Note: Descriptions are shown in the official language in which they were submitted.
CA 02328291 2000-10-11
- 1 -
5018548aus1
Boehringer Ingelheim Pharma KG Case 5/1239-FL
D-55216 INGELHEIM Foreign filing text
New substituted indolinones, the preparation thereof and
their use as pharmaceutical compositions
The present invention relates to new substituted indoli-
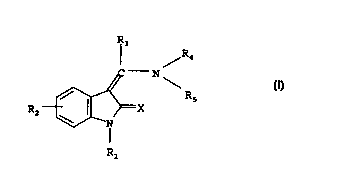
nones of general formula
R3
R4
- N
Rs
RZ g (I) ,
R1
the isomers thereof and the salts thereof, particularly the
physiologically acceptable salts thereof which have valu-
able properties.
.,... The above compounds of general formula I wherein R1 denotes
a hydrogen atom or a prodrug group have valuable pharmaco-
logical properties, particularly an inhibitory effect on
various kinases, particularly on complexes of CDKs (CDK1,
CDK2, CDK3, CDK4, CDK6, CDK7, CDK8 and CDK9) with their
specific cyclines (A, B1, B2, C, D1, D2, D3, E, F, G1, G2,
H, I and K) and viral cycline (cf. L. Mengtao in J. Viro-
logy .Z1(3), 1984-1991 (1997)), and the other compounds of
the above general formula I wherein R1 does not represent a
hydrogen atom or a prodrug group, are valuable intermediate
products for preparing the abovementioned compounds.
CA 02328291 2000-10-11
- 2 -
The present invention thus relates to the above compounds
of general formula I, whilst the compounds wherein R1
denotes a hydrogen atom or a prodrug group have valuable
pharmacological properties, the pharmaceutical compositions
containing the pharmacologically active compounds, their
use and processes for preparing them.
In the above general formula I
X denotes an oxygen or sulphur atom,
R1 denotes a hydrogen atom, a C1_4-alkoxy-carbonyl or
C2_4-alkanoyl group,
RZ denotes a carboxy or C1_4-alkoxy-carbonyl group or an
aminocarbonyl group optionally substituted by one or two
C1_3-alkyl groups, whilst the substituents may be identical
or different,
R3 denotes a hydrogen atom or a C1_6-alkyl group which may
be substituted at the 2 position, in relation to the carbon
atom of the R3-C (R4NR5) = group by a fluorine, chlorine or
bromine atom, by a hydroxy, C1_3-alkoxy, Cl_3-alkylsulphenyl,
C1_3-alkylsulphinyl, Cl_3-alkylsulphonyl, phenylsulphenyl,
phenylsulphinyl, phenylsulphonyl, amino, C1_3-alkylamino,
di- (C1_3-alkyl) -amino, CZ_5-alkanoylamino or N- (C1_3-al-
kylamino) -Cz_5-alkanoylamino group,
R4 denotes a hydrogen atom, a C1_6-alkyl group or a CS_.,-cyc-
loalkyl group optionally substituted by a C1_3-alkyl group
wherein a methylene group in the 3 or 4 position in rela-
tion to the carbon atom of the R3-C (R4NR5) = group may be
substituted by an imino group optionally substituted by a
C1_3-alkyl group,
a phenyl or naphthyl group which may be substituted
CA 02328291 2000-10-11
- 3 -
by a fluorine, chlorine, bromine or iodine atom,
by a methoxy group optionally substituted by 1 to 3
fluorine atoms,
by a C2_3-alkoxy which may be substituted in the 2 or 3
position by a Cl_3-alkyl amino, di- (C1_3-alkyl) -amino or 5-
to 7-membered cycloalkyleneimino group, whilst additio-
nally an alkyl moiety in the abovementioned alkylamino and
dialkylamino groups may be substituted by a phenyl group,
by a trifluoromethyl, amino, C1_3-alkylamino, di-
(C1_3-alkyl) -amino, C~_5-al kanoylamino, N- (C1_3-alkyl) -
Cz_5-alkanoylamino, C1_5-alkylsulphonylamino, N- (C1_3-alkyl) -
C1_5-alkylsulphonylamino, phenylsulphonylamino,
N-(C1_3-alkyl)-phenylsulphonylamino, aminosulphonyl,
C1_3-alkylaminosulphonyl or di- (C1_3-alkyl) -aminosulphonyl
group, whilst additionally an alkyl moiety in the above-
mentioned alkylamino and dialkylamino groups may be sub-
stituted by a phenyl group,
by a carbonyl group which is substituted by a hydroxy,
Cl_3-alkoxy, amino, C._3-alkyl amino or N- (C1_5-alkyl) -
C1_3-alkylamino group, whilst additionally an alkyl
moiety in the abovementioned groups may be substituted
by a carboxy, C1_3-alkoxycarbonyl or phenyl group or in
the 2 or 3 position by a di-(C1_3-alkyl)-amino, pipera-
zino, N-(C1_3-alkyl)-piperazino or 5- to 7-membered cyc-
loalkyleneimino group,
by a C1_3-alkyl group which is substituted by an amino,
C1_.,-alkyl amino, CS_-,-cycloalkylamino, CS_.,-cycloalkyl-
C1_3-alkyl amino or phenyl-Cl_3-alkyl amino group which may
additionally be substituted at the amino nitrogen atom
by a C1_3-alkyl group wherein the hydrogen atoms are
CA 02328291 2000-10-11
- 4 -
wholly or partially replaced by fluorine atoms, by a
CS_.,-cycloalkyl, CZ_4-alkenyl or Cl_4-alkyl group, whilst
the abovementioned C1_4-alkyl substituent may in each
case be additionally mono-, di- or trisubstituted by a
cyano, carboxy, C1_3-alkoxycarbonyl, pyridyl, imidazo-
lyl, benzo[1,3]dioxole or phenyl group, wherein the
phenyl group may be substituted by fluorine, chlorine
or bromine atoms, by methyl, methoxy, trifluoromethyl,
cyano or nitro groups and the substituents may be
identical or different, or may be substituted in the
2, 3 or 4 position by a hydroxy group,
by a C1_3-alkyl group which may be substituted by a
hydroxy, carboxy, thiomorpholino, 1-oxido-thiomorpho-
lino, 1,1-dioxido-thiomorpholino, piperazino, N-(C1_3-al-
kyl)-piperazino or N-phenyl-piperazino group, by a 5- to
7-membered cycloalkenyleneimino group or by a 4- to 7-
membered cycloalkyleneimino group, wherein the abovemen-
tinned 5- to 7-membered cycloalkyleneimino groups may be
substituted by one or two C1_3-alkyl groups, by a
CS_,-cycloalkyl or phenyl group, by a C1_3-alkyl,
CS_,-cycloalkyl, phenyl, carboxy or Cl_4-alkoxy-carbonyl
group and by a hydroxy group and in the abovementioned
cycloalkyleneimino groups a methylene group adjacent to
the nitrogen atom may be replaced by a carbonyl group,
by a C1_3-alkyl group which is substituted by a 5- to 7-
membered cycloalkyleneimino group, whilst a phenyl group
optionally mono- or disubstituted by fluorine, chlorine
or bromine atoms or by methyl or methoxy groups, wherein
the substituents may be identical or different, or an
oxazolo, imidazolo, thiazolo, pyridino, pyrazino or
pyrimidino group, optionally substituted by a fluorine,
chlorine, bromine or iodine atom or by a methyl, methoxy
or amino group, may be fused to the abovementioned 5- to
CA 02328291 2000-10-11
- 5 -
7-membered cycloalkyleneimino groups via two adjacent
carbon atoms,
whilst additionally the abovementioned monosubstituted
phenyl groups may be substituted by a fluorine, chlorine
or bromine atom or by a methyl, methoxy or nitro group,
a 5-membered heteroaromatic group which contains an imino
group, an oxygen or sulphur atom or an imino group, an
oxygen or sulphur atom and one or two nitrogen atoms, or
a 6-membered heteroaromatic group which contains one, two
or three nitrogen atoms, whilst the abovementioned 5- and
6-membered heteroaromatic groups may additionally be sub-
stituted by a chlorine or bromine atom or by a methyl
group, or a phenyl ring may be fused to the abovementioned
5- or 6-membered heteroaromatic groups via two adjacent
carbon atoms, and
RS denotes a hydrogen atom or a C1_3-alkyl group.
Furthermore, the carboxy, amino or imino groups present in
a compound of the above general formula I may be substitu-
ted by groups which can be cleaved in vivo.
In addition to the alkoxycarbonyl and alkanoyl groups al
ready mentioned above, groups which can be cleaved in vivo
include an acyl group such as the benzoyl, pyridinoyl, pen-
tanoyl or hexanoyl group, an allyloxycarbonyl group, a
C1_ls-alkoxycarbonyl group such as the pentoxycarbonyl,
hexyloxycarbonyl, octyloxycarbonyl, nonyloxycarbonyl,
decyloxycarbonyl, undecyloxycarbonyl, dodecyloxycarbonyl or
hexadecyloxycarbonyl group, a phenyl-C1_6-alkoxycarbonyl
group such as the benzyloxycarbonyl, phenylethoxycarbonyl
or phenylpropoxycarbonyl group, a C1_3-alkylsulphonyl-C2_4-
CA 02328291 2000-10-11
- 6 -
alkoxycarbonyl, C1_3-alkoxy-Cz_4-alkoxy-Cz_4-alkoxycarbonyl or
R~CO-O- (RdCRe) -O-CO-group wherein
R~ denotes a Cl_e-alkyl, CS_.,-cycloalkyl, phenyl or phenyl-
s C1_3-alkyl group,
Re denotes a hydrogen atom, a C1_3-alkyl, CS_.,-cycloalkyl or
phenyl group and
Rd denotes a hydrogen atom or a C1_3-alkyl group or the
R~CO-O- (RdCRe) -O- group,
whilst the abovementioned ester groups may also be used as
a group which can be converted in vi.vo into a carboxy
group.
Preferred compounds of general formula I, however, are
those wherein
X denotes an oxygen atom,
R1 denotes a hydrogen atom,
",~ RZ denotes an aminocarbonyl group,
R3 denotes a hydrogen atom or a C1_4-alkyl group which may
be substituted, at the 2 position in relation to the carbon
atom of the R3-C (R4NR5) = group by a chlorine or bromine atom
or by a phenylsulphonyl group,
R4 denotes a hydrogen atom, a C1_3-alkyl group or a cyclo-
pentyl or cyclohexyl group optionally substituted by a
methyl group, whilst in the cyclopentyl and cyclohexyl
group a methylene group in the 3 or 4 position in relation
to the carbon atom of the R;-C(R4NR5)= group may be replaced
by an imino group optionally substituted by a methyl group,
CA 02328291 2000-10-11
_ 7 _
a phenyl group which is substituted
by a fluorine, chlorine, bromine or iodine atom,
by a methoxy group optionally substituted by 1 to 3
fluorine atoms,
by a Cz_3-alkoxy which is substituted in the 2 or 3
position by methylamino, dimethylamino or 5- to 7-
membered cycloalkyleneimino group, whilst additionally a
methyl group in the abovementioned amino groups may be
substituted by a phenyl group,
by a trifluoromethyl, amino, C2_5-alkanoylamino,
N- (C1_3-alkyl) -CZ_5-alkanoylamino, C.1_5-alkylsulpho-
nylamino, N- (Cl_3-alkyl) -Cl_5-alkylsulphonylamino, phe-
nylsulphonylamino, N-(C1_3-alkyl)-phenylsulphonylamino or
aminosulphonyl group, whilst additionally an alkyl moie-
ty in the abovementioned alkylamino and dialkylamino
groups may be substituted by a phenyl group,
by a carbonyl group which is substituted by a hydroxy,
C1_3-alkoxy, amino, C=_3-alkyl amino or N- (C1_5-alkyl) -
C1_3-alkylamino group, whilst additionally an alkyl
moiety in the abovementioned groups may be substituted
by a carboxy, C1_3-alkoxycarbonyl or phenyl group or in
the 2 or 3 position by a di-(C1_3-alkyl)-amino, pipera-
zino, N-(C1_3-alkyl)-piperazino or 5- to 7-membered cyc-
loalkyleneimino group,
by a C1_,-alkyl group which is substituted by an amino,
C1_.,-alkyl amino, CS_.,-cycloalkylamino, CS_,-cycloalkyl-
C1_3-alkylamino or phenyl-C1_3-alkylamino group which may
additionally be substituted at the amino nitrogen atom
by a C1_3-alkyl group wherein the hydrogen atoms are
CA 02328291 2000-10-11
- g _
wholly or partially replaced by fluorine atoms, by a
CS_.,-cycloalkyl, C2_4-alkenyl or Cl_4-alkyl group, whilst
the abovementioned C1_4-alkyl substituent may in each
case be additionally substituted by a cyano, carboxy,
C1_3-alkoxycarbonyl, pyridyl, imidazolyl, benzo[1,3)di-
oxole or phenyl group, wherein the phenyl group may be
monosubstituted by a fluorine, chlorine or bromine
atom or by a methyl, methoxy, cyano, trifluoromethyl
or nitro group, or di- or trisubstituted by fluorine,
chlorine or bromine atoms or by methyl or methoxy
"' groups, and the substituents may be identical or dif-
ferent, or may be substituted in the 2, 3 or 4 posi-
tion by a hydroxy group,
by a C1_3-alkyl group which may be substituted by a
hydroxy, carboxy, thiomorpholino, 1-oxido-thiomor-
pholino, 1,1-dioxido-thiomorpholino, piperazino,
N-(C1_3-alkyl)-piperazino or N-phenyl-piperazino group,
by a 5- to 7-membered cycloalkenyleneimino group or by a
4- to 7-membered cycloalkyleneimino group, wherein the
abovementioned 5- to 7-membered cycloalkyleneimino-
groups may be substituted by one or two C1_3-alkyl
groups, by a cyclohexyl or phenyl group, by a C1_3-alkyl,
cyclohexyl, phenyl, carboxy or C1_,,-alkoxy-carbonyl group
and by a hydroxy group and in the abovementioned
cycloalkyleneimino groups a methylene group adjacent to
the nitrogen atom may be replaced by a carbonyl group,
by a C1_3-alkyl group which is substituted by a 5- to 7-
membered cycloalkyleneimino group, whilst a phenyl group
optionally mono- or disubstituted by fluorine, chlorine
or bromine atoms or by methyl or methoxy groups, wherein
the substituents may be identical or different, or a py-
razino or thiazolo group, optionally substituted by an
amino group, may be fused to the abovementioned 5- to 7-
CA 02328291 2000-10-11
- g _
membered cycloalkyleneimino groups via two adjacent
carbon atoms,
whilst additionally the abovementioned monosubstituted
phenyl groups may be substituted by a fluorine, chlorine
or bromine atom or by a methyl, methoxy or nitro group,
a pyridyl group optionally substituted by a chlorine or
bromine atom or by a methyl group,
an oxazolyl, isoxazolyl, imidazolyl or thiazolyl group
"' optionally substituted by a methyl group, to which a
phenyl ring may be fused via two adjacent carbon atoms,
and
RS denotes a hydrogen atom or a C1_3-alkyl group,
particularly those compounds of general formula I wherein
R1 to R3 and RS are as hereinbefore defined and
R4 denotes a hydrogen atom, a C1_6-alkyl group or a CS_,-cyc-
loalkyl group optionally substituted by a C1_3-alkyl group
wherein a methylene group in the 3 or 4 position in rela-
tion to the carbon atom of the R3-C (R4NR5) = group may be
replaced by an imino group optionally substituted by a
C1_3-alkyl group,
a phenyl or naphthyl group which may be substituted
by a fluorine, chlorine or iodine atom, by a C1_3-alkoxy,
amino, C1_3-alkylamino, di- (C1_,-alkyl) -amino, Cz_5-al-
kanoylamino, N- (C1_3-alkylamino) -C2_5-alkanoylamino,
C1_5-alkylsulphonylamino, N- (C1_3-alkyl) -C1_5-alkylsul-
phonylamino, phenylsulphonylamino or N-(Cl_3-alkyl)-phenyl-
sulphonylamino group or by a C1_3-alkyl group which may be
substituted by a C1_5-alkylamino, di- (C1_5-alkyl) -amino,
thiomorpholino, 1-oxido-thiomorpholino, 1,1-dioxido-thio-
CA 02328291 2000-10-11
- 10 -
morpholino, piperazino, N-(Cl_3-alkyl)-piperazino, N-phenyl-
piperazino, CS_,-cycloalkenyleneimino group or by a C4_,-cyc-
loalkyleneimino group, whilst the abovementioned CS_.,-cyc-
loalkyleneimino groups may be substituted by one or two
C1_3-alkyl groups, by a CS_.,-cycloalkyl or phenyl group, by a
Cl_3-alkyl, CS_.,-cycloalkyl, phenyl, carboxy or Cl_4-alkoxy-
carbonyl group and by a hydroxy group,
the isomers and salts thereof.
Particularly preferred compounds of general formula I are
"' those wherein R1 to RS are as hereinbefore defined and RZ
is in the 5 position,
particularly those compounds wherein
X denotes an oxygen atom,
R1 denotes a hydrogen atom,
RZ in the 5 position denotes an aminocarbonyl group,
R3 denotes a hydrogen atom or a C1_4-alkyl group which may
be terminally substituted by a chlorine or bromine atom or
by a phenylsulphonyl group,
R4 denotes a hydrogen atom, a C1_3-alkyl group or a cyclo-
pentyl or cyclohexyl group optionally substituted by a
methyl group, whilst in the cyclohexyl group a methylene
group in the 4 position in relation to the carbon atom of
the R3-C (R4NR5) = group may be replaced by an imino group
optionally substituted by a methyl group,
a phenyl group which may be substituted
by a fluorine, chlorine, bromine or iodine atom,
CA 02328291 2000-10-11
- 11 -
by a methyl or ethyl group, which may in each case be
substituted by a Cl_3-alkylamino, di- (C1_3-alkyl) -amino,
thiomorpholino, 1-oxido-thiomorpholino, 1,1-dioxido-thio-
morpholino, N-phenyl-piperazino, 5- to 6-membered cyclo-
alkenyleneimino group or by a 5- to 7-membered cycloalky-
leneimino group, whilst the abovementioned 5- to 7-membered
cycloalkyleneimino groups may be substituted by one or two
methyl groups, by a cyclohexyl or phenyl group, by a
methyl, cyclohexyl or phenyl group and by a hydroxy group,
or
by a methyl or ethyl group which may be substituted by a
phenyl group which is substituted by a 5 to 7-membered
cycloalkyleneimino group, whilst additionally a phenyl
ring is fused to the abovementioned cycloalkyleneimino
groups via 2 adjacent carbon atoms,
by a methyl or ethyl group substituted by an amino,
methylamino or ethylamino group, each of which is additi-
onally substituted at the amino nitrogen atom by a benzyl
or phenylethyl group, wherein the phenyl moiety in the
abovementioned groups may be monosubstituted by a fluo-
rine, chlorine or bromine atom or by a methyl, methoxy,
'i 25 cyano, trifluoromethyl or nitro group or di- or tri-
substituted by fluorine, chlorine or bromine atoms or by
methyl or methoxy groups, and the substituents may be
identical or different,
whilst additionally the abovementioned monosubstituted
phenyl groups may be substituted by a fluorine, chlorine
or bromine atom or by a methyl, methoxy or nitro group,
and
RS denotes a hydrogen atom or a C1_4-alkyl group,
CA 02328291 2000-10-11
- 12 -
the isomers and the salts thereof.
Most particularly preferred compounds of general formula I
are those wherein
X denotes an oxygen atom,
R1 denotes a hydrogen atom,
RZ in the 5 position denotes an aminocarbonyl group,
R3 denotes a hydrogen atom or a C1_4-alkyl group,
R4 denotes a phenyl group which may be substituted
by a fluorine, chlorine, bromine or iodine atom,
by a methyl or ethyl group, which may be substituted in
each case by a C1_3-alkyl amino, di- (C1_3-alkyl) -amino,
thiomorpholino, 1-oxido-thiomorpholino, 1,1-dioxido-
thiomorpholino, N-phenyl-piperazino, 5- to 6-membered
cycloalkenyleneimino group or by a 5- to 7-membered cyclo-
alkyleneimino group, whilst the abovementioned 5- to 7-
membered cycloalkyleneimino groups may be substituted by
one or two methyl groups, by a cyclohexyl or phenyl group,
by a methyl, cyclohexyl or phenyl group and by a hydroxy
group, or
by a methyl or ethyl group which may be substituted by a
phenyl group which is substituted in the 4 position by a 5
to 7-membered cycloalkyleneimino group, whilst additio-
nally a phenyl ring is fused to the abovementioned cyclo-
alkyleneimino groups via 2 adjacent carbon atoms,
by a methyl or ethyl group substituted by an amino,
methylamino or ethylamino group, each of which is
CA 02328291 2000-10-11
- 13 -
additionally substituted at the amino nitrogen atom by a
benzyl or phenylethyl group, and wherein the phenyl moiety
may be monosubstituted by a fluorine, chlorine or bromine
atom or by a methyl, methoxy, cyano, trifluoromethyl or
nitro group, disubstituted by methyl or methoxy groups or
trisubstituted by methyl or methoxy groups, and the sub-
stituents may be identical or different,
whilst additionally the abovementioned monosubstituted
phenyl groups may be substituted by a fluorine, chlorine
or bromine atom or by a methyl, methoxy or nitro group,
and
RS denotes a hydrogen atom or a Cl_4-alkyl group,
the isomers and the salts thereof.
The following are mentioned as examples of particularly
preferred compounds:
(a) 3-Z-[1-(4-piperidinomethyl-phenylamino)-1-methyl-methy-
lene]-5-amido-2-indolinone,
(b) 3-Z-[1-(4-bromo-phenylamino)-1-methyl-methylene]-5-
amido-2-indolinone,
(c) 3-Z-[1-(4-piperidinomethyl-phenylamino)-1-butyl-
methylene]-5-amido-2-indolinone,
(d) 3-Z-[1-(4-chlorophenylamino)-1-methyl-methylene]-5-
amido-2-indolinone and
(e) 3-Z-(1-phenylamino-methylene)-5-amido-2-indolinone
(f) 3-Z-[1-(4-(N-benzyl-N-methyl-aminomethyl)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone,
CA 02328291 2000-10-11
- 14 -
(g) 3-Z-[1-(4-(N-(4-chlorobenzyl)-aminomethyl)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone,
(h) 3-Z-[1-(4-(N-benzyl-N-ethyl-aminomethyl)-phenylamino]-
1-methyl-methylene]-5-amido-2-indolinone,
(i) 3-Z-[1-(4-(N-benzyl-aminomethyl)-phenylamino)-1-
methyl-methylene]-5-amido-2-indolinone,
(j) 3-Z-[1-(4-(N-benzyl-N-methyl-aminomethyl)-phenyl-
amino)-methylene]-5-amido-2-indolinone,
(k) 3-Z-[1-(4-(2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone,
(1) 3-Z-[1-(4-piperidinomethyl-3-nitro-phenylamino)-
1-methyl-methylene]-5-amido-2-indolinone and
(m) 3-Z-[1-(4-methyl-3-nitro-phenylamino)-1-methyl-
methylene)-5-amido-2-indolinone
as well as the isomers and the salts thereof.
According to the invention, the new compounds may be ob-
tained, for example, according to the following processes
known in principle from the literature:
a. reacting a compound of general formula
CA 02328291 2000-10-11
- 15 -
R3
Z1
R2' / I ~X (II)
N
Rs
wherein
X and R3 are as hereinbefore defined,
R2' has the meanings given for R2 hereinbefore,
R6 denotes a hydrogen atom or a protecting group for the
nitrogen atom of the lactam group, whilst one of the groups
RZ' or R6 may also denote a bond to a solid phase optionally
formed via a spacer and the other group R2' or R6 is as
hereinbefore defined, and
Z1 denotes a halogen atom, a hydroxy, alkoxy or aralkoxy
group, e.g. a chlorine or bromine atom or a methoxy, ethoxy
or benzyloxy group,
with an amine of general formula
Rs
H - N\ (III),
\ R4
wherein
R4 and RS are as hereinbefore defined,
and if necessary subsequently cleaving any protecting
groups used for the nitrogen atom of the lactam group or
from a solid phase.
A suitable protecting group for the nitrogen atom of the
lactam group might be, for example, an acetyl, benzoyl,
ethoxycarbonyl, tert.butyloxycarbonyl or benzyloxycarbonyl
group and
CA 02328291 2000-10-11
- 16 -
A suitable solid phase might be, for example, a resin such
as a 4-(2',4'-dimethoxyphenylaminomethyl)-phenoxy resin,
the bonding preferably taking place via the amino group, or
a p-benzyloxybenzylalcohol resin, the bonding preferably
taking place via an intermediate member such as a 2,5-di-
methoxy-4-hydroxy-benzyl derivative.
The reaction is appropriately carried out in a solvent such
as dimethylformamide, toluene, acetonitrile, tetrahydro-
furan, dimethylsulphoxide, methylene chloride or mixtures
thereof, optionally in the presence of an inert base such
°° as triethylamine, N-ethyl-diisopropylamine or sodium
hydrogen carbonate at temperatures between 20 and 175°C,
whilst any protecting group used may be cleaved at the same
time as a result of transamidation.
If Z1 in a compound of general formula II denotes a ha-
logen atom, the reaction is preferably carried out in the
presence of an inert base at temperatures of between 20
and 120°C.
If Z1 in a compound of general formula II denotes a
hydroxy, alkoxy or aralkoxy group, the reaction is
preferably carried out at temperatures between 20 and
200°C.
The subsequent cleaving of any protecting group used, if
necessary, is appropriately carried out either hydrolyti-
cally in an aqueous or alcoholic solvent, e.g. in metha-
nol/water, ethanol/water, isopropanol/water, tetrahydro-
furan/water, dioxan/water, dimethylformamide/water, metha-
nol or ethanol in the presence of an alkali metal base
such as lithium hydroxide, sodium hydroxide or potassium
hydroxide at temperatures between 0 and 100°C, preferably
at temperatures between 10 and 50°C,
CA 02328291 2000-10-11
- 17 -
or more advantageously by transamidation with an organic
base such as ammonia, methylamine, butylamine, dimethyl-
amine or piperidine in a solvent such as methanol, etha-
nol, dimethylformamide and mixtures thereof or in an ex-
cess of the amine used, at temperatures of between 0 and
100°C, preferably at temperatures between 10 and 50°C.
Any solid phase used is preferably cleaved by means of
trifluoroacetic acid and water at temperatures of between
0 and 35°C, preferably at ambient temperatures.
b. in order to prepare a compound of general formula I
wherein R~ denotes one of the abovementioned aminocarbonyl
groups:
amidating a compound of general formula
R3
R4
- N
Rs
HO-CO O (IV),
R1
wherein R1 and R, are as hereinbefore defined, or the
reactive derivatives thereof, with an amine of general
formula
H - (R.,NRB) (V)
wherein R., and R~ which may be identical or different
denote hydrogen atoms or C1_~-alkyl groups.
CA 02328291 2000-10-11
- 18 -
The amidation is preferably carried out in a solvent such
as methylene chloride, diethyl ether, tetrahydrofuran,
toluene, dioxan, acetonitrile, dimethylsulphoxide or di-
methylformamide, optionally in the presence of an inor-
ganic or tertiary organic base, preferably at temperatures
between 20°C and the boiling temperature of the solvent
used. The amidation is carried out with a corresponding
acid, preferably in the presence of a dehydrating agent,
e.g. in the presence of isobutyl chloroformate, tetraethyl
orthocarbonate, trimethyl orthoacetate, 2,2-dimethoxypro-
pane, tetramethyloxysilane, thionylchloride, trimethyl-
chlorosilane, phosphorus trichloride, phosphorus pentoxi-
de, N,N'-dicyclohexylcarbodiimide, N,N'-dicyclohexylcar-
bodiimide/N-hydroxysuccinimide, N,N'-dicyclohexylcarbo-
diimide/1-hydroxy-benzotriazole, 2-(1H-benzotriazol-1-yl)-
1,1,3,3-tetramethyluronium tetrafluoroborate, 2-(1H-benzo-
triazol-1-yl)-1,1,3,3-tetramethyluronium tetrafluorobo-
rate/1-hydroxy-benzotriazole, N,N'-carbonyldiimidazole or
triphenylphosphine/carbon tetrachloride, and optionally
with the addition of a base such as pyridine, 4-dimethyl-
aminopyridine, N-methylmorpholine or triethylamine, appro-
priately at temperatures between 0 and 150°C, preferably
at temperatures between 0 and 100°C, and the acylation is
carried out with a corresponding reactive compound such as
......
the anhydride, ester, imidazolide or halide thereof, op-
tionally in the presence of a tertiary organic base such
as triethylamine, N-ethyldiisopropylamine or N-methylmor-
pholine at temperatures between 0 and 150°C, preferably at
temperatures between 50 and 100°C.
If according to the invention a compound of general
formula I is obtained which contains an alkoxycarbonyl
group, this may be converted by hydrolysis into a
corresponding carboxy compound, or
CA 02328291 2000-10-11
- 19 -
if a compound of general formula I is obtained which
contains an amino or alkylamino group, this may be
converted by alkylation or reductive alkylation into a
corresponding alkylamino or dialkylamino compound, or
if a compound of general formula I is obtained which
contains an amino or alkylamino group, this may be
converted by acylation into a corresponding acyl compound,
or
if a compound of general formula I is obtained which
contains a carboxy group, this may be converted by
esterification or amidation into a corresponding ester or
aminocarbonyl compound, or
if a compound of general formula I is obtained which
contains a nitro group, this may be converted by reduction
into a corresponding amino compound.
The subsequent hydrolysis is preferably carried out in an
aqueous solvent, e.g. in water, isopropanol/water, tetra-
hydrofuran/water or dioxan/water, in the presence of an
acid such as trifluoroacetic acid, hydrochloric acid or
sulphuric acid or in the presence of an alkali metal base
such as lithium hydroxide, sodium hydroxide or potassium
hydroxide at temperatures between 0 and 100°C, preferably
at temperatures between 10 and 50°C.
The subsequent reductive alkylation is preferably carried
out in a suitable solvent such as methanol, methanol/wa-
ter, methanol/water/ammonia, ethanol ether, tetrahydrofu-
ran, dioxan or dimethylformamide, optionally with the
addition of an acid such as hydrochloric acid in the
presence of catalytically activated hydrogen, e.g. hy-
drogen in the presence of Raney nickel, platinum or
palladium/charcoal, or in the presence of a metal hydride
CA 02328291 2000-10-11
- 20 -
such as sodium borohydride, lithium borohydride or lithium
aluminium hydride at temperatures between 0 and 100°C,
preferably at temperatures between 20 and 80°C.
The subsequent alkylation is carried out with an alkyla-
ting agent such as an alkyl halide or dialkylsulphate such
as methyl iodide, dimethylsulphate or propyl bromide, pre-
ferably in a solvent such as methanol, ethanol, methylene
chloride, tetrahydrofuran, toluene, dioxan, dimethylsulph-
oxide or dimethylformamide, optionally in the presence of
an inorganic or tertiary organic base such as triethylami-
ne, N-ethyl-diisopropylamine or dimethylaminopyridine,
preferably at temperatures between 20°C and the boiling
temperature of the solvent used.
The subsequent acylation is preferably carried out in a
solvent such as methylene chloride, diethyl ether, tetra-
hydrofuran, toluene, dioxan, acetonitrile, dimethylsulph-
oxide or dimethyl formamide, optionally in the presence of
an inorganic or tertiary organic base, preferably at tem-
peratures between 20°C and the boiling temperature of the
solvent used. The acylation is carried out with a cor-
responding acid, preferably in the presence of a dehydra-
"~ ting agent, e.g. in the presence of isobutyl chlorofor-
mate, tetraethyl orthocarbonate, trimethyl orthoacetate,
2,2-dimethoxypropane, tetramethoxysilane, thionylchloride,
trimethylchlorosilane, phosphorus trichloride, phosphorus
pentoxide, N,N'-dicyclohexylcarbodiimide, N,N'-dicyclohe-
xylcarbodiimide/N-hydroxysuccinimide, N,N'-dicyclohexyl-
carbodiimide/1-hydroxy-benzotriazole, 2-(1H-benzotriazol-
1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate, 2-(1H-
benzotriazol-1-yl)-1,1,3,3-tetramethyluronium tetrafluo-
roborate/1-hydroxy-benzotriazole, N,N'-carbonyldiimidazole
or triphenylphosphine/carbon tetrachloride, and optionally
with the addition of a base such as pyridine, 4-dimethyl
amino-pyridine, N-methylmorpholine or triethylamine,
CA 02328291 2000-10-11
- 21 -
appropriately at temperatures of between 0 and 150°C,
preferably at temperatures of between 0 and 100°C, and the
acylation is carried out with a corresponding reactive
compound such as an anhydride, ester, imidazole or halide
thereof, optionally in the presence of a tertiary organic
base such as triethylamine, N-ethyl-diisopropylamine or N-
methyl-morpholine at temperatures of between 0 and 150°C,
preferably at temperatures of between 50 and 100 C.
The subsequent esterification or amidation is appropri-
ately carried out by reacting a corresponding reactive
carboxylic acid derivative with a corresponding alcohol or
amine as described hereinbefore.
The subseqent reduction of a nitro group is preferably
carried out by hydrogenolysis, e.g. with hydrogen in the
presence of a catalyst such as palladium/charcoal or Raney
nickel in a solvent such as methanol, ethanol, ethyl ace-
tate, dimethylformamide, dimethylformamide/acetone or gla-
cial acetic acid, optionally with the addition of an acid
such as hydrochloric acid or glacia'~ acetic acid at tempe-
ratures between 0 and 50°C, but preferably at ambient tem-
perature, and at a hydrogen pressure of from 1 to 7 bar,
but preferably from 3 to 5 bar.
In the reactions described hereinbefore, any reactive
groups present such as carboxy, amino, alkylamino or imino
groups may be protected during the reaction by conven-
tional protecting groups which are cleaved again after the
reaction.
For example, a protecting group for a carboxy group may be
a trimethylsilyl, methyl, ethyl, tert.butyl, benzyl or
tetrahydropyranyl group and
CA 02328291 2000-10-11
- 22 -
protecting groups for an amino, alkylamino or imino group
may be an acetyl, trifluoroacetyl, benzoyl, ethoxycar-
bonyl, tert.butoxycarbonyl, benzyloxycarbonyl, benzyl,
methoxybenzyl or 2,4-dimethoxybenzyl group and
additionally, for the amino group, a phthalyl group.
Any protecting group used is optionally subsequently
cleaved for example by hydrolysis in an aqueous solvent,
e.g. in water, isopropanol/water, tetrahydrofuran/water or
dioxan/water, in the presence of an acid such as trifluo-
roacetic acid, hydrochloric acid or sulphuric acid or in
the presence of an alkali metal base such as lithium
hydroxide, sodium- hydroxide or potassium hydroxide, at
temperatures between 0 and 100°C, preferably at tempera-
tures between 10 and 50°C.
However, a benzyl, methoxybenzyl or benzyloxycarbonyl
group is cleaved, for example hydrogenolytically, e.g.
with hydrogen in the presence of a catalyst such as
palladium/charcoal in a suitable solvent such as methanol,
ethanol, ethyl acetate, dimethylformamide, dimethylform-
amide/acetone or glacial acetic acid, optionally with the
addition of an acid such as hydrochloric acid or glacial
acetic acid at ~.emperatures between 0 and 50°C, but pre-
ferably at ambient temperature, and at a hydrogen pressure
of 1 to 7 bar, but preferably 3 to 5 bar.
A methoxybenzyl group may also be cleaved in the presence
of an oxidising agent such as cerium(IV)ammonium nitrate
in a solvent such as methylene chloride, acetonitrile or
acetonitrile/water at temperatures of between 0 and 50°C,
preferably at ambient temperature.
A 2,4-dimethoxybenzyl group, however, is preferably
cleaved in trifluoroacetic acid in the presence of
anisole.
CA 02328291 2000-10-11
- 23 -
A tert.butyl or tert.butyloxycarbonyl group is preferably
cleaved by treating with an acid such as trifluoroacetic
acid or hydrochloric acid, optionally using a solvent such
as methylene chloride, dioxan, ethyl acetate or ether.
A phthalyl group is preferably cleaved in the presence of
hydrazine or a primary amine such as methylamine, ethyl-
amine or n-butylamine in a solvent such as methanol,
ethanol, isopropanol, toluene/water or dioxan at tempera-
tures between 20 and 50°C.
Moreover, chiral compounds of general formula I obtained
may be resolved intc their enantiomers and/or diastere-
omers.
Thus, for example, the compounds of general formula I
obtained which occur as racemates may be separated by
methods known per se (cf. Allinger N. L. and Eliel E. L.
in "Topics in Stereochemistry", Vol. 6, Wiley Inter-
science, 1971) into their optical antipodes and compounds
of general formula I with at least 2 asymmetric carbon
atoms may be resolved into their diastereomers on the
basis of their physical-chemical differences using methods
known per se, e.g. by chromatography and/or fractional
crystallisation, and, if these compounds are obtained in
racemic form, they may subsequently be resolved into the
enantiomers as mentioned above.
The enantiomers are preferably separated by column sepa-
ration on chiral phases or by recrystallisation from an
optically active solvent or by reacting with an optically
active substance which forms salts or derivatives such as
e.g. esters or amides with the racemic compound, parti-
cularly acids and the activated derivatives or alcohols
thereof, and separating the diastereomeric mixture of
CA 02328291 2000-10-11
- 24 -
salts or derivatives thus obtained, e.g. on the basis of
their differences in solubility, whilst the free antipodes
may be released from the pure diastereomeric salts or
derivatives by the action of suitable agents. Optically
active acids in common use are e.g. the D- and L-forms of
tartaric acid or dibenzoyltartaric acid, di-o-tolyltar-
taric acid, malic acid, mandelic acid, camphorsulphonic
acid, glutamic acid, aspartic acid, N-acetyl-aspartic acid
or quinic acid. An optically active alcohol may be for
example (+) or (-)-menthol and an optically active acyl
group in amides, for example, may be a (+)- or (-)-men-
thyloxycarbonyl group.
Furthermore, the compounds of formula I may be converted
into the salts thereof, particularly for pharmaceutical
use into the physiologically acceptable salts with in-
organic or organic acids. Acids which may be used for this
purpose include for example hydrochloric acid, hydrobromic
acid, sulphuric acid, phosphoric acid, fumaric acid, suc-
cinic acid, lactic acid, citric acid, tartaric acid, ma-
lefic or methanesulphonic acid.
Moreover, if the new compounds of formula I contain a
carboxy group, they may subsequently, if desired, be
converted into the salts thereof with inorganic or organic
bases, particularly for pharmaceutical use into the
physiologically acceptable salts thereof. Suitable bases
for this purpose include for example sodium hydroxide,
potassium hydroxide, arginine, cyclohexylamine, etha-
nolamine, diethanolamine and triethanolamine.
The compounds of general formulae I to V used as starting
materials are known from the literature in some cases or
are described in the Examples.
CA 02328291 2000-10-11
- 25 -
As already mentioned, the new compounds of general formula
I wherein R1 denotes a hydrogen atom or a prodrug group
have valuable pharmacological properties, particularly an
inhibitory effect on various kinases and cycline/CDK
complexes, on the proliferation of cultivated human tumour
cells and, when administered orally, on the growth of
tumours in nude mice which have been infected with human
tumour cells.
For example, the compounds listed in Table 1 were tested
for their biological properties as follows:
Tnhihit-in_n_ of car .lc- inP/CDK en2~r~,~, in vitz'o actiy~ty
High Fiver insect cells (BTI-TN-5B1-4) which had been in-
fected with a high titre of recombinant baculovirus were
used to produce active human cycline/CDK holoenzymes. By
using a baculovirus vector which contained two promoters
(polyhedrin enhancer promoter, P10 enhancer promoter),
GST-tagged cyclines (e. g. cycline D1 or cycline D3) with
the corresponding His6-tagged CDK subunit (e. g. for CDK4
or CDK6) were expressed in the same cell. The active ho-
loenzyme was isolated by affinity chromatography on glu-
tathione sepharose. Recombinant GST-tagged pRB (aa 379-
928) was produced in E. coli and purified by affinity
chromatography on glutathione sepharose.
The substrates used for the kinase assays depended on the
specific kinases. Histone H1 (Sigma) was used as the
substrate for cycline E/CDK2, cycline A/CDK2, cycline
B/CDK1 and for v-cycline/CDK6. GST-tagged pRB (aa 379-
928) was used as substrate for cycline D1/CDK4, cycline
D3/CDK4, cycline D1/CDK6 and for cycline D3/CDK6.
CA 02328291 2000-10-11
- 26 -
Lysates of the insect cells infected with recombinant
baculovirus or recombinant kinases (obtained from the
lysates by purification) were incubated together with
radiolabelled ATP in the presence of a suitable substrate
with various concentrations of the inhibitor in a 1% DMSO
(dimethyl sulphoxide) solution for 45 minutes at 30°C.
The substrate proteins with associated radioactivity were
precipitated with 5o TCA (trichloroacetic acid) in water-
repellent PVDF mufti-well microtitre plates (Millipore) or
with 0.5o phosphoric acid solution on Whatman P81 filters.
After the addition of scintillation liquid the radioacti-
vity was measured in a Wallace 1450 Microbeta Liquid Scin-
tillation Counter. For each concentration of the substance
double measurements were carried out; ICsovalues were cal-
culated for the enzyme inhibition.
Tnhi hi t-i nn o h - ~ rnl_iferat-ion of rmltivated hLman tLmour
~Pll~
Cells of the Leiomyosarcoma tumour cell line SK-UT-1B
(obtained from the American Type Culture Collection
(ATCC)) were cultivated in Minimum Essential Medium with
'~ 25 non-essential amino acids (Gibco), supplemented with
sodium pyruvate (1 mmol), glutamine (2 mmol) and 10%
foetal calf serum (Gibco) and harvested during the log-
growth phase. Then the SK-UT-1B cells were added to
Cytostar~ mufti-well plates (Amersham) at a density of
4000 cells per well and incubated overnight in an
incubator. Various concentrations of the compounds
(dissolved in DMSO; final concentration: <la) were added
to the cells. After 48 hours' incubation 14C-thymidine
(Amersham) was added to each well and incubation was
continued for a further 24 hours. The quantity of 14C-
thymidine incorporated into the tumour cells in the
CA 02328291 2000-10-11
- 27 -
presence of the inhibitor and representing the number of
cells in the S phase was measured in a Wallace 1450 Mi-
crobeta Liquid Scintillation Counter. ICSo values for the
inhibition of proliferation (= inhibition of incorporated
14C-thymidine) were calculated, correcting for the back-
ground radiation. All the measurements were done twice.
In vivo P_f_fects on tLmoL_r-bPar;nc~ n ~d mi
°' 106 cells [SK-UT-1B, or non-small cell lung tumour NCI-
H460 (obtained from ATCC)] in a volume of 0.1 ml were
injected subcutaneously into male and/or female nude mice
(NMRI nu/nu; 25-35g; N = 10-20); alternatively, small
fragments of SK-UT-1B or NCI-H460 cell clumps were im-
planted subcutaneously. One to three weeks after the
injection or implantation a kinase inhibitor was admi-
nistered daily by oral route for a period of 2 to 4 weeks
(by oesophageal tube). The size of the tumour was mea-
sured three times a week using a digital sliding gauge.
The effect of a kinase inhibitor on the tumour growth was
determined as a percentage inhibition compared with a
control group treated with placebo.
Table 2 which follows contains the results obtained in in
vitro test 2:
CA 02328291 2000-10-11
- 28 -
Compound Inhibition of SKUT
(example no.) -1B proliferation
ICSO [:Ml
1(11) 0.032
1 (8) 0.060
1 (26) 0.036
1 (3) 0.040
1(1) 0.100
1(96) 0.005
1(91) 0.010
1(95) 0.008
1(51) 0.013
1(105) 0.019
1(110) 0.020
1(117) 0.020
1(71) 0.030
In view of their biological properties, the new compounds
of general formula I, their isomers and physiologically
acceptable salts are suitable for the treatment of
diseases characterised by excessive or abnormal cell
proliferation.
Such diseases include (with no claim to completeness):
viral infections (e. g. HIV and Kaposi's sarcoma);
inflammation and autoimmune diseases (e. g. colitis,
arthritis, Alzheimer's disease, glomerulonephritis and
wound healing); bacterial, fungal and/or parasitic
infections; leukaemias, lymphoma and solid tumours; skin
diseases (e. g. psoriasis); bone diseases; cardiovascular
diseases (e. g. restenosis and hypertrophy). They are also
useful for protecting proliferating cells (e. g. hair,
intestinal, blood and progenitor cells) against DNA damage
caused by radiation, UV treatment and/or cytostatic
treatment.
CA 02328291 2000-10-11
- 29 -
The new compounds may be used for the short-term or long-
term treatment of the abovementioned diseases, optionally
in conjunction with other 'state of the art' compounds
such as other cytostatics.
The dosage required to achieve such an effect is appropri-
ately 0.1 to 30 mg/kg, preferably 0.3 to 10 mg/kg by in-
travenous route, and 0.1 to 100 mg/kg, preferably 0.3 to
30 mg/kg by oral route, in each case administered 1 to 4
times a day. For this purpose, the compounds of formula I
prepared according to the invention may be formulated,
optionally together with other active substances, with one
or more inert conventional carriers and/or diluents, e.g.
with corn starch, lactose, glucose, microcrystalline
cellulose, magnesium stearate, polyvinylpyrrolidone, cit-
ric acid, tartaric acid, water, water/ethanol, water/gly-
cerol, water/sorbitol, water/polyethylene glycol, propy-
lene glycol, cetylstearyl alcohol, carboxymethylcellulose
or fatty substances such as hard fat or suitable mixtures
thereof, to produce conventional galenic preparations such
as plain or coated tablets, capsules, powders, suspensions
or suppositories.
The Examples which follow are intended to illustrate the
invention:
CA 02328291 2000-10-11
- 30 -
Meth,~rl 'I -_acet~l - -i ndol i none-S- _arbo girl a P
10.5 g of methyl 2-indolinone-5-carboxylate (prepared
analogously to Ogawa, Hidenori et al. in Chem.Pharm.Bull
~6. 2253-2258 (1988)) are stirred in 30 ml of acetic
anhydride for 4 hours at 140°C. The mixture is then left
to cool, poured onto ice water and the precipitate is
suction filtered. The product is washed with water once
more, then taken up in methylene chloride, dried over
sodium sulphate and concentrated by evaporation.
Yield: 11 g (86 0 of theory),
Rf value: 0.63 (silica gel, methylene chloride/methanol -
50:1)
Methyl 1-acetyl-3-(1-ethoxy-1-butyl-methylene]-2-
indolinone-5-carbox~rlate
11 g of methyl 1-acetyl-2-indolinone-5-carboxylate are
stirred in 110 m1 of acetic anhydride and 30 ml of
triethyl orthovalerate for 2 hours at 100°C. Then it is
concentrated by rotary evaporation, the residue is washed
'~ 25 with ether and suction filtered.
Yield: 11.5 g (67 0 of theory),
Rf value: 0.55 (silica gel, methylene chloride/petroleum
ether/ethyl acetate = 4:5:1)
The following compounds are prepared analogously to
Example II:
(1) methyl 1-acetyl-3-(1-ethoxy-methylene]-2-indolinone-5-
carboxylate
Prepared from methyl 1-acetyl-2-indolinone-5-carboxylate
and trimethyl orthoformate
CA 02328291 2000-10-11
- 31 -
(2) methyl 1-acetyl-3-(1-ethoxy-1-methyl-methylene]-2-
indolinone-5-carboxylate
Prepared from methyl 1-acetyl-2-indolinone-5-carboxylate
and triethyl orthoacetate
(3) methyl 1-acetyl-3-(1-ethoxy-1-ethyl-methylene]-2-
indolinone-5-carboxylate
Prepared from methyl 1-acetyl-2-indolinone-5-carboxylate
and triethyl orthopropionate
28.0 a of Rink resin (MBHA resin made by Novobiochem) were
allowed to swell in 330 m of dimethylformamide. Then 330
ml of 30% piperidine in dimethyl formamide were added and
the mixture was shaken for 7 minutes to cleave the 9H-
fluoren-9-yl-methoxycarbonyl group. Then the resin was
washed several times with dimethylformamide. Finally, 7.3
g 10.5 g of 2-indolinone-5-carboxylic acid (prepared
analogously to Ogawa, Hidenori et ate., Chem. Pharm. Bull
3~, 2253-2258 (1988)), 5.6 g of hydroxybenzotriazole, 13.3
g of O-(benzotriazol-1-yl)-N,N,N',N~-tetramethyluronium
tetrafluoroborate and 5.7 ml of N-ethyl-diisopropylamine
in 300 ml of dimethylformamide were added and the mixture
was shaken for 1 hour. The solution was suction filtered
and the resin was washed five times with 300 ml of dime-
thylformamide and three times with 300 ml of methylene
chloride. To dry it, nitrogen was blown through the
resin.
Yield: 20 g of charged resin
Fxam~lP TV
0.4 g of the charged resin prepared in Example III were
stirred with 2.5 ml of acetic anhydride at 90°C for 1
CA 02328291 2000-10-11
- 32 -
hour. Then 2.5 ml of trimethyl orthovalerate were added
and the mixture was shaken for a further 3 hours at 110°C.
Then the resin was suction filtered and washed with
dimethylformamide, methanol and finally with methylene
chloride.
Yield: 0.6 g of moist resin
The following charged resins were prepared analogously to
Example IV:
(1) resin charged with 3-Z-(1-ethoxy-methylene)-5-amido-2-
indolinone by reacting the product of Example I and
triethyl orthoformate
(2) resin charged with 3-Z-(1-methoxy-1-methyl-methylene)-
5-amido-2-indolinone by reacting the product of Example I
and trimethyl orthoformate
(3) resin charged with 3-Z-(1-methoxy-1-ethyl-methylene)-5-
amido-2-indolinone by reacting the product of Example I and
trimethyl orthopropionate
(4) resin charged with 3-Z-(1-methoxy-1-propyl-methylene)-
5-amido-2-indolinone by reacting the product of Example I
and trimethyl orthobutyrate
(5) resin charged with 3-Z-(1-methoxy-1-ethenyl-methylene)-
5-amido-2-indolinone by reacting the product of Example I
and 3,3,3-triethoxyprop-1-ene
(6) resin charged with 3-Z-(1-methoxy-1-(3-bromo-propyl)-
methylene)-5-amido-2-indolinone by reacting the product of
Example I and trimethyl 4-bromo-orthobutyrate
CA 02328291 2000-10-11
- 33 -
(7) resin charged with 3-Z-(1-methoxy-1-(2-phenylsulphonyl-
ethyl)-methylene)-5-amido-2-indolinone by reacting the
product of Example I and triethyl 3-phenylsulphonyl-
orthopropionate
10 6 g of 4-nitrobenzylbromide are dissolved in 25 ml of
ethanol, mixed with 25 ml of 10% ethanolic ethylamine
solution and refluxed for 2 hours. Then the solution is
concentrated by rotary evporation, the residue is taken up
in methylene chloride and washed with dilute sodium hy-
droxide solution. Then the organic phase is concentrated
by evaporation.
Yield: 2.3 g (46 % of theory),
Rf value: 0.20 (silica gel, methylene chloride/methanol =
9:1)
The following compounds are prepared analogously to
Example V:
4-[N-(4-chlorophenyl-methyl)-aminomethyl)-nitrobenzene
4-(N-cyclohexyl-aminomethyl)-nitrobenzene
4-(N-isopropyl-aminomethyl)-nitrobenzene
4-(N-butyl-aminomethyl)-nitrobenzene
4-(N-methoxycarbonylmethyl-aminomethyl)-nitrobenzene
4-(N-benzyl-aminomethyl)-nitrobenzene
4-(pyrrolidino-methyl)-nitrobenzene
CA 02328291 2000-10-11
- 34 -
4-(morpholino-methyl)-nitrobenzene
4-(piperidino-methyl)-nitrobenzene.
4-(hexamethyleneimino-methyl)-nitrobenzene
4-(4-hydroxy-piperidino-methyl)-nitrobenzene
4-(4-methyl-piperidino-methyl)-nitrobenzene
'~ 4-(4-ethyl-piperidino-methyl)-nitrobenzene
4-(4-isopropyl-piperidino-methyl)-nitrobenzene
4-(4-phenyl-piperidino-methyl)-nitrobenzene
4-(4-benzyl-piperidino-methyl)-nitrobenzene
4-(4-ethoxycarbonyl-piperidino-methyl)-nitrobenzene
4-(dimethylamino-methyl)-nitrobenzene
4-(di-n-propylamino-methyl)-nitrobenzene
4-(4-tert.butoxycarbonyl-piperazino-methyl)-nitrobenzene
3-(dimethylamino-methyl)-nitrobenzene
4-(2-diethylamino-ethyl)-nitrobenzene
4-(2-morpholino-ethyl)-nitrobenzene
4-(2-pyrrolidino-ethyl)-nitrobenzene
4-(2-piperidino-ethyl)-nitrobenzene
CA 02328291 2000-10-11
- 35 -
4-(N-ethyl-N-benzyl-aminomethyl)-nitrobenzene
4-(N-n-propyl-N-benzyl-aminomethyl)-nitrobenzene
4-[N-methyl-N-(4-chlorophenylmethyl)-aminomethyl]-
nitrobenzene
4-[N-methyl-N-(4-bromophenylmethyl)-aminomethyl]-
nitrobenzene
'""~ 4- [N-methyl-N- (3-chlorophenylmethyl) -aminomethyl] -
nitrobenzene
4-[N-methyl-N-(3,4-dimethoxyphenylmethyl)-aminomethyl]-
nitrobenzene
4-[N-methyl-N-(4-methoxyphenylmethyl)-aminomethyl)-
nitrobenzene
4- [N- (2, 2, 2-trifluoroethyl) -N-benzyl-aminomethyl] -
nitrobenzene
4-[N-(2,2,2-trifluoroethyl)-N-(4-chlorophenylmethyl)-
aminomethyl]-nitrobenzene
4-(2,6-dimethyl-piperidino-methyl)-nitrobenzene
4-(thiomorpholino-methyl)-nitrobenzene
4-(S-oxido-thiomorpholino-methyl)-nitrobenzene
4-(S,S-dioxido-thiomorpholino-methyl)-nitrobenzene
4-(azetidino-methyl)-nitrobenzene
CA 02328291 2000-10-11
- 36 -
4-(2,5-dihydropyrrol-1-yl-methyl)-nitrobenzene
4-(3,6-dihydro-2H-pyridin-1-yl-methyl)-nitrobenzene
4-(2-methoxycarbonyl-pyrrolidino-methyl)-nitrobenzene
4-(3,5-dimethyl-piperidino-methyl)-nitrobenzene
4-(4-phenyl-piperazinyl-methyl)-nitrobenzene
4-(4-phenyl-4-hydroxy-piperidino-methyl)-nitrobenzene
4-[N-(3,4,5-trimethoxy-benzyl)-N-methyl-aminomethyl]-
nitrobenzene
4-[N-(3,4-dimethoxy-benzyl)-N-ethyl-aminomethyl]-
nitrobenzene
4-[N-(3-chlorobenzyl)-N-methyl-aminomethyl]-nitrobenzene
4-[N-(2,6-dichlorobenzyl)-N-methyl-aminomethyl]-
nitrobenzene
4-[N-(4-trifluoromethylbenzyl)-N-methyl-aminomethyl]-
nitrobenzene
4-(N-benzyl-N-isopropyl-aminomethyl)-nitrobenzene
4-(N-benzyl-N-tert.butyl-aminomethyl)-nitrobenzene
4-(diisopropylamino-methyl)-nitrobenzene
4-(di-n-propylamino-methyl)-nitrobenzene
4-(diisobutylamino-methyl)-nitrobenzene
CA 02328291 2000-10-11
- 37 -
4-(2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-methyl)-
nitrobenzene
4-(2,3-dihydro-isoindol-2-yl-methyl)-nitrobenzene
4-(6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinolin-2-yl-
methyl)-nitrobenzene
4-(1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-
nitrobenzene
4-[N-(2-hydroxyethyl)-N-benzyl-aminomethyl]-nitrobenzene
4-[N-(1-ethyl-pentyl)-N-(pyridin-2-yl-methyl)-
aminomethyl]-nitrobenzene
4-(N-phenethyl-N-methyl-aminomethyl)-nitrobenzene
4-[N-(3,4-dihydroxy-phenethyl)-N-methyl-aminomethyl]-
nitrobenzene
4-[N-(3,4,5-trimethoxy-phenethyl)-N-methyl-aminomethyl]-
nitrobenzene
4-[N-(3,4-dimethoxy-phenethyl)-N-methyl-aminomethyl]-
nitrobenzene
4-[N-(4-nitro-phenethyl)-N-methyl-aminomethyl]-
nitrobenzene
4-(N-phenethyl-N-benzyl-aminomethyl)-nitrobenzene
4-(N-phenethyl-N-cyclohexyl-aminomethyl)-nitrobenzene
4-[N-(2-(pyridin-2-yl)-ethyl)-N-methyl-aminomethyl]-
nitrobenzene
CA 02328291 2000-10-11
- 38 -
4 - [N- ( 2 - (pyridin-4 -yl ) -ethyl ) -N-methyl -aminomethyl ] -
nitrobenzene
4-[N-(pyridin-4-yl-methyl)-N-methyl-aminomethyl]-
nitrobenzene
4-(dibenzylamino-methyl)-nitrobenzene
4-[N-(4-nitro-benzyl)-N-propyl-aminomethyl]-nitrobenzene
4-[N-benzyl-N-(3-cyano-propyl)-aminomethyl]-nitrobenzene
4-(N-benzyl-N-allyl-aminomethyl)-nitrobenzene
4-[N-(benzo(1,3)dioxol-5-yl-methyl)-N-methyl-aminomethyl]-
nitrobenzene
4-(7-chloro-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-nitrobenzene
4-(7,8-dichloro-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-nitrobenzene
4-(7-methoxy-2,3,4,5-tetrahydro-benzo(d)azepin-3-y1-
methyl)-nitrobenzene
4-(7-methyl-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-nitrobenzene
4-(7,8-dimethoxy-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-nitrobenzene
4-(6,7-dichloro-1,2,3,4-tetrahydro-isoquinolin-2-yl-
methyl)-nitrobenzene
CA 02328291 2000-10-11
- 39 -
4-(6,7-dimethyl-1,2,3,4-tetrahydro-isoquinolin-2-yl-
methyl)-nitrobenzene
4-(6-chloro-1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-
nitrobenzene
4-(7-chloro-1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-
nitrobenzene
4-(6-methoxy-1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-
nitrobenzene
4-(7-methoxy-1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-
nitrobenzene
4-(2,3,4,5-tetrahydro-azepino(4,5-b)pyrazin-3-yl)-methyl)-
nitrobenzene
4-(7-amino-2,3,4,5-tetrahydro-azepino(4,5-b)pyrazin-3-yl-
methyl)-nitrobenzene
4-(2-amino-5,6,7,8-tetrahydro-azepino(4,5-d)thiazol-6-yl-
methyl)-nitrobenzene
4-(5,6,7,8-tetrahydro-azepino(4,5-d)thiazol-6-yl-methyl)-
nitrobenzene
4- (N-F'thvl -N- r .bmroxy .ar rbon~rl-ami_nometh~rl ) -ni rob n .ene
2.2 g of 4-(N-ethyl-aminomethyl)-nitrobenzene are
dissolved in 50 ml of ethyl acetate and stirred with 2.6 g
of di-tert.butyl-dicarbonate for 30 minutes at ambient
temperature. Then the solution is washed with water and
concentrated by evaporation.
CA 02328291 2000-10-11
- 40 -
Yield: 3.4 g (97 0 of theory),
Rf value: 0.90 (silica gel, methylene chloride/methanol -
9:1)
The following compounds are prepared analogously to
Example VI:
4-[N-(4-chlorophenylmethyl)-N-tert.butoxycarbonyl-
aminomethyl]-nitrobenzene
4-(N-cyclohexyl-N-tert.butoxycarbonyl-aminomethyl)-
nitrobenzene
4-(N-isopropyl-N-tert.butoxycarbonyl-aminomethyl)-
nitrobenzene
4-(N-butyl-N-tert.butoxycarbonyl-aminomethyl)-nitrobenzene
4-(N-methoxycarbonylmethyl-N-tert.butoxycarbonyl-
aminomethyl)-nitrobenzene
4-(N-benzyl-N-tert.butoxycarbonyl-aminomethyl)-
nitrobenzene
4-(N-ethyl-N-tert.butoxycarbonyl-aminomethyl)-nitrobenzene
4- (N-Eth~,l -N- r _b ~ ox~-arbon~rl -aminomPrh~rl ~-aniline
6.4 g of 4-(N-ethyl-N-tert.butoxycarbonyl-aminomethyl)-
nitrobenzene are dissolved in 60 ml of methanol and
hydrogenated with 1.5 g of Raney nickel at ambient
temperature under 3 bar. The catalyst is then filtered off
and the solution is evaporated down.
Yield: 4.78 g
CA 02328291 2000-10-11
- 41 -
Rf value: 0.70 (silica gel, methylene chloride/methanol =
50:1)
The following compounds are prepared analogously to
Example VII:
4-[N-(4-chlorophenylmethyl)-N-tert.butoxycarbonyl-
aminomethyl)-aniline
4-(N-cyclohexyl-N-tert.butoxycarbonyl-aminomethyl)-aniline
4-(N-isopropyl-N-tert.butoxycarbonyl-aminomethyl)-aniline
4-(N-butyl-N-tert.butoxycarbonyl-aminomethyl)-aniline
4-(N-methoxycarbonylmethyl-N-tert.butoxycarbonyl-
aminomethyl)-aniline
4-(N-benzyl-N-tert.butoxycarbonyl-aminomethyl)-aniline
4-(pyrrolidino-methyl)-aniline
4-(morpholino-methyl)-aniline
4-(piperidino-methyl)-aniline
4-(hexamethyleneimino-methyl)-aniline
4-(4-hydroxy-piperidino-methyl)-aniline
4-(4-methyl-piperidino-methyl)-aniline
4-(4-ethyl-piperidino-methyl)-aniline
4-(4-isopropyl-piperidino-methyl)-aniline
CA 02328291 2000-10-11
- 42 -
4-(4-phenyl-piperidino-methyl)-aniline
4-(4-benzyl-piperidino-methyl)-aniline
4-(4-ethoxycarbonyl-piperidino-methyl)-aniline
4-(dimethylamino-methyl)-aniline
4-(di-n-propylamino-methyl)-aniline
4-(4-tert.butoxycarbonyl-piperazino-methyl)-aniline
3-(dimethylamino-methyl)-aniline
4-(2-diethylamino-ethyl)-aniline
4-(2-morpholino-ethyl)-aniline
4-(2-pyrrolidino-ethyl)-aniline
4-(2-piperidino-ethyl)-aniline
4-(N-ethyl-N-benzyl-aminomethyl)-aniline
4-(N-propyl-N-benzyl-aminomethyl)-aniline
4-(N-methyl-N-(4-chlorophenylmethyl)-aminomethyl)-aniline
4-(N-methyl-N-(4-bromophenylmethyl)-aminomethyl)-aniline
4-(N-methyl-N-(3-chlorophenylmethyl)-aminomethyl)-aniline
4-(N-methyl-N-(3,4-dimethoxyphenylmethyl)-aminomethyl)-
aniline
4-(N-methyl-N-(4-methoxyphenylmethyl)-aminomethyl)-aniline
CA 02328291 2000-10-11
- 43 -
4-[N-(2,2,2-trifluoroethyl)-N-benzyl-aminomethyl]-aniline
4-[N-(2,2,2-trifluoroethyl)-N-(4-chlorophenylmethyl)-
aminomethyl]-aniline
4-(2,6-dimethyl-piperidino-methyl)-aniline
4-(thiomorpholino-methyl)-aniline
4-(S-oxido-thiomorpholino-methyl)-aniline
4-(S,S-dioxido-thiomorpholino-methyl)-aniline
4-(azetidino-methyl)-aniline
4-(2,5-dihydropyrrol-1-yl-methyl)-aniline
4-(3,6-dihydro-2H-pyridin-1-yl-methyl)-aniline
4-(2-methoxycarbonyl-pyrrolidino-methyl)-aniline
4-(3,5-dimethyl-piperidino-methyl)-aniline
4-(4-phenyl-piperazino-methyl)-aniline
4-(4-phenyl-4-hydroxy-piperidino-methyl)-aniline
4-[N-(3,4,5-trimethoxybenzyl)-N-methyl-aminomethyl]-
aniline
4-[N-(3,4-dimethoxybenzyl)-N-ethyl-aminomethyl]-aniline
4-(N-benzyl-N-ethyl-aminomethyl)-aniline
4-[N-(3-chlorobenzyl)-N-methyl-aminomethyl]-aniline
CA 02328291 2000-10-11
- 44 -
4-[N-(2,6-dichlorobenzyl)-N-methyl-aminomethyl]-aniline
4-[N-(4-trifluoromethylbenzyl)-N-methyl-aminomethyl)-
aniline
4-(N-benzyl-N-isopropyl-aminomethyl)-aniline
4-(N-benzyl-N-tert.butyl-aminomethyl.)-aniline
4-(diisopropylamino-methyl)-aniline
4-(di-n-propylamino-methyl)-aniline
4-(diisobutylamino-methyl)-aniline
4-(2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-methyl)-aniline
4-(2,3-dihydro-isoindol-2-yl-methyl)-aniline
4-(6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinolin-2-yl-
methyl) -aniline
4-(1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-aniline
4-[N-(2-hydroxyethyl)-N-benzyl-aminomethyl]-aniline
4-[N-(1-ethyl-pentyl)-N-(pyridin-2-yl-methyl)-
aminomethyl]-aniline
4-(N-phenethyl-N-methyl-aminomethyl)-aniline
4-[N-(3,4-dihydroxy-phenethyl)-N-methyl-aminomethyl)-
aniline
CA 02328291 2000-10-11
- 45 -
4-[N-(3,4,5-trimethoxy-phenethyl)-N-methyl-aminomethyl]-
aniline
4-[N-(3,4-dimethoxy-phenethyl)-N-methyl-aminomethyl)-
aniline
4-[N-(4-nitro-phenethyl)-N-methyl-aminomethyl)-aniline
4-(N-phenethyl-N-benzyl-aminomethyl)-aniline
4-(N-phenethyl-N-cyclohexyl-aminomethyl)-aniline
4 - [N- ( 2 - (pyridin-2 -yl ) -ethyl ) -N-methyl-aminomethyl ] -
aniline
4- [N- (2- (pyridin-4-yl) -ethyl) -N-methyl-aminomethyl] -
aniline
4-[N-(pyridin-4-yl-methyl)-N-methyl-aminomethyl]-aniline
4-(dibenzylamino-methyl)-aniline
4-[N-(4-nitro-benzyl)-N-propyl-aminomethyl]-aniline
4-[N-benzyl-N-(3-cyano-propyl)-aminomethyl)-aniline
4-(N-benzyl-N-allyl-aminomethyl)-aniline
4-[N-benzyl-N-(2,2,2-trifluoroethyl)-aminomethyl]-aniline
4-[N-(benzo(1,3)dioxol-5-yl-methyl)-N-methyl-aminomethyl]-
aniline
4-(7-chloro-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-aniline
CA 02328291 2000-10-11
- 46 -
4-(7,8-dichloro-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-aniline
4-(7-methoxy-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-aniline
4-(7-methyl-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-aniline
4-(7,8-dimethoxy-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-aniline
4-(6,7-dichloro-1,2,3,4-tetrahydro-isoquinolin-2-yl-
methyl) -aniline
4-(6,7-dimethyl-1,2,3,4-tetrahydro-isoquinolin-2-yl-
methyl)-aniline
4-(6-chloro-1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-
aniline
4-(7-chloro-1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-
aniline
4-(6-methoxy-1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-
aniline
4-(7-methoxy-1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-
aniline
4-(2,3,4,5-tetrahydro-azepino(4,5-b)pyrazin-3-yl-methyl)-
aniline
4-(7-amino-2,3,4,5-tetrahydro-azepino(4,5-b)pyrazin-3-yl-
methyl)-aniline
CA 02328291 2000-10-11
- 47 -
4-(2-amino-5,6,7,8-tetrahydro-azepino(4,5-d)thiazol-6-yl-
methyl)-aniline
4-(5,6,7,8-tetrahydro-azepino(4,5-d)thiazol-6-yl-methyl)-
aniline
CA 02328291 2000-10-11
- 48 -
Preparation of the end products:
~z hPn~rl mi no-~ -b yrl -mPt-h~rl enP) -5-ami do-2-i ndol i nonP
600 g of a resin prepared according to Example IV were
suspended in 3 ml of dimethylformamide and shaken with 0.4
g of aniline for 10 hours at 70°C. Then the mixture was
filtered and the resin was washed several times with
methylene chloride, methanol and dimethylformamide. Then
3 ml of methanolic ammonia were added for 2 hours in order
to cleave the acetyl group. Finally, after further washing
with dimethylformamide and methylene chloride, 4 ml of 10%
trifluoroacetic acid in methylene chloride were added over
90 minutes, the resin was separated off and the solution
was concentrated by evaporation. The residue was taken up
in a little 1N sodium hydroxide solution and extracted with
a little methylene chloride. The organic phase was dried
over sodium sulphate and concentrated by rotary
evaporation.
Yield: 37 mg
Rf value: 0.6 (silica gel, methylene chloride/methanol =
9:1)
CzoHziNs~z
mass spectrum: m/z = 335 (M~)
The following compounds are prepared analogously to Example
1:
(1) 3-Z-(1-phenylamino-methylene)-5-amido-2-indolinone
Prepared from the resin prepared according to Example IV(1)
and aniline
Rf value: 0.59 (silica gel, methylene chloride/methanol -
9:1)
3 5 C16H13N30z
mass spectrum: m/z = 279 (M')
CA 02328291 2000-10-11
- 49 -
(2) 3-Z-[1-(4-methyl-phenylamino)-1-methyl-methylene]-5-
amido-2-indolinone
Prepared from the resin according to Example IV(2) and 4-
methylaniline
Rf value: 0.44 (silica gel, methylene chloride/methanol -
9:1)
ClaHmN3~z
mass spectrum: m/z = 307 (M+)
(3) 3-Z-[1-(4-Chloro-phenylamino)-1-methyl-methylene]-5-
'"' amido-2-indolinone
Prepared from the resin according to Example IV(2) and 4-
chloroaniline
Rf value: 0,45 (silica gel, methylene chloride/methanol =
9:1)
C1~H14C1N30z
mass spectrum: m/z = 327/329 (M')
(4) 3-Z-[1-(4-ethyl-phenylamino)-1-methyl-methylene]-5-
amido-2-indolinone
Prepared from the resin according to Example IV(2) and 4-
ethylaniline
Rf value: 0.43 (silica rel, methylene chloride/methanol -
9:1)
C19H19N3~2
mass spectrum: m/z = 321 (M')
(5) 3-Z-[1-(4-methoxy-phenylamino)-1-methyl-methylene]-5-
amido-2-indolinone
Prepared from the resin according to Example IV(2) and 4-
methoxyaniline
Rf value: 0.46 (silica gel, methylene chloride/methanol =
9:1)
3 5 C18H1.,N303
mass spectrum: m/z = 323 (M')
CA 02328291 2000-10-11
- 50 -
(6) 3-Z- [1- (4-iodo-phenyl amino) -1-methyl-methylene] -5-
amido-2-indolinone
Prepared from the resin according to Example IV(2) and 4-
iodoaniline
R4 value: 0.36 (silica gel, methylene chloride/methanol -
9:1)
CmHia IN3~z
mass spectrum: m/z = 419 (M+)
(7) 3-Z-[1-(4-fluoro-phenylamino)-1-methyl-methylene]-5-
°"' amido-2-indolinone
Prepared from the resin according to Example IV(2) and 4-
fluoroaniline
Rf value: 0.60 (silica gel, methylene chloride/methanol =
9:1)
CmHiaFNs~z
mass spectrum: m/z = 311 (M')
(8) 3-Z-[1-(4-bromo-phenylamino)-1-methyl-methylene]-5-
amido-2-indolinone
Prepared from the resin according to Example IV(2) and 4-
bromoaniline
Rf value: 0.53 (silica gel, methylene chloride/methanol =
9:1)
C1~H14BrN30z
mass spectrum: m/z = 371/373 (M')
(9) 3-Z-(1-phenylamino-1-methyl-methylene)-5-amido-2-
indolinone
Prepared from the resin according to Example IV(2) and
aniline
Rf value: 0,58 (silica gel, methylene chloride/methanol =
9:1)
3 5 C1~H15N30z
mass spectrum: m/z = 293 (M')
CA 02328291 2000-10-11
- 51 -
(10) 3-Z-(1-amino-1-methyl-methylene)-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and
ammonia
Rf value: 0,23 (silica gel, methylene chloride/methanol =
9:1)
CiiHiiN30z
mass spectrum: m/z - 217 (M')
(11) 3-Z-[1-(4-piperidinomethyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and
4-(piperidinomethyl)-aniline
Rf value: 0,31 (silica gel, methylene chloride/methanol =
9:1)
CzaHzsN40z
mass spectrum: m/z - 390 (M~)
(12) 3-Z-[1-(4-pyrrolidinomethyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and 4-
pyrrolidinomethyl-aniline
Rf value: 0.20 (silica gel, methylene chloride/methanol =
4:1)
2 5 CzzHz4N4~z
mass spectrum: m/z = 376 (M')
(13) 3-Z-[1-(4-Dipropylaminomethyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin according to Example II(2) and 4-
dipropylaminomethyl-aniline
Rf value: 0.71 (silica gel, methylene chloride/methanol =
4:1)
C24H30N4~2
mass spectrum: m/z = 406 (M')
CA 02328291 2000-10-11
- 52 -
(14) 3-Z-[1-[4-(2-piperidinoethyl)-phenylamino]-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and 4-
(2-piperidinoethyl)-aniline
Rf value: 0.38 (silica gel, methylene chloride/methanol =
4:1)
Cz4H2aN4~z
mass spectrum: m/z = 404 (M+)
(15) 3-Z-[1-[4-(2-diethylaminoethyl)-phenylamino]-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and 4-
(2-diethylaminoethyl)-aniline
Rf value: 0.33 (silica gel, methylene chloride/methanol =
4:1)
C23H28N4~2
mass spectrum: m/z = 393 (M')
(16) 3-Z-[1-(4-Hexamethyleneiminomethyl-phenylamino)-1-
methyl-methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and
4-hexamethyleneiminomethyl-aniline
Rf value: 0.34 (silica gel, methylene chloride/methanol -
4:1)
C24H2eNa~2
mass spectrum: m/z = 404 (M+)
(17) 3-Z-[1-[4-(N-methyl-N-methansulphonyl-amino)-
phenylamino]-1-methyl-methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and
4-(N-methyl-N-methanesulphonyl-amino)-aniline
Rf value: 0.36 (silica gel, methylene chloride/methanol =
9:1)
CisH2aNa0ns
mass spectrum: m/z - 400 (M')
CA 02328291 2000-10-11
- 53 -
(18) 3-Z-[1-(4-methanesulphonylamino-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and
4-methanesulphonylamino-aniline
Rf value: 0.31(silica gel, methylene chloride/methanol -
9:1)
CiaHiaN4~4s
mass spectrum: m/z = 386 (M+)
(19) 3-Z-[1-(4-Bromophenylamino)-1-ethyl-methylene]-5-
amido-2-indolinone
-- Prepared from the resin according to Example IV(3) and
4-bromoaniline
Rf value: 0.52 (silica gel, methylene chloride/methanol -
9:1)
CiaHisBrN30z
mass spectrum: m/z = 385/387 (M'/M+2+)
(20) 3-Z-[1-(4-piperidinomethyl-phenylamino)-1-ethyl-
methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(3) and
4-piperidinomethyl-aniline
Rf value: 0.42 (silica gel, methylene chloride/methanol -
4:1)
' 25 Cz4HzeN4~z
mass spectrum: m/z = 404 (M')
(21) 3-Z-[1-(4-piperidinomethyl-phenylamino)-1-propyl-
methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(4) and
4-piperidinomethyl-aniline
Rf value: 0.49 (silica gel, methylene chloride/methanol -
4:1)
CzsHaoNa~z
mass spectrum: m/z = 418 (M+)
CA 02328291 2000-10-11
- 54 -
(22) 3-Z-[1-(4-Bromophenylamino)-1-propyl-methylene]-5-
amido-2-indolinone
Prepared from the resin according to Example IV(4) and
4-bromoaniline
Rf value: 0,53 (silica gel, methylene chloride/methanol =
9:1)
C19H18BrN3~2
mass spectrum: m/z = 399/401 (M*/M+2*)
(23) 3-Z-[(4-Bromophenylamino)-methylene]-5-amido-2-
indolinone
._ Prepared from the resin prepared according to Example IV(1)
and 4-bromoaniline
C1sH12Bz'N302
mass spectrum: m/z = 357/359 (M*/M+~*)
(24) 3-Z-[(4-piperidinomethyl-phenylamino)-methylene]-5-
amido-2-indolinone
Prepared from the resin prepared according to Example IV(1)
and 4-piperidinomethyl-aniline
C22H24N4~2
mass spectrum: m/z = 376 (M*)
(25) 3-Z-[1-(4-Bromophenylamino)-1-butyl-methylene]-5-
amido-2-indolinone
Prepared from the resin according to Example IV and
4-bromoaniline
Rf value: 0,53 (silica gel, methylene chloride/methanol -
9:1)
3 0 C2oH2oBrN302
mass spectrum: m/z = 413/415 (M*/M+2*)
(26) 3-Z-[1-(4-piperidinomethyl-phenylamino)-1-butyl-
methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV and
4-piperidinomethyl-aniline
CA 02328291 2000-10-11
- 55 -
Rf value: 0.48 (silica gel, methylene chloride/methanol =
4:1)
CzsH3zNa~z
mass spectrum: m/z = 432 (M~)
(27) 3-Z-[1-(4-piperidinomethyl-phenylamino)-1-ethenyl-
methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(5) and
4-piperidinomethyl-aniline
(28) 3-Z-[1-(4-piperidinomethyl-phenylamino)-1-(3-
bromopropyl)-methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(6) and
4-piperidinomethyl-aniline
(29) 3-Z-[1-(4-piperidinomethyl-phenylamino)-1-(2-
phenylsulphonylethyl)-methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(7) and
4-piperidinomethyl-aniline
(30) 3-Z-[1-[4-(2,6-dimethylpiperidinomethyl)-phenylamino]-
1-methyl-methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and
4-(2,6-dimethylpiperidinomethyl)-aniline
(31) 3-Z-[1-(4-Thiomorpholinomethyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and
4-thiomorpholinomethyl-aniline
Rf value: 0.53 (silica gel, methylene chloride/methanol =
9:1)
CzzHzaN4~zs
mass spectrum: m/z = 408 (M')
(32) 3-Z-[1-[(4-Thiomorpholino-S-oxido-methyl)-
phenylamino]-1-methyl-methylene]-5-amido-2-indolinone
CA 02328291 2000-10-11
- 56 -
Prepared from the resin according to Example IV(2) and
4-(thiomorpholino-S-oxido-methyl)-aniline
Rf value: 0.21 (silica gel, methylene chloride/methanol =
9:1)
CzzHz4N4~3S
mass spectrum: m/z = 424 (M+)
(33) 3-Z-[1-[4-(Thiomorpholino-S,S-dioxido-methyl)-
phenylamino]-1-methyl-methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and
4-(thiomorpholino-S,S-dioxido-methyl)-aniline
(34) 3-Z-[1-(4-Azetidionomethyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and
4-azetidionomethyl-aniline
(35) 3-Z-[1-[4-(2,5-Dihydropyrrol-1-yl-methyl)-
phenylamino]-1-methyl-methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and
4-(2,5-dihydropyrrol-1-yl-methyl)-aniline
Rf value: 0.10 (silica gel, methylene chloride/methanol =
9:1)
CzzHzzN4~z
mass spectrum: m/z - 375 (M+H~)
(36) 3-Z-[1-[4-(3,6-Dihydro-2H-pyridin-1-yl-methyl)-
phenylamino]-1-methyl-methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and
4-(3,6-dihydro-2H-pyridin-1-yl-methyl)-aniline
Rf value: 0.20 (silica gel, methylene chloride/methanol =
9:1)
C23H24N4~2
mass spectrum: m/z - 389 (M+H)'
CA 02328291 2000-10-11
_ 57 _
(37) 3-Z-[1-[4-(2-ethoxycarbonyl-pyrrolidinomethyl)-
phenylamino]-1-methyl-methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and
4-(2-ethoxycarbonyl-pyrrolidinomethyl)-aniline
Rf value: 0.50 (silica gel, methylene chloride/methanol -
9:1)
C24H26N4~4
mass spectrum: m/z = 435 (M+H)+
(38) 3-Z- [1- [4- (3, 5-dimethyl-piperidinomethyl) -
phenylamino]-1-methyl-methylene]-5-amido-2-indolinone
- Prepared from the resin according to Example IV(2) and
4-(3,5-dimethyl-piperidinomethyl)-aniline
Rf value: 0.16 (silica gel, methylene chloride/methanol -
9:1)
CzsH3oN4~2
mass spectrum: m/z = 418 (M*)
(39) 3-Z-[1-[4-(4-phenyl-piperazinylmethyl)-phenylamino]-
1-methyl-methylene]-5-amido-2-indolinone-trifluoroacetate
Prepared from the resin according to Example IV(2) and
4-(4-phenyl-piperazinylmethyl)-aniline
Rf value: 0.40 (silica gel, methylene chloride/methanol -
9:1)
C28Hz9Ns~2
mass spectrum: m/z = 468 (M+H)+
(40) 3-Z-[1-[4-(4-phenyl-4-hydroxy-piperidinylmethyl)
phenylamino]-1-methyl-methylene]-5-amido-2-indolinone
trifluoroacetate
Prepared from the resin according to Example IV(2) and
4-(4-phenyl-4-hydroxy-piperidinylmethyl)-aniline
C29H30N4~3
mass spectrum: m/z = 483 (M+H)'
CA 02328291 2000-10-11
- 58 -
(41) 3-Z-[1-(3-methoxy-phenylamino)-1-methyl-methylene]-5-
amido-2-indolinone
Prepared from the resin according to Example IV(2) and
3-methoxy-aniline
Rf value: 0.40 (silica gel, methylene chloride/methanol -
9:1)
CiBH17N3~3
Mass spectrum: m/z = 323 (M+)
(42) 3-Z-[1-(3-ethoxycarbonyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and
ethyl 3-amino-benzoate
C20H19N3O4
Mass spectrum: m/z = 365 (M+)
(43) 3-Z-[1-(4-dimethylaminomethyl-phenylamino)-1-
methylmethylen]-5-amido-2-indolinone trifluoroacetate
Prepared from the resin according to Example IV(2) and
4-dimethylaminomethyl-aniline
2 0 CZOH22N40z
Mass spectrum: m/z = 351 (M+H')
(44) 3-Z-[1-[4-(4-Cyclohexyl-piperidinylmethyl)-
phenylamino]-1-methyl-methylene]-5-amido-2-indolinone
"°~"' 25 Prepared from the resin according to Example IV(2) and
4-(4-cyclohexyl-piperidinylmethyl)-aniline
(45) 3-Z-[1-(4-morpholinyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
30 Prepared from the resin according to Example IV(2) and
4-morpholinyl-aniline
CziHz2Na~3
Mass spectrum: m/z = 378 (M')
35 (46) 3-Z-[1-(N-methyl-piperidin-4-ylamino)-1-methyl-
methylene)-5-amido-2-indolinone
CA 02328291 2000-10-11
- 59 -
Prepared from the resin according to Example IV(2) and
4-amino-N-methyl-piperidine
CmHzzNn~z
Mass spectrum: m/z = 314 (M')
(47) 3-Z-[1-(4-methylcyclohexylamino)-1-methyl-methylene]-
5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and
4-methyl-cyclohexylamine
C18Hz3N30z
Mass spectrum: m/z - 313 (M+)
(48) 3-Z-(1-Cyclopentylamino-1-methyl-methylene)-5-amido-
2-indolinone
Prepared from the resin according to Example IV(2) and
cyclopentylamine
Rf value: 0.70 (silica gel, methylene chloride/methanol -
4:1)
CisHi9N30z
Mass spectrum: m/z = 285 (M+)
(49) 3-Z-(1-isopropylamino-1-methyl-methylene)-5-amido-
2-indolinone
Prepared from the resin according to Example IV(2) and
"~ 25 isopropylamine
CiaHmN3~z
Mass spectrum: m/z - 259 (M+)
(50) 3-Z-[1-(4-ethoxycarbonylmethylaminomethyl-phe-
nylamino)-1-methyl-methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and
4-(ethoxycarbonylmethyl-N-tert.butyloxycarbonyl-
aminomethyl)-aniline
CziHzzNa~a
Mass spectrum: m/z - 394 (M')
CA 02328291 2000-10-11
- 60 -
(51) 3-Z-[1-(4-benzylaminomethyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin according to Example IV(2) and
4-(N-benzyl-N-tert.butyloxycarbonyl-aminomethyl)-aniline
Rf value: 0.24 (silica gel, methylene chloride/methanol =
9:1)
C25H24N4~2
Mass spectrum: m/z = 412 (M+)
(52) 3-Z-[1-(4-Butylaminomethyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone trifluoroacetate
~- Prepared from the resin according to Example IV(2) and
4-(N-butyl-N-tert.butyloxycarbonyl-aminomethyl)-aniline
Rf value: 0.40 (silica gel, methylene chloride/methanol -
4:1)
C22H2sN4~2
Mass spectrum: m/z = 378 (M')
(53) 3-Z-[1-(4-ethylaminomethyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone trifluoroacetate
Prepared from the resin according to Example IV(2) and
4-(N-ethyl-N-tert.butyloxycarbonyl-aminomethyl)-aniline
Rf value: 0.20 (silica gel, methylene chloride/methanol =
4:1)
".- 25 C2oH2zN40z
Mass spectrum: m/z = 351 (M+H+)
(54) 3-Z-[1-(4-Cyclohexylaminomethyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone trifluoroacetate
Prepared from the resin according to Example IV(2) and
4-(cyclohexyl-(N-tert.butyloxycarbonyl-amino-methyl)-
aniline
C24HzeN4~z
Mass spectrum: m/z = 405 (M')
CA 02328291 2000-10-11
- 61 -
(55) 3-Z-[1-(4-isopropylaminomethyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone trifluoroacetate
Prepared from the resin according to Example IV(2) and
4-(N-isopropyl-N-tert.butyloxycarbonyl-aminomethyl)-aniline
CZ1H24N4~2
Mass spectrum: m/z = 365 (M')
(56) 3-Z-[1-(4-trifluoromethoxy-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 4-
trifluoromethoxy-aniline
".~.. C18H14F3N3~3
mass spectrum: m/z = 377 (M~)
(57) 3-Z-[1-(4-difluoromethoxy-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 4-
difluoromethoxy-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol -
9:1 )
ClaHisF2Na~3
mass spectrum: m/z = 359 (M+H')
(58) 3-Z-[1-(4-bromo-3-chloro-phenylamino)-1-methyl-
- 25 methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 4-
bromo-3-chloro-aniline
C1.,H13BrC1N302
mass spectrum: m/z = 405/407/409 (M'/M+2+/M+4')
(59) 3-Z-[1-(4-trifluoromethyl-3-bromo-phenylamino)-1-
methyl-methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 4-
trifluoromethyl-3-bromo-aniline
3 5 C18H1sBrF3N30z
mass spectrum: m/z = 439/441 (M'/M+2')
CA 02328291 2000-10-11
- 62 -
(60) 3-Z-[1-(4-chloro-phenylamino)-methylene]-5-amido-2-
indolinone
Prepared from the resin produced in Example IV(1) and 4-
chloro-aniline
CisHizC1N30z
mass spectrum: m/z = 312/314 (M')
(61) 3-Z-[1-(3-bromo-phenylamino)-1-methyl-methylene]-5-
amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 3-
bromo-aniline
C1~H14BrN30z
mass spectrum: m/z = 371/373 (M')
(62) 3-Z-[1-(3-chloro-phenylamino)-1-methyl-methylene]-5-
amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 3-
chloro-aniline
2 0 C1~H14C1N302
mass spectrum: m/z = 327/329 (M~)
(63) 3-Z-[1-(2-chloro-phenylamino)-1-methyl-methylene]-5-
amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 2-
chloro-aniline
C1.,H14C1N30z
mass spectrum: m/z = 327/329 (M')
(64) 3-Z-[1-(4-bromo-3-methyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 4-
bromo-3-methyl-aniline
Rf value: 0.60 (silica gel, methylene chloride/methanol -
9:1 )
CisHisBrN30z
CA 02328291 2000-10-11
- 63 -
mass spectrum: m/z = 385/387 (M+)
(65) 3-Z-[1-(4-bromo-3-methoxy-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 4-
bromo-3-methoxy-aniline
Rf value: 0.60 (silica gel, methylene chloride/methanol =
9:1 )
ClaHlsBrN303
mass spectrum: m/z = 401/403 (M+)
(66) 3-Z-[1-(4-fluoro-3-nitro-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 4-
fluoro-3-nitro-aniline
RF value: 0.40 (silica gel, methylene chloride/methanol =
9:1 )
C17H13 FN4O4
mass spectrum: m/z = 356 (M')
(67) 3-Z-[1-(4-bromo-3-nitro-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 4-
bromo-3-nitro-aniline
'"" 25 Rf value: 0.50 (silica gel, methylene chloride/methanol =
9:1 )
C1.,H13BrN404
mass spectrum: m/z - 416/418 (M')
(68) 3-Z-[1-(4-Ethyl-3-nitro-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 4-
ethyl-3-nitro-aniline
Rf value: 0.70 (silica gel, methylene chloride/methanol -
9:1 )
C19H1BN4O4
CA 02328291 2000-10-11
- 64 -
mass spectrum: m/z = 366 (M')
(69) 3-Z-[1-(4-chloro-3-nitro-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 4-
chloro-3-nitro-aniline
C17H13 C1N4O4
mass spectrum: m/z = 371/373 (M+)
(70) 3-Z-[1-(3-Nitro-phenylamino)-1-methyl-methylene]-5-
amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 3-
nitro-aniline
C17H14N4O4
mass spectrum: m/z = 338 (M+H~)
(71) 3-Z-[1-(4-Methyl-3-vitro-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 4-
methyl-3-vitro-aniline
Rf value: 0.50 (silica gel, methylene chloride/methanol =
9:1 )
CleHlsN4~a
mass spectrum: m/z = 352 (M')
"~ 2 5
(72) 3-Z-[1-(4-bromo-3-methoxycarbonyl-phenylamino)-1-
methyl-methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and
methyl 2-bromo-5-aminobenzoate
Rf value: 0.50 (silica ael, methylane chloride/methanol =
9:1 )
C19H16BrN304
mass spectrum: m/z = 429/431 (M+H')
(73) 3-Z-[1-(4-Carbamoyl-phenylamino)-1-methyl-methylene]-
5-amido-2-indolinone
CA 02328291 2000-10-11
- 65 -
Prepared from the resin produced in Example IV(2) and 4-
aminobenzamide
Rf value: 0,20 (silica gel, methylene chloride/methanol -
9:1 )
C18H1sN4~3
mass spectrum: m/z = 336 (M')
(74) 3-Z-[1-(4-(Piperidino-carbonyl)-phenylamino)-1-
methyl-methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 1-
(4-amino-benzoyl)-piperidine
Rf value: 0.50 (silica gel, methylene chloride/methanol -
9:1 )
Cz3Hz4N4~3
mass spectrum: m/z = 404 (M+)
(75) 3-Z-[1-(4-(2-(diethylamino)-ethyl-carbamoyl)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone-
trifluoracetate
Prepared from the resin produced in Example IV(2) and 4-
amino-N- [2- (diethyl amino) -ethyl] -benzamide
Rf value: 0.30 (silica gel, methylene chloride/methanol =
9:1 )
C24H29N5~3
mass spectrum: m/z - 436 (M+H')
(76) 3-Z-[1-(4-trifluoromethyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example Iv(2) and 4-
trifluoromethyl-aniline
C18H14F3N3O2
mass spectrum: m/z - 361 (M')
(77) 3-Z-[1-(3-Hydroxymethyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
CA 02328291 2000-10-11
- 66 -
Prepared from the resin produced in Example IV(2) and 3-
aminobenzylalcohol
Ci8H17N3~3
mass spectrum: m/z = 323 (M')
(78) 3-Z-[1-(4-(Hydroxycarbonyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 4-
aminobenzoic acid
Rf value: 0.20 (silica gel, methylene chloride/methanol -
4:1 )
,..-, C18H15N3v4
mass spectrum: m/z = 336 (M-H+)
(79) 3-Z-[1-(4-Ethoxycarbonylmethyl-3-nitro-phenylamino)-
1-methyl-methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and
ethyl 4-amino-2-nitro-phenylacetate
Rf value: 0.70 (silica gel, methylene chloride/methanol -
9:1 )
C21H2oNa~s
mass spectrum: m/z = 424 (M+)
(80) 3-Z-[1-(3-Methoxycarbonyl-4-methyl-phenylamino)-1-
methyl-methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and
methyl 3-amino-6-methyl-benzoate
Rf value: 0.70 (silica gel, methylene chloride/methanol -
9:1 )
3 0 CzoH19N304
mass spectrum: m/z = 365 (M')
(81) 3-Z-[1-(3-Diethylcarbamoyl-4-methyl-phenylamino)-1-
methyl-methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 3-
amino-6-methyl-benzoic acid diethylamide
CA 02328291 2000-10-11
- 67 -
Rf value: 0.50 (silica gel, methylene chloride/methanol =
9:1 )
C23H26N4~3
mass spectrum: m/z = 406 (M+)
(82) 3-Z-[1-(3-Ethylcarbamoyl-4-methyl-phenylamino)-1-
methyl-methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 3-
amino-6-methyl-benzoic acid ethylamide
Rf value: 0.40 (silica gel, methylene chloride/methanol =
9:1)
.~ C21H22N4~3
mass spectrum: m/z - 378 (M+)
(83) 3-Z-[1-(3-Sulphamoyl-4-methyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 3-
amino-6-methyl-phenylsulphonic acid amide
Rf value: 0.30 (silica gel, methylene chloride/methanol -
9:1)
C18H18N4O4S
mass spectrum: m/z - 386 (M')
(84) 3-Z-[1-(3-Acetylamino-4-methyl-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 4-
amino-2-acetylamino-toluene
Rf value: 0.65 (silica gel, methylene chloride/methanol =
9:1)
3 0 C2oHzoNa~s
mass spectrum: m/z = 364 (M')
(85) 3-Z-[1-(4-(2-Dimethylamino-ethoxy)-phenylamino)-1-
methyl-methylene]-5-amido-2-indolinone-trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
(2-dimethylamino-ethoxy)-aniline
CA 02328291 2000-10-11
- 68 -
Rf value: 0,10 (silica gel, methylene chloride/methanol =
4:1 )
C21H24N4O3
mass spectrum: m/z - 380 (M+)
(86) 3-Z-[1-(4-(2-Piperidino-ethoxy)-phenylamino)-1-
methyl-methylene]-5-amido-2-indolinone-trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
(2-piperidino-ethoxy)-aniline
Rf value: 0.70 (silica gel, methylene chloride/methanol -
4:1 )
... Cz4H2eNa0s
mass spectrum: m/z = 420 (M')
(87) 3-Z-[1-(4-(3-Dimethylamino-propoxy)-phenylamino)-1-
methyl-methylene]-5-amido-2-indolinone-trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
(3-dimethylamino-propoxy)-aniline
Rf value: 0.10 (silica gel, methylene chloride/methanol -
4:1 )
C22H26N4~3
mass spectrum: m/z = 394 (M+)
(88) 3-Z-[1-(4-(3-Piperidino-propoxy)-phenylamino)-1-
methyl-methylene]-5-amido-2-indolinone-trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
(3-piperidino-propoxy)-aniline
Rf value: 0.20 (silica gel, methylene chloride/methanol =
4:1 )
3 0 C25H3oN403
mass spectrum: m/z = 434 (M')
(89) 3-Z-[1-(4-(3-(N-Benzyl-N-methylamino)-propoxy)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone-
trifluoroacetate
CA 02328291 2000-10-11
- 69 -
Prepared from the resin produced in Example IV(2) and 4-
[3-(N-benzyl-N-methylamino)-propoxy]-aniline
Rf value: 0.60 (silica gel, methylene chloride/methanol =
4:1 )
C28H3oN403
mass spectrum: m/z = 470 (M')
(90) 3-Z-[1-(4-(N-benzyl-aminomethyl)-phenylamino)-
methylene]-5-amido-2-indolinone-trifluoroacetate
Prepared from the resin produced in Example IV(1) and 4-
(N-benzyl-N-tert.butoxcarbonyl-aminomethyl)-aniline
C24H22N4~2
mass spectrum: m/z = 399 (M+H+)
(91) 3-Z-[1-(4-(N-(4-chlorobenzyl)-aminomethyl)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone-
trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
[N-(4-chlorobenzyl-N-tert.butoxycarbonyl-aminomethyl)-
aniline
Rf value: 0.40 (silica gel, methylene chloride/methanol =
9:1)
C2sH2sC1N402
mass spectrum: m/z = 446/448 (M')
(92) 3-Z-[1-(4-(N-(3,4,5-Trimethoxybenzyl)-N-methyl-
aminomethyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone-trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
[N-((3,4,5-trimethoxy-benzyl)-N-methyl-aminomethyl]-
aniline
Rf value: 0.50 (silica gel, methylene chloride/methanol -
9:1)
Cz9Ha2N4~s
mass spectrum: m/z = 516 (M')
CA 02328291 2000-10-11
- 70 -
(93) 3-Z-[1-(4-(N-(3,4-Dimethoxy-benzyl)-N-methyl-amino-
methyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone-trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
[N-(3,4-dimethoxy-benzyl)-N-methyl-aminomethyl]-aniline
Rf value: 0.40 (silica gel, methylene chloride/methanol =
9:1)
C28H30N4O4
mass spectrum: m/z = 486 (M*)
(94) 3-Z-[1-(4-(N-(3,4-Dimethoxy-benzyl)-N-ethyl-
aminomethyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone-trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
[N-(3,4-dimethoxy-benzyl)-N-ethyl-aminomethyl]-aniline
Rf value: 0.40 (silica gel, methylene chloride/methanol -
9:1)
C29H32N40a
mass spectrum: m/z = 500 (M*)
(95) 3-Z-[1-(4-(N-Benzyl-N-ethyl-aminomethyl)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone-
trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
(N-benzyl-N-ethyl-aminomethyl)-aniline
Rf value: 0.50 (silica gel, methylene chloride/methanol -
9:1)
Cz~H2aNa02
mass spectrum: m/z = 440 (M*)
(96) 3-Z-[1-(4-(N-Benzyl-N-methyl-aminomethyl)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone-
trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
(N-benzyl-N-methyl-aminomethyl)-aniline
CA 02328291 2000-10-11
- 71 -
Rf value: 0,55 (silica gel, methylene chloride/methanol =
9:1)
C26H26N4~2
mass spectrum: m/z = 426 (M+)
(97) 3-Z-[1-(4-(N-Benzyl-N-methyl-aminomethyl)-
phenylamino)-1-ethyl-methylene]-5-amido-2-indolinone-
trifluoroacetate
Prepared from the resin produced according to Example
IV(3) and 4-(N-benzyl-N-methyl-aminomethyl)-aniline
Rf value: 0.50 (silica gel, methylene chloride/methanol =
9:1)
C2~HzeN402
mass spectrum: m/z - 440 (M')
(98) 3-Z-[1-(4-(N-Benzyl-N-methyl-aminomethyl)-
phenylamino)-1-propyl-methylene]-5-amido-2-indolinone-
trifluoroacetate
Prepared from the resin produced according to Example
IV(4) and 4-(N-benzyl-N-methyl-amino-methyl)-aniline
Rf value: 0.50 (silica gel, methylene chloride/methanol =
9:1)
CzeH3oNa02
mass spectrum: m/z - 454 (M+)
(99) 3-Z- [1- (4- (N- (4-chlorobenzyl) -N-methyl-aminomethyl) -
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone-
trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
[N-(4-chlorobenzyl)-N-methyl-aminomethyl]-aniline
Rf value: 0.40 (silica gel, methylene chloride/methanol =
9:1)
C26H25C1N4~2
mass spectrum: m/z = 460/462 (M')
CA 02328291 2000-10-11
- 72 -
(100) 3-Z-[1-(4-(N-(3-chlorobenzyl)-N-methyl-aminomethyl)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone-
trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
[N-(3-chlorobenzyl)-N-methyl-aminomethyl]-aniline
Rf value: 0.40 (silica gel, methylene chloride/methanol =
9:1)
CzsHzsC1N40z
mass spectrum: m/z = 460/462 (M*)
(101) 3-Z- [1- (4- (N- (2, 6-dichlorobenzyl) -N-methyl-
.-. aminomethyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone-trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
[N-(2,6-dichlorobenzyl)-N-methyl-aminomethyl]-aniline
Rf value: 0,38 (silica gel, methylene chloride/methanol =
9:1)
C26H24C12N4~2
mass spectrum: m/z = 494/496/498 (M+2'/M+4')
(102) 3-Z- [1- (4- (N- (4-trifluoromethylbenzyl) -N-methyl-
aminomethyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone-trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
[N-(4-trifluoromethylbenzyl)-N-methyl-aminomethyl]-aniline
Rf value: 0,38 (silica gel, methylene chloride/methanol =
9:1)
Cz~HzsFsN4~z
mass spectrum: m/z = 494 (M*)
(103) 3-Z-[1-(4-(N-Benzyl-N-isopropyl-aminomethyl)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone-
trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
(N-benzyl-N-isopropyl-aminomethyl)-aniline
CA 02328291 2000-10-11
- 73 -
Rf value: 0.50 (silica gel, methylene chloride/methanol =
9:1)
C28H30N4~2
mass spectrum: m/z = 454 (M')
(104) 3-Z-[1-(4-(N-benzyl-N-tert.butyl-aminomethyl)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone-
trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
(N-benzyl-N-tert.butyl-aminomethyl)-aniline
Rf value: 0.50 (silica gel, methylene chloride/methanol -
"~ 9:1)
C29H32N4O2
mass spectrum: m/z = 468 (M')
(105) 3-Z-[1-(4-(N-benzyl-N-methyl-aminomethyl)-
phenylamino)-methylene]-5-amido-2-indolinone-
trifluoroacetate
Prepared from the resin produced in Example IV(1) and 4-
(N-benzyl-N-methyl-aminomethyl)-aniline
C2sF"~zaNa~2
mass spectrum: m/z = 413 (M+H')
(106) 3-Z-[1-(4-(N-benzyl-N-ethyl-aminomethyl)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone-
trifluoroacetate
Prepared from the resin produced in Example IV(1) and 4-
(N-benzyl-N-ethyl-aminomethyl)-aniline
C26H26N4O2
mass spectrum: m/z = 427 (M+H')
(107) 3-Z-[1-(4-(Diisopropylamino-methyl)-phenylamino)-
1-methyl-methylene]-5-amido-2-indolinone-trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
(diisopropylamino-methyl)-aniline
CA 02328291 2000-10-11
- 74 -
Rf value: 0.50 (silica gel, methylene chloride/methanol =
4:1)
C24H30N4O2
mass spectrum: m/z = 406 (M')
(108) 3-Z-[1-(4-(Di-n-propylamino-methyl)-phenylamino)-
methylene]-5-amido-2-indolinone-trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
(di-n-propylamino-methyl)-aniline
Cz3HzeN402
mass spectrum: m/z = 393 (M+H~)
(109) 3-Z-[1-(4-(diisobutylamino-methyl)-phenylamino)-
1-methyl-methylene]-5-amido-2-indolinone-trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
(diisobutylamino-methyl)-aniline
Rf value: 0.50 (silica gel, methylene chloride/methanol =
9:1)
C26H34N4~2
mass spectrum: m/z = 434 (M+)
(110) 3-Z-[1-(4-(2,3,4,5-Tetrahydro-benzo(d)azepin-3-yl-
methyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone-trifluoroacetate
"' 25 Prepared from the resin produced in Example IV(2) and 4-
(2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-methyl)-aniline
R~ value: 0.30 (silica gel, methylene chloride/methanol =
9:1)
CzaHzeN4~z
mass spectrum: m/z = 452 (M')
(111) 3-Z-[1-(4-(1,3-Dihydro-isoindol-2-yl-methyl)-phenyl-
amino)-1-methyl-methylene]-5-amido-2-indolinone-trifluo-
roacetate
Prepared from the resin produced in Example IV(2) and 4-
(1,3-dihydro-isoindol-2-yl-methyl)-aniline
CA 02328291 2000-10-11
- 75 -
Rf value: 0,35 (silica gel, methylene chloride/methanol =
9:1)
C26H24N4~2
mass spectrum: m/z = 425 (M+H')
(112) 3-Z-[1-(4-(6,7-Dimethoxy-1,2,3,4-tetrahydro-iso-
quinolin-2-yl-methyl)-phenylamino)-1-methyl-methylene]-5-
amido-2-indolinone-trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
(6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinolin-2-yl-
methyl)-aniline
.-, Rf value: 0.50 (silica gel, methylene chloride/methanol =
9:1)
C29H30N4O4
mass spectrum: m/z = 499 (M+H+)
(113) 3-Z-[1-(4-(1,2,3,4-tetrahydro-isoquinolin-2-yl-
methyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone-trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4
(1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-aniline
(114) 3-Z-[1-(4-(N-(Ethoxycarbonylmethyl)-N-benzyl-
aminomethyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
"'~ 25 indolinone-trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
[N-(ethoxycarbonylmethyl)-N-benzyl-aminomethyl)-aniline
Rf value: 0.60 (silica gel, methylene chloride/methanol =
9:1)
3 0 C29H3oN404
mass spectrum: m/z = 498 (M+)
(115) 3-Z- [1- (4- (N- (2-Hydroxyethyl) -N-benzyl-aminomethyl) -
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone-
35 trifluoroacetate
CA 02328291 2000-10-11
- 76 -
Prepared from the resin produced in Example IV(2) and 4-
[N- (2-hydroxyethyl) -N-benzyl-aminomethyl) -aniline
Rf value: 0.40 (silica gel, methylene chloride/methanol =
9:1)
Cz7HzsNa03
mass spectrum: m/z = 456 (M+)
(116) 3-Z-[1-(4-(N-(1-Ethyl-pentyl)-N-(pyridin-2-yl-
methyl)-aminomethyl)-phenylamino)-1-methyl-methylene]-5-
amido-2-indolinone-trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
"' [N-(1-ethyl-pentyl)-N-(pyridin-2-yl-methyl)-aminomethyl]-
aniline
Rf value: 0,45 (silica gel, methylene chloride/methanol =
9:1)
C31H37NSO2
mass spectrum: m/z = 511 (M+)
(117) 3-Z-[1-(4-(Piperidino-methyl)-3-nitro-phenylamino)-
1-methyl-methylene]-5-amido-2-indolinone
Prepared from the resin produced in Example IV(2) and 4-
(piperidino-methyl)-3-nitro-aniline
Rf value: 0.70 (silica gel, methylene chloride/methanol =
9:1)
Cz3HzsNsC4
mass spectrum: m/z = 436 (M+H')
(118) 3-Z-[1-(4-(N-Phenethyl-N-methyl-aminomethyl)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone-
trifluoroacetate
Prepared from the resin produced in Example IV(2) and 4-
(N-phenethyl-N-methyl-aminomethyl)-aniline
Rf value: 0.50 (silica gel, methylene chloride/methanol =
9:1)
3 5 Cz7HzeNa~z
mass spectrum: m/z = 441 (M+H')
L
CA 02328291 2000-10-11
_ 77 _
(119) 3-Z-[1-(4-(N-phenethyl-N-ethyl-aminomethyl)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone
(120) 3-Z-[1-(4-(N-(3,4-dihydroxy-phenethyl)-N-methyl-
amino-methyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone
(121) 3-Z-[1-(4-(N-(3,4,5-trimethoxy-phenethyl)-N-methyl-
aminomethyl)-phenylamino)-1-methyl-methylene]-5-amido-
2-indolinone
(122) 3-Z-[1-(4-(N-(3,4-dimethoxy-phenethyl)-N-methyl-
amino-methyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone
(123) 3-Z-[1-(4-(N-(4-nitro-phenethyl)-N-methyl-
aminomethyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone
(124) 3-Z-[1-(4-(N-phenethyl-N-benzyl-aminomethyl)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone
C33H32N4~2
mass spectrum: m/z = 517 (M+H')
Rf value: 0,43 (silica gel, methylene chloride/methanol =
9:1)
(125) 3-Z-[1-(4-(N-phenethyl-N-cyclohexyl-aminomethyl)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone
(126) 3-Z-[1-(4-(N-(4-nitro-phenethyl)-N-isopropyl-
aminomethyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone
(127) 3-Z- [1- (4- (N- (2- (pyridin-2-yl) -ethyl) -N-methyl-
amino-methyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
CA 02328291 2000-10-11
- 78 _
indolinone
C26H27N5~2
mass spectrum: m/z = 441 (M+)
Rf value: 0,51 (silica gel, methylene chloride/methanol =
4:1)
(128) 3-Z- [1- (4- (N- (2- (pyridin-4-yl) -ethyl) -N-methyl-
amino-methyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone
(129) 3-Z-[1-(4-(N-(pyridin-2-yl-methyl)-N-methyl-amino-
""' methyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone
(130) 3-Z-[1-(4-(N-(pyridin-3-yl-methyl)-N-methyl-amino-
methyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone
(131) 3-Z-[1-(4-(N-(pyridin-4-yl-methyl)-N-methyl-amino-
methyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone
(132) 3-Z-[1-(4-(dibenzylamino-methyl)-phenylamino)-1-
methyl-methylene]-5-amido-2-indolinone
.~ 25 Ca2HsoNa02
mass spectrum: m/z = 503 (M+H')
Rf value: 0,47 (silica gel, methylene chloride/methanol =
9:1)
(133) 3-Z-[1-(4-(N-(4-nitro-benzyl)-N-propyl-aminomethyl)
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone
(134) 3-Z- [1- (4- (N-benzyl-N- (3-cyano-propyl) -aminomethyl) -
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone
CA 02328291 2000-10-11
- 79 _
(135) 3-Z-[1-(4-(N-benzyl-N-allyl-aminomethyl)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone
CzaHzsN40z
mass spectrum: m/z = 451 (M+H')
Rf value: 0,53 (silica gel, methylene chloride/methanol =
9:1)
(136) 3-Z-[1-(4-(imidazol-1-yl-methyl)-phenylamino)-1-
methyl-methylene]-5-amido-2-indolinone
CzlHzoNsOz
mass spectrum: m/z = 389 (M+H~)
'"~ Rf value: 0,20 (silica gel, methylene chloride/methanol =
4:1)
(137) 3-Z-[1-(4-(imidazol-2-yl-amino-methyl)-phenylamino)-
1-methyl-methylene]-5-amido-2-indolinone
(138) 3-Z-[1-(4-(N-benzyl-N-(2,2,2-trifluoroethyl)-
aminomethyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone
(139) 3-Z-[1-(4-(N-(benzo(1,3)dioxol-5-yl-methyl)-N-
methyl-aminomethyl)-phenylamino)-1-methyl-methylene]-5-
amido-2-indolinone
...
Cz~HzsN404
mass spectrum: m/z = 470 (M+H')
Rf value: 0,50 (silica gel, methylene chloride/methanol = 9:1)
(140) 3-Z-[1-(4-(7-chloro-2,3,4,5-tetrahydro-
benzo(d)azepin-3-yl-methyl)-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
(141) 3-Z-[1-(4-(7,8-dichloro-2,3,4,5-tetrahydro-
benzo(d)azepin-3-yl-methyl)-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
CA 02328291 2000-10-11
- 80 -
(142) 3-Z-[1-(4-(7-bromo-2,3,4,5-tetrahydro-
benzo(d)azepin-3-yl-methyl)-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
(143) 3-Z-[1-(4-(7-fluoro-2,3,4,5-tetrahydro-
benzo(d)azepin-3-yl-methyl)-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
(144) 3-Z-[1-(4-(7-methoxy-2,3,4,5-tetrahydro-
benzo(d)azepin-3-yl-methyl)-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
(145) 3-Z-[1-(4-(7-methyl-2,3,4,5-tetrahydro-
benzo(d)azepin-3-yl-methyl)-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
(146) 3-Z-[1-(4-(7,8-dimethoxy-2,3,4,5-tetrahydro-
benzo(d)azepin-3-yl-methyl)-phenylamino)-1-methyl-
methylene]-5-amido-2-indolinone
(147) 3-Z-[1-(4-(6,7-dichloro-1,2,3,4-tetrahydro-
isoquinolin-2-yl-methyl)-phenylamino)-1-methyl-methylene]-
5-amido-2-indolinone
(148) 3-Z-[1-(4-(6,7-dimethyl-1,2,3,4-tetrahydro-
isoquinolin-2-yl-methyl)-phenylamino)-1-methyl-methylene]-
5-amido-2-indolinone
(149) 3-Z-[1-(4-(6,7-difluoro-1,2,3,4-tetrahydro-
isoquinolin-2-yl-methyl)-phenylamino)-1-methyl-methylene]-
5-amido-2-indolinone
C2~HzsNa~z
mass spectrum: m/z = 439 (M+H')
Rf value: 0,43 (silica gel, methylene chloride/methanol -
9:1)
CA 02328291 2000-10-11
- 81 -
(150) 3-Z-[1-(4-(6-chloro-1,2,3,4-tetrahydro-isoquinolin-
2-yl-methyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone
(151) 3-Z-[1-(4-(7-chloro-1,2,3,4-tetrahydro-isoquinolin-
2-yl-methyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone
(152) 3-Z-[1-(4-(6-methoxy-1,2,3,4-tetrahydro-isoquinolin-
2-yl-methyl)-phenylamino)-1-methyl-methylene]-5-amido-
2-indolinone
(153) 3-Z-[1-(4-(7-methoxy-1,2,3,4-tetrahydro-isoquinolin-
2-yl-methyl)-phenylamino)-1-methyl-methylene]-5-amido-2-
indolinone
(154) 3-Z-[1-(4-(2,3,4,5-tetrahydro-azepino(4,5-b)pyrazin-
3-yl-methyl)-phenylamino)-1-methyl-methylene]-5-amido-
2-indolinone
(155) 3-Z-[1-(4-(7-Amino-2,3,4,5-tetrahydro-azepino(4,5-
b)pyrazin-3-yl-methyl)-phenylamino)-1-methyl-methylene]-5-
amido-2-indolinone
(156) 3-Z-[1-(4-(2-amino-5,6,7,8-tetrahydro-azepino(4,5-
d)thiazol-6-yl-methyl)-phenylamino)-1-methyl-methylene]-5-
amido-2-indolinone
(157) 3-Z-[1-(4-(5,6,7,8-tetrahydro-azepino(4,5-d)thiazol-
6-yl-methyl)-phenylamino)-1-methyl-methylene]-5-amido-
2-indolinone
(158) 3-Z-[1-(pyridin-3-yl-amino)-1-methyl-methylene]-5-
amido-2-indolinone
CA 02328291 2000-10-11
- 82 -
(159) 3-Z-[1-(thiazol-2-yl-amino)-1-methyl-methylene]-5-
amido-2-indolinone
(160) 3-Z-[1-(benzimidazol-2-yl-amino)-1-methyl-
methylene]-5-amido-2-indolinone
(161) 3-Z-[1-(5-methyl-isoxazol-3-yl-amino)-1-methyl-
methylene]-5-amido-2-indolinone
(162) 3-Z-[1-(imidazol-2-yl-amino)-1-methyl-methylene]-
5-amido-2-indolinone
(163) 3-Z-[1-(5-methyl-pyridin-2-yl-amino)-1-methyl-
methylene]-5-amido-2-indolinone
(164) 3-Z-(1-(5-bromo-pyridin-2-yl-amino)-1-methyl-
methylene]-5-amido-2-indolinone
(165) 3-Z-[1-(2-chloro-pyridin-5-yl-amino)-1-methyl-
methylene]-5-amido-2-indolinone
(166) 3-Z-[1-(4-(N-butyl-N-methyl-aminomethyl)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone
C23HzeNaOz
mass spectrum: m/z = 392 (M+)
(167) 3-Z-[1-(4-(N-isobutyl-aminomethyl)-phenylamino)-1-
methyl-methylene]-5-amido-2-indolinone
C22H2bN4~2
mass spectrum: m/z = 378 (M+)
(168) 3-Z-[1-(4-(N-cyclohexylmethyl-aminomethyl)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone
C25H30N402
mass spectrum: m/z = 418
CA 02328291 2000-10-11
- 83 -
Rf value: 0,26 (silica gel, methylene chloride/methanol =
4:1)
3-Z-[1-(4-Diethylcarbamoyl-phenylamino)-1-methyl-
m~th~rlene] -S-amido-2-indolinone
2 g of the resin prepared in Example IVb are reacted
analogously to Example 1 with 2 g of ethyl 4-aminobenzoate
in dimethylformamide at 110°C. The moist charged resin is
°""~ suspended in 15 ml of dioxane and 15 ml of methanol and
stirred with 12 ml of 1N sodium hydroxide solution for 40
hours. Then the mixture is neutralised with dilute hy-
drochloric acid and washed with methylene chloride,
methanol and dimethylformamide. 300 mg of the resin are
then suspended in 3 ml of dimethylformamide and left to
stand with 0.2 ml of diethylamine, 0.5 g of TBTU (O-
benzotriazol-1-yl-N,N,N',N'-tetramethyluronium-tetra-
fluoroborate), and 0.8 ml of N-ethyl-diisopropylamine for
40 hours at ambient temperature. Finally, the product is
cleaved from the resin as described in Example 1.
Yield: 61 mg,
Rf value: 0.30 (silica gel, methylene chloride/methanol =
9:1)
C22H24N4O3
mass spectrum: m/z = 392 (M')
The following compounds are prepared analogously to
Example 2:
(1) 3-Z-[1-(4-benzylcarbamoyl-phenylamino)-1-phenyl-
methylene]-5-amido-2-indolinone
Prepared analogously to Example 2 with benzylamine
Rf value: 0.30 (silica gel, methylene chloride/methanol -
9:1)
CA 02328291 2000-10-11
- 84 -
CzsHzzNa~3
mass spectrum: m/z = 426 (M*)
(2) 3-Z-[1-(4-(N-methoxycarbonylmethyl-carbamoyl)-
phenylamino)-1-phenyl-methylene]-5-amido-2-indolinone
Prepared analogously to Example 2 with glycinethyl ester
Rf value: 0.50 (silica gel, methylene chloride/methanol =
9:1)
CziHzoNa~s
mass spectrum: m/z = 408 (M*)
""' (3) 3-Z-[1-(4-dimethylcarbamoyl-phenylamino)-1-phenyl-
methylene]-5-amido-2-indolinone
Prepared analogously to Example 2 with dimethylamine
Rf value: 0.40 (silica gel, methylene chloride/methanol =
9:1)
C2oHzoN403
mass spectrum: m/z = 364 (M*)
(4) 3-Z-[1-(4-(N-(2-piperidino-ethyl)-carbamoyl)
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone-
trifluoroacetate
Prepared analogously to Example 2 with 1-(2-amino-ethyl)-
piperidine
Rf value: 0.30 (silica gel, methylene chloride/methanol -
4:1)
CzsH29Ns~3
mass spectrum: m/z = 448 (M+H*)
(5) 3-Z-[1-(4-(N-methyl-piperazino-carbamoyl)-
phenylamino)-1-phenyl-methylene]-5-amido-2-indolinone-
trifluoroacetate
Prepared analogously to Example 2 with N-methyl-piperazine
Rf value: 0.40 (silica gel, methylene chloride/methanol -
4:1)
C23H2sNsO3
CA 02328291 2000-10-11
_ 85 _
mass spectrum: m/z = 419 (M')
(6) 3-Z- [1- (4- (N- (2-Diethyl amino-ethyl) -N-methyl-
carbamoyl)-phenylamino)-1-phenyl-methylene]-5-amido-2-
indolinone-trifluoroacetate
Prepared analogously to Example 2 with N,N-diethyl-N'-
methyl-ethylenediamine
Rf value: 0.20 (silica gel, methylene chloride/methanol -
4:1)
Cz5H31Ns03
mass spectrum: m/z = 449 (M+)
(7) 3-Z-[1-(4-Butylcarbamoyl-phenylamino)-1-phenyl-
methylene]-5-amido-2-indolinone
Prepared analogously to Example 2 with butylamine
Rf value: 0.80 (silica gel, methylene chloride/methanol =
4:1)
C22H24N4O3
mass spectrum: m/z = 392 (M+)
3-Z-[1-(4-(N-methyl-N-benzoyl-amino)-phenylamino)-1-
merh~rl -~m ~,h~rlene,l-S-ami do- -i ndol i non
4.5 g of a resin prepared according to Example IVb are
reacted analogously to Example 1 with 3.4 g of 4-(9H-
fluoren-9-yl-methoxycarbonyl)-methylamino)-aniline in
dimethylformamide. Then the 9H-fluoren-9-yl-
methoxycarbonyl group is cleaved with 40 ml of 300
piperidine in dimethylformamide and the resin is washed
several times. Then 400 mg of the resin are suspended in 4
ml of dimethylformamide and 0.3 ml of triethylamine and
reacted with 0.3 ml of benzoylchloride for one hour at
ambient temperature. Finally, the product is cleaved from
CA 02328291 2000-10-11
- 86 -
the resin with trifluoroacetic acid as described in
Example 1.
Yield: 25 mg,
Rf value: 0.51 (silica gel, methylene chloride/methanol =
9:1)
C30H24N4~3
mass spectrum: m/z - 426 (M+)
The following compounds are prepared analogously to
Example 3:
'° (1) 3-Z-[1-(4-(N-methyl-N-propionyl-amino)-phenylamino)-
1-methyl-methylene]-5-amido-2-indolinone
Prepared analogously to Example 3 with propionic acid
chloride
Rf value: 0.52 (silica gel, methylene chloride/methanol =
9:1)
C21H22N4~3
mass spectrum: m/z = 378 (M')
(2) 3-Z-[1-(4-(N-methyl-N-butyryl-amino)-phenylamino)-
1-methyl-methylene]-5-amido-2-indolinone
Prepared analogously to Example 3 with butyric acid
chloride
Rf value: 0.28 (silica gel, methylene chloride/methanol =
9:1)
C22H24N4~3
mass spectrum: m/z = 392 (M+)
(3) 3-Z-[1-(4-(N-methyl-N-ethanesulphonyl-amino)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone
Prepared analogously to Example 3 with ethanesulphonic
acid chloride
Rf value: 0.30 (silica gel, methylene chloride/methanol =
9:1)
CzoHzzN404s
CA 02328291 2000-10-11
- 87 _
mass spectrum: m/z = 413 (M-H')
(4) 3-Z-[1-(4-(N-methyl-N-propanesulphonyl-amino)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone
Prepared analogously to Example 3 with propanesulphonic
acid chloride
Rf value: 0.31 (silica gel, methylene chloride/methanol =
9:1)
C21H24N4O4s
mass spectrum: m/z = 451 (M+Na')
(5) 3-Z-[1-(4-(N-methyl-N-phenylsulphonylamino)-
phenylamino-1-methyl-methylene]-5-amido-2-indolinone
Prepared analogously to Example 3 with phenylsulphonic
acid chloride
Rf value: 0.46 (silica gel, methylene chloride/methanol =
9:1)
C24H22N4O4'S
mass spectrum: m/z = 462 (M')
(6) 3-Z-[1-(4-(N-methyl-N-acetyl-amino)-phenylamino)-1-
methyl-methylene]-5-amido-2-indolinone
Prepared analogously to Example 3 with acetylchloride
Rf value: 0.20 (silica gel, methylene chloride/methanol =
9:1)
CzoHzoN4~a
mass spectrum: m/z = 364 (M')
(7) 3-Z-[1-(4-(N-methyl-N-phenylmethylsulphonyl-amino)-
phenylamino)-1-methyl-methylene]-5-amido-2-indolinone
Prepared analogously to Example 3 with
phenylmethanesulphonic acid chloride
Rf value: 0.43 (silica gel, methylene chloride/methanol =
9:1)
3 5 C25H24N4~4S
mass spectrum: m/z = 475 (M-H')
CA 02328291 2000-10-11
_ 88 _
Methyl 3-Z-[1-(4-(N-benzyl-N-methyl-aminomethyl)-
~n~rl ami no) -'I -m th~rl -me~h~rl~n~] -2-i nclol i none-5-
carY~ox~rl a -P
8.0 g (28 mmol) of methyl 1-acetyl-3-(1-ethoxy-1-methyl-
methylene)-2-indolinone-5-carboxylate are dissolved in 60
ml of dimethylformamide and stirred with 6.3 g (28 mmol)
°'"' of 4-(N-benzyl-N-methyl-aminomethyl)-aniline for 6 hours
at 80°C. Then 30 ml of conc. ammonia are added and the
mixture is left to stand for 2 hours at 45°C. The solution
is evaporated down and the residue is washed with ethanol
and ether. Then it is chromatographed over a small silica
gel column with ethyl acetate/ethanol (9:1).
Yield: 8.6 g (70 % of theory),
melting point: 150-152 °C
2 0 C27H27Na~3
mass spectrum: m/z = 442 (M+)
The following compounds are prepared analogously to
Example 4:
(1) methyl 3-Z-[1-(4-(piperidino-methyl)-phenylamino)-1-
methyl-methylene]-2-indolinone-5-carboxylate
C24H27N3~3
mass spectrum: m/z = 406 (M+H')
(2) methyl 3-Z-[1-(4-bromo-phenylamino)-1-methyl-
methylene]-2-indolinone-5-carboxylate
CieHisBrN203
mass spectrum: m/z = 386/388 (M')
CA 02328291 2000-10-11
- 89 -
(3) methyl 3-Z-[1-(4-chloro-phenylamino)-1-methyl-
methylene]-2-indolinone-5-carboxylate
CieHisC1N203
mass spectrum: m/z = 342/344 (M~)
(4) methyl 3-Z-[1-(4-(N-methyl-N-methylsulphonyl-amino)-
phenylamino)-1-ethyl-methylene]-2-indolinone-5-carboxylate
C2oHziN3~ss
mass spectrum: m/z = 415 (M+)
(5) methyl 3-Z- [1- (4- (2, 3, 4, 5-tetrahydro-benzo (d) azepin-3-
yl-methyl)-phenylamino)-1-methyl-methylene]-2-indolinone-
5-carboxylate
C29H29N3~2
mass spectrum: m/z = 467 (M')
3-Z-(1-(4-(N-benzyl-N-methyl-aminomethyl)-phenylamino)-
1-meth~rl_-meth~rlen 1 - -i ndol i non -~- rbc~ yl i a _i
2.3 g (5 mmol) of methyl 3-Z-[1-(4-(N-benzyl-N-methyl-
aminomethyl)-phenylamino)-1-methyl-methylene]-2-
indolinone-5-carboxylate are dissolved in 50 ml of
methanol and 50 ml of dioxane and stirred with 25 ml of 1N
sodium hydroxide solution for 1 hour at 70°C. Then the
mixture is neutralised with 25 ml of 1N hydrochloric acid
and evaporated to dryness. The residue is washed several
times with water and dried.
Yield: 1.9 g (85 0 of theory),
C26H2sN3~3
mass spectrum: m/z = 428 (M+H')
The following compounds are prepared analogously to
Example 5:
CA 02328291 2000-10-11
- 90 -
(1) 3-Z-[1-(4-(Piperidino-methyl)-phenylamino)-1-methyl-
methylene]-2-indolinone-5-carboxylic acid
C23H25N3o3
mass spectrum: m/z = 392 (M+H')
(2) 3-Z-[1-(4-bromo-phenylamino)-1-methyl-methylene]-2-
indolinone-5-carboxylic acid
(3) 3-Z-[1-(4-chloro-phenylamino)-1-methyl-methylene]-2-
indolinone-5-carboxylic acid
C17 H13C1N203
mass spectrum: m/z = 327/329 (M-H+)
(4) 3-Z-[1-(4-(N-methyl-N-methylsulphonyl-amino)-
phenylamino)-1-methyl-methylene]-2-indolinone-5-carboxylic
acid
C19H19N3~SS
mass spectrum: m/z = 401 (M+)
(5) 3-Z-[1-(4-(2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-phenylamino)-1-methyl-methylene]-2-indolinone-5-
carboxylic acid
C28H27N3~3
mass spectrum: m/z = 453 (M')
3-Z-[1-(4-(N-benzyl-N-methyl-aminomethyl)-phenylamino)-
1-meth~rl -~m ~h~rl enel -2-i ndol i nnna-~,-di th~rl rarhamo~r~-
3 0 i_ndoli_n_one
0.3 g of 3-Z-[1-(4-(N-benzyl-N-methyl-aminomethyl)-
phenylamino)-1-methyl-methylene]-2-indolinone-2-
indolinone-5-carboxylic acid are dissolved with 1.2 g of
N-ethyl-diisopropylethylamine in 8 ml of
dimethylformamide. Then 0.1 g of diethylamine and 0.4 g of
CA 02328291 2000-10-11
- 91 -
TBTU (O-benzotriazol-1-yl-N,N,N',N'-tetramethyluronium-
tetrafluoroborate) are added and the mixture is stirred
for 20 hours at ambient temperature. It is then evaporated
down and the residue is suspended in water and extracted
with methylene chloride. The organic phase is evaporated
down and chromatographed over a silica gel column with
methylene chloride/ethanol (19:1).
Yield: 0.2 g (68% of theory),
Rf value: 0.36 (silica gel, methylene chloride/ethanol =
19 : 1 )
CsoH34N4~z
mass spectrum: m/z = 482 (M')
The following compounds are prepared analogously to
Example 6:
(1) 3-Z-[1-(4-(Piperidino-methyl)-phenylamino)-1-methyl-
methylene]-2-indolinone-5-diethylcarbamoyl-2-indolinone
Prepared from the compound produced in Example 5(1) and
diethylamine
C27H34N4O2
mass spectrum: m/z = 446 (M+)
(2) 3-Z-[1-(4-(N-methyl-N-methylsulphonyl-amino)-
phenylamino)-1-methyl-methylene]-2-indolinone-5-
diethylcarbamoyl-2-indolinone
Prepared from the compound produced in Example 5(4) and
diethylamine
C23H28N4~4'S
mass spectrum: m/z = 457 (M+H+)
(3) 3-Z-[1-(4-(2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-phenylamino)-1-methyl-methylene]-5-
diethylcarbamoyl-2-indolinone
CA 02328291 2000-10-11
- 92 -
(4) 3-Z-[1-(4-(2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-phenylamino)-1-methyl-methylene]-5-
dimethylcarbamoyl-2-indolinone
(5) 3-Z-[1-(4-(N-Phenylmethyl-N-methylamino-methyl)-
phenylamino)-1-methyl-methylene]-5-methylcarbamoyl-2-
indolinone
(6) 3-Z-[1-(4-(N-Phenylmethyl-N-methylamino-methyl)-
phenylamino)-1-methyl-methylene]-5-dimethylcarbamoyl-2-
indolinone
(7) 3-Z-[1-(4-(N-Phenylmethyl-N-methylamino-methyl)-
phenylamino)-1-methyl-methylene]-5-diethylcarbamoyl-2-
indolinone
(8) 3-Z-[1-(4-(N-Phenylmethyl-N-methylamino-methyl)-
phenylamino)-1-methyl-methylene]-5- propylcarbamoyl-2-
indolinone
(9) 3-Z-[1-(4-(N-Phenylmethyl-N-methylamino-methyl)-
phenylamino)-1-methyl-methylene]-5-dipropylcarbamoyl-2-
indolinone
(10) 3-Z-[1-(4-(dimethylamino-methyl)-phenylamino)-1
methyl-methylene]-5-methylcarbamoyl-2-indolinone
(11) 3-Z-[1-(4-(dimethylamino-methyl)-phenylamino)-1-
methyl-methylene]-5-dimethylcarbamoyl-2-indolinone
(12) 3-Z-[1-(4-(dimethylamino-methyl)-phenylamino)-1-
methyl-methylene]-5-diethylcarbamoyl-2-indolinone
(13) 3-Z-[1-(4-(dimethylamino-methyl)-phenylamino)-1-
methyl-methylene]-5-propylcarbamoyl-2-indolinone
CA 02328291 2000-10-11
- 93 -
(14) 3-Z-[1-(4-(dimethylamino-methyl)-phenylamino)-1-
methyl-methylene]-5-dipropylcarbamoyl-2-indolinone
(15) 3-Z-[1-(3-(dimethylamino-methyl)-phenylamino)-1-
methyl-methylene]-5-methylcarbamoyl-2-indolinone
(16) 3-Z-[1-(3-(dimethylamino-methyl)-phenylamino)-1-
methyl-methylene]-5-dimethylcarbamoyl-2-indolinone
(17) 3-Z-[1-(3-(dimethylamino-methyl)-phenylamino)-1
methyl-methylene]-5-diethylcarbamoyl-2-indolinone
(18) 3-Z-[1-(3-(dimethylamino-methyl)-phenylamino-1-
methyl-methylene]-5-propylcarbamoyl-2-indolinone
(19) 3-Z-[1-(3-(dimethylamino-methyl)-phenylamino)-1-
methyl-methylene]-5-dipropylcarbamoyl-2-indolinone
(20) 3-Z-[1-(4-chloro-phenylamino)-1-methyl-methylene]-
5-methylcarbamoyl-2-indolinone
(21) 3-Z- [1- (4-chloro-phenyl amino) -1-methyl-methylene] -5-
dimethylcarbamoyl-2-indolinone
(22) 3-Z-[1-(4-chloro-phenylamino)-1-methyl-methylene]-5-
diethylcarbamoyl-2-indolinone
(23) 3-Z-[1-(4-chloro-phenylamino)-1-methyl-methylene]-5-
propylcarbamoyl-2-indolinone
(24) 3-Z-[1-(4-chloro-phenylamino)-1-methyl-methylene]-5-
dipropylcarbamoyl-2-indolinone
(25) 3-Z-(1-phenylamino-1-methyl-methylene)-5-
methylcarbamoyl-2-indolinone
CA 02328291 2000-10-11
- 94 -
(26) 3-Z-(1-phenylamino-1-methyl-methylene)-5-
dimethylcarbamoyl-2-indolinone
(27) 3-Z-(1-Phenylamino-1-methyl-methylene)-5-
diethylcarbamoyl-2-indolinone
3-Z-[1-(4-methyl-3-amino-phenylamino)-1-methyl-methylene]-
5-amid- -indolinonP
130 mg of 3-Z-[1-(4-methyl-3-nitro-phenylamino)-1-methyl-
°~ methylene]-5-amido-2-indolinone are dissolved in 9 ml of
methanol and hydrogenated with 50 mg of Raney nickel at
ambient temperature under 3 bar of hydrogen pressure. The
catalyst is filtered off and the solution is evaporated
down.
Yield: 97 mg (70 % of theory),
Rf value: 0.60 (silica gel, methylene chloride/Ethanol -
4:1)
2 0 ClgHiaNa~2
mass spectrum: m/z = 322 (M')
The following compound is prepared analogously to Example
7:
(1) 3-Z-[1-(4-(piperidino-methyl)-3-amino-phenylamino)-1-
methyl-methylene]-5-amido-2-indolinone
Prepared aus 3-Z-[1-(4-(piperidino-methyl)-3-nitro-phenyl-
amino)-1-methyl-methylene]-5-amido-2-indolinone
Rf value: 0,15 (silica gel, methylene chloride/ethanol -
9:1)
CzaHz~Ns~z
mass spectrum: m/z = 406 (M+H')
CA 02328291 2000-10-11
- 95 -
Fx~m
Dry ampoule containing 75 mg of active substance per 10 ml
Composition:
Active substance 75.0 mg
Mannitol 50.0 ma
water for injections ad 10.0 ml
Preparation:
Active substance and mannitol are dissolved in water.
After packaging the solution is freeze-dried. To produce
the solution ready for use, the product is dissolved in
water for injections.
Dry ampoule containing 35 mg of active substance per 2 ml
Composition:
Active substance 35.0 mg
Mannitol 100.0 mg
water for injections ad 2.0 ml
Preparation:
Active substance and mannitol are dissolved in water.
After packaging, the solution is freeze-dried.
To produce the solution ready for use, the product is
dissolved in water for injections.
CA 02328291 2000-10-11
- 96 -
Tablet containing 50 mg of active substance
Composition:
(1) Active substance 50.0 mg
(2) Lactose 98.0 mg
(3) Maize starch 50.0 mg
(4) Polyvinylpyrrolidone 15.0 mg
(5) Magnesium stearate ~_0 mg
215.0 mg
Preparation:
(1), (2) and (3) are mixed together and granulated with an
aqueous solution of (4). (5) is added to the dried
granulated material. From this mixture tablets are
pressed, biplanar, faceted on both sides and with a
dividing notch on one side.
Diameter of the tablets: 9 mm.
Tablet containing 350 mg of active substance
Composition:
(1) Active substance 350.0 mg
(2) Lactose 136.0 mg
(3) Maize starch 80.0 mg
(4) Polyvinylpyrrolidone 30.0 mg
(5) Magnesium stearate 4.0 ma
600.0 mg
CA 02328291 2000-10-11
_ 97 _
Preparation:
(1), (2) and (3) are mixed together and granulated with an
aqueous solution of (4). (5) is added to the dried
granulated material. From this mixture tablets are
pressed, biplanar, faceted on both sides and with a
dividing notch on one side.
Diameter of the tablets: 12 mm.
Capsules containing 50 mg of active substance
Composition:
(1) Active substance 50.0 mg
(2) Dried maize starch 58.0 mg
(3) Powdered lactose 50.0 mg
(4) Magnesium stearate 2..0 ma
160.0 mg
Preparation:
(1) is triturated with (3). This trituration is added to
the mixture of (2) and (4) with vigorous mixing.
This powder mixture is packed into size 3 hard gelatin
capsules in a capsule filling machine.
CA 02328291 2000-10-11
_ 98 _
Capsules containing 350 mg of active substance
Composition:
(1) Active substance 350.0 mg
(2) Dried maize starch 46.0 mg
(3) Powdered lactose 30.0 mg
(4) Magnesium stearate 4.0 mg
430.0 mg
Preparation:
(1) is triturated with (3). This trituration is added to
the mixture of (2) and (4) with vigorous mixing.
This powder mixture is packed into size 0 hard gelatin
capsules in a capsule filling machine.
Suppositories containing 100 mg of active substance
1 suppository contains:
Active substance 100.0 mg
Polyethyleneglycol (M.W. 1500) 600.0 mg
Polyethyleneglycol (M.W. 6000) 460.0 mg
Polyethylenesorbitan monostearate X40_0 ma
2,000.0 mg
Preparation:
The polyethyleneglycol is melted with the polyethylene-
sorbitan monostearate. The milled active substance is
CA 02328291 2000-10-11
_ 99 _
homogeneously dispersed in the melt at 40°C. The melt is
cooled to 38°C and poured into lightly pre-cooled
suppository moulds.