Note: Descriptions are shown in the official language in which they were submitted.
WO 99/50659 PCT/GB99/01001
Rapid Method for Detecting Micro-Organisms and Evaluating Antimicrobial
Activi
Field of the Invention
The present invention relates to methods and apparatus for the detection of
the
presence of specific micro-organisms in a fluid. It is particularly
applicable, but in no
way limited, to identifying microbial pathogens in liquid solution. The
invention also
relates to methods for antibiotic sensitivity testing, and in particular
methods for
assessing the effectiveness of antimicrobial agents, for screening compounds
for
antimicrobial activity, and for determining an appropriate means of treatment
for an
unknown microbial infection.
Background to the Invention
There are many applications in which it is important to be able to detect the
presence of a specific micro-organism. For example, in combating viral or
bacterial
infections, it is necessary to be able to identify the micro-organism
responsible. Also
in the brewing industry, it is important to be able to determine if a yeast is
present in
a solution and more particularly to determine its vitality and viability. !n
this context
the term micro-organism has a broad meaning. It encompasses bacteria, viruses
and fungi.
In both these examples speed of analysis is extremely important. For
instance, in diagnosis of a medical problem the medical practitioner needs to
know
what organism is causing the symptoms within hours rather than days. The most
appropriate treatment can then be started straight away, giving the patient
the best
chance of a speedy recovery. In some cases, such as meningitis, rapid and
accurate diagnosis is a matter of life and death.
1
CA 02326320 2000-09-27
WO 99/50659 PGTIGB99101001
Under present arrangements samples are usually sent to a pathology
laboratory for culture and subsequent identification. By the very nature of
the
procedure this takes days rather than hours. There may be more than one
organism present which requires a number of different cultures in different
media.
It is also known to detect micro-organisms by the use of specifrc probes that
are designed to attach themselves to the micro-organism by covalent bonding
and
thus to attach a marker to them so that they may be detected by some physical
property. Unlike the present invention such techniques are slow and can only
search for one micro-organism at a time.
The use of microelectro-kinetics to study yeast vitality and viability has
been
described by P. Brown in Brewer's Guardian, November 1997, pages 39-40.
However, yeasts are relatively large and particularly uniform in size. Even
so, it
proves difficult to distinguish between commercial yeasts, such as S.
cerevisiae and
S. carlsbergensis, and wild yeast. Such a result mitigates against being able
to
differentiate between much smaller organisms such as bacteria which are much
more variable in size and shape. In fact, zeta potentials of living organisms
are
poorly understood. The method is generally considered tedious, d~cult and
temperamental. Furthermore, it is not readily amenable to multiple sampling.
Similarly, when treating bacterial infections, it is important to determine
whether or not the causative micro-organism is susceptible to the chosen drug
or
antibiotic. However, present techniques for assessing the efficacy of a drug
or
antibiotic against a particular micro-organism can take several days to carry
out.
Current practice is to prescribe a general or best guess antibiotic to combat
the
symptoms of the infection. In other words, therapy of the infection begins
before
laboratory results are available, and antibiotic sensitivity testing provides
only a
2
CA 02326320 2000-09-27
fi 5-04-2000 G B 009901001
. . . .... .. . .. ..
.. .. .. . . . . . . .
. . . ... . . . . . . .
.
. . . . .... . . .
.
-. . . . . . . . ..
. ... ... .. . .. ..
supplementary role in confrming that the organism is susceptible to the
current
agent that is being used. Usually the laboratory report will influence
treatment only if
the patient is failing to respond. Sometimes it may allow the clinician to
change from
a toxic to a less toxic agent Antibiotic sensitivity testing may be defined as
a
method for determining the sensitivity of a micro-organism to a potential drug
treatment. The micro-organism may be a bacterium, a fungus, a yeast, a
parasite or
a virus.
Currently the antibiotic sensfivity of bacteria can be assessed in a variety
of
ways:
1. Agar Diffusion tests, in which the antibiotic is allowed to diffuse from a
point
source, commonly in the form of a filter paper disc, into an agar medium
which has been seeded with a test organism.
2. Broth dilution tests, in which serial dilutions of antibiotic in a suitable
fluid
medium are inoculated with the test organism. The highest dilution of the
antibiotic to inhibit growth after overnight incubation is the minimum
inhibitory
concentration (MIC).
3. Agar incorporation tests, which are essentially similar to broth dilution
tests
except that the antibiotic diiutions are incorporated i~ an agar medium in a
series of Pe#ri dishes. These are spot-inoculated with a number of test
organisms, usually be means of a semi-automatic inoculation device.
These methods, none of which are rapid, are described iri detail in
Antimicrobial Chemotherapy, edited by David Greenwood, published by Baiiliere
Tindail, London, pp 73 - 82,
Newer methods of assessing bacterial susceptibility to antibiotics are being
developed: the most rapid, are tests which detect resistance to Gi-lacfam
antibiotics
3
CA 02326320 2000-09-2~ AMENDED SHEET
15-04-2000 GB 009901001
.... .. . .. ..
.. .. .. . . . . . . .
. . . . ... . .. . . ..
. . . . .... . . . .
..
... ... .. . .. ..
by the demonstration of ~3-lactamase in bacteria. This can be accomplished in
a few
minutes once a bacterial culture is available. However, since a wide range of
~-
lactamases are encountered to which various ~-lactam agents display
differential
susceptibility, the tests are of limited value. Other methods employ
turbidometric
techniques to detect anfibacterial activity by comparing the growth of
bacteria
exposed to antibiotic with a drug-free control over a time span which for fast
growing
organisms can be as little as 2 or 3 hours. Turbidornetric results show
discrepancies with more traditional methods with certain bacterium/drug
combinations and there is controversy over the correct interpretation of these
results. Equivalent methods are available for viruses, fungi and other
parasites.
W079/00834 describes a system for analysing chemical and biological
samples using electrostatic fields.
Clearly, current approaches are less than ideal, and a rapid method for
determining
antibiotic susceptibility which can be widely applied and which provides
results consistent
with those obtained by conventional methods is highly desirable.
One of the first steps in drug discovery is to find one or more compounds that
show
a particular biological activiiyr of interest. These first compounds, called
leads, are usually
discovered by screening, which means testing tens of thousands of random
molecules for
signs of activity.
Recent developments in automation have made it possible to screen tens of
thousands of compounds per month, an effort that would have taken years
previously. This has meant that there has been an exploding demand for
"libraries°
of random molecules to test. Similar developments in automation and
combinatorial
chemistry have made it possible to synthesise thousands of distinct compounds
per
month, with a relatively small investment. These combinatorial libraries are
made by
forming all possible combinations of a series of sets of precursor molecules,
and
applying the same sequence of reactions to each combination. The distribution
of
4
CA 02326320 2000-09-2~ AMENDED SHEET
y ~,mn~~~:~-rn_M~'f:_'V_CHL_tV C_>.5 _ ' fi_= 7= Q____18_vdU__' _ +4~4 L7'?.?
F3fiEi8(;Fi-__ +4v 8J '.'.:.3;~'~49~bo:If 4
06-07-2000 GB 009901001
prac;ursors, and often the maintenance of reaction oondtdons, is carried out
by a
robo~c synthesis system.
Lead molecules are typically only weekly scdve, and may be to~ac or
otherwise unsuitable for uee as dnrgs. The next step in drug discovery is lead
expansion, ~ which hundreds or thousands of variants of the most promising
leads
are made. These are tested, and some era typically found to be maro active
andlor
less toxic than the origin8t leads. This process can be iterative. The
combinatorial
library approach is also suitable for this lead expansion phase.
It is an object of the present invention to provide apparatus and methods
i0 which overcome same or all of these diaadvsntagea.
~~,,~mmarv of the Invention
According to the pres0nc invention there is provided a method of identifying
one yr more micro-organisms in a fluid sample comprising the stops of:-
(i~ opdonally culturing the sample if necessary to increes~o the number of
micro-orgeniems for a pr~e~determined range;
iii) measuring the zeta poten~ata of any micro-organisms present;
(lily optionally normalising the measurements taken in step (ii) such that
they relate to stsndard conditions;
(iv) comparing said measured zeta poteMiaJs with a table of the zeta
potentials of known micro-organisms to determine which, tf any, of ~e
kno~m micro-organisms are present In the fluid.
By way of example there Is provided a method of determining the presence
of a speafic micro-orge~nism in a liquid solution which comprises applying an
s
CA 02326320 2000-09-2~. AMENDED SHEET
WO 99!50659 PCT/GB99/01001
electric field across the solution, opticafly measuring the speed of movement
of any
micro-organism suspended in the solution as a result of the applied electric
field,
and identifying the presence of one or more specific organisms by comparing
the
measured values with those of known micro-organisms which have been measured
under standard conditions.
The measured speed of movement or electrophoretic mobility is an indication
of the electro-kinetic potential, also termed zeta potential, of the micro-
organism and
is dependent upon several physical properties of the micro-organism including
size,
shape, overall charge and surface charge distribution.
As well as permitting a solution to be analysed for the presence of a specific
micro-organism, by comparing the zeta potentials measured during analysis of a
solution with a table of the zeta potentials of known micro-organisms, the
invention
can enable rapid analysis of a solution to determine what mixture of micro-
organisms are present. .
According to a second aspect of the invention, there is provided an
apparatus for determining the presence of a specific micro-organism in a
liquid
solution which comprises means for applying an electric field across a
measurement
cell containing the solution, a laser light source for illuminating the cell,
detecting
means for sensing light after impinging on a micro-organism suspended in the
solution, means for analysing the scattered light to provide a measurement of
the
speed of movement of the micro-organism by which the laser light was
scattered,
and means for identifying the presence of the specific micro-organism upon
measurement of a speed of movement matching that of the micro-organism.
The apparatus of the invention, as well as distinguishing between micro
organisms on the basis of their electo-kinetic potential, achieves improved
sensitivity
by allowing the scattering angle at which light is detected to be optimised
for the
6
CA 02326320 2000-09-27
WO 99750659 PCTIGB99lO1001
micro-organism of interest as it has been found in practice that light from
different
micro-organisms is scattered in different directions.
It is preferred for the laser light source to comprise a laser, a beam
splitter
for dividing the light emitted from the light source into two coherent beams,
means
for directing the light beams along different directions and means for
modulating the
length of one of the light paths to create a variable interference pattern
between the
two beams at the cell. The effect of modulating the length of one of the light
paths
will be that the interference pattern will oscillate along the cell and fight
scattered by
a stationary particle or micro-organism will pulsate at the frequency of the
modulation. !f the particle is moving however, it will move with the
interference in
one direction and in opposition to it in the opposite direction. Because of
the
Doppler effect, the frequency of modulation of the scattered light will differ
from the
frequency of modulation of the length of one of the light paths by an amount
indicative of the rate of movement of the particle in the cell.
The detected frequencies of light scattered by moving particles when the
applied electric field is reversed should be symmetrical about the modulation
frequency of the light path. If, however, the electrodes of the cell are
polarised then
reversal of the potential applied to the electrodes will not result in an
exact reversal
of the applied electric field and the measurements obtained will not be
symmetrical.
It is preferred to provide means for reversing the polarity of the electric
field
applied across the cell periodically to avoid polarisation of the electrodes
as a result
of an electrolytic reaction between the electrodes and ions in the solution.
The speed with which a given micro-organism will move through a solution
when an electric field is applied will depend on certain factors other than
the zeta
potential, such as the pH and temperature of the solution. It is preferred to
maintain
7
CA 02326320 2000-09-27
'V. VO' : EI'A-INUE\_CHE_\. Q_5 _. ~ ~_ __ ? = U _.__ ~ 8 v ~ 1 _ r _ +4~4
1?_~? _F3ES_t3B6_8-._ _ +4Ef 89 'd.39944~fi5 : # ..5
06-07-2000 . G B 009901001
these fact constant duHng measuroment but It is aitematively possible to
measure these parsme~rs and cornpensabe the measurement obtained.
Acxording to a third aspect of the present invention there is pnrrided a
method of identifying the cause of an infection in a sample from human or
animal
body ~:omprising #~e steps ois:
(a) culturing the sample if necessary to increase the number of miao-
or~ganixns to a pre-determined range;
(b) measuring the zete potential of said micro-organisms using the
method described herein;
(c) comparing the measured zeta potentials) with values obtained on
isolated micro-org~nisms.
This method can be used to din~ct a physician to an approp~ate course of
treatment. For example, not only can the method flag up the infecting spades,
but it
can also fist a range of possible drug treatments known to be effective
against the
micro-organisms which have been identified. The physiaan would then select the
appropriate treatment based on this inforrnatlon, the eymptoms exhibited by
the
patient and the physicians skiff and experienc$.
Accardir<g ~ a further aspect of the invention there Is provided a method for
antibiotic sensitivltyr testing.
In one embodiment, the method comprises:
(a) measuring the zeta potential o! one or more micro-organism in a
sample,
(b) adding to said sample an antirnicrobial agent,
B
CA 02326320 2000-09-2? AMENDED SHEET
WO 99/50659 PCT/GB99/01001
(c) measuring again the zeta potential of the said one or more micro-
organisms in said sample,
(d) determining the change in zeta potential between said measurements
and correlating said change to either:
(i) the antimicrobial sensitivity of said one or more micro-
organisms in said sample, or
(ii) the antimicrobial activity of said antimicrobial agent.
In a further embodiment, the step correlating said change in zeta potential to
either the antimicrobial sensitivity of a micro-organism or to the
antimicrobiai activity
of a an antimicrobial agent may be automated.
The present invention enables the following objectives and advantages to be
accomplished.
It is an object of the present invention to provide a method for rapidly
assessing the susceptibility of a patient or veterinary sample to a spectrum
of
antimicrobial agents in order to determine the most appropriate treatment to
be
initiated. Advantages of the present invention include avoidance of the
development
of drug-resistant strains; economic dispensing of medicines; and an increase
in the
quality of patient care.
Another object of the present invention is to provide a method for assessing
emerging drugs for antimicrobial activity. Advantages of the present invention
include determination of MIC values; investigation of the effects of the
candidate
drugs on microbial physiology; and determination of the mechanism of action of
the
candidate drug.
9
CA 02326320 2000-09-27
WO 99/50659 PCT/GB99/01001
A further object of the present invention is to provide a method for screening
for agents having antimicrobial activity. Advantages of the present invention
include
the ability quickly to perform large-scale, automated screening; to identify
drugs
which affect cell vitality but not cell viability.
A yet further object of the present invention is to provide an automated
method for screening agents for antimicrobial activity.
Description of the Drawings
The invention will be further described, by way of an example only, with
reference to the accompanying drawings wherein:-
Figure 1 illustrates a stylised diagram of a yeast cell;
Figure 2 illustrates graphically the diffuse double layer around a sphere of
moderate potential;
Figure 3 illustrates schematically a typical double beam apparatus for
measuring zeta potentials;
Figure 4 shows the Zeta potential profile of a control sample of S
pneumoniae;
Figure 5 shows the zeta potential profile of a sample of S pneumoniae
treated with penicillin.
Description of the Preferred Embodiments
The present aspects and embodiments represent currently the best ways
known to the applicant of putting the invention into practice. But they are
not the
only ways in which this could be achieved. They are illustrated, and they will
now be
described, by way of example only.
CA 02326320 2000-09-27
WO 99/50659 PCT/GB99101001
Techniques and apparatus for measuring electrophoretic mobility and zeta
potential are known per se and have been used in the analysis of certain
colloidal
suspensions, such as paints. The present invention is, however, based on the
discovery that the zeta potential of a micro-organism is a parameter which
under
properly controlled conditions, can act as a reliable indicator of the
presence of that
specific micro-organism.
Microelectrophoresis is a division of the science of electrophoresis and deals
specifically with particles in the region of less than 20 Nm and with a
minimum
dimension in the order of 0.01 irm. Below this limit, objects are considered
to be of
molecular dimensions. There is considerable blurring of this lower border
especially
when dealing with macromolecules. Electrophoresis has long been the method of
choice for identifying many charged molecular species, such as proteins,
nucleic
acid and amino acids. One much publicised type of electrophoresis is DNA
finger
printing which allows the separation and matching of two samples of DNA.
Micro-organisms of all types, from bacteria to viruses fall into the size
range
applicable to microelectrophoresis. Viruses are particularly interesting
because they
are composed mostly of protein and nucleic acid and tend to have a relatively
stable, uniform structure. As such, they provide a direct link with
conventional
electrophoresis techniques.
When any surface is placed in contact with an aqueous solution the surface
acquires a surface charge which is usually negative. The origins of this are
either
absorption of ions from solution or by the ionisation of charged groups on the
surface. In the case of micro-organisms both of these mechanisms can be seen
to
operate and the observed surface charges are usually negative, which is the
commonly observed sign. The use of this charge to identify specific micro-
organisms is what makes this technology both novel and powerful. Importantly,
the
11
CA 02326320 2000-09-27
WO 99/50659 PCT/GB99101001
method is not size dependent so both large and small micro-organisms can be
identified.
For purposes of microelectrophoresis Figure 1 describes how the micro-
organisms can be regarded from this view point. The shaded areas represent
areas
of hydrophobic surface at which charge builds up at the surface through
contact
absorption. Also shown at this surface are the charges native to the surface
of the
micro-organism and specifically absorbed at the surface. The charge produces
an
electrical double layer and the electrical potential varies as described by
Figure 2.
Fig. 2 shows the distribution of charge across a double layer at the surface
of a
hypothetical micro-organism. The charge at the shear surface is the zeta
potential;
beyond this the charge decays away rapidly in the bulk solution.
The surface charge of an idealised micro-organism arises from two sources.
First groups on the surface of the micro-organism, such as carboxylate groups,
may
be ionised. Secondly, ions may be adsorbed from solution onto the surface of
the
micro-organism, particularly at hydrophobic regions on the surface where
anionic
groups may be preferentially adsorbed.
Apparatus for measuring zeta potentials are known per se. One such
example is the ZETAS1ZER 2000 (TM) made by Malvern Instruments.
This instrument, as it name suggests, is used to measure particle size. It
utilises a dual laser beam and a photodetector is arranged to measure
deflected
light at a fixed scattering angle.
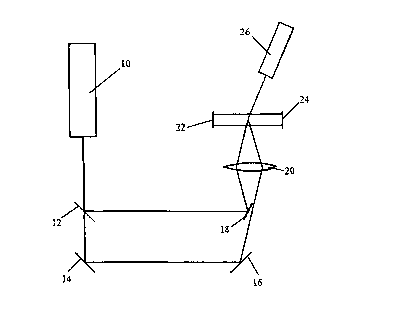
A typical double beam instrument is shown in Figure 3 which illustrates a
neon-helium laser 10 producing a red laser beam. The beam is incident upon a
beam sputter 12 that allows half the incident fight energy to pass through it
towards
a mirror and modulator 1 and reflects the other haff towards a mirror 18. The
mirror
and modulator 14 is a mirror vibrated by a piezoelectric vibrator that
reflects the
12
CA 02326320 2000-09-27
WO 99/50659 PCT/GB99/01001
incident beam towards a further stationary mirror 16. The two halves of the
split
laser beam are focused by a lens 20 an a cell 22 that contains a liquid to be
analysed. An electric field that is reversed periodically is applied across
the cell by
means of electrodes 22 and 24 and this causes micro-organisms in the liquid to
move with a characteristic velocity towards the electrode that instantaneously
has
the opposite polarity to the net charge on the micro-organism. When the micro-
organism is illuminated by the laser light it scatters the incident light in a
predetermined direction and this light is sensed by means of a detector 26.
The two halves of the split laser beam travel different distances before
reaching the cell 20. In the case of the half that is reflected by the mirror
and
modulator 14, the path length is modulated periodically. When they are
recombined,
the two halves of the split laser interfere with one another and the
interference
pattern will move across the cell. As a result, a stationary particle
suspended in the
cell will scatter light at a frequency related to the frequency of modulation
of the
mirror 14. If, however, a particle is moving towards one end of the cell 20 or
the
other, then while it is moving with the interference pattern, light scattered
from it will
have a lower frequency and conversely when the particle is moving in the
opposite
direction to the interterence pattern light scattered from it will have a
higher
frequency. By analysing the frequency of the scattered light sensed by the
detector
26 it is possible to determine the speed and direction of movement of the
particle.
The speed of movement is related to the distribution of charge about the
surface of the particle and is indicative of the so-called zeta potential.
Depending on
the shape of the particle, it will align itself differently in the applied
electric field and
for any specific micro-organism there will be an angle at which the intensity
of the
scattered light is a maximum. The apparatus may therefore be used to determine
the presence of a specific micro-organism in the solution being analysed by
setting
13
CA 02326320 2000-09-27
WO 99/50659 PGT/GB99101001
the detector 26 to the scattering angle associated with the micro-organism and
monitoring the output of the detector for the frequencies corresponding to the
previously determined speed of movement of the micro-organism in the applied
field.
It is preferred to take steps to prevent polarisation of the electrodes 22, 24
as
such polarisation may affect the electric field as sensed by particles
suspended in
the liquid in the cell 20. Polarisation may be prevented by periodically
switching the
polarity of the electric field. Such switching will in itself prevent
polarisation but
additionally it will allow polarisation to be detected as the detected
frequencies wi8
not be symmetrical about the frequency of movement of the bands of the
interference pattern.
The detector type used in this application can take a number of forms.
However, we have found that commercially available solid state photodiodes
such
as Radio Spares Model AEPX 65 (Stock No 8461749) work well.
The nature of the data captured takes the form of a series of exponential
decays. These represent the length of time taken for the scattered light to
decay to
zero, this is effectively the particle velocity. The exponential decays are
converted
to mean normal distributions. These are collected and transformed graphically
by
the method of Eishus to give mobilities and zeta potentials as follows:-
t m
g~'~(t) - 2 sinh ( AI') E G~ exp (-tl')
t 2 j-1
t - time
g~'~(t) - normalised electric field autocorrelation function
G; - normalisation constants and are obtained from fitting experimental
values of t to the summation part of the above equation.
Values of j are usually limited to 3 and are identified with the mean,
standard
deviation and skewness of the data.
14
CA 02326320 2000-09-27
WO 99/50659 PCT/GB99/01001
For the homodyne case the g~'~(t) is obtained from:
g~z~(t) - 1 + (g~'~(t)]Z where g~a?(t) is obtained from
g~2~(t) - 1 + a 2~t and is known as the homodyne autocorrelation
coefficient
(Ref: D.E. KOPPEL J Chem Phys 1972 57 4814)
This method of handling the data measured is contained in a software
programme as a means for analysing the scattered light. In addition, the
software
can contain a library or table of recorded measurements for known micro-
organisms.
A comparator programme can be run such that the operator receives as a print-
out
or screen message a list of those micro-organisms considered to be present.
In this context the term look-up table is used as a generic term to encompass
any compilation of zeta potential measurements obtained for known micro-
organisms. This look-up table can take a variety of forms. It may, for
example, be a
physical table whereby comparisons are made manually. Alternatively, and
preferably, the "table" can be a computer database of results. A comparator
programme then compares a measured value, or series of measured values,
against known results. Limits may be included within the programme such that a
"best ft" only answer is provided or a range of "possibilities" can be given.
The
comparator programme may also advantageously contain the necessary
calculations to normalise the measured zeta potential values such that they
relate to
standard conditions.
An apparatus of the general type described above has been used to
measure the electrophoretic mobility and zeta potentials of known micro-
organisms.
The machine was calibrated using a standard NaCI solution having a
conductivity of
0.15mS and a pH of approximately 7Ø Test samples were normalised to this
conductivity by use of the Briggs equation, which in this case takes the form:
CA 02326320 2000-09-27
WO 99/50659 PCTIGB99101001
Kao~ = (Kobe + Kat)IKat
Where the K's are solution, observed and standard respectively.
Most encouragingly, different samples of the same bacteria gave constant
results. Unexpectedly, the zeta potential peaks for different organisms
occurred at
different and distinguishable voltages. The sensitivity of the present system
is such
that different organisms can be identified uniquely, even from a mixture of
organisms. Typical results are shown in Table 1. This work shows how micro-
organisms can be identifed one from another in dilute aqueous solution at pH7.
These experiments were carried out by injecting a solution containing in the
region
of 100,000 micro-organisms per millilitre into an apparatus as shown in Figure
3 or
an apparatus of the type described above.
TABLE/
Micro-oroanism MeanIZeta PotentialStandard Deviation
(mV)
_
Campylobactor jejuni-7.5 0.945
Listeria monocytogenes-11.7 - 1.08
Salmonella (Wild) -9.76 1.00
E. Coli (0157) -18.6 _. __ _ _ 2.15
Shigella sonnei -27.2 1.1
Listeria innocula -11.9 0.81
Bacillus cereus -18.8 - - NIA
Staphylococcus aureus-17.8 NIA
Proteus mirabilis -21.5 NIA
Entrococcus faecalis-15.3 - NIA
Saccharomyces cerevisiae-23.4 0.5
S Carlburgensis -27.3 - 0.6
16
CA 02326320 2000-09-27
WO 99/50659 PCTIGB99/01001
TABLE l (Contd)
Micro-or4anism Mean/Zeta Potential- Standard Deviation
( mV)
Saccf~aromyces cerevisiae-3.0 0.4
(non-viable)
S Carlburgensis -2.0 0.2
(non-viable)
It is assumed that the data is normally distributed around the mean, which
has been established for yeasts in normal growth. This allows calculation of
the
goodness of separation which in this case it is represented by the chi squared
analysis shown in Table ll.
TABLE II
Chi sauared analysis of date in Table I
Micro-organismCampylobactorListeria SalmonellaE. Coli Shigella
(0157)
Campylobactor
Listeria .024
Monocytogenes
Salmonella 0.21 0.355
E. Coli (0157)0 0.019 0.001
Shigelia 0 0 0 0.015
Listeria Innocula0.013 0.949 0.49 0.002 0
Table II indicates that the only two members of the table that are not
separable are the two members of the listeria group of organisms.
The observation that two different strains of the Listeria bacteria cannot be
differentiated one from another does not greatly detract from this method. The
information that a patient is suffering from Listeriosis, as opposed to an E
Coli or
other bacterial infection, is of great assistance to a medical practitioner. A
course of
17
CA 02326320 2000-09-27
WO 99/50659 PCTIGB99101001
treatment can be started immediately which will combat all Listeria strains
which are
commonly found in the human population.
However, it is possible to further enhance the sensitivity of this method by
incorporating one or more of the additional steps, which make the
identification
process more discriminatory, or provide confirmation of micro-organism
identity:
1. Culturing the sample in a medium selective for a particular micro-organism
or
class of micro-organisms, whereby the micro-organism or micro-organisms
of interest grows in preference to other micro-organisms that may be
present. A wide range of selective media is available from Oxoid Ltd and is
described in their catalogue. This provides a method of distinguishing
between micro-organisms that otherwise have very similar zeta potentials.
2. Contacting the sample with an agent known to kill one or more of the micro-
organisms present, and measuring the zeta potential a second time. The
elimination of the zeta potential corresponding to the micro-organism of
interest is a confirmatory test if the agent is specific for the micro-
organism of
interest. The agent may be an antibiotic, antibacterial or other selective
poison. The same end may also be accomplished by contacting the sample
with an agent that specifically causes the agglomeration of the micro-
organism, such as an antibody or other immunological reagent, whereby the
micro-organism is precipitated out of solution.
3. Measuring the zeta potential at a second pH and comparing said zeta
potential with a second table of the zeta potentials of known micro-organisms
at this second pH. The diversity of the ionising groups on the surface of the
micro-organism, which contribute to the zeta potential, means that a
distinction can be made between two micro-organisms which have a similar
zeta potential at one pH, but not at another.
18
CA 02326320 2000-09-27
WO 99/50659 PC1'/GB99/01001
The facility to perform zeta potential measurements at different pH's is a key
aspect of one of the embodiments of the invention. It provides a means of
distinguishing between two micro-organisms which would othenrvise be difficult
or
impossible to separate.
In one embodiment this process can be automated. Having taken a zeta
potential measurement at a first pH, an aliquot of acid or base can be
introduced
into the measurement cell and the reading repeated. In fact, a series of pH's
can be
used by the incremental additions of acid or base.
Dosage means to infect a solution of acid or alkali is known per se to the
person skilled in the art. Typical bases which can be used are sodium or
potassium
hydroxide or an amine base such as triethylamine. Acids include hydrochloric
or
sulphuric acid or organic acids such as acetic or citric acid.
The apparatus may advantageously include a pH meter such that the pH of
the solution in the cell is measured and recorded automatically.
The uniformity of virus structures would give every indication that viruses
can
be distinguished one from another in a similar way.
As such, this method represents an important new method of diagnosis
which can be performed in vitro on a sample removed from the human body. The
method does not have to be performed by a medical practitioner but can be
readily
carried out by a technician. The technician need not have any medical
knowledge in
order to carry out this method effectively and requires only the most basic of
training.
The method provides the medic with a clear indication of which pathogenic
bacteria, or combination of bacteria, are present in the patient's sample.
From that
information and the symptoms experienced by the patient, the medic can then
19
CA 02326320 2000-09-27
WO 99/50659 PCT/GB99/01001
formulate the most appropriate treatment. It provides, in effect, an
intermediate
result upon which to decide on a suitable course of treatment.
Except as stated above, it is preferred that the pH value of the solution and
its temperature should always be the same during measurement as the pH may
affect the measured zeta potential but it is alternatively possible to
compensate the
obtained measurement for changes in pH and temperature.
However, an important advantage of the present invention is that while the
chemical composition of the micro-organism is the same whether it is alive or
dead,
the zeta potential changes when the micro-organism dies because the charge
distributed around the micro-organism is affected by the chemical processes
taking
place while the organism is alive, especially near its outer periphery. (f,
therefore,
steps have been taken to eliminate a micro-organism that is the cause of an
infection, the present invention can be used to determine if such steps have
been
effective.
The invention has been described in relation to identifying organisms in a
solution. In this context the term "solution" has a very broad meaning. It
refers to
any liquid, organic or inorganic, which may contain a micro-organism. It
includes, by
way of example only, notionally pure water, brewing worts, body fluids such as
urine
and diluted samples of these, plant extracts or swabbings and solutions
obtained by
bubbling a gas through a liquid in order to entrain any micro-organisms
present in
the gas. fn this way contaminations in gaseous fluids may also be investigated
using the present method.
This method is not limited to the foregoing micro-organisms in Table I.
Potentially any pathogenic micro-organism or class of micro-organism can be
identified using this method and the preferred treatments) selected.
CA 02326320 2000-09-27
WO 99/50659 PCT/GB99/01001
Where very small numbers of bacteria are present in the initial sample then it
may be necessary to culture the micro-organism using methods well-known in the
art.
The bacterial count in the urine from patients having urinary infections is in
the order of 108 to 10' cells per ml. These levels are easily and rapidly
detected
using the method of the present invention, and it is anticipated that an
indication of
the infecting pathogen will be provided within 5 minutes.
Food and water samples however tend to contain rather fewer bacteria per
ml, and for these the sample will need to be cultured. The sample may be mixed
with, for example, McConkey's broth. After a predetermined time, when the
number
of cells present per ml will have increased to 105 or more, the cultured
sample may
be analysed for the presence or absence of micro-organisms using the method of
the present invention. No filtration is necessary and the broth ingredients do
not
interfere with the analysis.
Food samples are prepared, for example, by swabbing the surface of the
food or macerating the food. Thus Salmonella could be detected in a chicken
neck
flap swab within 7h, and indications are that 200 cells per ml of Salmonella
(a level
which is considered to be infective) could be detected within 2.5h culture
time.
These timescales contrast markedly with the methods currently available
where results take days not hours to come through. Recent food poisoning and
meningitis scares have shown that patients can deteriorate very substantially
or
even die over this time period. Providing there are sufficient micro-organisms
in the
sample then a result can be obtained in minutes using the present method.
Importantly, the culture media used to multiply micro-organisms do not
interfere so
no fltration or only crude filtration is required.
21
CA 02326320 2000-09-27
WO 99150659 PCT/GB99/01001
This method, and particularly the method of data processing is applicable to
a wide variety of samples including human and animal body fluids, plant
extracts,
food, drink and notionally pure water.
In a further aspect of the invention, this method can be applied to provide a
method of antibiotic sensitivity testing. The method comprises measuring the
zeta
potential of a microbial sample, which may be a known micro-organism or a
clinical
or veterinary sample; the sample is then exposed to an antimicrobial agent;
subsequent measurement of the zeta potential indicates both the potency of the
antibacterial material, and the sensitivity of the microbial sample to that
material.
In another embodiment, the approach may be applied to assessing the
antimicrobial activity of a material. Without wishing to limit the scope of
the
invention, the material may be a compound or mixture of compounds isolated
from a
natural source, it may be a compound that is a derivative of a known family of
antimicrobial agents, or it may be one or more compounds from a library of
compounds produced by combinatorial chemistry techniques. Typically these
libraries contain between 5 and 50 variants of a candidate therapeutic agent.
In a further embodiment, the approach may be applied to determining the
antimicrobial susceptibility of a clinical or veterinary sample obtained from
a patient
or animal suspected of suffering from a microbial infection to determine the
most
effective treatment of the infection.
The following Example illustrates a particular embodiment of the invention:
Streptococcus pneumoniae was grown for about 4 h in faked horse blood medium.
Aliqouts, representing about 105 cells, were transferred to two tubes
containing
growth medium: one of these also contained penicillin at 0.01 glml. The zeta
potential of both samples was measured using a ZETAS1ZER 2000 (RTM) from
Malvern Instruments. The tubes were incubated at 37°C for 3 h, and
the zeta
22
CA 02326320 2000-09-27
WO 99/50659 PCT/GB99/01001
potential of each measured again. Figure 4 shows the zeta potential profile of
the
tube containing the growth medium and S pneumoniae only; Figure 5 shows the
zeta potential of the tube additionally containing penicillin. There is a
clear
difference in these profiles. In both Figures, the peak at 0 mV represents
dead cells.
The zeta potential of all dead micro-organisms is close to 0 mV: without
wishing to
be bound by a particular theory, it is believed that these changes are brought
about
by the vacuolation of the cells, resulting in the zeta potential decaying to
about 0 mv.
Living, healthy cells of S pneumoniae have a zeta potential of about -27.9 mV,
as
shown in Fig. 4 and Table lll. The other peak in Fig. 4 is believed also to be
cells of
S pneurnoniae, but which are non-viable.
Table III. Mean Zeta Potentials for S pneumonlae
Sam le ~ ~~~~~ - Mean Zeta Potential (mV)
Antibiotic Present -0.3
No Antibiotic Present -27.9
Thus the present invention is directed toward a method for assessing
antimicrobiat sensitivity.
In one aspect, the invention provides a method for rapidly assessing the
susceptibility of a patient or veterinary sample to a spectrum of
anttmicrobiaf agents
in order to determine the most appropriate treatment to be initiated. This
process
could be automated, so that a patient would have a sample taken during the
morning by a nurse or technician, the sample would be analysed according to
the
present invention, and a prescription for the most appropriate antibiotic
would be
available later that day.
A machine is envisaged in which a sample from a patient is divided into x
samples and each sample is incubated to provide about 105 cells per sample.
Each
sample is then treated with a different antibiotic and incubation continued.
After set
periods of time the zeta potential of each sample is measured.
23
CA 02326320 2000-09-27
WO 99/50659 PCT/GB99/0~001
The antibiotic associated with the sampfe whose zeta potential tends to zero
quickest is the treatment of choice. A printer associated with the machine can
then
print out a prescription for checking and signature by the medical
practitioner.
This method is not a method of diagnosis as such. It simply provides the
medical practitioner with information on which agents) kills the micro-
organisms)
most satisfactorily in vitro. It can be carried out by a technician with no
medical
knowledge and only basic training.
In another aspect the invention may be applied to assessing emerging drugs
for antfmicrobial activity.
In a further aspect the invention can be applied to screening for agents
having antimfcrobial activity.
The foregoing describes how the approach may be used for antibacterial
agents: the same approach may be applied to antifungal, antiviral and anti-
parasitic
agents.
24
CA 02326320 2000-09-27