Note: Claims are shown in the official language in which they were submitted.
24
What we claim is:
1. A method of treating or preventing infection caused by
bacteria, mycobacterium or fungi by administration of a
composition containing as an active agent a bacteria,
mycobacterium or yeast growth-inhibiting effective
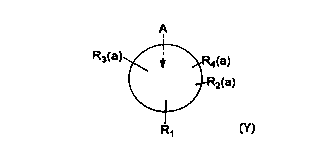
amount of a compound of the formula:
<IMG>
wherein A is a hydrocarbon aromatic ring system, R1 is
bound directly to an oxygen and is also bound to a
nitrogen through a saturated carbon or carbon chain,
with R1 being of the structure CHOZX wherein Z may be
hydrogen, a second bond to the oxygen, or may be
carboxyl, ether or ester moiety wherein the ether or ester
moiety may be alkyl of 1-8 carbons, phenyl, phenylalkyl,
wherein the alkyl moiety consists of 1-4 carbons
and wherein any said alkyl or phenyl group may,
additionally, be substituted with hydroxy, alkyl of 1-2
carbons, alkenyl of 2-3 carbons, halo, amino, or alkyl
amino group and X is (CH2)~ N((CH2)n(CH3))m wherein ~ is 1
to 3, n is ~6, m is 1 or 2 with the proviso that when
m is 2, at least one of n is < 3, or X may be (CH2)o J
wherein o is 0-4 and J is a saturated nitrogen-containing
ring system with up to 10 carbon atoms in the ring
system and may have up to 4 bridge carbons, and
wherein any saturated ring system may be substituted with
alkyl, alkenyl, halo, alkoxy or haloalkyl moieties of
1-5 carbons or with phenyl, phenoxy, phenylalkyl,
phenylalkoxy, carboxy or carbonyl groups, wherein the
carboxy or carbonyl groups, including keto or ester
25
moieties, with alkyl groups having 1-4 carbons, alkenyl
groups of 2-5 carbons or phenylalkyl wherein the alkyl
is of 1-3 carbons or phenylalkoxy wherein the alkyl is
of 1-3 carbons and, further, wherein X and Z may be
linked to form a heterocyclic ring system and (a) is
0-4 with the proviso that at least one of (a) is not 1,
R2, R3 and R4 may be alkyl (including cycloalkyl), a
saturated, nitrogen-containing ring of 4-10 atoms,
alkoxy, aryl, aryloxy, aryloxyalkyl, amino, amino-alkyl,
alkyl-aminoalkyl, arylamino, alkenyl, arylalkenyl,
arylalkylaminoalkyl, carboxyalkyl, hydroxy, halo,
alkenyl, or alkenyloxy, halo-substituted alkyl, wherein
any alkyl has 1-8 carbons, alkenyl has 2-8 carbons,
wherein halo is chloro, fluoro or bromo and aryl is a
ring system of 1-3 rings and wherein any alkyl or aryl
at R2, R3 and R4 may be further substituted with halo
(including multiple halo subtitutions), aryl of 1-2
rings, alkyl, haloalkyl or alkoxy, with the proviso
that at least one of R2, R3 and R4 is an electron-rich
substituent.
2. A method of claim 1 of treating or preventing infection
caused by bacteria, mycobacterium or fungi by administration
of a composition containing as an active agent
a bacteria, mycobacterium or yeast growth-inhibiting
effective amount of a compound of the formula:
<IMG>
wherein any of R2, R3, R4, R5, R6, R7 and R8 may be H,
alkyl (including cycloalkyl), a saturated,
nitrogen-containing ring of 4-10 atoms, alkoxy, aryl, aryloxy,
aryloxyalkyl, amino, amino-alkyl, alkyl-aminoalkyl,
arylamino, alkenyl, arylalkenyl, arylalkylaminoalkyl,
26
carboxyalkyl, hydroxy, halo, alkenyl, or alkenyloxy,
halo-substituted alkyl, wherein any alkyl has 1-8
carbons, alkenyl has 2-8 carbons, wherein halo is chloro,
fluoro or bromo and aryl is a ring system of 1-3 rings
and wherein any alkyl or aryl may be further substituted
with halo (including multiple halo subtitutions),
aryl of 1-2 rings, alkyl, haloalkyl or alkoxy, with the
proviso that at least one of R2, R3, R4, R5, R6, R7 and R8
is an electron-rich substituent and one of R1, R9 or R10
is bound directly to an oxygen and is also bound to a
nitrogen through a saturated carbon or carbon chain,
being of the structure CHOZX wherein Z may be hydrogen,
a second bond to the oxygen, or may be carboxyl, ether
or ester moiety wherein the ether or ester moiety may
be alkyl of 1-8 carbons, phenyl, phenylalkyl, wherein
the alkyl moiety consists of 1-4 carbons and wherein
any said alkyl or phenyl group may, additionally, be
substituted with hydroxy, alkyl of 1-2 carbons, alkenyl
of 2-3 carbons, halo, amino, or alkyl amino group and X
is (CH2)~ N((CH2)n(CH3))m wherein ~ is 1-3, n is ~6, m is
1 or 2 with the proviso that when m is 2, at least one
n is < 3, or X may be (CH2)o J wherein o is 0-4 and J is a
saturated nitrogen-containing ring system with up to 10
carbon atoms in the ring system and may have up to 4
bridge carbons, and wherein any saturated ring system
may be substituted with alkyl, alkenyl, halo, alkoxy or
haloalkyl moieties of 1-5 carbons or with phenyl,
phenoxy, phenylalkyl, phenylalkoxy, carboxy or carbonyl
groups, wherein the carboxy or carbonyl groups, including
keto or ester moieties, with alkyl groups having 1-4
carbons, alkenyl groups of 2-5 carbons or phenylalkyl
wherein the alkyl is of 1-3 carbons or phenylalkoxy
wherein the alkyl is of 1-3 carbons and, furthermore, X
and Z may be linked to form a heterocyclic ring system.
3. A method of claim 2 wherein at least two of R2, R3, R4,
27
R5, R6, R7 and R8 are Cl or F3C.
4. A method of claim 2 wherein Z=H, X=CH2-(2-piperidine)
R2 and R4 are Cl.
5. A method of claim 2 wherein R9 is CHOZX and Z=H,
X=CH2-N(C4H9)(C3H7) and R5 and R6 are Cl.
6. A method of claim 2 wherein R1 is CHOZX and Z=H,
X=(CH2)2NH(C3H7) and R3 is Cl.
7. A method of claim 2 wherein R1 is CHOZX and Z=H,
X=(CH2)2NH(C3H7), R3 is Cl and R5 is CF3.
8. A method of of claim 1 of treating or preventing
infection caused by bacteria, mycobacterium or fungi by
administration of a composition containing as an active
agent a bacteria, mycobacterium or yeast growth-inhibiting
effective amount of a compound of the formula:
<IMG>
wherein any of R2, R3, R4, R5, R6, R7 and R8 may be H,
alkyl (including cycloalkyl), a saturated,
nitrogen-containing ring of 4-10 atoms, alkoxy, aryl, aryloxy,
aryloxyalkyl, amino, amino-alkyl, alkyl-aminoalkyl,
arylamino, alkenyl, arylalkenyl, arylalkylaminoalkyl,
carboxyalkyl, hydroxy, halo, alkenyl, or alkenyloxy,
halo-substituted alkyl, wherein any alkyl has 1-8
carbons, alkenyl has 2-8 carbons, wherein halo is chloro,
fluoro or bromo and aryl is a ring system of 1-3 rings
and wherein any alkyl or aryl may be further
28
substituted with halo (including multiple halo subtitutions),
aryl of 1-2 rings, alkyl, haloalkyl or alkoxy, with the
proviso that at least one of R2, R3, R4, R5, R6, R7 and R8
is an electron-rich substituent and one of R1, is bound
directly to an oxygen and is also bound to a nitrogen
through a saturated carbon or carbon chain, being of
the structure CHOZX wherein Z may be hydrogen, a second
bond to the oxygen, or may be carboxyl, ether or ester
moiety wherein the ether or ester moiety may be alkyl
of 1-8 carbons, phenyl, phenylalkyl, wherein the alkyl
moiety consists of 1-4 carbons and wherein any said
alkyl or phenyl group may, additionally, be substituted
with hydroxy, alkyl of 1-2 carbons, alkenyl of 2-3
carbons, halo, amino, or alkyl amino group and X is
(CH2)~ N((CH2)n(CH3))m wherein ~ is 1-3, n is ~ 6, m is 1
or 2 with the proviso that when m is 2, at least one n
is < 3, or X may be (CH2)o J wherein o is 0-4 and J is a
saturated nitrogen-containing ring system with up to 10
carbon atoms in the ring system and may have up to 4
bridge carbons, and wherein any saturated ring system
may be substituted with alkyl, alkenyl, halo, alkoxy or
haloalkyl moieties of 1-5 carbons or with phenyl,
phenoxy, phenylalkyl, phenylalkoxy, carboxy or carbonyl
groups, wherein the carboxy or carbonyl groups, including
keto or ester moieties, with alkyl groups having 1-4
carbons, alkenyl groups of 2-5 carbons or phenylalkyl
wherein the alkyl is of 1-3 carbons or phenylalkoxy
wherein the alkyl is of 1-3 carbons and, furthermore, X
and Z may be linked to form a heterocyclic ring system.
9. A method of claim 8 wherein Z=H, X=CH2-(2-piperidine)
R6 = Cl, R7 = OCH3, R3 = 4-Cl-phenyl.
10. A method of claim 8 wherein Z=H, X=CH2-(2-piperidine)
R6 = Cl, R7 = OCH3, R3 = 3,4 dichloro-phenyl.
11. A method of claim 8 wherein Z=H, X=CH2-(2-piperidine)
29
R6 Cl, R7=CF3, R3=4-Cl-phenyl .
12. A method of claim 8 wherein Z=H, X=CH2-(2-piperidine)
R6 CF3, R7=OCH3, R3=3, 4-diclhoro-phenyl.
13. A method of claim 1 of treating or preventing infection
caused by bacteria, mycobacterium or fungi by administration
of a composition containing as an active agent
a bacteria, mycobacterium or yeast growth-inhibiting
effective amount of a compound of the formula:
<IMG>
wherein any of R2, R3, R4, R5, and R6, may be H, alkyl
(including cycloalkyl), a saturated, nitrogen-containing
ring of 4-10 atoms, alkoxy, aryl, aryloxy,
aryloxyalkyl, amino, amino-alkyl, alkyl-aminoalkyl,
arylamino, alkenyl, arylalkenyl, arylalkylaminoalkyl,
carboxyalkyl, hydroxy, halo, alkenyl, or alkenyloxy,
halo-substituted alkyl, wherein any alkyl has 1-8
carbons, alkenyl has 2-8 carbons, wherein halo is chloro,
fluoro or bromo and aryl is a ring system of 1-3 rings
and wherein any alkyl or aryl may be further substituted
with halo (including multiple halo subtitutions),
aryl of 1-2 rings, alkyl, haloalkyl or alkoxy, with the
proviso that at least one of R2, R3, R4, R5, R6, R7 and R8
is an electron-rich substituent and one of R1, is bound
directly to an oxygen and is also bound to a nitrogen
through a saturated carbon or carbon chain, being of
the structure CHOZX wherein Z may be hydrogen, a second
bond to the oxygen, or may be carboxyl, ether or ester
moiety wherein the ether or ester moiety may be alkyl
of 1-8 carbons, phenyl, phenylalkyl, wherein the alkyl
moiety consists of 1-4 carbons and wherein any said
alkyl or phenyl group may, additionally, be substituted
30
with hydroxy, alkyl of 1-2 carbons, alkenyl of 2-3
carbons, halo, amino, or alkyl amino group and X is
(CH2)~ N((CH2)n(CH3))m wherein ~ is 1-3, n is ~6, m is 1
or 2 with the proviso that when m is 2, at least one n
is < 3, or X may be (CH2)o J wherein o is 0-4 and J is a
saturated nitrogen-containing ring system with up to 10
carbon atoms in the ring system and may have up to 4
bridge carbons, and wherein any saturated ring system
may be substituted with alkyl, alkenyl, halo, alkoxy or
haloalkyl moieties of 1-5 carbons or with phenyl,
phenoxy, phenylalkyl, phenylalkoxy, carboxy or carbonyl
groups, wherein the carboxy or carbonyl groups,
including keto or ester moieties, with alkyl groups having
1-4 carbons, alkenyl groups of 2-5 carbons or phenylalkyl
wherein the alkyl is of 1-3 carbons or phenylalkoxy
wherein the alkyl is of 1-3 carbons, and, furthermore,
X and Z may be linked to form a heterocyclic ring
system.
14. A method of claim 13 wherein Z=H, X=CH2-(2-piperidine)
R3 and R5 are 4-Cl-phenyl.
15. A method of claim 13 wherein Z=H, X=CH2-(2-piperidine)
R3=Cl, R5=4-OCH3-phenyl.
16. A method of claim 13 wherein Z=H, X=CH2CH2(2-piperidine)
R3=Cl, R5=4-OCH3-phenyl
17. A method of of claim 1 of treating or preventing
infection caused by bacteria, mycobacterium or fungi by
administration of a composition containing as an active
agent a bacteria, mycobacterium or yeast growth-inhibiting
effective amount of a compound of the formula:
31
<IMG>
wherein any (a) is 1-3 and R2, and R3, may be H, alkyl
(including cycloalkyl), a saturated, nitrogen-containing
ring of 4-10 atoms, alkoxy, aryl, aryloxy, aryloxyalkyl,
amino, amino-alkyl, alkyl-aminoalkyl, arylamino,
alkenyl, arylalkenyl, arylalkylaminoalkyl, carboxyalkyl,
hydroxy, halo, alkenyl, or alkenyloxy, halo-substituted
alkyl, wherein any alkyl has 1-8 carbons,
alkenyl has 2-8 carbons, wherein halo is chloro, fluoro
or bromo and aryl is a ring system of 1-3 rings and
wherein any alkyl or aryl may be further substituted
with halo (including multiple halo subtitutions), aryl
of 1-2 rings, alkyl, haloalkyl or alkoxy, with the
proviso that at least one of R2, R3, R4, R5, R6, R7 and R8
is an electron-rich substituent and one of R1, is bound
directly to an oxygen and is also bound to a nitrogen
through a saturated carbon or carbon chain, being of
the structure CHOZX wherein Z may be hydrogen, a second
bond to the oxygen, or may be carboxyl, ether or ester
moiety wherein the ether or ester moiety may be alkyl
of 1-8 carbons, phenyl, phenylalkyl, wherein the alkyl
moiety consists of 1-4 carbons and wherein any said
alkyl or phenyl group may, additionally, be substituted
with hydroxy, alkyl of 1-2 carbons, alkenyl of 2-3
carbons, halo, amino, or alkyl amino group and X is
(CH2)~ N((CH2)n(CH3))m wherein ~ is 1-3, n is ~6, m is 1
or 2 with the proviso that when m is 2, at least one n
is < 3, or X may be (CH2)o J wherein o is 0-4 and J is a
saturated nitrogen-containing ring system with up to 10
32
carbon atoms in the ring system and may have up to 4
bridge carbons, and wherein any saturated ring system
may be substituted with alkyl, alkenyl, halo, alkoxy or
haloalkyl moieties of 1-5 carbons or with phenyl,
phenoxy, phenylalkyl, phenylalkoxy, carboxy or carbonyl
groups, wherein the carboxy or carbonyl groups, including
keto or ester moieties, with alkyl groups having 1-4
carbons, alkenyl groups of 2-5 carbons or phenylalkyl
wherein the alkyl is of 1-3 carbons or phenylalkoxy
wherein the alkyl is of 1-3 carbons, and, furthermore,
X and z may be linked to form a heterocyclic ring
system.
18. A method of claim 17 wherein the active agent is
desbutyl-halofantrine.
19. A method of claim 17 wherein the active agent is chosen
from among compounds wherein
Z X R2, n R3 n
H piperidinyl (#1) CF3 1 CF3 1
H piperidinyl (#2) Cl 1 CF3 1
H piperidinyl (#3) Cl 2 CF3 1
H piperidinyl (#4) Br 1 Br 1
H piperidinyl (#5) Cl 1 Cl 1
H CH2-piperidinyl (#6) Cl 1 Cl 1
H piperidinyl (#7) CF3 2 Cl 2
H piperidinyl (#8) CF3 1 CF3 1
H CH2-piperidinyl (#9) Cl 2 CF3 1
ZX= <IMG> CF3 1 CF3 1
H CH2NHCH(CH2CH3)2 (#11) CF3 1 CF3 1
H (CH2)NH(CH2)3CH3 (#12) CF3 1 Cl 2
33
20. A compound of the formula:
<IMG>
wherein A is a hydrocarbon aromatic ring system, R1 is
bound directly to an oxygen and is also bound to a nitrogen
through a saturated carbon or carbon chain, with R1
being of the structure CHOZX wherein Z may be hydrogen, a
second bond to the oxygen, or may be carboxyl, ether or
ester moiety.wherein the ether or ester moiety may be
alkyl of 1-8 carbons, phenyl, phenylalkyl, wherein the
alkyl moiety consists of 1-4 carbons and wherein any said
alkyl or phenyl group may, additionally, be substituted
with hydroxy, alkyl of 1-2 carbons, alkenyl of 2-3 carbons,
halo, amino, or alkyl amino group and X (CH2)oJ
wherein o is 2-4 and J is a saturated nitrogen-containing
ring system with up to 10 carbon atoms in the ring system
and may have up to 4 bridge carbons, and wherein any
saturated ring system may be substituted with alkyl,
alkenyl, halo, alkoxy or haloalkyl moieties of 1-5 carbons
or with phenyl, phenoxy, phenylalkyl, phenylalkoxy, carboxy
or carbonyl groups, wherein the carboxy or carbonyl
groups, including keto or ester moieties, with alkyl
groups having 1-4 carbons, alkenyl groups of 2-5 carbons
or phenylalkyl wherein the alkyl is of 1-3 carbons or
phenylalkoxy wherein the alkyl is of 1-3 carbons, and (a)
is 0-4 with the proviso that at least one of (a) is not 1,
R2, R3 and R4 may be alkyl (including cycloalkyl), a
saturated, nitrogen-containing ring of 4-10 atoms, alkoxy,
34
aryl, aryloxy, aryloxyalkyl, amino, amino-alkyl,
alkyl-aminoalkyl, arylamino, alkenyl, arylalkenyl, arylalkyl-
aminoalkyl, carboxyalkyl, hydroxy, halo, alkenyl, or
alkenyloxy, halo-substituted alkyl, wherein any alkyl has
1-8 carbons, alkenyl has 2-8 carbons, wherein halo is
chloro, fluoro or bromo and aryl is a ring system of 1-3
rings and wherein any alkyl or aryl at R2, R3 and R4 may be
further substituted with halo (including multiple halo
subtitutions), aryl of 1-2 rings, alkyl, haloalkyl or
alkoxy, with the proviso that at least one of R2, R3 and R4
is an electron-rich substituent.
21. A compound of claim 20 wherein A is a benzene ring.
22. A compound of claim 20 wherein A is a phenanthrene ring.
23. A compound of claim 20 wherein A is naphthalene ring.
24. A compound of claim 20 wherein A is anthracene ring.