Note: Descriptions are shown in the official language in which they were submitted.
CA 02324702 2000-10-27
REFORMATS PURIFICATION AND HEAT RECOVERY FOR FUEL CELL
FIELD OF THE INVENTION
The present invention relates to a fuel cell-based electrical generation
system which employs pressure swing adsorption for enhancing the efficiency
and
durability of the fuel cell.
BACKGROUND OF THE INVENTION
Fuel cells provide an environmentally friendly source of electrical current.
One form of fuel cell used for generating electrical power, particularly for
vehicle
propulsion and for smaller scale stationary power generation, includes an
anode
channel for receiving a flow of hydrogen gas, a cathode channel for receiving
a
flow of oxygen gas, and a polymer electrolyte membrane (PEM) which separates
the anode channel from the cathode channel. Oxygen gas which enters the
cathode
reacts with hydrogen ions which cross the electrolyte to generate a flow of
electrons. Environmentally safe water vapour is also produced as a byproduct.
External production, purification, dispensing and storage of hydrogen
(either as compressed gas or cryogenic liquid) requires costly infrastructure,
while
storage of hydrogen fuel on vehicles presents considerable technical and
economic
barriers. Accordingly, for stationary power generation, it is preferred to
generate
hydrogen from natural gas by steam reforming or partial oxidation followed by
water gas shift. For fuel cell vehicles using a liquid fuel, it is preferred
to
generate hydrogen from methanol by steam reforming or from gasoline by partial
oxidation or autothermal reforming, again followed by water gas shift.
However,
the resulting hydrogen contains carbon monoxide and carbon dioxide impurities
which cannot be tolerated respectively by the PEM fuel cell catalytic
electrodes in
more than trace levels.
CA 02324702 2000-10-27
-2-
The conventional method of removing residual carbon monoxide from the
hydrogen feed to PEM fuel cells has been catalytic selective oxidation, which
compromises efficiency as both the carbon monoxide and a fraction of the
hydrogen are consumed by low temperature oxidation, without any recovery of
the
heat of combustion. Palladium diffusion membranes can be used for hydrogen
purification, but have the disadvantages of delivery of the purified hydrogen
at low
pressure, and also the use of rare and costly materials.
Pressure swing adsorption systems (PSA) have the attractive features of
being able to provide continuous sources of oxygen and hydrogen gas, without
significant contaminant levels. PSA systems and vacuum pressure swing
adsorption systems (VPSA) separate gas fractions from a gas mixture by
coordinating pressure cycling and flow reversals over an adsorber or adsorbent
bed
which preferentially adsorbs a more readily adsorbed gas component relative to
a
less readily adsorbed gas component of the mixture. The total pressure of the
gas
mixture in the adsorber is elevated while the gas mixture is flowing through
the
adsorber from a first end to a second end thereof, and is reduced while the
gas
mixture is flowing through the adsorbent from the second end back to the first
end. As the PSA cycle is repeated, the less readily adsorbed component is
concentrated adjacent the second end of the adsorber, while the more readily
adsorbed component is concentrated adjacent the first end of the adsorber. As
a
result, a "light" product (a gas fraction depleted in the more readily
adsorbed
component and enriched in the less readily adsorbed component) is delivered
from
the second end of the adsorber, and a "heavy" product (a gas fraction enriched
in
the more strongly adsorbed component) is exhausted from the first end of the
adsorber.
However, the conventional system for implementing pressure swing
adsorption or vacuum pressure swing adsorption uses two or more stationary
adsorbers in parallel, with directional valuing at each end of each adsorber
to
connect the adsorbers in alternating sequence to pressure sources and sinks.
This
system is often cumbersome and expensive to implement due to the large size of
CA 02324702 2000-10-27
-3-
the adsorbers and the complexity of the valuing required. Further, the
conventional PSA system makes inefficient use of applied energy because of
irreversible gas expansion steps as adsorbers are cyclically pressurized and
depressurized within the PSA process. Conventional PSA systems could not be
applied to fuel cell power plants for vehicles, as such PSA systems are far
too
bulky and heavy because of their low cycle frequency and consequently large
adsorbent inventory.
Another problem is the need for air compression with a substantial
mechanical parasitic load to achieve high power density and high voltage
efficiency with PEM fuel cells, either in the absence of PSA in prior art fuel
cell
systems, or to a lesser extent with the use of PSA to increase oxygen
concentration. If as usual that mechanical power is provided by a electric
motor
powered by the fuel cell, significant efficiency losses are entailed in
electrical
power conversion and conditioning for variable speed compressor drive, and the
fuel stack must be substantially larger to support this parasitic load as well
as the
application load to which useful power is delivered. In prior art PEM fuel
cell
power plants for automotive and other transportation applications,
approximately
% of fuel cell gross power output is diverted to the parasitic load of air
20 compression.
Yet another problem arises in the need to provide heat for endothermic fuel
processing reactions to generate low purity reformate hydrogen from
hydrocarbon
fuels (e.g. natural gas, gasoline or diesel fuel) or oxygenate fuels (e.g.
methanol,
ethanol or dimethyl ether). In the prior art, the necessary heat is provided
for
steam reforming of natural gas or methanol at least in part by burning
hydrogen
provided as anode tail gas from the fuel cell. Especially in the case of
methanol
reforming which can be performed at relatively low temperature, combustion of
valuable hydrogen to generate such low grade heat is extremely detrimental to
overall exergetic efficiency.
CA 02324702 2000-10-27
-4-
Likewise, the necessary heat for processing of heavier fuels such as
gasoline is achieved by combustion of a portion of the fuel in a partial
oxidation
or autothermal reforming process. Again, a portion of the high grade fuel is
consumed to upgrade the remainder of that fuel to low purity hydrogen than can
be purified for use in the fuel cell. With a low temperature fuel cell,
thermal
efficiency of prior art fuel processing systems has been extremely low, as
high
grade fuel is consumed. No opportunity has been found for efficient thermal
integration between a high temperature fuel processor and a low temperature
fuel
cell in transport applications.
SUMMARY OF THE INVENTION
According to the invention, there is provided a fuel cell based electrical
generation system which addresses the deficiencies of the prior art fuel cell
electrical generation systems, particularly as to energy-efficient PSA oxygen
enrichment, PSA purification of reformate hydrogen, heat recovery form the
fuel
cell stack and/or from combustion of hydrogen PSA tail gas, and thermal
powering of air compression for the oxygen PSA and of any PSA vacuum
pumping so as to minimize the size of the costly fuel cell stack while
maximizing
overall exergetic efficiency of energy conversion from the raw fuel.
The electrical current generating system comprises a fuel cell, an oxygen
gas delivery system, and a hydrogen gas delivery system. The fuel cell
includes
an anode channel having an anode gas inlet for receiving a supply of hydrogen
gas, a cathode channel having a cathode gas inlet and a cathode gas outlet,
and an
electrolyte in communication with the anode and cathode channel for
facilitating
ion transport between the anode and cathode channel. The oxygen gas delivery
system is coupled to the cathode gas inlet and delivers air or oxygen (e.g.
oxygen
enriched air) to the cathode channel.
The oxygen gas delivery system may simply be a air blower. However,
for superior performance it incorporates an oxygen pressure swing adsorption
CA 02324702 2000-10-27
- 5 -
system, including a rotary module having a stator and a rotor rotatable
relative to
the stator, for enriching oxygen gas from air. The rotor includes a number of
flow paths for receiving adsorbent material therein for preferentially
adsorbing a
first gas component in response to increasing pressure in the flow paths
relative to
a second gas component. The pressure swing adsorption system also may include
compression machinery coupled to the rotary module for facilitating gas flow
through the flow paths for separating the first gas component from the second
gas
component. The stator includes a first stator valve surface, a second stator
valve
surface, and plurality of function compartments opening into the stator valve
surfaces. The function compartments include a gas feed compartment, a light
reflux exit compartment and a light reflux return compartment.
In one variation, the compression machinery comprises a compressor for
delivering pressurized air to the gas feed compartment, and a light reflux
expander
coupled between the light reflux exit compartment and the light reflux return
compartment. The gas recirculating means comprises a compressor coupled to the
light reflux expander for supplying oxygen gas, exhausted from the cathode gas
outlet, under pressure to the cathode gas inlet. As a result, energy recovered
from
the pressure swing adsorption system can be applied to boost the pressure of
oxygen gas delivered to the cathode gas inlet.
The oxygen gas delivery system is coupled to the cathode gas inlet and
delivers oxygen gas to the cathode channel. The hydrogen gas delivery system
supplies purified hydrogen gas to the anode gas inlet, and may have provision
for
recirculating hydrogen gas from the anode gas exit back to the anode gas inlet
with
increased purity so as to avoid accumulation of impurities in the anode
channel.
In a preferred embodiment, the oxygen gas separation system comprises an
oxygen pressure swing adsorption system, and the hydrogen gas separation
system
comprises a reactor for producing a first hydrogen gas feed from hydrocarbon
fuel, and a hydrogen pressure swing adsorption system coupled to the reactor
for
purifying hydrogen gas received from the first hydrogen gas feed. Hydrogen gas
CA 02324702 2000-10-27
-6-
from the anode exit may be recirculated to the hydrogen pressure swing
adsorption
system as a second hydrogen gas feed for a feed repressurization step of the
PSA
cycle. Both pressure swing adsorption systems include a rotary module having a
stator and a rotor rotatable relative to the stator. The rotor includes a
number of
flow paths for receiving adsorbent material therein for preferentially
adsorbing a
first gas component in response to increasing pressure in the flow paths
relative to
a second gas component. The function compartments include a gas feed
compartment and a heavy product compartment.
The feed gas to the hydrogen PSA system is reformate gas or "syngas",
generated in alternative fuel processing methods known to the art by steam
reforming (e.g. of methanol or natural gas or light hydrocarbons), or by
autothermal reforming or partial oxidation (e.g. of natural gas, gasoline or
diesel
fuel). The CO content of methanol reformate (generated by relatively low
temperature steam reforming of methanol) is typically about 1 % . Other fuel
processors (e.g. steam methane reformers, and POX or autothermal reformers
operating on any feedstock) operate at much higher temperature, and preferably
include a lower temperature water gas shift reactor stage to reduce to CO
content
to about 1 % .
The reformate gas contains hydrogen plus the basic impurity components of
C02, CO and water vapour. If generated by air-blown POX or autothermal
reforming, the reformate gas will also contain a large inert fraction of
nitrogen and
argon. The fraction of inert atmospheric gases can be greatly reduced if an
oxygen PSA system is used to supply the POX or autothermal reformer, either
directly from the PSA, or as humid and still oxygen enriched air that has been
passed through the fuel cell cathode channel which was directly fed oxygen-
enriched air from the PSA.
In one variation, the oxygen pressure swing adsorption system includes a
compressor coupled to the gas feed compartment for delivering pressurized air
to
the gas feed compartment, and a vacuum pump coupled to the compressor for
CA 02324702 2000-10-27
extracting nitrogen product gas from the heavy product compartment. The
reactor
comprises a steam reformer, including a burner, for producing syngas, and a
water gas shift reactor coupled to the steam reformer for converting some CO
to
hydrogen. The hydrogen pressure swing adsorption system includes a vacuum
pump for delivering fuel gas from the heavy product compartment to the burner.
The fuel gas is burned in the burner, and the heat generated therefrom is used
to
supply the endothermic heat of reaction necessary for the steam reformer
reaction.
The resulting reformate gas is delivered to the water gas shift reactor for
removal
of impurities, and then delivered as the impure hydrogen gas feed to the
hydrogen
pressure swing adsorption system.
In another variation, the invention includes a burner for burning fuel. The
reactor comprises an autothermal reformer for producing syngas, and a water
gas
shift reactor coupled to the autothermal reformer for converting the syngas to
the
second hydrogen gas feed. The compressor of the oxygen pressure swing
adsorption system delivers pressurized air to the burner, and the heavy
product gas
is delivered from the hydrogen pressure swing adsorption system as tail gas to
be
burned in the burner. The compression machine of the oxygen pressure swing
adsorption system also includes an expander coupled to the feed compressor for
driving the compressor from hot gas of combustion emitted from the burner. The
feed compressor with the expander may be on a common shaft with a motor drive,
or may constitute a free rotor similar to an automotive turbocharger. The same
expander or another expander may be coupled to a vacuum pump to assist the PSA
process. Again, the vacuum pump with its expander may be provided as a free
rotor similar to an automotive turbocharger. Heat from the burner may also be
applied to preheat air and/or fuel supplied to the autothermal reformer.
Independently of whether PSA is used for oxygen enrichment, the present
invention provides a hydrogen PSA apparatus for purifying the reformate. The
hydrogen PSA may be designed to deliver high purity hydrogen, or else may be
designed less stringently to achieve adequately high removal of noxious
components (harmful to the fuel cell) such as CO, H2S, halogens, methanol,
etc.
CA 02324702 2000-10-27
_ g _
In the latter case, the hydrogen PSA would in its first pass only achieve
partial
removal of less harmful constituents (e.g., N2, Ar and C02). In that case,
anode
tail gas would then preferably be recycled to the feed end of the PSA inlet
for use
in a feed pressurization step, thus avoiding any need for mechanical
recompression. Even when high hydrogen purity is specified for the PSA, this
feature enables a small bleed from the end of the anode channel back to the
feed
pressurization step of the hydrogen PSA, as would be desirable for avoiding a
strict dead-headed configuration with the risk of accumulation in the anode
channel
of any contaminant slip due to equipment imperfections or operational
transient
upsets.
Operating temperature of the adsorbers in the hydrogen PSA unit of the
invention will preferably be elevated well above ambient, as the reformate gas
is
supplied at a temperature after water gas shift of typically about
200°C, while
operating temperatures of PEM fuel cells may extend from 80°C to about
100°C.
Alternatively, the adsorbers may be operated at a lower temperature if the
reformate is cooled, thus providing an opportunity for partial removal of
water and
any methanol vapour by condensation before admission to the hydrogen PSA unit.
Advantages of operation at moderately elevated temperature are ( 1 ) reformate
coolers and water condensers upstream of the hydrogen PSA can be avoided, (2)
PSA removal of water vapour and C02 may be more readily achieved at
moderately elevated temperature compared to ambient temperature, (3) CO can be
more selectively adsorbed than C02 over Cu(I) loaded adsorbents particularly
at
elevated temperature, and (4) kinetics of CO sorption and desorption on CO-
selective sorbents will be greatly enhanced at higher temperature.
Consequently a
preferred operating temperature range for the adsorbers is from about
80°C to
about 200°C, and a more preferred operating range is from about
100°C to about
160°C .
The hydrogen PSA unit may be configured to support a temperature
gradient along the length of the flow channels, so that the temperature at the
first
CA 02324702 2000-10-27
-9-
end of the adsorbers is higher than the temperature at the second end of the
adsorbers.
Especially in the mode of design for low purity hydrogen with anode
recycle, the hydrogen PSA may use CO-selective adsorbents with CO-complexing
ions Cu(I) or Ag introduced by ion exchange or impregnation into a suitable
adsorbent carrier. Prior art CO-selective adsorbents have used a wide
diversity of
zeolites, alumina or activated carbon adsorbents as carriers. With CO-
selective
adsorbents, enhanced hydrogen recovery may be achieved while tolerating some
accumulation of non-CO impurities circulated through the fuel cell anode loop.
In the present invention, the active adsorbent preferably including a CO-
selective component is supported on thin adsorbent sheets which are layered
and
spaced apart by spacers defining flow channels, so as to provide a high
surface
area parallel passage support with minimal mass transfer resistance and flow
channel pressure drop. With crystalline adsorbents such as zeolites, and
amorphous adsorbents such as alumina gel or silica gel, the adsorbent sheet is
formed by coating or in-situ synthesis of the adsorbent on a reinforcement
sheet of
inert material, e.g. a wire mesh, a metal foil, a glass or mineral fiber
paper, or a
woven or nonwoven fabric. Active carbon adsorbent may also be coated onto a
reinforcement sheet of inert material, but adosrbent sheets of active carbon
may
also be provided as self supporting carbon fiber paper or cloth. Adsorbers of
the
layered adsorbent sheet material may be formed by stacking flat or curved
sheets;
or by forming a spiral roll, with the flow channels between the sheets
extending
from the first end of the adsorber to the second end thereof; to fill the
volume of
the adsorber housing of the desired shape. Typical thickness of the adsorbent
sheet may be in the range of about 100 to about 200 microns, while flow
channel
spacing between the sheets may be in the range of about 50 to about 200
microns.
In the present invention, the adsorbent material contacting the flow
channels between the first and second ends of the adsorbers may in general be
selected to be different in distinct zones of the flow channels, so that the
CA 02324702 2000-10-27
-10-
adsorbers would have a succession of zones (e.g. a first zone, a second zone,
a
third zone, a perhaps additional zones) with distinct adsorbents proceeding
along
the flow channels from the first end to the second end.
In a first variant configured to deliver high purity hydrogen, the adsorbent
in a first zone of the adsorbers adjacent the first end will be a dessicant to
achieve
bulk removal of water vapour in that first zone, the adsorbent in a second
zone in
the central portion of the adsorbers will be selected to achieve bulk removal
of
C02 and some removal of CO, and the adsorbent in a third zone of the adsorbers
will be selected to achieve final removal of CO and substantial removal of any
nitrogen and argon. A suitable dessicant for the first zone is alumina gel. A
suitable adsorbent for the second zone is 13X zeolite, or SA, or active
charcoal.
Suitable adsorbents for the third zone may be a strongly carbon monoxide and
nitrogen selective adsorbent selected from the group including but not limited
to
Na-LSX, Ca-LSX, Li-LSX, Li- exchanged chabazite, Ca- exchanged chabazite,
Sr- exchanged chabazite. The zeolite adsorbents of this group are
characterized
by strong hydrophilicity, corresponding to selectivity for polar molecules.
This
first variant relying on physical adsorption will operate most effectively at
relatively lower temperatures, unlikely to exceed much more than about
100°C
although certain adsorbents such as Ca- or Sr-exchanged chabazite would remain
adequately effective for CO and N2 removal at temperatures to about
150°C. The
adsorbent in the second or third zone may be a more strongly carbon monoxide
selective adsorbent such as a Cu(I)-exchanged zeolite such as an X or a Y
zeolite,
mordenite, or chabazite. Alternatively, the adsorbent in some or all zones of
the adsorbers may be a hydrophobic adsorbent selected from the group including
but not limited to active carbon, Y-zeolite and silicalite; and preferably
containing
Cu(I) for enhanced CO- selectivity in a zone adjacent the second end of the
adsorbers.
tivity.
In another variant configured to deliver at least partially purified hydrogen
with CO nearly completely removed, the adsorbent in the first or second zone
of
CA 02324702 2000-10-27
-11-
the adsorbers will include a component catalytically active at the operating
temperature of that zone for the water gas shift reaction. A portion of the
carbon
monoxide sorbed onto the catalytically active component may then react with
water vapour by the water gas shift reaction to generate carbon dioxide and
additional hydrogen.
Industrial H2 PSA is normally conducted at considerably elevated pressures
( > 10 tiara) to achieve simultaneous high purity and high recovery ( --- 80 %
-
85 % ). Fuel cell systems operating with pressurized methanol reformers or in
integration with gas turbine cycles may operate at relatively high pressures.
However, most PEM fuel cell systems operate at ambient to about 3 tiara
pressure.
As feed pressure and the overall working pressure ratio of the PSA are
reduced,
productivity and recovery of a simple cycle deteriorate. Under given pressure
conditions, use of CO-selective adsorbents should significantly improve
recovery
at specified product CO concentration, if hydrogen purity with respect to
other
impurities such as nitrogen and carbon dioxide can be relaxed.
At very low feed pressures (e.g. 2 - 3 tiara), the H2 PSA would need
supplemental compression to achieve high recovery. We may consider vacuum
pumping to widen the working pressure ratio, or alternatively "heavy reflux"
which is recompression and recycle to the PSA feed of a fraction of its
exhaust
stream at full pressure. Vacuum and heavy reflux options may be combined in
PSA systems for reformate purification. We have successfully used the heavy
reflux option, without CO-selective adsorbents or any vacuum pumping, in a lab
bench PSA device to achieve -- 95 % recovery from synthetic methanol reformate
at - 3 tiara feed pressure.
To get heavy reflux in a very low pressure PSA, the vacuum pump may be
configured so that part of its flow is reinjected into the PSA feed. Extremely
high hydrogen recovery can then be obtained (even at a fairly low overall
pressure
ratio) just by pumping enough heavy reflux. The vacuum level can be traded
against the mass flow of heavy reflux.
CA 02324702 2000-10-27
-12-
A fuel cell may be a stand-alone power plant, or else it may be integrated
with some type of combustion engine. In the case of a stand-alone fuel cell,
all
mechanical power for air handling compression and any oxygen and/or hydrogen
PSA units must be provided as electrical power by the appropriately sized fuel
cell
stack. In this case, tight constraints apply to the recovery level that must
be
achieved by the HZ PSA at specified purity. In the absence of any useful
export
use for high grade heat, an efficient heat balance requires that the heating
value of
combustible waste gases (HZ, CO and unreacted fuel) be matched to the heat
demand of the fuel processor. For a fuel cell with steam reforming (e.g.
methanol
or natural gas), nominal hydrogen recovery by the HZ PSA has to be about 75 %
to
80 % as the PSA tail gas is burned to heat the reformer; while for a POX or
autothermal reformer, hydrogen recovery by the PSA needs to be extremely high
(at least 90% to 95%) as such reformers can only use a limited amount of
external
combustion heat from burning PSA tail gas or fuel cell anode tail gas, e.g.
for
preheating feed oxygen/air and fuel reactants to the reformer.
Also to be considered are combined cycles where a combustion engine is
integrated with a fuel cell. Here, the above strict heat balance constraints
on
necessary hydrogen recovery to be achieved by the PSA may be relaxed in
designing for most desirable technical, emissions and economic performance of
the
power plant. The prior art includes combined cycle power plants with a gas
turbine cycle integrated with a fuel cell system. Fuel cell auxiliary power
units
have been proposed for automobiles and passenger railcars with internal
combustion engines as primary power plants.
Co-pending Canadian patent application No. 2,274,240 provides examples
of how PSA units may be integrated with gas turbine power plants, or with fuel
cell power plants having a gas turbine auxiliary engine. The gas turbine may
power all compressors and vacuum pumps for the 02 PSA, along with vacuum
pump and/or heavy reflux compression for the H2 PSA. This auxiliary gas
turbine cycle allows a heavy reflux vacuum pump and compressor to be driven by
the turboexpander which expands the products of hydrogen PSA tail gas
CA 02324702 2000-10-27
-13-
combustion. A feature here is integration of the vacuum pumps) with the gas
turbine powered by tail gas combustion. Either single or multiple spool gas
turbine configurations may be considered. Centrifugal or axial machines may be
used as the compressors and pumps. Approaches based on integration of gas
turbines and fuel cells are particularly favourable for larger power levels.
In order to achieve high process efficiency and high recovery of the PSA
units along with high overall efficiency of the fuel cell system, the hydrogen
PSA
tail gas may be burned in an auxiliary combustion engine to drive the air
handling
system compressor and any vacuum pumps for the oxygen and hydrogen PSA
units. For smaller plants, internal combustion engines may be attractive
relative to
gas turbine configurations. Either way, powering the compressor and vacuum
pumps) by burning tail gas avoids the cost penalty of a bigger fuel cell stack
in
order to run compression machinery as parasitic electrical loads. The engine
exhaust heat and/or cooling jacket heat may be further recovered to preheat
and
vaporize fuel reactants and to provide some or all of the heat of reforming
for a
methanol reformer.
The engine could be a reciprocator or a rotary engine. It may aspirate the
hydrogen PSA tail gas directly as fuel, or else be turbocharged to pull
greater
vacuum from the PSA exhaust. Modern Wankel derivative engines have
favourable specific displacement and power density. Thus an auxiliary internal
combustion engine could act as its own vacuum pump on tail gas being inducted
directly as fuel. Some oxygen enriched tail gas from the fuel cell cathode
could
be fed as a supplement to intake air to make up for the heavy load of C02. In
view of the hydrogen, water and carbon dioxide content of the tail gas fueling
this
engine, conditions are favourable for extremely low emissions of NOx and other
noxious contaminants.
CA 02324702 2000-10-27
-14-
Heat Recovery Methanol Reformer System for Low Pressure Fuel Cell
The conventional approach for methanol reforming is to pump the liquid
reactants to an elevated pressure for vaporization and the vapour phase
methanol
reforming reaction. This approach enables the reactor itself to be compact,
and
provides driving pressure for hydrogen purification by PSA or palladium
diffusion
membranes.
A novel low pressure process for steam methanol reforming can get
enhanced heat recovery from a low pressure fuel cell. More than 60% of the
endothermic heat of steam reforming methanol is in fact the heat of
vaporization to
boil the methanol and the water inputs. If the fuel cell is cooled to vaporize
feed
liquid fuel and water at the fuel cell stack working temperature, the system
could
be more efficient due to this heat recovery which liberates hydrogen to
generate
electricity while absorbing about 25 % of the stack cooling load. A water-rich
mix
of 14% methanol in water will boil at atmospheric pressure and 85°C to
generate a
50/50 vapour mix as required by stoichiometry, or at a modestly higher
temperature with a larger excess of water in the liquid phase to obtain a
small
excess of steam as actually required to ensure low CO concentration. Therefore
the liquid mixture of water containing a fraction of methanol may be
circulated as
fuel cell stack coolant, and then flash evaporated to generate a methanol-H20
vapour mix to be admitted into the reforming catalyst chamber at fuel cell
system
working pressure. If the fuel cell were operating at less than 85°C,
flash
evaporation would have to be performed under vacuum or else with a higher
concentration of methanol (as also desirable for antifreeze characteristics
for
winter conditions) so that only a fraction of the water required for methanol
reforming is provided by vaporization using fuel cell stack waste heat. As
higher
PEM fuel cell operating temperatures are considered, this concept becomes more
viable as permitting either atmospheric or higher pressure for flash
evaporation, or
else a larger temperature differential driving heat exchange in the stack
coolant
channels.
CA 02324702 2000-10-27
-15-
The above concept of stack heat recovery to boil the methanol reforming
reactants is more attractive for a relatively low pressure fuel cell, unless
the
working temperature were greatly increased. If all the steam feed to the
methanol
reformer is generated by stack heat recovery, some mechanical compression of
the
reformer reactant vapour mixture would be needed except for a very low
pressure
PEM fuel cell (e.g. operating at a pressure below 1.5 bars absolute). Such a
very
low pressure fuel cell would be expected to benefit greatly from PSA 02
enrichment as enabling high power density at low total pressure. However,
vacuum pumping would then be required for both the oxygen PSA and a hydrogen
PSA unit, particularly to obtain high recovery of hydrogen in the hydrogen
PSA.
An alternative approach within the invention is to operate the fuel cell at
somewhat higher pressure (e.g. operating at a pressure of about 2 or 3 bars
absolute), with the stack coolant liquid mixture of water and methanol
containing a
higher concentration of methanol, so that the vapour mixture thus generated
contains all the methanol vapour for the methanol reformer, plus only a
portion of
the steam required for reforming that methanol. Supplementary steam is then
generated by an alternative heat source, for example exhaust heat or cooling
jacket
heat from a combustion engine or turbine used to drive the feed air compressor
and any vacuum pumps required to operate the PSA equipment.
In the case of a POX or autothermal gasoline fuel processor, the
endothermic heat for the reforming reaction is generated by burning a portion
of
the fuel stream within the reforming reactor. Hence, there is at most a very
limited opportunity for burning the hydrogen PSA tail gas usefully to support
the
reforming process (e.g. to preheat incoming air and fuel streams), because
ample
high grade heat is generated within POX and autothermal reformers. If there is
no other use for combustion heat from burning the hydrogen PSA tail gas, it
will
be obvious that the hydrogen PSA would have to achieve extremely high hydrogen
recovery (in the range of e. g. 90 % to 99 % ) to achieve heat balance and
full
utilization of fuel. In the case of a methanol reformer with stack heat
recovery to
boil the reactants as provided above within the present invention, the
hydrogen
CA 02324702 2000-10-27
-16-
PSA would have to achieve very high hydrogen recovery ( -- 90 % ) in view of
the
substantial heat recovery from the stack to reduce the methanol reformer heat
demand.
The present invention therefore provides for an auxiliary combustion engine
or turbine, cooperating with the fuel cell power plant to at least assist the
feed air
compression and any vacuum pumping loads. Tail gas from the hydrogen PSA
unit is now usefully consumed as fuel for the auxiliary combustion engine or
turbine, so that the necessary hydrogen recovery achieved by the PSA unit may
be
relaxed to the range of e.g. 70% to 90% as the heat balance and fuel
utilization
constraints are opened. Hence, the need for heavy reflux compression and
vacuum pumping to assist the hydrogen PSA unit is reduced or eliminated.
Simultaneously, the auxiliary combustion engine or turbine unloads the PSA
compression and any vacuum pumping load from the fuel cell electrical output,
thus reducing the size and cost of the fuel cell.
The thermally integrated combination of the auxiliary combustion engine or
turbine with the fuel processor provides alternative waste heat sources for
vaporizing steam directly at the reforming pressure, for heating an
endothermic
reactor, and for recovering exothermic heat e.g. of water gas shift. A
thermally
integrated design can also be configured to minimize thermal inefficiencies,
e.g. of
heat loss by conduction to the environment, simply by placing hot components
of
the fuel processsor and the auxiliary heat engine within a common housing, and
with components at similar operating temperatures in close adjacent proximity.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 shows an axial section of a rotary PSA module.
Figs. 2 through SB show transverse sections of the module of Fig. 1.
CA 02324702 2000-10-27
-17-
Fig. 6 is a simplified schematic of a PEM fuel cell power plant with a
steam reforming fuel processor, a PSA unit for reformate hydrogen
purification,
and a VPSA unit for oxygen enrichment.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Fps. 1 - 5
Fig. 1 shows a rotary PSA module 1, which includes a number "N" of
adsorbers 3 in adsorber housing body 4. Each adsorber has a first end 5 and a
second end 6, with a flow path therebetween contacting a nitrogen-selective
adsorbent. The adsorbers are deployed in an axisymmetric array about axis 7 of
the adsorber housing body. The housing body 4 is in relative rotary motion
about
axis 7 with first and second functional bodies 8 and 9, being engaged across a
first
valve face 10 with the first functional body 8 to which feed gas mixture is
supplied
and from which the heavy product is withdrawn, and across a second valve face
11 with the second functional body 9 from which the light product is
withdrawn.
In preferred embodiments as particularly depicted in Figs. 1 - 5, the
adsorber housing 4 rotates and shall henceforth be referred to as the adsorber
rotor
4, while the first and second functional bodies are stationary and together
constitute a stator assembly 12 of the module. The first functional body shall
henceforth be referred to as the first valve stator 8, and the second
functional body
shall henceforth be referred to as the second valve stator 9.
In the embodiment shown in Figs. 1 - 5, the flow path through the
adsorbers is parallel to axis 7, so that the flow direction is axial, while
the first
and second valve faces are shown as flat annular discs normal to axis 7.
However, more generally the flow direction in the adsorbers may be axial or
radial, and the first and second valve faces may be any figure of revolution
centred on axis 7. The steps of the process and the functional compartments to
CA 02324702 2000-10-27
-18-
be defined will be in the same angular relationship regardless of a radial or
axial
flow direction in the adsorbers.
Figs. 2 - 5 are cross sections of module 1 in the planes defined by arrows
12 - 13, 14 - 15, and 16 - 17. Arrow 20 in each section shows the direction of
rotation of the rotor 4. Fig. 2 shows section 12 - 13 across Fig.l, which
crosses
the adsorber rotor. In this example, "N" = 72. The adsorbers 3 are mounted
between outer wall 21 and inner wall 22 of adsorber wheel 208. Each adsorber
comprises a rectangular flat pack 3 of adsorbent sheets 23, with spacers 24
between the sheets to define flow channels here in the axial direction.
Separators
25 are provided between the adsorbers to fill void space and prevent leakage
between the adsorbers. In other configurations, the adsorbent sheets may be
formed in curved packs or spiral rolls.
Satisfactory adsorbent sheets have been made by coating a slurry of zeolite
crystals with binder constituents onto the reinforcement material, with
successful
examples including nonwoven fibreglass scrims, woven metal fabrics, and
expanded aluminum foils. The adsorbent sheets comprise a reinforcement
material,
in preferred embodiments glass fibre, metal foil or wire mesh, to which the
adsorbent material is attached with a suitable binder. For applications such
as
hydrogen purification, some or all of the adsorbent material may be provided
as
carbon fibers, in woven or nonwoven form to serve as its own reinforcement
material. Spacers are provided by printing or embossing the adsorbent sheet
with
a raised pattern, or by placing a fabricated spacer between adjacent pairs of
adsorbent sheets. Alternative satisfactory spacers have been provided as woven
metal screens, non-woven fibreglass scrims, and metal foils with etched flow
channels in a photolithographic pattern.
Typical experimental sheet thicknesses have been 150 microns, with spacer
heights in the range of 100 to 150 microns, and adsorber flow channel length
approximately 20 cm. Using X type zeolites, excellent performance has been
CA 02324702 2000-10-27
-19-
achieved in oxygen separation from air and hydrogen purification from
reformate
at PSA cycle frequencies in the range of 30 to 150 cycles per minute.
As shown in Fig. 1, the adsorbers 3 comprise a plurality of distinct zones
between the first end 5 and the second end 6 of the flow channels, here shown
as
three zones respectively a first zone 26 adjacent the first end 5, a second
zone 27
in the middle of the adsorbers, and a third zone 28 adjacent the second end 6.
The first zone typically contains an adsorbent or dessicant selected for
removing
very strongly adsorbed components of the feed gas mixture, such as water or
methanol vapour, and some carbon dioxide. The second zone contains an
adsorbent typically selected for bulk separation of impurities at relatively
high
concentration, and the third zone contains an adsorbent typically selected for
polishing removal of impurities at relatively low concentration.
In embodiments with three zones, the first zone may be the first 10 % to
% of the flow channel length from the first end, the second zone may be the
next roughly 40 % to 50 % of the channel length, and the third zone the
remainder.
In embodiments with only two adsorber zones, the first zone may be the first
10 % to 30 % of the flow channel length from the first end, and the second
zone
20 the remainder. The zones may be formed by coating the different adsorbents
onto
the adsorbent support sheet material in bands of the same width as the flow
channel length of the corresponding zone. The adsorbent material composition
may change abruptly at the zone boundary, or may be blended smoothly across
the
boundary. Particularly in the first zone of the adsorber, the adsorbent must
be
compatible with significant concentrations of water vapour.
Fig. 3 shows the porting of rotor 4 in the first and second valve faces
respectively in the planes defined by arrows 14 - 15 , and 16 - 17. An
adsorber port
provides fluid communication directly from the first or second end of each
30 adsorber to respectively the first or second valve face.
CA 02324702 2000-10-27
-20-
Figs. 4A and 4B show the first stator valve face 100 of the first stator 8 in
the
first valve face 10, in the plane defined by arrows 14 - 15. Fluid connections
are
shown to a feed compressor 101 inducting feed gas from inlet filter 102, and
to an
exhauster 103 delivering second product to a second product delivery conduit
104.
Compressor 101 and exhauster 103 are shown coupled to a drive motor 105.
Arrow 20 indicates the direction of rotation by the adsorber rotor. In the
annular valve face between circumferential seals 106 and 107, the open area of
first
stator valve face 100 ported to the feed and exhaust compartments is indicated
by
clear angular segments 111 - 116 corresponding to the first functional ports
communicating directly to functional compartments identified by the same
reference
numerals 111 - 116. The substantially closed area of valve face 100 between
functional compartments is indicated by hatched sectors 118 and 119 which are
slippers with zero clearance, or preferably a narrow clearance to reduce
friction and
wear without excessive leakage. Typical closed sector 118 provides a
transition for
an adsorber, between being open to compartment 114 and open to compartment
115.
Gradual opening is provided by a tapering clearance channel between the
slipper and
the sealing face, so as to achieve gentle pressure equalization of an adsorber
being
opened to a new compartment. Much wider closed sectors (e.g. 119) are provided
to substantially close flow to or from one end of the adsorbers when
pressurization
or blowdown is being performed from the other end.
The feed compressor provides feed gas to feed pressurization compartments
111 and 112, and to feed production compartment 113. Compartments 111 and 112
have succcessively increasing working pressures, while compartment 113 is at
the
higher working pressure of the PSA cycle. Compressor 101 may thus be a
multistage or split stream compressor system delivering the appropriate volume
of
feed flow to each compartment so as to achieve the pressurization of adsorbers
through the intermediate pressure levels of compartments 111 and 112, and then
the
final pressurization and production through compartment 113. A split stream
compressor system may be provided in series as a multistage compressor with
interstage delivery ports; or as a plurality of compressors or compression
cylinders
CA 02324702 2000-10-27
-21 -
in parallel, each delivering feed air to the working pressure of a compartment
111 to
113. Alternatively, compressor 101 may deliver all the feed gas to the higher
pressure, with throttling of some of that gas to and 112 at their respective
intermediate pressures.
Similarly, exhauster 103 exhausts heavy product gas from countercurrent
blowdown compartments 114 and 115 at the successively decreasing working
pressures of those compartments, and finally from exhaust compartment 116
which
is at the lower pressure of the cycle. Similarly to compressor 101, exhauster
103
may be provided as a multistage or split stream machine, with stages in series
or in
parallel to accept each flow at the appropriate intermediate pressure
descending to the
lower pressure.
In the example embodiment of Fig. 4A, the lower pressure is ambient
pressure, so exhaust compartment 116 communicates directly to heavy product
delivery conduit 104. Exhauster 103 thus is an expander which provides
pressure
letdown with energy recovery to assist motor 105 from the countercurrrent
blowdown
compartments 114 and 115. For simplicity, exhauster 103 may be replaced by
throttling orifices as countercurrent blowdown pressure letdown means from
compartments 114 and 115.
In some preferred embodiments, the lower pressure of the PSA cycle is
subatmospheric. Exhauster 103 is then provided as a vacuum pump, as shown in
Fig.
4B. Again, the vacuum pump may be multistage or split stream, with separate
stages in series or in parallel, to accept countercurrent blowdown streams
exiting their
compartments at working pressures greater than the lower pressure which is the
deepest vacuum pressure. In Fig. 4B, the early countercurrent blowdown stream
from compartment 114 is released at ambient pressure directly to heavy product
delivery conduit 104. If for simplicity a single stage vacuum pump were used,
the
countercurrent blowdown stream from compartment 115 would be throttled down to
the lower pressure over an orifice to join the stream from compartment 116 at
the
inlet of the vacuum pump.
CA 02324702 2000-10-27
-22-
If the feed gas is provided at an elevated pressure at least equal to the
higher
pressure of the PSA cycle, as may conveniently be the case of a hydrogen PSA
operating with e.g. methanol reformate feed, compressor 101 would be
eliminated.
To reduce energy losses from irreversible throttling over orifices to supply
feed
pressurization compartments e.g. 111, the number of feed pressurization stages
may
be reduced, sot that adsorber repressurization is largely achieved by product
pressurization, by backfill from light reflux steps. Alternatively, compressor
101
may be replaced in part by an expander which expands feed gas to a feed
pressurization compartment e.g. 111 from the feed supply pressure of the
higher
pressure to the intermediate pressure of that compartment, so as to recover
energy for
driving a vacuum pump 103 which reduces the lower pressure below ambient
pressure
so as to enhance the PSA process performance.
Figs. SA and SB shows the second stator valve face, at section 16 - 17 of Fig.
1. Open ports of the valve face are second valve function ports communicating
directly to a light product delivery compartment 121; a number of light reflux
exit
compartments 122, 123, 124 and 125; and the same number of light reflux return
compartments 126, 127, 128 and 129 within the second stator. The second valve
function ports are in the annular ring defined by circumferential seals 131
and 132.
Each pair of light reflux exit and return compartments provides a stage of
light reflux
pressure letdown, respectively for the PSA process functions of supply to
backfill,
full or partial pressure equalization, and cocurrent blowdown to purge.
Illustrating the option of light reflux pressure letdown with energy recovery,
a split stream light reflux expander 140 is shown in Figs. 1 and SA to provide
pressure
let-down of four light reflux stages with energy recovery. The light reflux
expander
provides pressure let-down for each of four light reflux stages, respectively
between
light reflux exit and return compartments 122 and 129, 123 and 128, 124 and
127, and
125 and 126 as illustrated. The light reflux expander 140 may power a light
product
booster compressor 145 by drive shaft 146, which delivers the oxygen enriched
light
product to oxygen delivery conduit 147 and compressed to a delivery pressure
above
the higher pressure of the PSA cycle.
CA 02324702 2000-10-27
- 23 -
Since the light reflux and light product have approximately the same purity,
expander 140 and light product compressor 145 may be hermetically enclosed in
a
single housing which may conveniently be integrated with the second stator as
shown
in Fig. 1. This configuration of a "turbocompressor" light product booster
without a
separate drive motor is advantageous, as a useful pressure boost of the light
product
can be achieved without an external motor and corresponding shaft seals, and
can also
be very compact when designed to operate at very high shaft speeds.
Fig. SB shows the simpler alternative of using a throttle orifice 150 as the
pressure letdown means for each of the light reflux stages.
Turning back to Fig. 1, compressed feed gas is supplied to compartment 113
as indicated by arrow 125, while heavy product is exhausted from compartment
117
as indicated by arrow 126. The rotor is supported by bearing 160 with shaft
seal 161
on rotor drive shaft 162 in the first stator 8, which is integrally assembled
with the
first and second valve stators. The adsorber rotor is driven by motor 163 as
rotor drive
means.
As leakage across outer circumferential seal 131 on the second valve face 11
may compromise light product purity, and more importantly may allow ingress of
humidity into the second ends of the adsorbers which could deactivate the
nitrogen-
selective or CO-selective adsorbent, a buffer seal 170 is provided to provide
more
positive sealing of a buffer chamber 171 between seals 131 and 171. Even
though
the working pressure in some zones of the second valve face may be
subatmospheric
(in the case that a vacuum pump is used as exhauster 103), buffer chamber is
filled
with dry light product gas at a buffer pressure positively above ambient
pressure.
Hence, minor leakage of light product outward may take place, but humid feed
gas
may not leak into the buffer chamber. In order to further minimize leakage and
to
reduce seal frictional torque, buffer seal 171 seals on a sealing face 172 at
a much
smaller diameter than the diameter of circumferential seal 131. Buffer seal
170 seals
between a rotor extension 175 of adsorber rotor 4 and the sealing face 172 on
the
second valve stator 9, with rotor extension 175 enveloping the rear portion of
second
CA 02324702 2000-10-27
-24-
valve stator 9 to form buffer chamber 171. A stator housing member 180 is
provided
as structural connection between first valve stator 8 and second valve stator
9.
In the following figures of this disclosure, simplified diagrams will
represent
the PSA apparatus as described above. These highly simplified diagrams will
indicate
just a single feed conduit 181 to, and a single heavy product conduit 182
from, the
first valve face 10; and the light product delivery conduit 147 and a single
representative light reflux stage 184 with pressure let-down means
communicating to
the second valve face 11. Reference numerals pertaining to PSA units as
described
above will be unprimed for an oxygen enrichment PSA or VPSA unit, and primed
for
a hydrogen purification PSA or VPSA unit.
Figs. 6 - 8
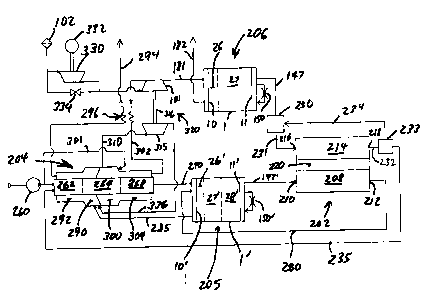
Figs. 6 - 8 show a fuel cell power plant 200, according to the present
invention, comprising a fuel cell 202, a steam reforming fuel processor 204, a
hydrogen purification PSA system 205, and an oxygen enrichment PSA or VPSA
system 206. The fuel cell comprises an anode channel 208 including an anode
gas
inlet 210 and an anode gas outlet 212, a cathode channel 214 including a
cathode
gas inlet 216 and a cathode gas outlet 218, and a PEM electrolyte membrane 220
cooperating with the anode channel 208 and the cathode channel 214 for
facilitating
ion exchange between the anode channel 208 and the cathode channel 214.
The oxygen PSA or VPSA system 206 extracts oxygen gas from feed air,
and comprises a PSA rotary module 1 and a compressor 101 for delivering
pressurized feed air to the feed compartments of the rotary module 1. Nitrogen
enriched gas as heavy product gas from the blowdown and exhaust compartments
of the rotary module 1 is withdrawn by conduit 182, either for discharge
directly
by atmosphere as in Fig. 6 or to a vacuum pump 103 for discharge as in Fig. 7.
The adsorbers 3 of rotary module 1 have a first zone 26 loaded with a suitable
dessicant such as alumina gel for removal of water vapour, and a second zone
27
loaded with a nitrogen-selective zeolite. Dry oxygen enriched air as the light
CA 02324702 2000-10-27
- 25 -
product gas of VPSA module 1 is delivered by conduit 147 to humidification
chamber 230 and thence by conduit 231 to cathode inlet 216. A portion of the
oxygen reacts with hydrogen ions when electric current is generated, to form
water
in the cathode. The cathode exhaust gas now containing a reduced amount of
oxygen (but still typically oxygen-enriched well above ambient air
composition)
plus water is withdrawn from cathode exit 218 by conduit 232 to separator 233.
In Figs. 6 and 7, a portion of the humid cathode exhaust gas (or water
condensate)is removed from separator 233 by conduit 234, which transfers water
and any recycle oxygen back to humidification chamber 230 for recirculation
through cathode channel 214. Any oxygen recirculation through conduit 234 must
be driven by appropriate recirculation pressure boost means, such as a blower
or an
ej ector.
If fuel processor 204 in Figs. 6 and 7 is a partial oxidation or autothermal
reformer, the remaining oxygen (plus any accumulated argon and nitrogen) and
the
fuel cell product water are delivered from separator 233 by conduit 235 to the
fuel
processor 204. If fuel processor 204 in Figs. 6 and 7 is a steam reforming
reactor,
the fuel cell product water as condensate is delivered from separator 233 by
conduit
235 to the fuel processor 204. In that event, accumulations of argon and
nitrogen
in the cathode channel must be recycled from separator 233 back to the oxygen
PSA unit as shown in Fig. 8 by conduit 236 to the first valve face 10 of PSA
module 1 as a feed pressurization stream at an intermediate pressure below the
higher pressure of the PSA cycle, or else purged to atmosphere.
A hydrocarbon fuel is supplied to the fuel processor 204 by a feed pump or
compressor 260, is combined with water from conduit 235, and is vaporized and
preheated in heat exchanger 262. The preheated stream of fuel and steam is
then
admitted to reforming catalytic chamber 264. In the example that the fuel is
CA 02324702 2000-10-27
-26-
methane, the following steam reforming reactions take place,
CH4 + HZO ~ CO + 3Hz
CH4 + 2H20 ~ COZ + 4H2
in addition to partial combustion in the case of an autothermal reformer:
CH4 + '/ZOZ -a CO + 2H,
The resulting reformate or "syngas" (dry composition approximately 70%
HZ with roughly equal amounts of CO and COZ as major impurities, and unreacted
CH4 and NZ as minor impurities) is cooled to about 250?C, and then passed to
the
water gas shift reaction zone 268 for reacting most of the CO with steam to
produce more HZ and CO2:
CO + H20 ~ COZ + HZ
The hydrogen rich reformate still contains about 1% to 2% CO after water
gas shift, along with substantial amounts of carbon dioxide and water vapour.
For
high performance and longevity of a PEM fuel cell, it is necessary that CO
concentration be reduced well below 100 ppm and preferably below 10 ppm.
Consequently, the impure reformate is admitted by conduit 270 to the higher
pressure feed port of hydrogen PSA unit 205, including rotary PSA module 1'.
The
adsorbers 3' of rotary module 1' have a first zone 26' loaded with a suitable
dessicant such as alumina gel for removal of water vapour, a second zone 27'
loaded with an adsorbent selective for CO removal and at least partial bulk
removal
of CO2, and a third zone 28' loaded with an adsorbent suitable for polishing
removal of CO and at least partial removal of other impurities such as N2. The
invention provides numerous combinations and variations of suitable adsorbents
for
the three zones of the hydrogen PSA adsorbers, as recited in our
simultaneously
filed copending patent application "carbon monoxide removal from fuel cell",
the
disclosure of which is incorporated herein by this reference thereto.
CA 02324702 2000-10-27
-27-
Purified hydrogen light product from the hydrogen PSA module 1 is
delivered by conduit 147' to anode inlet 216, passed through anode channel
208,
and then exhausted from anode exit 218 back to a feed presurization
compartment
in the first vavle surface 10' of hydrogen PSA module 1', so as to retain
hydrogen
within the fuel cell anode loop including conduits 147' and 280 and anode
channel
208, while using the hydrogen PSA unit to reject impurities that otherwise
would
accumulate on the anode.
Exhaust second product gas from the hydrogen PSA module contains water
vapour, C02, and combustible values including H2, CO and any unreacted fuel
from the reformer. This gas is exhausted from valve face 10' by conduit 285 to
low pressure burner 290 where this fuel is oxidized completely, preferably
over a
suitable catalyst to ensure stable combustion of this low BTU gas and to
suppress
NOx formation. Burner 290 delivers hot products of combustion to heat
exchange channel 292, which is in countercurrent thermal contact for heat
recovery
to reformer reactor zone 264 and preheater zone 262. After cooling in channel
292 and further cooling in heat exchanger 296, the flue gas from burner 290 is
discharged to atmosphere by exhaust conduit 294.
Fuel processor 204 is also thermally integrated with a high pressure burner
300, to which a portion of the fuel from fuel pump 260 may be introduced by
conduit 301. Compressed air is supplied to burner 300 from feed compressor 101
through conduit 302, heat exchanger 296 (for recuperative heat exchange from
exhaust flue gas) and heat exchange channel 304 which is in countercurrent
thermal
contact for heat recovery from water gas shift reaction zone 268 and reformer
reactor zone 264 if the reforming reaction includes partial oxidation for net
exothermicity. Hot products of combustion [including nitrogen and unreacted
oxygen) from combustion chamber 300 are conveyed by conduit 310 to expander
turbine 315, coupled by shaft 316 to compressor 101. The combination of
compressor 101 and expander 315 are shown as a free rotor turbocompressor 320,
similar to an automotive turbocharger. [Alternatively a drive motor or a
generator may be coupled to shaft 316, for starting, power assist, or net
energy
CA 02324702 2000-10-27
-28-
delivery.] In Figs. 6 and 7, a blower 330 driven by motor 332 is provided to
boost the inlet pressure to compressor 101, if desired to assist the
compression of
feed air in normal operation, but preferably only as a starting device to
initiate
rotation of turbocompressor 320 in which case bypass valve 334 is opened
during
normal operation after starting.
The still hot gas discharged by expander 315 is discharged by conduit 336
to low pressure burner 290, providing heat and oxygen to support catalytic
combustion therein. Supplemental air or oxygen may be provided to low pressure
burner 290 if required during starting or any phase of normal operation.
While Fig. 6 shows an embodiment whose lower working pressure is
atmospheric, Fig. 7 shows an embodiment with vacuum applied to the oxygen and
hydrogen PSA units to improve their performance, perhaps to enable a reduced
working pressure of the fuel cell. Vacuum pump 338 receives the second product
exhaust gases at subatmospheric pressure from both the oxygen PSA and the
hydrogen PSA by respectively conduits 182 and 182', and delivers the combined
stream to the catalytic low pressure burner 290 by conduit 285. Vacuum pump
338
is provided as a turbocompressor 340 with expander 345 driving pump 338
through
shaft 346. Expander 345 is arranged in parallel or series with expander 315 to
expand hot gas delivered by conduit 310 from high pressure burner 300.
The combustion turbine embodiments for powering auxiliary compression
machinery have the important advantage of using readily available and low cost
turbocharger equipment. Fig. 8 shows an alternative embodiment using a rotary
internal combustion engine 400 to power the compressor 101 and optional vacuum
pump 103 of the oxygen PSA 206 by shaft coupling 405, while itself providing
vacuum suction if desired for the hydrogen PSA 205. Engine 400 is fueled at
least in part by the hydrogen PSA tail gas, and has a starter motor 410 (or
supplemental power output generator 410).
CA 02324702 2000-10-27
-29-
Engine 400 may be any type of internal combustion engine, but is here
shown as a Wankel engine. Working chambers 412 are defined between rotor 414
and casing 415. The rotor is coupled to drive shaft 405 by internal gear 416.
An
intake port 421, exhaust port 422 and spark plugs 423 are provided in casing
415.
A water cooling jacket 425 is provided. The engine has an air filter 426
delivering
air to carburetor 427, and to intake port 422. The carburetor mixes the air
with
hydrogen PSA exhaust gas delivered by exhaust conduit 182' to carburetor 427.
Fig. 8 shows details of an illustrative water management system. Product
water of fuel cell 202 is captured in separator 233 which has a cooling coil
430,
and is delivered to liquid water manifold 432. A portion of the water is
delivered
from manifold 432 to pump 435, and thence by flow control 436 to the oxygen
humidification chamber 230 and by flow control 437 to engine cooling jacket
425.
Hot water from the engine cooling jacket is flash evaporated and delivered
through
depressurization orifice 485 and conduit 486 to methanol reforming reactor
catalyst
zone 264, which in turn is in heat exchange contact with the engine exhaust in
channel 440. Engine exhaust is delivered from exhaust port 422 to channel 440
for
exhaust heat recovery to the endothermic methanol vapour phase reforming
reaction
in reactor zone 264, and then through emission control after-treatment
catalyst 443
and exhaust pipe 444 to atmosphere.
Reformate hydrogen is delivered from reactor zone 264 by conduit 450 to
feed hydrogen PSA unit 205. A portion of the reformate may be diverted to
carbuertor 427 from conduit 450 by flow control 452 as supplemental fuel for
engine 400.
A portion of the water condensate in water manifold 434 is delivered by
pump 460 to liquid fuel mixing chamber 465, which also receives liquid
methanol
fuel delivered by fuel pump 260. The flow rates of pumps 260 and 460 are
adjusted to achieve a desired concentration ratio of the water/methanol
mixture
exiting the mixing chamber 465 by conduit 466 delivering this mixture as fuel
cell
stack coolant circulated through cooling passage 468 through the fuel cell
stack
CA 02324702 2000-10-27
-30-
202. The coolant pressure is maintained high enough to maintain it in the
liquid
phase within the cooling passage. A portion of the water/methanol mixture
coolant
exiting cooling passage 468 is flash evaporated in separator 474 by
depressurization
valve 475 to approximately the working pressure of reforming reactor zone 264,
and the resulting vapour mixture is delivered by conduit 480 to the reforming
reactor catalytic zone 264. The balance of the water/methanol mixture coolant
is
repressurized and recirculated by pump 470 through cooling radiator 471 to
reject
fuel cell stack heat that has not been recovered to vaporize the water and
methanol
reactants.