-dihydrothiazol-4-yl,
4,5-dihydrothiazol-5-yl, 2,5-dihydrothiazol-2-yl,
2,5-dihydrothiazol-4-yl, 2,5-dihydrothiazol-5-yl,
2,3-dihydroimidazol-2-yl, 2,3-dihydroimidazol-4-yl,
2,3-dihydroimidazol-5-yl, 4,5-dihydroimidazol-2-yl,
4,5-dihydroimidazol-4-yl, 4,5-dihydro-imidazol-5-yl,
2,5-dihydroimidazol-2-yl, 2,5-dihydroimidazol-4-yl,
2,5-dihydroimidazol-5-yl, 2-morpholinyl, 3-morpholinyl,
2-piperidinyl, 3-piperidinyl, 4-piperidinyl,
3-tetrahydropyridazinyl, 4-tetrahydropyridazinyl,
2-tetrahydropyrimidinyl, 4-tetrahydropyrimidinyl,
5-tetrahydropyrimidinyl, 2-tetrahydropyrazinyl,
1,3,5-tetrahydrotriazin-2-yl, 1,2,4-tetrahydrotriazin-3-yl,
1,3-dihydrooxazin-2-yl, 1,3-dithian-2-yl, 2-tetrahydropyranyl,
1,3-dioxolan-2-yl, 3,4,5,6-tetrahydropyridin-2-yl,
0050/48891 CA 02323588 2000-09-13
34
4H-1,3-thiazin-2-yl, 4H-3,1-benzothiazin-2-yl,
1,1-dioxo-2,3,4,5-tetrahydrothien-2-yl, 2H-1,4-benzothiazin-3-yl,
2H-1,4-benzoxazin-3-yl, 1,3-dihydrooxazin-2-yl, 1,3-dithian-2-yl.
The specification "partially or fully halogenated" is meant to
express that some or all of the hydrogen atoms in the groups thus
characterized may be replaced by identical or different halogen
atoms as mentioned above.
When preparing the mixtures, it is preferred to employ the pure
active ingredients I and II to V, with which further active
ingredients against harmful fungi or other pests, such as
insects, arachnids or nematodes, or else herbicidal or
growth-regulating active ingredients or fertilizers can be
admixed.
The mixtures of the compounds I and at least one compound II to V
can be applied simultaneously, that is joined or separately, and
have outstanding action against a wide range of phytopathogenic
fungi, in particular from the classes of the Ascomycetes,
Basidiomycetes, Phycomycetes and Deuteromycetes. Some of them act
systematically and are therefore also suitable for use as folio
and soil-acting fungicides.
They are especially important for controlling a large number of
fungi in a variety of crop plants, such as cotton, vegetable
species (for example cucumbers, beans, tomatoes, potatoes and
cucurbits), barley, grass, oats, bananas, coffee, maize, fruit
species, rice, rye, soya, grapevine, wheat, ornamentals, sugar
cane, and a variety of seeds.
They are particularly suitable for controlling the following
phytopathogenic fungi: Erysiphe graminis (powdery mildew) in
cereals, Erysiphe cichoracearum and Sphaerotheca fuliginea in
cucurbits, Podosphaera leucotricha in apples, Uncinula necator in
grapevines, Puccinia species in cereals, Rhizoctonia species in
cotton, rice and lawns, Ustilago species in cereals and sugar
cane, Venturia inaequalis (scab) in apples, Helminthosporium
species in cereals and rice, Septoria nodorum in wheat, Botrytis
cinera [sic] (gray mold) in strawberries, vegetables, ornamentals
and grapevines, Cercospora arachidicola in groundnuts,
Pseudocercosporella herpotrichoides in wheat and barley,
Pyricularia oryzae in rice and lawns, Phytophthora infestans in
potatoes and tomatoes, Plasmopara viticola in grapevines,
Pseudoperonospora species in hops and cucumbers, Alternaria
0050/48891 CA 02323588 2000-09-13
species in vegetables and fruit, Mycosphaerella species in
bananas and Fusarium and Verticillium species.
The mixtures according to the invention are particularly
5 preferably utilizable for controlling Pyricularia oryzae.
The compounds I and at least one of the compounds II to V can be
applied simultaneously, either together or separately, or in
10 succession, the sequence, in the case of separate application,
generally not having any effect on the control results.
Depending on the nature of the desired effect, the application
rates of the mixtures according to the invention are, in
15 Particular in agricultural crops, from 0.01 to 8 kg/ha,
preferably from 0.1 to 5 g/ha [sic], in particular from 0.5 to
3.0 kg/ha.
In the case of the compounds I, the application rates are from
20 0.01 to 2.5 kg/ha, preferably from 0.05 to 2.5 kg/ha, in
particular from 0.1 to 1.0 kg/ha.
Correspondingly, in the case of the compounds II to V, the
application rates are from 0.001 to 5 kg/ha, preferably from
25 p,005 to 2 kg/ha, in particular from 0.01 to 1.0 kg/ha.
For seed treatment, the application rates of the mixture are
generally from 0.001 to 250 g/kg of seed, preferably 0.01 to
30 100 g/kg, in particular 0.01 to 50 g/kg.
If phytopathogenic harmful fungi are to be controlled, the
separate or joint application of the compounds I and at least one
of the compounds II to V is effected by spraying or dusting the
35 seeds, the plants or the soils before or after sowing the plants,
or before or after plant emergence.
The fungicidal synergistic mixtures according to the invention
can be formulated for example in the form of ready-to-spray
solutions, powders and suspensions or in the form of highly
concentrated aqueous, oily or other suspensions, dispersions,
emulsions, oil dispersions, pastes, dusts, materials for
broadcasting or granules, and applied by spraying, atomizing,
dusting, broadcasting or watering. The use form depends on the
intended purpose; in any case, it should ensure as fine and
0050/48891 CA 02323588 2000-09-13
36
uniform as possible a distribution of the mixture according to
the invention.
The formulations are prepared in a known manner, for example by
expanding the active ingredient with solvents and/or carriers, if
desired by use of emulsifiers and dispersants. If the diluent
used is water, it is also possible to use other organic solvents
as auxiliary solvents. Suitable auxiliaries are essentially:
solvents, such as aromatics (for example xylene), chlorinated
aromatics (for example chlorobenzenes), paraffins (for example
mineral oil fractions), alcohols (for example methanol, butanol),
ketones (for example cyclohexanone), amines (for example
ethanolamine, dimethylformamide) and water; carriers, such as
natural ground minerals (for example kaolins, clays, talc, chalk)
and ground synthetic minerals (for example finely divided silica
gel, silicates); emulsifiers, such as nonionic and anionic
emulsifiers (for example polyoxyethylene fatty alcohol ethers,
alkylsulfonates and arylsulfonates), and dispersants, such as
ligninsulfite waste liquors and methylcellulose.
Suitable surfactants are the alkali metal salts, alkaline earth
metal salts and ammonium salts of aromatic sulfonic acids, e.g.
ligno-, phenol-, naphthalene- and dibutylnaphthalenesulfonic
acid, and of fatty acids, alkyl- and alkylarylsulfonates, alkyl,
lauryl ethers and fatty alcohol sulfates, and salts of sulfated
hexa-, hepta- and octadecanols, or of fatty alcohol glycol
ethers, condensates of sulfonated naphthalene and its derivatives
with formaldehyde, condensates of naphthalene or of the
naphthalenesulfonic acids with phenol and formaldehyde,
polyoxyethylene octylphenol ether, ethoxylated isooctyl-, octyl-
or nonylphenol, alkylphenol polyglycol ethers, tributylphenyl
polyglycol ethers, alkylaryl polyether alcohols, isotridecyl
alcohol, fatty alcohol ethylene oxide condensates, ethoxylated
castor oil, polyoxyethylene alkyl ethers or polyoxypropylene
[sic], lauryl alcohol polyglycol ether acetate, sorbitol esters,
lignosulfite waste liquors or methylcellulose.
Powders, materials for broadcasting and dusts can be prepared by
mixing or jointly grinding the compounds I and at least one of
the compounds II to V or the mixture of the compounds I and at
least one of the compounds II to V with a solid carrier.
Granules (eg. coated granules, impregnated granules or
homogeneous granules) are usually prepared by binding the active
ingredient, or active ingredients, to a solid carrier.
0050/48891 CA 02323588 2000-09-13
37
Fillers or solid carriers are, for example, mineral earths, such
as silica gel, silicic acids, silica gels [sic], silicates, talc,
kaolin, limestone, lime, chalk, bole, loess, clay, dolomite,
diatomaceous earth, calcium sulfate, magnesium sulfate, magnesium
oxide, ground synthetic materials, and fertilizers, such as
ammonium sulfate, ammonium phosphate, ammonium nitrate, ureas,
and products of vegetable origin, such as cereal meal, tree bark
meal, wood meal and nutshell meal, cellulose powders or other
solid carriers.
The formulations generally comprise 0.1 to 95~ by weight,
preferably 0.5 to 90$ by weight, of one of the compounds I and at
least one of the compounds II to V or of the mixture of the
compounds I and at least one of the compounds II to V. The active
ingredients are employed in a purity of from 90~ to 100,
preferably 95~ to 100 (according to NMR or HPLC spectrum [sic].
The corresponding formulations are applied by treating the
harmful fungi, their habitat or the plants, seeds, soils, areas,
materials or spaces to be kept free from them with a fungicidally
effective amount of the mixture, or of the compounds I and at
least one of the compounds II to V in the case of separate
application.
Application can be effected before or after infection by the
harmful fungi.
Examples of such preparations comprising the active ingredients
are:
I. A solution of y0 parts by weight of the active ingredients
and 10 parts by weight of N-methylpyrrolidone; this
solution is suitable for use in the form of microdrops;
II. A mixture of 20 parts by weight of the active ingredients,
80 parts by weight of xylene, 10 parts by weight of the
adduct of 8 to 10 mol of ethylene oxide and 1 mol of oleic
acid N-monoethanolamide, 5 parts by weight of the calcium
salt of dodecylbenzenesulfonate, 5 parts by weight of the
adduct of 40 mol of ethylene oxide and 1 mol of castor oil;
a dispersion is obtained by finely distributing the
solution in water;
III. An aqueous dispersion of 20 parts by weight of the active
ingredients, 40 parts by weight of cyclohexanone, 30 parts
by weight of isobutanol, 20 parts by weight of the adduct
of 40 mol of ethylene oxide and 1 mol of castor oil;
0050/48891 CA 02323588 2000-09-13
38
IV. An aqueous dispersion of 20 parts by weight of the active
ingredients, 25 parts by weight of cyclohexanol, 65 parts
by weight of a mineral oil fraction of boiling point 210 to
280~C, and 10 parts by weight of the adduct of 40 mol of
ethylene oxide and 1 mol of castor oil;
V. A mixture, ground in a hammer mill, of 80 parts by weight
of the active ingredients, 3 parts by weight of the sodium
salt of diisobutylnaphthalene-I-sulfonic acid, 10 parts by
weight of the sodium salt of a lignosulfonic acid from a
sulfite waste liquor and 7 parts by weight of pulverulent
silica gel; a spray mixture is obtained by finely
distributing the mixture in water;
VI. An intimate mixture of 3 parts by weight of the active
ingredients and 97 parts by weight of finely divided
kaolin; this dust comprises 3% by weight of active
ingredient;
VII. An intimate mixture of 30 parts by weight of the active
ingredients, 92 parts by weight of pulverulent silica gel
and 8 parts by weight of paraffin oil which had been
sprayed onto the surface of this silica gel; this
formulation imparts good adhesion to the active ingredient;
VIII. A stable aqueous dispersion of 40 parts by weight of the
active ingredients, 10 parts by weight of the sodium salt
of a phenolsulfonic acid/urea/formaldehyde condensate, 2
' parts by weight of silica gel and 48 parts by weight of
water; this dispersion may be diluted further;
IX. A stable oily dispersion of 20 parts by weight of the
active ingredients, 2 parts by weight of the calcium salt
of dodecylbenzenesulfonic acid, 8 parts by weight of fatty
alcohol polyglycol ether, 20 parts by weight of the sodium
salt of a phenolsulfonic acid/urea/formaldehyde condensate
and 88 parts by weight of a paraffinic mineral oil.
The synergistic activity of the mixtures according to the
invention can be demonstrated by the following experiments:
The active ingredients, separately or together, are formulated as
a 10% emulsion in a mixture of 63% by weight of cyclohexanone and
27% by weight of emulsifier, and correspondingly diluted with
water to the desired concentration.
Evaluation is carried out by determining the infected leaf areas
in percent. These percentages are converted into efficacies. The
efficacy (W) is calculated as follows using Abbot's formula:
0050/48891 CA 02323588 2000-09-13
39
W = (1 - a) -100/(3
a corresponds to the fungal infection of the treated plants in
% and
corresponds to the fungal infection of the untreated
(control) plants in %
An efficacy of 0 means that the infection level of the treated
plants corresponds to that of the untreated control plants; an
efficacy of 100 means that the treated plants were not infected.
The expected efficacies of the mixtures of the active ingredients
were determined using Colby's formula [R.S. Colby, Weeds 15,
20-22 (1967)] and compared with the observed efficacies.
Colby's formula: E = x + y - x~y/100
E expected efficacy, expressed in % of the untreated control,
when using the mixture of the active ingredients A and B at
the concentrations a and b
x efficacy, expressed in % of the untreated control, when using
active ingredient A at the concentration a
y efficacy, expressed in % of the untreated control, when using
active ingredient B at the concentration b
Use Example 1 - Activity against Pyricularia oryzae (protective)
Leaves of potted rice seedlings c.v. "Tai-Nong 67" were sprayed
to runoff point with an aqueous preparation of active ingredient
prepared using a stock solution comprising 10% of active
ingredient, 63% of cyclohexanone and 27% of emulsifier. The
following day, the plants were inoculated with an aqueous spore
suspension of Pyricularia oryzae. The test plants were
subsequently placed in climatized chambers at 22-24~C and 95-99%
relative atmospheric humidity for 6 days. The extent of the
development of the disease on the leaves was then determined
visually.
The visually determined values for the percentage of diseased
leaf areas were converted into efficacies as percent of the
untreated control. An efficacy of 0 is the same disease level as
in the untreated control, an efficacy of 100 is 0% disease. The
expected efficacies for the active ingredient combinations were
determined using Colby's formula (Colby, S. R. (Calculating
0050/48891 CA 02323588 2000-09-13
synergistic and antagonistic responses of herbicide
combinations", Weeds, 15, pp. 20-22, 1967) and compared with the
observed efficacies.
5 The compound I' below was employed as component a):
CH3
H3C~~~N~ ~N~O /
CH w N CH3NH N,O-CH3
3
H C'~ C
3
15 The test results are shown in Tables 1 and 2 below:
Table 1:
Efficacy in % of
Ex. Active ingredient conc. in ppm the untreated
20 control
1C without (100% disease) 0
2.0 20
2C Compound I'
0.5 0
2.0 20
253C Compound II
0.5 0
Table 2:
Mixture according to the Observed Calculated
30Ex. invention (conc. in ppm) efficacy efficacy*
4 2 ppm I' + 2 ppm II 99 36
25 0
5 0.5 ppm I' + 0.5 ppm II
35 * Calculated using Colby's formula
The test results show that for all mixing ratios the observed
efficacy is higher than the efficacy which had been calculated
beforehand using Colby's formula.
45