Some of the information on this Web page has been provided by external sources. The Government of Canada is not responsible for the accuracy, reliability or currency of the information supplied by external sources. Users wishing to rely upon this information should consult directly with the source of the information. Content provided by external sources is not subject to official languages, privacy and accessibility requirements.
Any discrepancies in the text and image of the Claims and Abstract are due to differing posting times. Text of the Claims and Abstract are posted:
| (12) Patent Application: | (11) CA 2323671 |
|---|---|
| (54) English Title: | CONTINUOUS NON-POLLUTING LIQUID PHASE TITANIUM DIOXIDE PROCESS |
| (54) French Title: | PROCEDE DE PRODUCTION DE DIOXYDE DE TITANE EN PHASE LIQUIDE, NON POLLUANT ET EN CONTINU |
| Status: | Deemed Abandoned and Beyond the Period of Reinstatement - Pending Response to Notice of Disregarded Communication |
| (51) International Patent Classification (IPC): |
|
|---|---|
| (72) Inventors : |
|
| (73) Owners : |
|
| (71) Applicants : |
|
| (74) Agent: | SMART & BIGGAR LP |
| (74) Associate agent: | |
| (45) Issued: | |
| (86) PCT Filing Date: | 1998-04-01 |
| (87) Open to Public Inspection: | 1998-12-03 |
| Examination requested: | 2000-09-13 |
| Availability of licence: | N/A |
| Dedicated to the Public: | N/A |
| (25) Language of filing: | English |
| Patent Cooperation Treaty (PCT): | Yes |
|---|---|
| (86) PCT Filing Number: | PCT/US1998/006499 |
| (87) International Publication Number: | WO 1998054095 |
| (85) National Entry: | 2000-09-13 |
| (30) Application Priority Data: | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
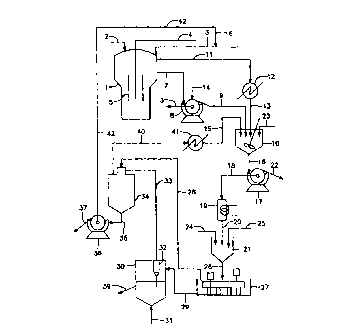
A process and apparatus for producing titanium dioxide from titanium ore or
slag is disclosed. The ore or slag (2) is reacted with sulfuric acid (3) in a
reactor (1) in which hot gas for agitation (4) is used. Makeup acid from line
(6) is fed to line (3). Together with recovered acid from the spray evaporator
(34) reacted product overflows from the reactor to rotary filter (8). The
filter cake is washed in the rotary filter whose water comes in through line
(14). The washed filter cake is delivered to dissolving tank (10).
Concentrated acid together with wash water is returned to the reactor through
line (3). Filtrate is hydrolized in hydrolysis tank (21) to form titanium
dioxide hydrate which is then calcined in a fluid bed calciner (30) to form
titanium dioxide.
L'invention a trait à un procédé, ainsi qu'à l'appareil correspondant, permettant de produire du dioxyde de titane à partir de minerai ou de crasses de titane. On fait réagir le minerai ou les crasses (2) avec de l'acide sulfurique (3) dans un réacteur (1) dans lequel on utilise un gaz chaud pour mise en suspension. De l'acide d'appoint provenant d'un conduit (6) est amené sur un conduit (3). Le produit réagi déborde, en compagnie de l'acide récupéré de l'évaporateur à pulvérisation (34), du réacteur vers un filtre à tambour (8). Le gâteau de filtration est lavé dans le filtre à tambour recevant de l'eau par un conduit (14). Le gâteau de filtration lavé est dirigé sur une cuve de dissolution (10). L'acide concentré et l'eau de lavage sont renvoyés au réacteur par un conduit (3). Le filtrat est hydrolysé dans un réservoir hydrolytique (21) pour former un hydrate de dioxyde de titane qui est ensuite calciné dans un four à calciner à lit fluidisé (30) afin de former du dioxyde de titane.
Note: Claims are shown in the official language in which they were submitted.
Note: Descriptions are shown in the official language in which they were submitted.
2024-08-01:As part of the Next Generation Patents (NGP) transition, the Canadian Patents Database (CPD) now contains a more detailed Event History, which replicates the Event Log of our new back-office solution.
Please note that "Inactive:" events refers to events no longer in use in our new back-office solution.
For a clearer understanding of the status of the application/patent presented on this page, the site Disclaimer , as well as the definitions for Patent , Event History , Maintenance Fee and Payment History should be consulted.
| Description | Date |
|---|---|
| Inactive: IPC from MCD | 2006-03-12 |
| Inactive: IPC from MCD | 2006-03-12 |
| Inactive: IPC from MCD | 2006-03-12 |
| Application Not Reinstated by Deadline | 2005-10-21 |
| Inactive: Dead - No reply to s.30(2) Rules requisition | 2005-10-21 |
| Deemed Abandoned - Failure to Respond to Maintenance Fee Notice | 2005-04-01 |
| Inactive: Abandoned - No reply to s.30(2) Rules requisition | 2004-10-21 |
| Inactive: IPRP received | 2004-06-16 |
| Inactive: S.30(2) Rules - Examiner requisition | 2004-04-21 |
| Letter Sent | 2004-04-20 |
| Reinstatement Requirements Deemed Compliant for All Abandonment Reasons | 2004-03-26 |
| Deemed Abandoned - Failure to Respond to Maintenance Fee Notice | 2003-04-01 |
| Inactive: Cover page published | 2000-12-11 |
| Inactive: First IPC assigned | 2000-12-06 |
| Letter Sent | 2000-12-01 |
| Inactive: Acknowledgment of national entry - RFE | 2000-11-30 |
| Application Received - PCT | 2000-11-27 |
| All Requirements for Examination Determined Compliant | 2000-09-13 |
| Request for Examination Requirements Determined Compliant | 2000-09-13 |
| Application Published (Open to Public Inspection) | 1998-12-03 |
| Abandonment Date | Reason | Reinstatement Date |
|---|---|---|
| 2005-04-01 | ||
| 2003-04-01 |
The last payment was received on 2004-03-26
Note : If the full payment has not been received on or before the date indicated, a further fee may be required which may be one of the following
Please refer to the CIPO Patent Fees web page to see all current fee amounts.
| Fee Type | Anniversary Year | Due Date | Paid Date |
|---|---|---|---|
| MF (application, 2nd anniv.) - small | 02 | 2000-04-03 | 2000-09-13 |
| Registration of a document | 2000-09-13 | ||
| Request for examination - small | 2000-09-13 | ||
| Reinstatement (national entry) | 2000-09-13 | ||
| Basic national fee - small | 2000-09-13 | ||
| MF (application, 3rd anniv.) - small | 03 | 2001-04-02 | 2001-04-02 |
| MF (application, 4th anniv.) - small | 04 | 2002-04-02 | 2002-04-02 |
| 2004-03-26 | |||
| Reinstatement | 2004-03-26 | ||
| MF (application, 5th anniv.) - small | 05 | 2003-04-01 | 2004-03-26 |
| MF (application, 6th anniv.) - small | 06 | 2004-04-01 | 2004-03-26 |
Note: Records showing the ownership history in alphabetical order.
| Current Owners on Record |
|---|
| KEMICRAFT OVERSEAS LIMITED |
| Past Owners on Record |
|---|
| BRIAN RICHARD DAVIS |
| ERIC JAMES MADSEN |
| JORGE MILLER |
| JOSEPH ALOYSIUS RAHM |