Note: Descriptions are shown in the official language in which they were submitted.
CA 02325643 2000-11-10
2
CROSSLINKED POLYSACCHARIDE, OBTAINED BY CROSSLINKING
WITH SUBSTITUTED POLYETHYLENE GLYCOL, AS
SUPERABSORBENT
The present invention relates to new reticulated polysaccharides obtained
from polysaccharides by crosslinking with at least one crosslinker selected in
the
group constituted by substituted (preferably halogenosubstituted, more
preferably
substituted by Br, Cl or I) polyethyleneglycols.
The present invention also relates to processes for preparing said crosslinked
polysaccharides.
The present invention also relates to the use of the crosslinked
polysaccharides
of the invention as superabsorbents, more particularly as inexpensive and/or
hypoallergenic biodegradable superabsorbents.
The invention also relates to superabsorbents mixtures comprising at least one
of crosslinked polysaccharides of the invention.
BACKGROUND OF THE INVENTION
CA 02325643 2000-11-10
3
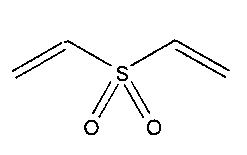
Anbergen and Oppermann' have studied the elasticity and the swelling behaviour
of sodium carboxymethylcellulose and hydroxyethylcellulose, chemically
crosslinked
with divinylsulfone 1.
In a patent issued in 1995, Kabraz reported the sorption capacity of
hydroxypropylcellulose, crosslinked with different concentration of
divinylsulfone (from
0.28 to 2.98 weight %). His best result showed a water sorption capacity of 44
g/g with
a crosslink of 0.91 weight %. The author also mentions that other
hydrophobically
modified carbohydrate polymers can be chosen, such as hydroxypropylstarch.
More recently, a patent has been granted to SCA Hygiene Products AB3 which
extents the study with divinylsulfone to low-cost, readily available,
renewable starting
materials such as carboxymethylcellulose, carboxymethylstarch, and others.
According
to the authors, results may be obtained with a mixture of
carboxymethylcellulose:
hydroxyethylcellulose (3 : 1 ) which absorbs close to 95 g of synthetic urine
per g of
polymer after free swelling for 120 min. In this patent, however, the quantity
of
divinylsulfone used is not reported. The same company has extended their work
with
divinylsulfone to other polysaccharides containing acidic groups4. It appears
that the best
result was obtained with carboxymethylcellulose crosslinked with 14 mol% of
divinylsulfone. This results in a centrifugal retention capacity of 111 g/g
with synthetic
urine. On page 6 of the patent4, it mentionned that the superabsorbent
polysaccharides
combine high absorption capacity with control of bacterial growth and control
of odour,
as well as with biodegradability. There is however no evidence that such
compounds
CA 02325643 2000-11-10
4
would be biodegradable, particularly since it is well known that
carboxymethylcellulose
and carboxymethylstarch are not completely biodegradable. Moreover, it appears
that
the diethylsulfone diether linkage is not biocompatible.
Therefore, there is still a need for new polysaccharide-based superabsorbents
with
a significant biodegradability.
DESCRIPTION OF THE INVENTION
The new reticulated polysaccharides according to the invention are obtained by
crosslinking of a polysaccharide with at least one substituted polyethylene
glycol.
A preferred embodiment of the invention is constituted by reticulated starches
obtained by crosslinking starches with at least one polyethylene glycol. As a
matter of
exemplification, starches crosslinked with dichloropolyethylene oxide are
preferred
particularly since starch is a renewable and inexpensive starting material.
The reticulated starches according to the invention are characterized by
reticulation occuring at first on the OH group on the C6 carbon atom of the
polymeric
unit, then on a C2 or C3 carbon atom of the polymeric polysaccharide.
CA 02325643 2000-11-10
The crosslinker used for preparing the crosslinked starches of the invention
is a
substituted polyethylene glycol .
Polyethylene glycol (PEG 2,) has been used in blended starches for specific
applications
5-9. Starch can react with ethylene glycol in the presence of an acid catalyst
at elevated
temperature to yield crude mixtures of glycol glucosides by cleavage of
glycosidic
bonds'°°". By high energy radiation, it is possible to link
polyethylene oxide) to starch'z.
It has also been reported that starch dialdehyde reacts with polyethylene
glycol to give
an acetal functionality'3; Ethylene oxide can be grafted to starch by anionic
polymerization'4~' S and polyethylene glycol chloroformate derivative'6 or
ethylene glycol
chloroformate" can also be used to crosslink starch. Moreover, diglycidyl
ether '8,
polyglycidyl ethers'9 and ethylene glycol dimethacrylatez° are among
known
crosslinkers.
Polyethylene glycols are biodegradable aerobically and anaerobicallyz'
Starches crosslinked with dichloro polyethylene oxides 3 appear to be
biodegradable. The study of the crosslinker length was performed by preparing
dichloro
derivatives of di 4, tri 5 and tetra ethylene oxide 6 (SOCI.,, pyridine,
benzene, reflux).
The experimental result thereby obtained show that carboxymethylstarch
crosslinked
with 0.62% of divinylsulfone 7, gives a water retention of 23 g/g in 0.9%
saline solution,
compared to 30 g/g when crosslinked with 9.85% of dichlorotriethylene dioxide
8. The
starch-citraconic half ester crosslinked with 0.6% of divinylsulfone 9 were
found to
CA 02325643 2000-11-10
6
exhibit a good water retention (25 g/g). The effect of divinylsulfone and
dichlorotriethylene dioxide concentrations on the water retention of
crosslinked
carboxymethylstarch in 0.9% saline solution, are shown on figures 1 and 2.
Even if 15
times more quantity of dichlorotriethylene dioxide is required to reach the
maximum
water retention, the choice of the former is still advantageous since
divinylsulfone is very
expensive.
Crosslinked carboxymethylstarches (figures 1 and 2) are prepared in two steps.
Starch is first alkylated with chloroacetic acid, thus the alkylated starch
thereby
obtained is crosslinked.
The applicant has also discover that it is possible to perform the
crosslinking
before the alkylation without negative effect on the water retention.
One important aspect is to prepare starch derivatives without starch
gelatinisation. This result was achieved with a lower pH with NaOH 3%, lower
temperature and by adding sodium chloride solution.
Other polyethylene glycol derivatives may be used as crosslinkers. For
instance,
other dihalides (Br, I) could be prepared as well as dichloroformates
derivatives 10;
diacyl chloride derivatives 11 and; diglycidyl derivatives 12. Moreover,
carboxymethylstarch could be replaced by maleate 13, succinate 14, 15,
citraconic 16,
CA 02325643 2000-11-10
7
phthalates half esters 17, sulfate 18, sulfonate 19, phosphate starches 20 and
cationic
starches 21 22 as well. Other examples are carboxylate acetal 23, citrate 24,
acognitate
25, N,N-dicarboxymethylamine 26, N,N,N-tricarboxymethylammonium 27, citrate
28;
the last 3 compounds utilising epichlorohydrin as linker arm; and
ethylenediamitetraacetate (EDTA) conjugate 29.
Examples of starches useful as starting materials are: corn, wheat, rice,
potato,
tapioca, waxy maize, sorghum, sago, waxy sorghum, physically modified starches
and
non-gelatinized starches. Other polysaccharides can also be used such as
cellulose, gums,
dextrines, polygalactomannan, and chitosan. In all cases, anionic and cationic
functionalizations of the selected polysaccharide could be introduced before
or after the
crosslinking
Water Retention Unit (WRU)
The Water Retention Unit (WRU) has been measured by the following procedure.
Two empty 15 ml test tubes (duplicata) are weighted (Te). Samples around 0.3g
~
O.OOSg (S) are introduced into both tubes. Saline solution ( 10 ml, 0.9%) is
added and the
gel is vortexed for 1 minute then allowed to stand for 15 minutes. Tubes are
centrifuged
at 2000RPM for 5 minutes and the upper aquous layer is decanted at 30°
angle for 5
seconds and tubes are weighted again (TS). In case there is no aquous layer,
the
CA 02325643 2000-11-10
8
procedure is repeted with 0.2g ~ 0.005g samples. WRU is calculated according
to the
equation (1) and is expressed in g of saline solution per g of absorbent.
WRU = Ts-Te-S ( 1 )
S
Those skilled in the art will gain further and better understanding of this
invention
and the new and important advantages, which is offered from the following
illustrative,
but not limiting, examples of this invention as it has been carried out.
EXAMPLE 1
Preparation of the crosslinker 1,5-dichloro-3-oxapentane (dichlorodiethylene
oxide 4).
lO.Og (0.094 mol) of diethylene glycol were dissolved in 100 ml benzene. To
this
solution, 30.8 ml (4eq.) of pyridine were added, followed by a dropwise
addition of 27.5
ml (4eq.) of thionylchloride. The reaction mixture was heated at reflux for 24
hours. At
room temperature, the organic layer was decanted from the pyridinium
hydrochloride
salt, washed with 150 ml of water, dried on anhydrous sodium sulfate, filtered
and
evaporated to dryness to give 8.4g ( 65% yield) of the dichloride as a light
yellow liquid,
used without further purification. Infrared spectroscopy showed the absence of
hydroxyl
band.
IR (neat): 2964, 2865, 1450, 1125, 747, 669 cm '.
CA 02325643 2000-11-10
9
EXAMPLE 2
Preparation ofthe cross-linker 1,8-dichloro-3,6-dioxaoctane
(dichlorotriethylene
dioxide 5).
lO.Og (0.067 mol) of triethylene glycol were treated as example 1 with 22 ml
(4eq.) of
pyridine and 19m1 (4eq.) of thionylchloride to give 8.8 g (62% yield) of the
dichloride
as a yellow oil, used without further purification. Infrared spectroscopy
showed the
absence of hydroxyl band.
IR (neat): 2962, 2870, 1452, 1123, 747, 666 cni'.
EXAMPLE 3
Preparation of the crosslinker 1,11-dichloro-3,6,9-trioxaundecane
(dichlorotetraethylene trioxyde 6).
lO.Og (0.052 mol) of tetraethylene glycol were treated as example 1 with 17 ml
(4eq. )
of pyridine and 15 ml (4eq.) of thionylchloride to give 7.2g (61 % yield) of
the dichloride
CA 02325643 2000-11-10
as a yellow oil, used without further purification. Infrared spectroscopy
showed the
absence of hydroxyl band.
IR (neat): 2951, 2870, 1459, 1118, 746, 665 cm'.
5
EXAMPLE 4
Preparation of a carboxymethylstarch, crosslinked with divinylsulfone (0.62%),
compound 7.
2.Og (0.0123 mol) of wheat starch A ( Supercell 1201-C, ADM/Ogilvie) was
suspended
in 40 ml of deionized water. Under stirring, 3.5 ml 30% NaOH (0.0263 mol, 2.1
eq.) was
added dropwise and the solution stirred at room temperature for 1 hour.
Chloroacetic
acid ( 1.16 g, 0.0123 mol, l eq), dissolved in 10 ml of deionized water and
neutralized with
1.6 ml 30% NaOH (0.0123 mol, 1 eq.) was added dropwise and the reaction
mixture was
heated at 70°C for 24 hours. At room temperature, l2mg (0.62 weight %)
of
divinylsulfone dissolved in l Oml acetone, was added dropwiseand the solution
was stirred
for 2 hours. The polymer was precipitated with 100 ml of methanol, triturated
in a
blender, washed with 3 portions of 60 ml methanol, filtered and dry at
60°C for 16 hours
to give 1.97g of a white solid. The solid was grinded with a coffee grinder to
get a fine
powder.
IR (KBr): 3428, 2928, 1611, 1430, 1159, 1083, 1020, 762, 711, 577 crri'.
CA 02325643 2000-11-10
11
EXAMPLE 5
Preparation of a carboxymethylstarch, crosslinked with divinylsulfone
(39.38%),
compound 7.
2.Og (0.0123 mol) of wheat starch A ( Supercell 1201-C, ADM/Ogilvie) was
suspended
in 40 ml of deionized water. Under stirring, 3.5 ml 30% NaOH (0.0263 mol, 2.1
eq.) was
added dropwise and the solution stirred at room temperature for 1 hour.
Chloroacetic
acid ( 1.16 g, 0.0123 mol, l eq), dissolved in 10 ml of deionized water and
neutralized with
1.6 ml 30% NaOH (0.0123 mol, 1 eq.) was added dropwise and the reaction
mixture was
heated at 70°C for 24 hours. At room temperature, 0.784g (39.38% weight
%) of
divinylsulfone dissolved in lOml acetone, was added dropwise and the solution
was
stirred for 2 hours. The polymer was precipitated with 100 ml of methanol,
triturated in
a blender, washed with 3 portions of 60 ml methanol, filtered and dry at
60°C for 16
hours to give 2.35g of a white solid. The solid was grinded with a coffee
grinder to get
a fine powder of compound 7.
IR (KBr): , 3427, 2927, 1603, 1415, 1321, 1154, 1083, 1025, 712, 578 crri'.
EXAMPLE 6
Preparation of a starch cicatronic half esther, crosslinked with
divinylsulfone
(0.62%), compound 9.
CA 02325643 2000-11-10
12
2.Og (0.0123 mol) of wheat starch A ( Supercell 1201-C, ADM/Ogilvie) was
suspended
in 40 ml of deionized water. Under stirring, 5.0 ml 30% NaOH (0.0375 mol,
3eq..) was
added dropwise and the solution stirred at room temperature for 1 hour.
Citraconic
anhydride (1.73g, 0.0133 mol, l.leq.), dissolved in lOml acetone was added
dropwise
and the reaction mixture was stirred at room temperature for 2 hours. l2mg
(0.62%) of
divinylsulfone, dissolved in lOml acetone, was added dropwise and the solution
was
stirred for 2 hours. The polymer was precipitated with 100 ml of methanol,
triturated in
a blender, washed with 3 portions of 60 ml methanol, filtered and dry at
60°C for 16
hours to give 1.92g of a white solid. The solid was grinded with a coffee
grinder to get
a fine powder of compound 9.
IR (KBr): 3399, 2929, 1715, 1644, 1571, 1446, 1407, 1276, 1153, 1081, 1026,
930, 853,
762, 710, 579, 530 cm'.
EXAMPLE 7
Preparation of carboxymethylstarch, crosslinked with dichlorotriethylene
dioxyde
(9.85%), compound 8.
2.Og (0.0123 mol) of wheat starch A ( Supercell 1201-C, ADM/Ogilvie) was
suspended
in 40 ml of deionized water. Under stirring, 3.5 ml 30% NaOH (0.0263 mol, 2.1
eq.) was
added dropwise and the solution stirred at room temperature for 1 hour.
Chloroacetic
acid ( 1.16 g, 0.0123 mol, l eq), dissolved in 10 ml of deionized water and
neutralized with
CA 02325643 2000-11-10
13
1.6 ml 30% NaOH (0.0123 mol, 1 eq.) was added dropwise and the reaction
mixture was
heated at 70°C for 24 hours. At room temperature, 0.197g (9.85% weight
%) of
dichlorotriethylene dioxide dissolved in lOml acetone, was added dropwise and
the
solution was heated at 70°C for 24 hours. The polymer was precipitated
with 100 ml of
methanol, triturated in a blender, washed with 3 portions of 60 ml methanol,
filtered and
dry at 60°C for 16 hours to give 1.95g of a white solid. The solid was
grinded with a
coffee grinder to get a fine powder of compound 8.
IR (KBr): 3408, 2929, 1607, 1423, 1327, 1158, 1083, 1021, 937, 849, 762, 710,
581,
530c1ri'.
CA 02325643 2000-11-10
14
EXAMPLE 8
Preparation of carboxymethylstarch, crosslinked with dichlorotriethylene
dioxyde
(40%), compound 8.
2.Og (0.0123 mol) of wheat starch A ( Supercell 1201-C, ADM/Ogilvie) was
suspended
in 40 ml of deionized water. Under stirring, 3.5 ml 30% NaOH (0.0263 mol, 2.1
eq.) was
added dropwise and the solution stirred at room temperature for 1 hour.
Chloroacetic
acid ( 1.16 g, 0.0123 mol, l eq), dissolved in 10 ml of deionized water and
neutralized with
1.6 ml 30% NaOH (0.0123 mol, 1 eq.) was added dropwise and the reaction
mixture was
heated at 70°C for 24 hours. At room temperature, 0.80g (40 weight %)
of
dichlorotriethylene dioxide dissolved in lOml acetone, was added dropwise and
the
solution was heated at 70°C for 24 hours. The polymer was precipitated
with 100 ml of
methanol, triturated in a blender, washed with 3 portions of 60 ml methanol,
filtered and
dry at 60°C for 16 hours to give 2.06g of a white solid. The solid was
grinded with a
coffee grinder to get a fine powder of compound 8.
IR (KBr): 3404, 2928, 1607, 1424, 1327, 1155, 1084, 1020, 934, 849, 762, 710,
580,
530crri '.
REFERENCES
1. Andergen U. and Oppermann W. Polymer, 1990, 31, 1854-1858.
2. Kabra WO 95/31500, Nov., 1995
3. Annergren et al. WO 00/21581, Apr., 2000.
CA 02325643 2000-11-10
4. Thornton et al. WO 00/35504, June, 2000
5. Cunningham R. L. et al., J. Appl. Polym. Sci., 1998,
69, 957-964.
6. Alafi R. et al., J. Appl. Polym. Sci., 1998, 68, 739-745.
7. Shi. B. and Seid P. A. J. Macromol. Sci. Pure Appl.
Chem., 1996, A33, 655-
5 671.
8. Palardy US. Pat. 5,843,238, Dec., 1998.
9. Tsukasa et al. Eur. Pat., 0 474 173 A1.
10. Carr M. E. and Cunningham R. L., Polym. Prep. (Am.
Chem. Soc., Div. Polym.
Chem.), 1992, 33, 946-947.
10 11. Leithaiser R. B., Pap. Meet.-Am. Chem. Soc. Div. Org.
Plast. Chem., 1996, 26,
44-54.
12. Asserson and King, US. Pat. 3,898,143, Aug., 1975.
13. Ezra G. and Zilkha A. J. Macromol. Sci.-Chem., 1970,
A4, 957-963.
14. Tahan M. and Zilkha A., J. Polym. Sci., PartA-l, 1969,
7, 1815-1824.
15 15. Tahan M. and Zilkha A., J. Polym. Sci., PartA-1, 1969,
7, 1825-1837.
16. Ezra G. and Zilkha A., J. Appl. Polym. Sci., 1969,
13, 1493-1496.
17. Jarowenko US. Pat. 3,284,442, Nov., 1966.
18. Cottrell et al., US. Pat. 5,801,116, Sep., 1998.
19. Engelhardt et al., US. Pat. 5,731,365, Mar., 1998.
20. Curme et al., GB 1 490 128, May, 1975.
21. Kawai F. Crit. Rev. Biotech., 1987, 6, 273-307.