Note: Descriptions are shown in the official language in which they were submitted.
CA 02323866 2000-09-12
WO 99/47080 PCT/US99/05661
BONE XENOGRAFTS
FIELD OF THE INVENTION
The present invention relates to the field of bone transplantation, and in
particular, to replacement and repair of damaged or defective human bone using
a
substantially immunologically compatible bone from a non-human animal.
BACKGROUND OF THE INVENTION
Human bone, a hard connective tissue consisting of cells embedded in an
extracellular matrix of mineralized ground substance and collagen fibers,
(Stedman's
Medical Dictionary, Williams & Wilkins, Baltimore, MD (1995)), is the most
frequently transplanted tissue in humans. J.M. Lane et al., Current Approaches
to
Experimental Bone Grafting, 18 Orthopedic Clinics of North America (2) 213
(1987). In the United States alone more than 100,000 bone graft or implant
procedures are performed every year to repair or to replace osseous defects
resulting
from trauma, infection, congenital malformation, or malignancy. Id.
Bone grafts and implants are often formed of autologous bone. Id.
Transplantable autologous bone tissue for large defects, particularly in
children, is
often unavailable, however. Id. In addition, autologous bone transplantation
may
result in postoperative morbidity such as pain, hemorrhage, wound problems,
cosmetic disability, infection or nerve damage at the donor site. Id. Further,
difficulties in fabricating the desired functional shape from the transplanted
autologous bone tissue may result in less than optimal filling of the bone
defect Id.
Alternatively, much of the structure and many of the properties of original
bone tissue may be retained in transplants through use of heterograft or
xenograft
materials, that is, tissue from a different species than the graft recipient.
In the area
of soft tissues, for example, tendons or ligaments from cows or other animals
are
covered with a synthetic mesh and transplanted into a heterologous host in
U.S. Pat.
No. 4,400,833. Flat tissues such as pig pericardia are also disclosed as being
suitable
for heterologous transplantation in U.S. Pat. No. 4,400,833. Bovine peritoneum
fabricated into a biomaterial suitable for prosthetic heart valves, vascular
grafts, burn
and other wound dressings is disclosed in U.S. Pat. No. 4,755,593. Bovine,
ovine, or
-1-
CA 02323866 2000-09-12
WO 99/47080 PCT/US99/05661
porcine blood vessel xenografts are disclosed in WO 84/03036. None of these
disclosures describe the use of a xenograft for bone replacement, however.
Once implanted in an individual, a xenograft provokes immunogenic
reactions such as chronic and hyperacute rejection of the xenograft, however.
In
particular, bone xenografts may result in increased rates of fracture,
resorption and
nonunion secondary to immunologic rejection.
The term "chronic rejection", as used herein, refers to an immunological
reaction in an individual against a xenograft being implanted into the
individual.
Typically, chronic rejection is mediated by the interaction of IgG natural
antibodies
in the serum of the individual receiving the xenograft and carbohydrate
moieties
expressed on cells, and/or cellular and/or extracellular matrices of the
xenograft. For
example, transplantation of cartilage xenografts from nonprimate mammals
(e.g.,
porcine or bovine origin) into humans is primarily prevented by the
interaction
between the IgG natural anti-Gal antibody present in the serum of humans with
the
carbohydrate structure Galal-3Gal~i1-4GlcNAc-R (a-galactosyl or a-gal epitope)
expressed in the xenograft. K.R. Stone et al., Porcine and bovine cartilage
transplants in cynomolgus monkey: I. A model for chronic xenograft rejection,
63
Transplantation 640-645 ( 1997); U. Galili et aL, Porcine and bovine cartilage
transplants in cynomolgus monkey: ll. Changes in anti-Gal response during
chronic
rejection, 63 Transplantation 646-651 (1997). In chronic rejection, the immune
system typically responds within one to two weeks of implantation of the
xenograft.
In contrast with "chronic rejection", "hyper acute rejection" as used herein,
refers to the immunological reaction in an individual against a xenograft
being
implanted into the individual, where the rejection is typically mediated by
the
interaction of IgM natural antibodies in the serum of the individual receiving
the
xenograft and carbohydrate moieties expressed on cells. This interaction
activates
the complement system causing lysis of the vascular bed and stoppage of blood
flow
in the receiving individual within minutes to two to three hours.
The term "extracellular matrix or matrices", as used herein, refer to an
extracellular bone matrix of mineralized ground substance and collagen fibers.
Stedman's Medical Dictionary, Williams & Wilkins, Baltimore, MD (1995).
Xenograft materials may be chemically treated to reduce immunogenicity
-2-
CA 02323866 2000-09-12
WO 99/47080 PCTNS99/05661
prior to implantation into a recipient. For example, glutaraldehyde is used to
cross-
link or "tan" xenograft tissue in order to reduce its antigenicity, as
described in detail
in U.S. Pat. No. 4,755,593. Other agents such as aliphatic and aromatic
diamine
compounds may provide additional crosslinking through the side chain carboxyl
groups of aspartic and glutamic acid residues of the collagen polypeptide.
Glutaraldehyde and diamine tanning also increases the stability of the
xenograft
tissue.
Xenograft tissues may also be subjected to various physical treatments in
preparation for implantation. For example, U.S. Pat. No. 4,755,593 discloses
subjecting xenograft tissue to mechanical strain by stretching to produce a
thinner
and stiffer biomaterial for grafting. Tissue for allograft transplantation is
commonly
cryopreserved to optimize cell viability during storage, as disclosed, for
example, in
U.S. Pat. No. 5,071,741; U.S. Pat. No. 5,131,850; U.S. Pat. No. 5,160,313; and
U.S.
Pat. No. 5,171,660. U.S. Pat. No. 5,071,741 discloses that freezing tissues
causes
mechanical injuries to cells therein because of extracellular or intracellular
ice crystal
formation and osmotic dehydration.
SUMMARY OF THE INVENTION
The present invention provides a substantially non-immunogenic bone
xenograft for implantation into a human in need of bone repair or replacement.
The
invention further provides methods for processing xenogeneic bone with reduced
immunogenicity but with substantially native elasticity and load-bearing
capabilities
for xenografting into humans.
As used herein, the term "xenograft" is synonymous with the term
"heterograft" and refers to a graft transferred from an animal of one species
to one of
another species. Stedman's Medical Dictionary, Williams & Wilkins, Baltimore,
MD ( 1995).
As used herein, the term "xenogeneic", as in xenogeneic graft, bone, etc.,
refers to a graft, bone, etc., transferred from an animal of one species to
one of
another species. Id.
The methods of the invention, include, alone or in combination, treatment
with radiation, one or more cycles of freezing and thawing, treatment with a
chemical
-3-
CA 02323866 2000-09-12
WO 99/47080 PGT/US99/OS661
cross-linking agent, treatment with alcohol or ozonation. In addition to or in
lieu of
these methods, the methods of the invention include a cellular disruption
treatment
and optionally glycosidase digestion of carbohydrate moieties of the xenograft
optionally followed by treatment of carbohydrate moieties of the xenograft
with
capping molecules. Still another method of the invention includes cellular
disruption
and sterilization treatments. After one or more of the above-described
processing
steps, the methods of the invention provide a xenograft having substantially
the same
mechanical properties as a native bone.
As used herein, the term "cellular disruption" as in, for example, cellular
disruption treatment, refers to a treatment for killing cells.
As used herein, the term "capping molecules)", refers to molecules) which
link with carbohydrate chains such that the xenograft is no longer recognized
as
foreign by the subject's immune system.
In one embodiment, the invention provides an article of manufacture
comprising a substantially non-immunogenic bone xenograft for implantation
into a
human.
In another embodiment, the invention provides a method of preparing a bone
xenograft for implantation into a human, which includes removing at least a
portion
of a bone from a non-human animal to provide a xenograft; washing the
xenograft in
water and alcohol; and subjecting the xenograft to at least one treatment
selected
from the group consisting of exposure to ultraviolet radiation, immersion in
alcohol,
ozonation, and freeze/thaw cycling, whereby the xenograft has substantially
the same
mechanical properties as a corresponding portion of a native bone.
As used herein, the term "portion", as in, for example, a portion of bone or
second surface carbohydrate moieties, refers to all or less than all of the
respective
bone or second surface carbohydrate moieties.
In another embodiment, the invention provides a method of preparing a bone
xenograft for implantation into a human, which includes removing at least a
portion
of a bone from a non-human animal to provide a xenograft; washing the
xenograft in
water and alcohol; subjecting the xenograft to a cellular disruption
treatment; and
digesting the xenograft with a glycosidase to remove first surface
carbohydrate
moieties, whereby the xenograft has substantially the same properties as a
-4-
CA 02323866 2000-09-12
WO 99/47080 PCT/US99/05661
corresponding portion of a native bone.
In still another embodiment, this method can include the additional step of
treating second surface carbohydrate moieties on the xenograft with capping
molecules to cap at least a portion of the second surface carbohydrate
moieties.
In yet another embodiment, the invention provides a method of preparing a
bone xenograft for implantation into a human, which includes removing at least
a
portion of a bone from a non-human animal to provide a xenograft; washing the
xenograft in water and alcohol; and subjecting the xenograft to sterilization
and
cellular disruption treatments, whereby the xenograft has substantially the
same
properties as a corresponding portion of a native bone and is substantially
non-
immunogenic.
As used herein, the terms "to cap" or "capping", refer to linking a capping
molecule such as a carbohydrate unit to the end of a carbohydrate chain, as
in, for
example, covalently linking a carbohydrate unit to surface carbohydrate
moieties on
the xenograft.
In further embodiments, the invention provides articles of manufacture
including substantially non-immunogenic bone xenografts for implantation into
humans produced by the above-identified methods of the invention.
In still a further embodiment, the invention provides a bone xenograft for
implantation into a human which includes a portion of a bone from a non-human
animal, wherein the portion has substantially no surface carbohydrate moieties
which
are susceptible to glycosidase digestion, and whereby the portion has
substantially
the same mechanical properties as a corresponding portion of a native bone.
In yet a further embodiment, the invention provides a bone xenograft for
implantation into a human which includes a sterilized, cellular disrupted
portion of a
bone from a non-human animal, whereby the portion has substantially the same
mechanical properties as a corresponding portion of a native bone and is
substantially
non-immunogenic.
BRIEF DESCRIPTION OF THE DRAWINGS
The various features of the invention may be more fully understood from the
-5-
CA 02323866 2000-09-12
WO 99/47080 PCTlUS99/05661
following description when read together with the accompanying drawings.
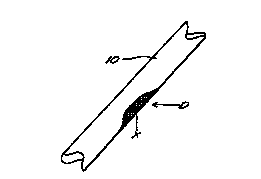
FIG. 1 shows a portion of a bone having a defect; and
FIG. 2 shows the bone portion of FIG. 1 with a xenograft of the invention in
the defect.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention is directed against the chronic rejection of xenografts
for implantation into humans. Accordingly, the bone xenograft produced in
accordance with the method of the invention is substantially non-immunogenic,
while generally maintaining the mechanical properties of a native bone.
The bone xenograft may be cut into segments, each of which may be
implanted into the recipient as set forth below.
The invention provides, in one embodiment, a method for preparing or
processing a xenogeneic bone for engraftment into humans. The bone may be
harvested from any non-human animal to prepare the xenografts of the
invention.
Bone from transgenic non-human animals or from genetically altered non-human
animals may also be used as xenografts in accordance with the present
invention.
Preferably, bovine, ovine, or porcine bones serve as sources of the bone used
to
prepare the xenografts. More preferably, immature pig, calf or lamb bones are
the
sources of the bone, since the bone of younger animals consists of more
cancellous
bone and may be less brittle than that of older animals. Most preferably, the
age of
the source animal is between six and eighteen months at time of slaughter.
In the first step of the method of the invention, an intact bone portion is
removed from a bone of a non-human animal. The source of the bone should be
collected from freshly killed animals and preferably immediately placed in a
suitable
sterile isotonic or other tissue preserving solution. Harvesting of the bone
portions
should occur as soon as possible after slaughter of the animal and preferably
should
be performed in the cold, i. e. , in the approximate range of about 5 °
C to about 20 ° C,
to minimize enzymatic degradation of the bone tissue.
The bone portions are harvested in the cold, under strict sterile technique
following known surgical procedures. The harvested bone portion is cut up into
strips or blocks and provided with and without cancellous bone attached to
cortical
-6-
CA 02323866 2000-09-12
WO 99/47080 PCTNS99/05661
bone.
The resultant xenograft is washed in about ten volumes of sterile cold water
to remove residual blood proteins and water soluble materials. The xenograft
is then
immersed in alcohol at room temperature for about five minutes, to sterilize
the bone
and to remove non-collagenous materials.
After alcohol immersion, the xenograft may be subjected to at least one of the
following treatments: radiation treatment, treatment with alcohol, ozonation,
one or
more cycles of freezing and thawing, and/or treatment with a chemical cross-
linking
agent. When more than one of these treatments is applied to the xenograft, the
treatments may occur in any order.
In one embodiment of the method of the invention, the xenograft may be
treated by exposure to ultraviolet radiation for about fifteen minutes or
gamma
radiation in an amount of about .5 to 3 MegaRad.
In another embodiment, the xenograft may be treated by again being placed in
an alcohol solution. Any alcohol solution may be used to perform this
treatment.
Preferably, the xenograft is placed in a 70% solution of isopropanol at room
temperature.
In still another embodiment, the xenograft may be subjected to ozonation.
In a further embodiment of the method of the invention, the xenograft may be
treated by freeze/thaw cycling. For example, the xenograft may be frozen using
any
method of freezing, so long as the xenograft is completely frozen, i.e., no
interior
warm spots remain which contain unfrozen tissue. Preferably, the xenograft is
dipped into liquid nitrogen for about five minutes to perform this step of the
method.
More preferably, the xenograft is frozen slowly by placing it in a freezer. In
the next
step of the freeze/thaw cycling treatment, the xenograft is thawed by
immersion in an
isotonic saline bath at room temperature (about 25 °C) for about ten
minutes.
In yet a further embodiment, the xenograft may optionally be exposed to a
chemical agent to tan or crosslink the proteins within the extracellular
matrix, to
further diminish or reduce the immunogenic determinants present in the
xenograft.
Any tanning or crosslinking agent may be used for this treatment, and more
than one
crosslinking step may be performed or more than one crosslinking agent may be
used
in order to ensure complete crosslinking and thus optimally to reduce the
CA 02323866 2000-09-12
WO 99/47080 PCT/US99/05661
immunogenicity of the xenograft. For example, aldehydes such as
glutaraldehyde,
formaldehyde, adipic dialdehyde, and the like, may be used to crosslink the
collagen
within the extracellular matrix of the xenograft in accordance with the method
of the
invention. Other suitable crosslinking agents include aliphatic and aromatic
diamines, carbodiimides, diisocyanates, and the like.
When glutaraldehyde is used as the crosslinking agent, for example, the
xenograft may be placed in a buffered solution containing about 0.05 to about
5.0%
glutaraldehyde and having a pH of about 7.4. Any suitable buffer may be used,
such
as phosphate buffered saline or trishydroxymethylaminomethane, and the like,
so
long as it is possible to maintain control over the pH of the solution for the
duration
of the crosslinking reaction, which may be from one to fourteen days, and
preferably
from three to five days.
Alternatively, the xenograft can be exposed to a crosslinking agent in a vapor
form, including, but not limited to, a vaporized aldehyde crosslinking agent,
such as,
for example, vaporized formaldehyde. The vaporized crosslinking agent can have
a
concentration and a pH and the xenograft can be exposed to the vaporized
crosslinking agent for a period of time suitable to permit the crosslinking
reaction to
occur. For example, the xenograft can be exposed to vaporized crosslinking
agent
having a concentration of about .OS to about 5.0% and a pH of about 7.4, for a
period
of time which can be from one to fourteen days, and preferably from three to
five
days. Exposure to vaporized crosslinking agent can result in reduced residual
chemicals in the xenograft from the crosslinking agent exposure.
The crosslinking reaction should continue until the immunogenic
determinants are substantially removed from the xenogeneic tissue, but the
reaction
should be terminated prior to significant alterations of the mechanical
properties of
the xenograft. When diamines are also used as crosslinking agents, the
glutaraldehyde crosslinking should occur after the diamine crosslinking, so
that any
unreacted diamines are capped. After the crosslinking reactions have proceeded
to
completion as described above, the xenograft should be rinsed to remove
residual
chemicals, and 0.01-0.05 M glycine may be added to cap any unreacted aldehyde
groups which remain.
In addition to or in lieu of the above treatments, the xenograft can be
_g_
CA 02323866 2000-09-12
WO 99/47080 PCT/US99/05661
subjected to a cellular disruption treatment to kill the xenograft's cells.
Optionally,
the cellular disruption treatment can precede or follow digestion of the
xenograft
with glycosidases to remove surface carbohydrate moieties from the xenograft.
According to a further option, the glycosidase digestion in turn can be
followed by
linkage with capping molecules to cap surface N-acetyllactosamine ends of
carbohydrate chains of the xenograft.
In an embodiment of this method of the invention, the xenograft is subjected
to a cellular disruption treatment to kill the cells of the bone prior to in
vitro
digestion of the xenograft with glycosidases. Typically after surface
carbohydrate
moieties have been removed from nucleated cells and the extracellular matrix,
nucleated, i.e., living cells re-express the surface carbohydrate moieties. Re-
expression of antigenic moieties of a xenograft can provoke continued
immunogenic
rejection of the xenograft. In contrast, non-nucleated, i.e., dead cells, are
unable to
re-express surface carbohydrate moieties. Removal of antigenic surface
carbohydrate
moieties from the non-nucleated cells and extracellular matrix of a xenograft
substantially permanently eliminates antigenic surface carbohydrate moieties
as a
source of immunogenic rejection of the xenograft.
Accordingly, in the above-identified embodiment, the xenograft of the present
invention is subjected to freeze/thaw cycling as discussed above to disrupt,
i.e., to
kill the cells of the bone. Alternatively, the xenograft of the present
invention is
treated with gamma radiation having an amount of .2 MegaRad up to about 3
MegaRad. Such radiation kills the bone cells and sterilizes the xenograft.
Once
killed, the bone cells are no longer able to re-express antigenic surface
carbohydrate
moieties such a-gal epitopes which are factors in the immunogenic rejection of
the
transplanted xenografts.
In another embodiment of the invention, either before or after the bone cells
are killed, the xenograft is subjected to a sterilization treatment. The
sterilization
treatment includes further immersion in alcohol and or other types of
detergents
which sterilize the xenograft and optionally remove at least some of the fatty
components and/or antigens of the xenograft.
In another embodiment of the invention, either before or after the bone cells
are killed, the xenograft is subjected to in vitro digestion of the xenograft
with
-9-
CA 02323866 2000-09-12
WO 99/47080 PCT/US99/05661
glycosidases, and specifically galactosidases, such as a-galactosidase, to
enzymatically eliminate antigenic surface carbohydrate moieties. In
particular, a-gal
epitopes are eliminated by enzymatic treatment with a-galactosidases, as shown
in
the following reaction:
a-galactosidase
Gal al-3Gal~i1-4GlcNAc-R ------------------> Gal~il-4GlcNAc-R+Gal
a-gal epitope N acetyllactosamine
The N acetyllactosamine residues are epitopes that are normally expressed on
human
and mammalian cells and thus are not immunogenic. The in vitro digestion of
the
xenograft with glycosidases is accomplished by various methods. For example,
the
xenograft can be soaked or incubated in a buffer solution containing
glycosidase. In
addition, the xenograft can be pierced to increase permeability, as further
described
below. Alternatively, a buffer solution containing the glycosidase can be
forced
under pressure into the xenograft via a pulsatile lavage process.
Elimination of the a-gal epitopes from the xenograft diminishes the immune
response against the xenograft. The a-gal epitope is expressed in nonprimate
mammals and in New World monkeys (monkeys of South America) as 1x106-35x106
epitopes per cell, as well as on macromolecules such as proteoglycans of the
extracellular matrix. U. Galili et al., Man, apes, and Old World monkeys
differ from
other mammals in the expression of a galactosyl epitopes on nucleated cells,
263 J.
Biol. Chem. 17755 (1988). This epitope is absent in Old World primates
(monkeys
of Asia and Africa and apes) and humans, however. Id. Anti-Gal is produced in
humans and primates as a result of an immune response to a-gal epitope
carbohydrate structures on gastrointestinal bacteria. U. Galili et al.,
Interaction
between human natural anti-a galactosyl immunoglobulin G and bacteria of the
human flora, 56 Infect. Immun. 1730 (1988); R.M. Hamadeh et al., Human natural
anti-Gal IgG regulates alternative complement pathway activation on bacterial
surfaces, 89 J. Clin. Invest. 1223 ( 1992). Since nonprimate mammals produce a-
gal
epitopes, xenotransplantation of xenografts from these mammals into primates
results in rejection because of primate anti-Gal binding to these epitopes on
the
-10-
CA 02323866 2000-09-12
WO 99/47080 PCTNS99/05661
xenograft. The binding results in the destruction of the xenograft by
complement
fixation and by antibody dependent cell cytotoxicity. U. GaliIi et aL,
Interaction of
the natural anti-Gal antibody with a galactosyl epitopes: A major obstacle for
xenotransplantation in humans, 14 Immunology Today 480 (1993); M. Sandrin et
al., Anti pig IgM antibodies in human serum react predominantly with Gal al -
3Gal
epitopes, 90 Proc. Natl. Acad. Sci. USA 11391 (1993); H. Good et aL,
Identification
of carbohydrate structures which bind human anti porcine antibodies:
implications
for discordant grafting in man. 24 Transplant. Proc. 559 (1992); B.H. Collins
et al.,
Cardiac xenografts between primate species provide evidence for the importance
of
the a galactosyl determinant in hyperacute rejection, 154 J. Immunol. 5500 (
1995).
Furthermore, xenotransplantation results in major activation of the immune
system to
produce increased amounts of high affinity anti-Gal. Accordingly, the
substantial
elimination of a-gal epitopes from bone cells and the extracellular matrix,
and the
prevention of re-expression of cellular a-gal epitopes can diminish the immune
response against the xenograft associated with anti-Gal antibody binding with
a-gal
epitopes.
Following treatment with glycosidase, the remaining carbohydrate chains
(e.g., glycosaminoglycans) of the xenograft are optionally treated with
capping
molecules to cap at least a portion of the remaining carbohydrate chains.
Treatment
with capping molecules is applicable to both glycosidase-treated and non-
glycosidase-treated xenografts, however. For example, xenografts from knock
out
animals which may lack a-gal epitopes may be treated with capping molecules to
cap
carbohydrate moieties on the xenograft, thereby reducing the xenograft's
immunogenicity. Examples of capping molecules used in the present invention
include fucosyl and n-acetyl glucosamine.
Prior to treatment, the outer lateral surface of the xenograft may optionally
be
pierced to increase permeability to agents used to render the xenograft
substantially
non-immunogenic. A sterile surgical needle such as an 18 gauge needle may be
used
to perform this piercing step, or, alternatively a comb-like apparatus
containing a
plurality of needles may be used. The piercing may be performed with various
patterns, and with various pierce-to-pierce spacings, in order to establish a
desired
access to the interior of the xenograft. Piercing may also be performed with a
laser.
-11-
CA 02323866 2000-09-12
WO 99/47080 PCT/US99/05661
In one form of the invention, one or more straight lines of punctures about
three
millimeters apart are established circumferentially in the outer lateral
surface of the
xenograft.
Prior to implantation, the bone xenograft of the invention may be treated with
limited digestion by prateolytic enzymes such as ficin or trypsin to increase
tissue
flexibility, or coated with anticalcification agents, antithrombotic coatings,
antibiotics, growth factors, or other drugs which may enhance the
incorporation of
the xenograft into the recipient joint. The bone xenograft of the invention
may be
further sterilized using known methods, for example, with additional
glutaraldehyde
or formaldehyde treatment, ethylene oxide sterilization, propylene oxide
sterilization,
or the like. The xenograft may be stored frozen until required for use.
Further, the bone xenograft of the invention can be treated with an
osteoinductive factor in an effective amount to stimulate the conversion of
soft tissue
cells to osseous tissue formers. Alternatively or additionally, the
osteoinductive
factor can be administered directly to the target defect. As used herein, the
term
"osteoinductive factor" refers to a protein which stimulates the
differentiation of
uncommitted connective tissue cells into bone-forming cells. J.M. Lane et al.,
Current Approaches to Experimental Bone Grafting, 18 Orthopedic Clinics
ofNorth
America (2) 214 ( 1987).
Methods of preparing and administering the osteoinductive factor to a graft or
to the target defect are known in the prior art such as, for example, Sharon
Stevenson, D.V.M., Ph.D., et al., The Effect of Osteogenic (A Bone
Morphogenetic
Protein) on the Formation of Bone in Orthotopic Segmental Defects in Rats, 76,
A.
The Journal of Bone And Joint Surgery No. I 1, 1676-1687 (1994) and John E.
Feighan, et al., Induction of Bone by a Demineralized Bone Matrix Gel: A Study
in a
Rat Femoral Defect Model, 13 Journal of Orthopaedic Research 881-889 (1995).
For
example, osteoinductive factor in the form of a gel, in the presence of a
synthetic
carrier, such as a hydroxyapatite ceramic cylinder, or using a carrier, such
as
polyethylene glycol (PEG) or glycerol, or a buffer, such as phosphate, and/or
any
combination of the above can be administered to the defect site and/or used to
impregnate the xenograft.
The osteoinductive factor is added to the interstices of the xenograft and/or
to
-12-
CA 02323866 2000-09-12
WO 99/47080 PCTNS99/05661
the defect site in an amount effective to induce bone formation. For example,
doses
of about 10 mg to about 200 mg of osteoinductive factor can be added. Examples
of
osteoinductive factors which can be used in the present invention include bone
morphogenic proteins (BMP) and associated noncollagenous proteins (NCP). Such
osteoinductive factors are commercially available from, for example, Creative
Biomolecules, Inc., Hopkington, Massachusetts.
The bone xenograft of the invention also can be treated with a
demineralization agent in an effective amount to remove substantially minerals
such
as, for example, calcium from the extracellular matrix of the xenograft. For
example,
the xenograft of the invention can be soaked in a solution containing
demineralization agents, such as, hydrochloric acid, and other
demineralization
agents known to those of ordinary skill in the art, at a predetermined
concentration to
demineralize substantially the xenograft of the invention. Once the minerals
are
removed from the xenograft, a porous volume matrix is formed with pores
ranging in
size from about 50 microns to about 500 microns. It is theorized that the
collagen of
demineralized extracellular bone matrix serves as an osteoconductive
scaffolding and
facilitates the migration of bone forming components once bone graft is
implanted.
J.M. Lane et al., Current Approaches to Experimental Bone Grafting, 18
Orthopedic
Clinics ofNorth America (2) 220 (1987). It is further theorized that
demineralized
bone possesses greater osteoinductive activity than, for example, autologous
bone,
because bone mineral impedes the release of osteoinductive proteins from
extracellular bone matrix. Id. at 218. According to this theory,
demineralization
enlarges the access of surrounding responsive cells to osteoinductive proteins
and
augments the potential of the osteoinductive proteins. Such demineralization
agents
are commercially available from, for example, Sigma, Inc., St. Louis,
Missouri.
In addition, a binding agent can be added into the bone xenograft of the
present invention. As used herein, a binding agent is an adhesion molecule, or
adhesive portion or analog thereof, which aids in bone formation by providing
a
tacky surface to which bone forming cells can stick. The binding agent is
added in
an effective amount to facilitate the attachment of mesenchymal and other
differentiated bone forming cells to the extracellular matrix of the bone
xenograft.
-13-
CA 02323866 2000-09-12
WO 99/47080 PCT/US99/OS661
Examples of binding agents useful in the present invention include bone
cement;
fibrin glue; mussel glue, such as mussel glue containing bioadhesive
polyphenolic
proteins derived from several species of the mussel genus Mytilus (see e.g.,
U.S.
Patent No. 4,585,585); chondronectin; osteonectin; and fibronectin and
arginine-
glycine-aspartic acid (RGD) peptide (see e.g., U.S. Patent No. 5,681,353), a
portion
of which can be conjugated to, for example, chondroitin sulfate, and other
binding
agents known to those persons of ordinary skill in the art. Such binding
agents are
commercially available from, for example, Telios Pharmaceuticals, Inc., San
Diego,
California.
The bone xenograft of the invention, or a segment thereof, may be implanted
into damaged human joints by those of skill in the art using known
arthroscopic
surgical techniques. Holes in bones are manually packed with bone according to
standard surgical techniques. Specific instruments for performing surgical
techniques, which ensure accurate and reproducible placement of bone implants
are
known to those of skill in the art.
This invention is further illustrated by the following Examples which should
not be construed as limiting. The contents of all references and published
patents
and patent applications cited throughout the application are hereby
incorporated by
reference.
EXAMPLE 1: Assessment Of Response In Mice To Implanted Bone Treated With a-
Galactosidase And Demineralized Bone Matrix Gel Containing Osteoinductive
Factor
In this example, porcine bone implants are treated with a-galactosidase to
eliminate a-galactosyl epitopes and impregnated with demineralized bone matrix
gel
containing osteoinductive factor to stimulate the conversion of soft tissue
formers in
the target defect to osseous tissue formers. The implants are transplanted
into mice
and the response to the implants is assessed. An exemplary bone portion 10
with a
defect D is shown in FIG. 1.
Porcine bone implants are sterilely prepared and surrounding attached soft
tissues surgically removed. The bone specimens are washed for at least five
minutes
-14-
CA 02323866 2000-09-12
WO 99/47080 PCT/US99/05661
with an alcohol, such as ethanol or isopropanol, to remove synovial fluid and
lipid
soluble contaminants.
The bone specimens are frozen at a temperature ranging from about -35
°C to
about - 90°C, and preferably at a temperature up to about -70°C,
to disrupt, that, is to
S kill, the specimens' bone cells.
Each bone specimen is cut into two portions. The first bone portion is
immersed in a buffer solution containing a-galactosidase at a predetermined
concentration. The first bone portion is allowed to incubate in the buffer
solution for
a predetermined time period at a predetermined temperature. The second bone
portion is incubated under similar conditions as the first bone portion in a
buffer
solution in the absence of a-galactosidase and serves as the control.
At the end of the incubation, the bone portions are washed under conditions
which allow the enzyme to diffuse out. Assays are performed to confirm the
complete removal of the a-gal epitopes.
The a-galactosidase first bone portions disclosed above are then impregnated
with demineralized bone matrix gel containing osteoinductive factor prepared
according to methods known in the prior art, such as, for example, John E.
Feighan,
et al., Induction ofBone by a Demineralized Bone Matrix Gel: A Study in a Rat
Femoral Defect Model, 13 Journal of Orthopaedic Research 881-889 (1995).
The bone samples are implanted in subcutaneous tissues of mice under
general inhalation anesthesia following known surgical procedures. Bone is
implanted in subcutaneous tissues to evaluate the osteoinductive properties of
bone.
Any bone formed is evidence of osteoinductive properties. Osteoconductive
properties of bone xenograft are evaluated after the xenograft is implanted
using
bone defective models such as the long bone drill hole model. The implantation
procedure is performed under sterile surgical technique, and the wounds are
closed
with 3-0 vicryl or a suitable equivalent known to those of ordinary skill in
the art.
FIG. 2 shows the bone portion 10 with the xenograft X (shown crosshatched) in
place at the defect D. The animals are permitted unrestricted cage activity
and
monitored for any sign of discomfort, swelling, infection, or rejection. Blood
samples (e.g., 2 ml) are drawn periodically (e.g., every two weeks) for
monitoring of
antibodies.
-15-
CA 02323866 2000-09-12
WO 99/47080 PCTNS99/05661
The occurrence of an immune response against the xenograft is assessed by
determining anti-Gal and non-anti-Gal anti-bone xenograft antibodies (i.e.,
antibodies binding to antigens other than the a-gal epitopes) in serum samples
from
the transplanted mice. Blood samples are drawn from the transplanted mice on
the
day of implant surgery and at periodic (e.g., two week) intervals post-
transplantation.
The blood samples are centrifuged and the serum samples are frozen and
evaluated
for the anti-Gal and other non-anti-Gal anti-bone xenograft antibody activity.
Anti-Gal activity is determined in the serum samples in ELISA with a-gal-
BSA as solid phase antigen, according to methods known in the prior art, such
as, for
example, the methods described in Galili et al., Porcine and bovine cartilage
transplants in cynomolgus monkey: Il. Changes in anti-Gal response during
chronic
rejection, 63 Transplantation 645-651 (1997).
Assays are conducted to determine whether a-galactosidase treated
xenografts induce the formation of anti-bone xenograft antibodies. For
measuring
anti-bone xenograft antibody activity, an ELISA assay is performed according
to
methods known in the prior art, such as, for example, the methods described in
K.R.
Stone et al., Porcine and bovine cartilage transplants in cynomolgus monkey.'
1. A
model for chronic xenograft rejection, 63 Transplantation 640-645 ( 1997).
The bone xenografts are optionally explanted at one to two months post-
transplantation, sectioned and stained for histological evaluation of
inflammatory
infiltrates. Post-transplantation changes in anti-GaI and other anti-bone
xenograft
antibody activities are correlated with the inflammatory histologic
characteristics
(i.e., granulocytes or mononuclear cell infiltrates) within the explanted
bone, one to
two months post-transplantation, using methods known in the art, as, for
example,
the methods described in K.R. Stone et al., Porcine and bovine cartilage
transplants
in cynomolgus monkey: 1. A model for chronic xenograft rejection, 63
Transplantation 640-645 ( 1997).
Where the bone xenograft is explanted, the bone xenograft is aseptically
harvested, using anesthetic procedure, removal of the implant and closure of
the soft
tissue. Tissue is harvested for possible immunologic testing if the gross and
histopathologic evaluation of the transplants indicate good performance of the
transplanted bone. The xenograft samples are collected, processed, and
examined
-16-
CA 02323866 2000-09-12
WO 99/47080 PCT/US99/05661
microscopically. A portion of the implant and surrounding tissue is frozen in
an
embedding medium for frozen tissue specimens in embedding molds for
immunohistochemistry evaluation according to the methods known in the prior
art.
"TISSUE-TEK~" O.C.T. compound which includes 10.24% w/w polyvinyl alcohol,
4.26% w/w polyethylene glycol, and 86.60% w/w nonreactive ingredients, and is
manufactured by Sakura FinTek, Torrence, California, is a non-limiting example
of a
possible embedding medium for use with the present invention. Other embedding
mediums known to those of ordinary skill in the art may also be used. The
remaining
implant and surrounding tissue is collected in 10% neutral buffered formalin
for
histopathologic examination.
EXAMPLE 2: Assessment Of Primate Response To Implanted Bone Treated With
a-Galactosidase and Demineralized Bone Matrix Gel Containing Osteoinductive
Factor
In this example, porcine bone implants are treated with a-galactosidase and
demineralized bone matrix gel containing osteoinductive factor, the implants
are
transplanted into cynomolgus monkeys, and the primate response to the bone
implants is assessed, as described in Example 1. After the bone xenografts are
explanted and tissue is harvested for possible immunologic testing, the
animals are
allowed to recover and are monitored closely until the incisions have healed
and the
gait of the animals is normal.
EXAMPLE 3: Assessment Of Response In Mice To Implanted Bone Treated With a-
Galactosidase, Demineralized Bone Matrix Gel Containing Osteoinductive Factor,
Fucosyl and Fucosyltransferase
In this example, porcine bone implants are treated with a-galactosidase to
eliminate a-gal epitopes, as described in Example 1. The implants are further
treated
with fucosyl and fucosyl transferase to cap carbohydrate chains with fucosyl.
Fucosyltransferase facilitates the transfer of fucosyl to the xenograft. The
fucosyl
links to and thus caps the carbohydrate chains. Capping with fucosyl
interferes with
-17-
CA 02323866 2000-09-12
WO 99/47080 PCT/US99/05661
the ability of the subject's immune system to recognize the xenograft as
foreign. The
implants are transplanted into mice, and the response to the bone implants is
assessed.
Porcine bone implants are prepared as described in Example 1 including the
a-galactosidase treatment. Prior to implantation into the mice, however, the
implants
are further treated with a predetermined amount of fucosyl and
fucosyltransferase, at
specified concentrations for a predetermined time and at a predetermined
temperature, to cap carbohydrate chains with fucosyl. For example, the samples
are
immersed in a buffer solution at predetermined concentrations of fucosyl and
fucosyl
transferase. The samples are incubated for a predetermined time period at a
predetermined temperature.
Other molecules, such as n-acetyl glucosamine in combination with the
corresponding glycosyltransferase, can also be used for capping the
carbohydrate
chains of the implants.
Subsequently, the samples are washed to remove the enzyme and implanted
into the mice, and the occurrence of an immune response against the xenograft
is
assessed as described above in Example 1.
EXAMPLE 4: Assessment Of Primate Response To Implanted Bone Treated With a-
Galactosidase, Demineralized Bone Matrix Gel Containing Osteoinductive Factor;
Fucosyl and Fucosyltransferase
In this example, porcine bone implants are treated with a-galactosidase,
demineralized bone matrix gel containing osteoinductive factor, fucosyl and
fucosyltransferase; the implants are transplanted into cynomolgus monkeys and
the
primate response to the bone implants is assessed, as described above in
Examples 1,
2 and 3.
EXAMPLE 5: Assessment Of Response In Mice To Implanted Bone Treated With
Alcohol and Freeze/'Thaw Cycling
In this example, porcine bone implants are treated with alcohol and
-18-
CA 02323866 2000-09-12
WO 99/47080 PCT/US99/05661
freeze/thaw cycling prior to their transplantation in mice and the response of
the mice
to the implants is assessed.
Porcine bone implants are sterilely prepared and surrounding attached soft
tissues surgically removed. The resultant xenograft is washed in about ten
volumes
of sterile cold water to remove residual blood,proteins and water soluble
materials.
The xenograft is then immersed in alcohol at room temperature for about five
minutes, to sterilize the bone and to remove non-collagenous materials.
After the alcohol washing, the xenograft is treated by again placing the
xenograft in an alcohol solution of 70% isopropanol at room temperature for at
least
five minutes.
Following the alcohol treatment step, the bone implants are placed in a
freezer until the xenograft is completely frozen, i.e., no interior warm spots
remain
which contain unfrozen tissue. Each bone implant is then thawed by immersion
in an
isotonic saline bath at room temperature (about 25 °C) for about ten
minutes.
It should be understood that the bone implants alternatively can be subjected
to the above-described freeze/thaw cycling treatment prior to the alcohol
treatment
step.
The implants are then implanted in the mice and the occurrence of an immune
responses against the xenograft is assessed as described above in Example 1.
EXAMPLE 6: Assessment Of Primate Response To Implanted Bone Treated With
Alcohol and Freeze/Thaw Cycling
In this example, porcine bone implants are treated with alcohol and
freeze/thaw cycling prior to their transplantation in cynomolgus monkeys and
the
primate response to the implants is assessed as described above in Examples 1-
S.
Those of skill in the art will recognize that the invention may be embodied in
other specific forms without departing from the spirit or essential
characteristics
thereof. The presently described embodiments are therefore to be considered in
all
respects as illustrative and not restrictive, the scope of the invention being
indicated
by the appended claims rather than by the foregoing description, and all
variations of
-19-
CA 02323866 2000-09-12
WO 99/47080 PCTlUS99/OS661
the invention which are encompassed within the meaning and range of
equivalency
of the claims are therefor intended to be embraced therein.
-20-