Note: Descriptions are shown in the official language in which they were submitted.
CA 02325043 2000-09-20
WO 99/49984 PCT/IB99/00474
NASAL SPRAY DEVICE WITH ELASTOMERIC VALVE
Field Qf the Invention
The present invention relates to a spray device adapted to provide multiple,
unit
doses of a fluid, the device comprising an elastomeric, self sealing valve
which
achieves a clean stop-start flow of the fluid at low fluid volumes. More
particularly,
the invention relates to a device delivering unit volumes in the range from
about 1 to
about 100 pl.
Background of the Invention
Treatment of maladies affecting the nasal region, such as hay fever or
congestion due
to colds, has long been effected by means of a nasal spray device. More
recently it
has been recognised that the mucous membranes of the nasal cavity can be used
as a
convenient delivery site for drugs targeted at other areas of the body. See
for
example WO 92/11049 which discloses a pen shaped device for nasal
administration
of, particularly, insulin. A spray form is often convenient for such
treatments.
Treatment of the eyes can also conveniently be erected by a spray device.
Preferred
volume doses for such applications are generally low, down to less than 10 pl.
Typically, repeat dosing will be required in order to make a treatment fully
effective.
Achieving clean stop-start flow from spray devices delivering such low volumes
can
be difficult. Typical problems encountered include residual weeping from a
valve
after application, with the potential for back contamination, and valve
clogging.
It has now been found that an elastomeric valve, particularly a slit valve,
provides
clean stop-start flow without clogging, even if the sprayed fluid comprises a
finely
divided particulate solid.
Accordingly, it is an object of the present invention to provide a spray
device which
efficiently delivers multiple unit doses at low unit volume.
It is yet a further object of this invention to provide a spray cut-off device
which
provides a clean cut-off of the spray and is not susceptible to clogging.
Summar~r of the Invention
According to the present invention there is provided a spray device, adapted
to
provide one or more unit doses of a fluid, the or each dose having a volume in
the
CA 02325043 2000-09-20
WO 99/49984 PCT/IB99/004?4
2
range from about 1 to about 100 pl, the device comprising an elastomeric, self
sealing valve having a fluid side and a delivery side, the valve opening to
allow
passage of the fluid when pressure is applied to fluid on the fluid side and
sealing
when the pressure is removed.
Preferably the spray device is an electrostatic spray device which charges the
spray
before entry into the nostrils.
Detailed Description of the Invention
Fluff s
The spray device of the invention preferably comprises a fluid reservoir
containing a
pharmaceutically acceptable fluid, the fluid comprising a pharmaceutically
acceptable
treatment agent selected from medicaments, flavours, salts, surfactants and
mixtures
thereof. The fluid optionally comprises other adjuvants dissolved or dispersed
within
it. The fluid can be aqueous or non-aqueous. Suitable aqueous fluids include
water
and mixtures of water with water-miscible solvents such as glycerol, propylene
1 S glycol, or alcohols such as ethanol or isopropyl alcohol. Aqueous
emulsions can also
be used, either water-in-oil or oil-in water emulsions. Preferably the fluid
is an
aqueous solution, dispersion or oil-in-water emulsion. Suitable non-aqueous
fluids
comprise polyethylene glycols, glycerol, propylene glycol, dimethyl
isosorbide,
silicone oils, ketones, ethers and mixtures thereof.
Although not limited to any particular range of resistivity, the invention has
particular
application to low resistivity fluids, especially those having a bulk
resistivity of less
than 1 x 108 ohm.cm, preferably those having a resistivity of less than 1 x
104
ohm.cm, more preferably less than 1 x 103 ohm.cm. If necessary, the fluid may
comprise a resistivity modifier, such as a pharmaceutically acceptable salt,
in order to
bring the bulk resistivity within the required range.
The fluid is preferably a pharmaceutically acceptable intranasal carrier.
Preferably,
the nasal composition is isotonic, i.e., it has the same osmotic pressure as
blood and
lacrimal fluid. The desired isotonicity of the compositions of this invention
may be
accomplished using, for example, the sodium chloride already present, or other
pharmaceutically-acceptable agents such as dextrose, boric acid, citric acid,
sodium
tartrate, sodium citrate, sodium phosphate, potassium phosphate, propylene
glycol or
other inorganic or organic solutes. Sodium chloride is preferred particularly
for
buffers containing sodium ions. Further examples of sodium chloride
equivalents are
CA 02325043 2000-09-20
WO 99/49984 PCT/I899/00474
3
disclosed in ReminQton's Pharmaceutical Sciences pp. 1491-1497 (Alfonso
Gennaro
18th ed. 1990), which is herein incorporated by reference.
Medicaments
The fluid can comprise a wide range of medicaments. By "medicament" is meant a
drug or other substance intended to have a therapeutic effect on the body.
Suitable
levels of the medicament are from 0.001 to 20%, preferably from 0.01 to 5%,
more
preferably from 0.1 to 5%. It will be appreciated that the levels of specific
medicaments will depend on many factors including their potency, safety
profile,
solubility / ease of dispersion and intended effect. The medicament, when
used, can
be one which is intended to have an effect at the site of application, such as
a
decongestant, antihistamine or anti-inflammatory drug, or it may be intended
for
systemic absorption such as an anti-viral, anti-depressant, anti-emetic, anti-
pyretic
medicament or a hormone or such-like. The medicament can be soluble in the
fluid
or can be an insoluble, finely divided particulate liquid or solid dispersed
within the
fluid.
Suitable decongestants include oxymetazoline, tramazoline, xylometazoline,
naph-
azoline, tetrahydrazoline, pseudoephedrine, ephedrine, phenylephrine, their
pharmaceutically acceptable salts, such as the hydrochlorides, and mixtures
thereof.
Preferred decongestants are selected from oxymetazoline, xylometazoline, their
pharmaceutically acceptable salts and mixtures thereof. Especially preferred
for use
herein is oxymetazoline hydrochloride which is soluble in water. When used in
the
compositions of the present invention, the decongestant is preferably present
at a
concentration of from about 0.01 % to about 3.0%, more preferably from about
0.01 % to about 1 %.
Antihistamines useful to the present invention include, but are not limited
to, fast-
acting, histamine H-1 receptor antagonists. Such H-1 receptor antihistamines
may be
selected from among the following groups of antihistamines: alkylamines,
ethanol-
amines, ethylenediamines, piperazines, phenothiazines, piperidines. Examples
of
useful fast acting antihistamines include acrivastine, carbinoxamine,
diphenhydramine,
chloropheniramine, brompheniramine, dexchloropheniramine, doxylamine,
clemastine, promethazine, trimeprazine, methdilazine, hydroxyzine, pyrilamine,
tripelennamine, meclizine, triprolidine, azatadine, cyproheptadine, rocastine,
phenindamine or pharmaceutically acceptable salts and mixtures thereof. Other
useful antihistamines include terfenadine, azelastine, cetirizine, astemizole,
ebastine,
CA 02325043 2000-09-20
WO 99/49984 PCT/IB99/00474
4
ketotifen, lodoxamide, loratadine, levocabastine, mequitazine, oxatomide,
setastine,
tazifylline, temelastine or pharmaceutically acceptable salts and mixtures
thereof.
When used in the compositions of the present invention, the antihistamine
component
is preferably present at a concentration of from about 0.01 % to about 3.0%,
more
preferably from about 0.01 % to about 1 %.
The medicament can also be an anti-inflammatory agent such as a
corticosteroid.
Particularly preferred agents within this class are glucocorticoids selected
from the
group consisting of beclomethasone, flunisolide, fluticasone, memetasone,
budesonide, pharmaceutically acceptable salts thereof and mixtures thereof.
When
used in the compositions of the present invention, the anti-inflammatory agent
is
preferably present at a concentration of from about 0.001 % to about 0.1 %,
more
preferably from about 0.01 % to about 0.1 %.
Also useful herein are xanthine derivatives such as caffeine and
methylxanthine and
the like; antiallergics; mucolytics; anticholinergics; non-opiate analgesics
such as
acetaminophen, acetylsalicylic acid, ibuprofen, etodolac, fenbuprofen,
fenoprofen,
ketorolac, flurbiprofen, indomethacin, ketoprofen, naproxen, pharmaceutically
acceptable salts thereof and mixtures thereof; opiate analgesics such as
butorphanol;
leukotriene receptor antagonists; mast cell stabilisers such as cromolyn
sodium,
nedocromil and lodoxamide; and lipoxygenase inhibiting compounds.
Further examples of suitable medicaments can be found in W097146243,
EP-A-780127, US-A-5,124,315, US-A-5,622,724, US-A-5,656,255 and
US-A-5, 705,490
Flavours
Various flavouring and/or aromatic components {e.g., aldehydes and esters) can
be
used in the fluids of the invention. These include, for example, menthol,
camphor,
eucalyptol, benzaldehyde (cherry, almond); citral (lemon, lime); neral;
decanal
(orange, lemon); aldehyde C-8, aldehyde C-9 and aldehyde C-12 (citrus fruits);
toiyl
aldehyde (cherry, almond); 2,6-dimethyl-octanal (green fruit); 2-dodecenal
(citrus,
mandarin); and herbal components such as thyme, rosemary and sage oils.
Additional
aromatic components suitable for use in the present invention include those
described
in U.S. Patent 4,,136163 to Watson et a(., U.S. Patent 4.459.425 to Amano et
al.,
and U.S. Patent 4.230,688 to Rowsell et al.; all of which are herein
incorporated by
reference. Mixtures of these aromatics can also be used.
CA 02325043 2000-09-20
WO 99/49984 PCT/IB99/00474
Surfactants
The fluid can also comprise one or more pharmaceutically acceptable
surfactants.
Such surfactants can be useful for dispersing or emulsifying medicaments or
flavours,
for enhancing absorption across the nasal membrane or as treatment agents in
their
5 own right, such as for softening earwax. The surfactants can be anionic,
nonionic,
cationic or amphoteric, preferably they are nonionic. Typical nonionic
surfactants
useful herein include: polyoxyethylene derivatives of fatty acid partial
esters of
sorbitol anhydrides such as polysorbate 80; polyoxyethylene derivatives of
fatty acids
such as po(yoxyethylene 50 stearate, as well as oxyethylated tertiary octyl
phenol
formaldehyde polymer (available from Sterling Organics as Tyloxapol) or
mixtures
thereof. The usual concentration is from 0.1% to 3% by weight.
alts
The fluid can also comprise one or more pharmaceutically acceptable salts. The
salt
can mineral salts such as e.g. sodium chloride, or salts of organic acids such
as
sodium citrate.
Other adjuvants
The fluid can further comprise other ingredients such as thickeners,
humectants,
suspending aids, encapsulating aids, chelating agents and preservatives.
The viscosity of the compositions may be maintained at a selected level using
a
pharmaceutically-acceptable thickening agent. Suitable thickening agents
include, for
example, xanthan gum, methyl cellulose, microcrystalline cellulose,
carboxymethyl
cellulose, chitosan, hydroxypropyl cellulose, hydroxypropyl methyl cellulose,
hydroxymethyl cellulose, hydroxyethyl cellulose, carboxyvinyl polymer,
carbomer,
and the like or pharmaceutically acceptable salts thereof. Mixtures of such
thickening
agents may also be used. The preferred concentration of the thickener will
depend
upon the agent selected. The important point is to use an amount which will
achieve
the selected viscosity. Viscous compositions are normally prepared from
solutions by
the addition of such thickening agents.
Fluids useful in the present invention can also comprise from about 0.01 % to
about
5% of a humectant to inhibit drying of the mucous membrane and to prevent
irritation. Any of a variety of pharmaceutically-acceptable humectants can be
employed including, for example sorbitol, propylene glycol, polyethylene
glycol,
glycerol or mixtures thereof. As with the thickeners, the concentration will
vary with
CA 02325043 2000-09-20
WO 99/49984 PCT/1B99/00474
6
the selected agent, although the presence or absence of these agents, or their
concentration is not an essential feature of the invention.
A pharmaceutically-acceptable preservative is generally employed to increase
the
shelf life of the compositions of the present invention. A variety of
preservatives
including, for example, benzyl alcohol, parabens, phenylethyl alcohol,
thimerosal,
chlorobutanol, chlorhexidine gluconate, or benzalkonium chloride can be
employed.
The most preferred preservative system for use herein comprises a combination
of
benzalkonium chloride, chlorhexidine gluconate and disodium EDTA as a
chelating
agent. A suitable concentration of the preservative will be from 0.001% to 2%
based
on the total weight, although there may be appreciable variation depending
upon the
agent selected.
Spray devices and saray characteristics
The spray device will generally comprise a means for controlling the dosage of
the
dispensed fluid. This can be as simple as an on-off switch which allows the
user to
control the dosage according to his or her needs. The spray device is adapted
to
provide one or more unit fluid doses, preferably multiple fluid doses, each
with a
volume in the range of from about 1 to about 100 ~I, preferably from about 1
to
about 20, more preferably from about 5 to about 15 ~1. The dose volume is
preferably pre-set but it can also be adjusted by the user to a desired
volume. The
device suitably comprises a reservoir for holding the fluid and a dosing means
for
selectively delivering a unit dose from the reservoir to the spray generator.
The
dosing means can be, for example, a metered valve or a syringe pump.
The device is preferably adapted to produce a spray having a fluid ligament,
the
ligament extending from the nosepiece and having a nosepiece end and a
delivery
end, the spray further comprising a spray cone diverging from the delivery end
of the
ligament. By "nosepiece end" is meant the point at which a plane (hereinafter
the
nosepiece plane) drawn perpendicular to the axis of the ligament and just
touching
the exterior of the nosepiece would intersect the centre of the ligament. The
ligament
preferably has a length of from about 1 to about 20 mm, more preferably from
about
1 to about 10 mm, yet more preferably from about 2 to about 8 mm, and
especially
from about 3 to about 6mm from the nosepiece end to the delivery end.
In preferred embodiments the spray cone has a cone angle of from about 10 to
about
90°, preferably 20 to about 50°, more preferably from about 30
to about 40°. In
electrostatic devices, the length of the ligament and the spray cone angle can
be
CA 02325043 2000-09-20
WO 99/49984 PCT/IB99/00474
7
adjusted by varying the viscosity or surface tension of the fluid, by varying
the fluid
flow rate or exit velocity, or by varying the electrical field strength
through applied
voltage, potential gradient or by use of a field intensifying electrode.
The total length of the ligament can be, and preferably is, longer than the
length from
the nosepiece end to the delivery end since the ligament preferably originates
from a
point on the device side of the nosepiece plane, such as from an 'elastomeric,
self
sealing valve as disclosed herein, and passes through a passage in the
nosepiece.
Suitably the distance from the point of origin of the ligament to the
nosepiece plane is
in the range from about 2 mm to about 15 mm, preferably from about 3 to about
10
mm and more preferably from about 5 to about 9 mm. In this way the nosepiece
can
be employed as a field intensifier which helps to control the ligament length.
For this
purpose the nosepiece is preferably a non-conducting material such as a
plastic which
can be e.g. polypropylene but is preferably a soft thermoplastic eiastomer
which
provides for greater comfort if held against the nose. Elastomers described
herein for
the self sealing valve are also suitable for the nosepiece.
Electrostatic devices suitable for ligament mode spraying are described in
WO 96/40441, in EP-A-501,725 and in co-pending application PCT/GB97/02746.
Preferably the present device is a device according to embodiments of EP-A-
501,725
or PCT/GB97/02746 in which a jet is created by mechanical means and an applied
high voltage leads to the jet or ligament breaking up into a spray cone. The
jet can be
generated by, for example, a syringe pump. Suitable jet velocities are from
about 0.5
to about 8, preferably from about 1 to about 3 ms''.
A suitable high voltage is in the range from about 1 kV up to about 15 kV,
preferably
from about 2kV to about IOkV and more preferably from about 2kV to about SkV.
The voltage can conveniently be applied, even within the constraints of a
small hand-
held device, from a low voltage (1.SV is sufficient) battery coupled to a step-
up
transformer. The battery is preferably of the long-life type and can be
rechargeable.
If a metal syringe pump is used, the voltage can be applied to the fluid
through the
pump which is preferably encased in an insulating plastic housing.
Alternatively, the
fluid can be charged by an electrode inserted into the fluid. The generator
can be
activated by the user by means of an external switch which can also be used to
mechanically prime the pump. Preferably the switch includes a metal portion by
means of which the user completes an earth return path to the high voltage
circuit. A
suitable arrangement for the overall device construction is described in
PCT/GB97/02746. In this way the user does not acquire a net charge. Alternate
CA 02325043 2000-09-20
WO 99/499$4 PCT/IB99/00474
8
arrangements, whereby an alternating voltage is applied, can also be used to
prevent
charge build-up.
The device is activated to deliver the spray. The ligament of the spray
extends
through the nostril opening, into the vestibule and preferably to within a
short
distance of the nasal valve opening, before breaking up to form the spray
cone. The
device can be constructed simultaneously to dispense two sprays, directed to
each of
two nostrils. The two sprays can be generated from two separate dosing means
or
can be provided from a single source such as by using a 'Y' shaped valve to
split a
single jet into two.
In order to provide clean cut-off at low unit volumes the device comprises an
elastomeric, self sealing exit valve having a fluid side and a delivery side,
the valve
opening to allow passage of the fluid when pressure is applied to fluid on the
fluid
side and sealing when the pressure is removed. By "exit valve" is meant that
the
elastomeric valve is the final dispensing valve and that there are no other
elements of
the device which mechanically, restrict or modify the flow of the fluid on the
downstream side of the valve. In highly preferred embodiments herein the valve
is a
slit valve. The valve can comprise a single slit or tow or more intersecting
slits, to
form a cross shape for example. Preferably, however, the valve comprises a
single
slit. Although the valve can be flat it is preferably dome-shaped by which is
meant a
non-planar valve having a recess such as with a hemispherical or frustoconical
dome.
In preferred embodiments the valve is essentially in the form of a
hemispherical dome
having a flange along its perimeter so that a collar can be fitted to retain
the valve in
the device. The diameter of the valve, including the flange, is typically from
about 2
to about 6 mm with the dome portion having a diameter of from about 1 to about
4
mm, typically about 2.Smm and a thickness from inside to out of from about 0.5
to
about 1.5 mm, suitably about 1 mm. The valve need not be of uniform thickness.
In
preferred embodiments the valve dome's exterior surface is hemispherical
whereas the
internal surface is formed with a small flat at the top of the dome where the
slit is
formed. Suitable slit widths are from about 50 to about 400 Vim, preferably
from
about 150 to about 250 um. It is to be understood that the slit width refers
to the
longest dimension of the slit when first created. The term "elastomer" herein
refers
to a material which is both elastically compressible and elastically
extensible. A wide
range of elastomers can be used, including but not limited to polyurethanes;
chloroprene, butyl, butadiene and styrene-butadiene rubbers, and silicone
elastomers
such as 2 part room temperature vulcanising (RTV) silicones. Preferred for use
CA 02325043 2000-09-20
WO 99/49984 PCT/IB99/00474
9
herein are the 2 part silicone RTVs. Suitable silicone RTVs are available
under the
trademark NuSil and have a hardness of from about 30 to about 80 Shore A,
preferably from about 40 to about 70 Shore A. The elastomers can optionally be
mixed with a suitable plasticiser or foaming agent to make them more
compressible.
The elastomer may also have other materials dispersed within it in order to
modify
the its properties, such as its conductivity. If elastomers of low tear
strength are
employed, the slit width may grow if the slit is held open for a prolonged
period of
time. The slit valve can be formed by piercing an injection moulded
elastomeric seal,
of the same dimensions and shape as the intended valve, with a pin having a
sharpened tip. The slit width is roughly proportional to the pin width. The
pin can
be a flat blade or can have a polygonal or round cross-section. The pin
preferably has
a polygonal or, especially, a round cross-section. It has been found that
sharp-edged
pins create a cut which behaves in use as a flap rather than a hole. This can
lead to a
jet which is not straight or even to the creation of two or more jets which
may lead to
I S unreliable or unpredictable spraying. Suitable pin diameters are from
about 100 to
350, more preferably from 150 to 250 ~tm in diameter. When silicone elastomers
are
used it is preferred that the piercing pin is withdrawn rapidly after forming
the slit to
avoid undesired slit growth. It has further been found that the geometry of
the
elastomeric seal and the method of piercing have a significant effect on the
effectiveness and reproducibility of slit formation. More reliable slit
formation is
obtained if the seal is pierced from the inside of the dome rather than from
the
outside.
The valve opens when pressure is applied to fluid on the fluid side and seals
when the
pressure is removed. As mentioned earlier, pressure can be applied to the
fluid by of
a dosing means such as a syringe pump. The applied pressure is suitably in the
range
from about 200 to about 5000 mbar (20 to 500 kPa), preferably from about 500
to
about 3000 mbar (50 to 300 kPa). The flow rate through the valve is generally
proportional to the pressure applied and is suitably in the range from about 5
to about
50, more preferably from about 5 to about 30 ~Is'~. At such pressures and with
the
disclosed valve type and slit dimensions a straight fluid ligament with an
exit velocity
of from about 0.5 to about 8 preferably from about 1 to about 3 ms ~ is
obtained.
The diameter of the issuing ligament is partly determined by the flow rate and
is
generally less than the width of the slit valve. Depending on the flow rate,
ligament
diameters of less than 50 p.m can be achieved, even when a valve slit width of
200
Itm is employed. The ligament diameter strongly influences the particle size
of the
CA 02325043 2000-09-20
WO 99/49984 PCT/IB99/00474
spray after break-up into the spray cone, the particle size being broadly
similar to the
ligament diameter. It is a feature of the ligament mode electrostatic spraying
herein
that a tightly distributed, almost mono-disperse spray is obtained. It is thus
possible
to achieve a spray with a median droplet size of from 20 to about 80,
preferably from
5 about 30 to about 70 pm, more preferably from about 40 to about 60 p,m, the
particle
size distribution generally having a standard deviation of less than 5,
typically less
than 2 Vim, and preferably less than 1 ~tm. It is commonly understood that,
for nasal
spraying, a particle size of 10 pm or less is desirable so that the particles
are not
carried' through into the lungs. It is believed, however, that having an
electrostatic
10 charge on the spray particles makes them much less likely to be carried
beyond the
nose since the charged particles tend to find an earthed surface rather
quickly.
The clean stop performance of the tip valve can be further improved by
introducing a
pressure relief feature behind the valve. This would take the form of a by-
pass to the
fluid reservoir so that any residual fluid pressurised by relaxation of the
elastomer
would return to a reservoir rather than dripping from the valve exit.
If necessary, several self sealing valves can be used in tandem to achieve
higher
volume throughputs whilst retaining the advantageously small spray particle
sizes.
The seals from which the valves are made can conveniently be manufactured by a
conventional injection moulding process.
Methods
The spray device herein is suitable for spraying into a bodily cavity,
particularly into
the nose, mouth or ears of a human. The low volume and gentle spray also make
it
suitable for e.g. ophthalmic spraying. Preferably the device is a nasal spray
device. A
preferred method of administering a fluid to the nasal cavity from the spray
device
comprises spraying the fluid into the nasal cavity without substantial
penetration of
the device into the nostrils. By "without substantial penetration into the
nostrils"
herein is meant that there is no insertion of a nozzle or such-like into the
nasal
vestibule. In use, the nosepiece of the device is preferably placed in contact
with the
nostril opening to obtain the full benefit of the field intensifying effect
described
herein in relation to the nosepiece. If pressure is applied by the user, for
certainty of
contact or to assist in orientation there may be some flaring of the nostril
or overlap
with the septum cartilage but nevertheless the nosepiece will not be
completely
surrounded by the nostril.
CA 02325043 2000-09-20
WO 99/49984 PCT/IB99/00474
11
The invention will now be described by way of example only, with reference to
the
accompanying drawings in which:
Fig. 1 is a sectional view through a human nose.
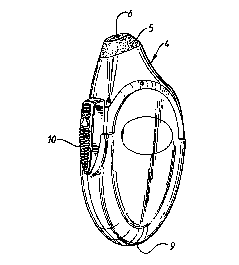
Fig. 2 is a perspective view of a spray device according to the invention.
Fig. 3 is a greatly simplified sectional view of the spray device of Figure 2
showing
the relationship of the elastomeric nozzle to the nosepiece and dosing means.
Fig. 4 is a schematic part section showing a spray issuing from a device
according to
the invention.
Fig. 5 is a enlarged sectional view of an elastomeric exit valve according to
the
invention.
Refernng to Fig. 1, the nose comprises a nasal cavity 1 in which are located
the
turbinates 2, convoluted finger-Iike structures covered with epithelium and
which are
susceptible to inflammation. The exterior opening to the nasal cavity is
through two
nostrils 3. Since the turbinates are located in the posterior part of the
nasal cavity it
has generally been difficult to spray them effectively without inserting a
spray device
into the nostrils.
Referring to Figures 2 and 3, there is shown a spray device 4 fitted with a
soft
elastomeric nosepiece 5 which is designed to fit against a user's nostril
opening
without being inserted into the nostril. The nosepiece is provided with a
passage 6
which connects to a syringe pump 7, the interior detail of which is not shown,
which
is used to generate the ligament of the spray through elastomeric exit valve
8. The
insulating casing 9 of the device further encloses (not shown) a replaceable
fluid
reservoir, battery, transformer and electronic circuitry for supplying a high
voltage
from the transformer to the fluid. The device is supplied with an actuating
mechanism 10 which mechanically primes the pump and triggers its release to
deliver
a pre-set unit dose of the fluid. The actuating mechanism includes a metal
portion to
provide an earth return from the user to the high voltage circuitry.
Referring now to Figure 4, a spray comprises ligament 11 and spray cone 12
having a
cone angle B. In practice the cone angle can be measured by spraying from a
fixed
distance onto disclosing paper and then measuring the diameter of the spray
pattern
or by direct viewing of the spray using high speed video photography with back
lighting. The ligament has a delivery end 13 where the ligament breaks up into
the
spray cone and a nosepiece end 14 defined as the point where plane A-A
intersects
CA 02325043 2000-09-20
WO 99/49984 PCT/IB99/00474
12
ligament 11. Plane A-A is drawn perpendicular to the axis of the ligament,
just
touching the tip of nosepiece 5.
Figure 5 illustrates an elastomeric exit valve 8 according to the invention.
The
nozzle, which is circularly symmetrical, comprises a flange 15 for securing
the nozzle
to the spray device, a domed head 16, a recess 17 and a slit 18 extending from
the
recess to the top of the dome. The nozzle is formed by injection moulding a
seal of
essentially the same construction and then piercing the dome with a sharpened
pin, of
round cross-section and 200 ~m diameter, from the inside of the recess.
Examples
The following examples further describe and demonstrate embodiments within the
scope of the present invention. The examples are given solely for the purpose
of
illustration and are not to be construed as limitations of the present
invention.
Example 1
A fluid of the present invention is prepared by combining the following
components
utilising conventional mixing techniques similar to that described below.
Component Wt
oxymetazoline hydrochloride 0.31
sodium citrate dehydrate 1.75
citric acid 0.35
Tyloxapol 0.70
chlorhexidine digluconate 0.054
benzalkonium chloride 0.02
camphor 0.04
eucalyptol 0.02
disodium EDTA dehydrate 0.01
distilled water q.s. 100m1
In an appropriately sized vessel, the above listed ingredients are added one
at a time
to water with mixing, allowing each to dissolve before adding the next. After
all the
ingredients have been added, purified water is used to bring the batch to the
appropriate weight. The solution has a bulk resistivity of 120 ohm.cm. The
solution
CA 02325043 2000-09-20
WO 99/49984 PCT/IB99/00474
13
is charged into a flexible laminate reservoir and fitted into an electrostatic
spray
device of the kind indicated in figure 2. The device further comprises an
elastomeric
valve as shown in Fig. 5, the valve being held in place by a screw collar
clamping the
flange. The nosepiece of the device is held against the nostril and the device
directed
such that the spray ligament will enter the nostril. On actuation of the
device, the
syringe pump builds pressure behind the valve to about 100 kPa (1000 mbar),
opening the slit valve to a width of about 50 um and dispensing 8 ~tl of the
solution
over a period of about I second. When the fluid is dispensed the valve closes
completely, preventing further fluid egress and contamination of the fluid in
the
reservoir. The dispensed fluid provides relief from nasal decongestion without
causing noticeable wetness either on or inside the nose. The iow dose volume
and
gentle delivery have the result that the user does not sense any appreciable
physical
impact from the spray.
Example II
A further fluid is prepared by combining the following components utilising
conventional mixing techniques similar to that described in Example I.
Component V~rgt "~o
flunisolide 0.025
chlorpheniramine 0.350
levocabastine 0.0125
propylene glycol 2.000
polyethylene glycol 1.000
ethylenediamine tetraacetic acid 0.050
benzalkonium chloride 0.010
distilled water q.s. 100m1
Intranasal spray administration, in the manner of Example I, of approximately
10 ~tl
of the fluid composition is used to provide relief from allergy or allergy-
like
symptoms.
Example III
A fluid of the present invention is prepared by combining the following
components
utilising conventional mixing techniques similar to that described in Example
I.
CA 02325043 2000-09-20
WO 99/49984 PCT/IB99/00474
14
Component art oho
beclomethasone diproprionate, monohydrate 0.042
chlorpheniramine 0.500
Avicel RC - 5911 0.200
dextrose 5. I 00
polysorbate 80 0.050
benzalkonium chloride 0.020
phenylethyl alcohol 0.025
distilled water q.s. 100m1
lmicrocrystalline cellulose and sodium carboxymethyl cellulose, supplied FMC
Corporation.
Intranasal spray administration, in the manner of Example I, of approximately
10 ~tl
of the fluid composition is used to provide relief from allergy or allergy-
like
symptoms.