Note: Descriptions are shown in the official language in which they were submitted.
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
TITLE OF THE INVENTION
ANTIDIABETIC AGENTS
B CKGROUND OF THE INVENTION
Insulin is a hormone that is necessary for normal
carbohydrate, protein and fat metabolism in mammals. Insulin is
known to bind to the extracellular domain (a-subunits) of its specific
receptor. Following insulin binding, conformational changes in the
insulin receptor lead to autophosphorylation of the intracellular (3-
subunits and stimulation of the receptor's intrinsic tyrosine kinase
activity and activation of insulin signal transduction pathway. The
activated insulin receptor tyrosine kinase phosphorylates several
intermediate substrates (e.g. IRS-1 and SHC). These proximal events
lead to activation of additional signaling intermediates such as PI-3-
kinase and MAP kinase. Through an unknown series of additional
steps, modulation of key cellular components (e.g. glucose transporter
translocation, activation of glycogen synthase, inhibition of
gluconeogenic enzymes) coordinate stimulation of glucose disposal and
inhibition of hepatic glucose output. Considerable evidence suggests
that insulin receptor tyrosine kinase activity is essential for many, if not
all of the biological effects of insulin. However, the precise biochemical
mechanisms linking receptor kinase-mediated tyrosine phosphorylation
to the regulation of cellular metabolic pathways are not completely
defined.
Two major forms of diabetes mellitus are now recognized.
Type I diabetes, or insulin-dependent diabetes, is the result of an
absolute deficiency of insulin, the hormone which regulates glucose
utilization, and patients with Type I diabetes are dependent on
exogenous insulin for survival. Type II diabetes, or non-insulin-
dependent diabetes (NIDDM), often occurs in the face of normal, or even
elevated levels of insulin and appears to be the result of the inability of
tissues to respond appropriately to insulin (i.e. insulin resistance).
Insulin resistance is a major susceptibility trait for NIDDM and is also a
-1-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
contributing factor in atherosclerosis, hypertension, lipid disorders and
polycystic ovarian syndrome.
Over time, many individuals with NIDDM show decreased
insulin production, which requires supplemental insulin for adequate
blood glucose control, especially during times of stress or illness. An
exogenous insulin regimen is often required in the treatment of
secondary diabetes, i.e., diabetes occurring in relation to other disease
states such as pancreatic disease. Insulin is also used in some cases of
gestational diabetes to obtain optimum blood glucose control. The
conventional route of insulin administration is subcutaneously via a
needle and syringe. Continuous subcutaneous insulin infusion with an
infusion pump is an alternative to conventional injection therapy for
achieving normalized levels of blood glucose.
Conventional treatments for NIDDM, which have not
changed substantially in many years, have significant limitations.
While physical exercise and a reduction in dietary intake of calories
could improve the diabetic condition, compliance with this treatment is
generally poor. Increasing the plasma level of insulin by administration
of sulfonylureas (e.g, tolbutamide, glipizide) which stimulate the
pancreatic ~3-cells to secrete more insulin, or by injection of insulin after
the response to sulfonylureas fails, will result in insulin concentrations
that stimulate even highly insulin-resistant tissues. However,
dangerously low levels of plasma glucose can result from these last two
treatments, and increasing insulin resistance due to the even higher
plasma insulin levels could theoretically occur. The biguanides
increase insulin sensitivity resulting in some correction of
hyperglycemia. However, the two biguanides, phenformin and
metformin, can induce lactic acidosis and nausea/diarrhea,
respectively.
Thiazolidinediones (glitazones) have been recently described
as a class of compounds with a mechanism of action which ameliorates
many symptoms of NIDDM. These agents substantially increase
insulin sensitivity in muscle, liver and adipose tissue in several NIDDM
animal models, resulting in the correction of elevated plasma levels of
_2_
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
glucose, triglycerides and nonesterified fatty acids without the
occurrence of hypoglycemia. However, undesirable effects associated
with the glitazones have occurred in animal and human studies,
including cardiac hypertrophy, hemadilution and liver toxicity.
Accordingly, there exists a continuing need for novel
therapeutic agents for ameliorating the symptoms of diabetes mellitus,
particularly for controlling the blood glucose level in patients, and for the
prevention of the onset of diabetes. In addition, there is a need for new
therapeutic agents for treating or overcoming insulin resistance in
cases where it contributes to the pathogenesis of diseases or disorders.
~UMM_AI~Y OF THE INVENTION
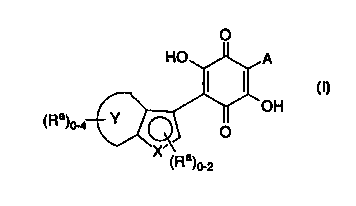
The present invention relates to compounds represented by
formula I:
~Ra)
(Ra)o-2
I
H
as well as tautomers, pharmaceutically acceptable salts, hydrates,
prodrugs and reduced forms thereof wherein:
ring Y represents a 5-6 membered aryl or heteroaryl fused
ring, which is optionally substituted with 1-4 groups selected from Ra;
X represents O, S(O)m or N, wherein m is 0, 1 or 2;
A represents a member selected from the group consisting
0~ (a) a 6-10 membered mono-or bicyclic aryl group;
(b) a 5-6 membered isolated monocyclic heteroaryl group;
-3-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
(c) a 9-10 membered bicyclic heteroaryl group, attachment
to which is through a 6 membered ring, or
(d) an 8- membered bicyclic heteroaryl group,
the heteroaryl groups having 1-4 heteroatoms selected from O, S(O)m
and N,
said aryl and heteroaryl groups being optionally substituted
with 1-3 Ra groups;
each Ra is independently selected from the group consisting
of:
halo, -OH, -C1_12 alkyl(Rb)3, -C2-10 alkenyl(Rb)3, -C2-10
alkynyl(Rb)3, -Cg_10 m'3'1(Rb)3~ -heteroaryl(Rb)3 ,-heterocyclyl(Rb)3,
-NH2, -NHC1_g alkyl(Rb)3, -N(C1_g alkyl(Rb)3)2, -N3, -OC1_6 alkyl(Rb)3,
-S(O)mH, -S(O)mC1_g alkyl(Rb)3, -CHO, -C(O)C1-g alkyl(Rb)3, -C02H,
-C(O)OC1_g alkyl(Rb)3, -C(O)SC1_s alkyl(Rb)3, -C(O)NH2, -C(O)NHC1_g
alkyl(Rb)3, -NHC(O)C1-g alkyl(Rb)3, -S(O)mNH2, -NHS(O)mCl_6
alkyl(Rb)3, -S(O)mNHCl_g alkyl(Rb)3 and -S(O)mN(C1_g alkyl(Rb)3)2~
and each Rb is independently selected from: H, OH, halo, -C1_4 alkyl,
-C2_4 alkenyl, -C2_4 alkynyl, -CF3, -OCF3, -N02, -N3, -CHO, -OC1_6
alkyl, -S(O)mCl_6 alkyl, -NH2, -NHC1_g alkyl, -N(C1_g alkyl)2, -C(O)C1_6
alkyl, -C02H, -C02C1_g alkyl, -C(O)NH2, -C(O)NHC1_g alkyl, -C(O)N(C1_
g alkyl)2, -OC(O)C1_g alkyl, -NHC(O)C1-g alkyl, -S(O)mNH2,
-S(O)mNHCI_g alkyl, -S(O)m(C1-g alkyl)2, aryl, heteroaryl and
heterocyclyl.
Pharmaceutical compositions and methods of treatment are
also included.
DETAILED DESCRIPTION OF T~EiE INVENTION
The present invention relates to compounds of formula I, as
well as tautomers, salts, hydrates and prodrugs thereof.
-4-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
The invention is described herein in detail using
terms that are defined below unless otherwise specified.
The term "alkyl" and the alkyl portions of aralkyl, alkoxy
and the like refer to a monovalent alkane (hydrocarbon) derived
radical containing from 1 to 15 carbon atoms unless otherwise
defined. It may be straight, branched or cyclic. Preferred straight or
branched alkyl groups include methyl, ethyl, propyl, isopropyl, butyl
and t-butyl. Preferred cycloalkyl groups include cyclopentyl and
cyclohexyl.
Alkyl also includes a straight or branched alkyl group
which contains or is interrupted by a cycloalkylene portion.
Examples include the following:
and -(CH ~ CH
- (CI-~2)x~~./~' (CH2)y 2)w ~ ( 2)z
wherein: x and y = from 0-10; and w and z = from 0-9.
The alkylene and monovalent alkyl portions) of the alkyl
group can be attached at any available point of attachment to the
cycloalkylene portion.
When substituted alkyl is present, this refers to a
straight, branched or cyclic alkyl group as defined above, substituted
with 1-3 groups as defined with respect to each variable.
The term "alkenyl" refers to a hydrocarbon radical
straight, branched or cyclic containing from 2 to 15 carbon atoms and
at least one carbon to carbon double bond. Preferably one carbon to
carbon double bond is present, and up to four non-aromatic (non-
resonating) carbon-carbon double bonds may be present. Preferred
alkenyl groups include ethenyl, propenyl, butenyl and cyclohexenyl.
As described above with respect to alkyl, the straight, branched or
cyclic portion of the alkenyl group may contain double bonds and may
be substituted when a substituted alkenyl group is provided.
The term "alkynyl" refers to a hydrocarbon radical straight,
branched or cyclic, containing from 2 to 15 carbon atoms and at least one
_5_
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
carbon to carbon triple bond. Up to three carbon-carbon triple bonds may
be present. Preferred alkynyl groups include ethynyl, propynyl and
butynyl. As described above with respect to alkyl, the straight, branched
or cyclic portion of the alkynyl group may contain triple bonds and may
be substituted when a substituted alkynyl group is provided.
The term "alkoxy" refers to those groups of the designated
carbon length in either a straight or branched configuration attached
through an oxygen linkage and if two or more carbon atoms in length,
they may include a double or a triple bond. Exemplary of such alkoxy
groups are methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy,
tertiary butoxy, pentoxy, isopentoxy, hexoxy, isohexoxy, allyloxy,
propargyloxy, and the like.
The term halo as used herein means fluoro, chloro, bromo
or iodo.
Aryl refers to aromatic rings e.g., phenyl, substituted
phenyl and like groups as well as rings which are fused, e.g., naphthyl
and the like. Aryl thus contains at least one ring having at least 5
atoms, with up to two such rings being present, containing up to 10
atoms therein, with alternating (resonating) double bonds between
adjacent carbon atoms. The preferred aryl groups are phenyl and
naphthyl. Aryl groups may likewise be substituted. Preferred
substituted aryls include phenyl and naphthyl substituted with up to
three Ra groups.
Heteroaryl is a group containing from 5 to 10 atoms, 1-4 of
which are heteroatoms, 0-4 of which heteroatoms are N and 0-1 of which
are O or S(O)m, said heteroaryl group being unsubstituted or substituted
with up to 3 Ra groups; examples are pyrrolyl, furanyl, thienyl, pyridyl,
quinolinyl, purinyl, imidazolyl, imidazopyridyl and pyrimidinyl.
Tautomers as used herein, refer to the following structures:
O O OH
HO _ O O
~~OH O \ O
O O OH
-6-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
Additionally, in the compound of formula I when X equals N, the
following structure is an example of a tautomer that is included:
A
OH
(Ra)o-t
These and others are included in the present invention.
Reduced forms of the compounds refer to the following
structure:
OH
HO /
-
OH
OH
These are also included in the present invention.
Prodrugs as used herein refer to C1_4 alkoxy, C1_4 acyloxy,
carboxylic acid and phosphate derivatives of the compounds of formula I
as well as other compounds which generate quinones in vivo. Examples
of prodrugs include the following:
O
RPO
-ORP
O
wherein at least one RP represents C1_4 alkyl, C1_4 acyl, C02H, a
phosphate group, a metal complex, such as a chelating metal, or
another group which generates the quinone in vivo.
_7_
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
A subset of compounds that is of particular interest is
described with reference to formula I wherein:
O
represents a phenyl ring. Within this subset of compounds, all other
variables are as originally defined.
Another subset of compounds that is of particular interest is
described with reference to formula I wherein:
O
represents a pyrrole ring. Within this subset of compounds, all
other variables are as originally defined.
Another subset of compounds that is of particular interest is
described with reference to formula I wherein:
Y O~ ~
N
represents H . Within this subset, all other
variables are as originally defined.
Another subset of compounds that is of particular interest is
described with reference to formula I wherein:
1-4 Ra groups are present, and each Ra is independently
selected from the group consisting of
halo, -OH, -C1_12 alkyl(Rb)3, -C2-10 ~kenyl(Rb)g, -C2_10
alkynyl(Rb)3, -Cg_10 ~'3'1(Rb)3~ -heteroaryl(Rb)3 ,-heterocyclyl(Rb)3,
-NH2, -NHC1_g alkyl(Rb)3, -N(C1_6 alkyl(Rb)3)2, -N3, -OC1_g alkyl(Rb)3,
-S(O)mH, -S(O)mCl_g alkyl(Rb)3, -CHO, -C(O)C1_g alkyl(Rb)3, -C02H,
-C(O)OC1_g alkyl(Rb)3, -C(O)SC1_g alkyl(Rb)3, -C(O)NH2, -C(O)NHC1_g
alkyl(Rb)3, -NHC(O)C1_6 alkyl(Rb)3, -S(O)mNH2, -NHS(O)mCl_6
alkyl(Rb)3, -S(O)mNHCl_g alkyl(Rb)3 and -S(O)mN(C1_g alkyl(Rb)g)2,
and each Rb is independently selected from: H, OH, halo,
-C1_4 alkyl, -C2_4 alkenyl, -C2-4 alkynyl, -CF3, -OCF3, -N02, -N3, -CHO,
_g_
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
-OC1_g alkyl, -S(O)mCl_g alkyl, -NH2, -NHC1_g alkyl, -N(C1_s alkyl)2,
-C(O)C1_g alkyl, -C02H, -C02C1_g alkyl, -C(O)NH2, -C(O)NHC1_6 alkyl,
-C(O)N(C1_g alkyl)2, -OC(O)C1_s alkyl, -NHC(O)C1_g alkyl, -S(O)mNH2,
-S(O)mNHCl_g alkyl, -S(O)m(C1_6 alkyl)2, aryl, heteroaryl and
heterocyclyl. Within this subset of compounds, all other variables are as
originally defined.
Another subset of compounds that is of particular interest is
described with reference to formula I wherein:
A represents a member selected from the group consisting
of:
a 5-10 membered mono-or bicyclic aryl group or
a 9-10 membered bicyclic heteroaryl group, attachment to
which is through a 6 membered ring, the heteroaryl groups having 1-4
heteroatoms selected from O, S(O)m and N,
said aryl and heteroaryl groups being optionally substituted
with 1-3 Ra groups. Within this subset of compounds, all other variables
are as originally defined.
More particularly, a subset of compounds that is of
particular interest is described with reference to formula I wherein A
represents an aryl group selected from phenyl and naphthyl, optionally
substituted with 1-3 Ra groups. Within this subset, all other variables
are as originally defined.
More particularly, another subset of compounds that is of
particular interest is described with reference to formula I wherein A
represents a 9-10 membered bicyclic heteroaryl group, attachment to
which is through a fi membered ring, the heteroaryl group having 1-4
heteroatoms selected from O, S(O)m and N,
said heteroaryl group being optionally substituted with 1-3
Ra groups. Within this subset of compounds, all other variables are as
originally defined. Examples of preferred values of A which are 9-10
membered bicyclic heteroaryl groups include the following:
_g_
CA 02328607 2000-09-27
WO 99/51225 PCTNS99/06767
N
I \ / \ / Nw
\ / w I / , \ I /
' N
N N
I ~ / \ / \
\ / , ,~ J \ I
N N ~ v ~N
/ / ~N i \ O
\ I ~N \ I ~ ~ /N
J , /
' N
H
\ N'
N
' / /
H
/ I /
\ ~ \ NH
, ~..... , \ N
H H
N
/ N ~ N
\ I y / i l/
H \
""" \
NH / N
/ I I ~ / N
\ \ N , \ I
' H ~ 'S
-10-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
N H
N
\ I ~ / ~ NH N/ I
' \N \ ' \ N~
H H
N H
~N N ~ N
\ I \~ Ni I N I /
H , ~N ,
NH
Ni I Ni I \~ / \
N N and
~ N H ~N S
More particularly, another subset of compounds that is of
particular interest is described with reference to formula I wherein A
represents a 5-6 membered isolated monocyclic heteroaryl group, having
1-3 heteroatoms selected from O, S(O)m and N, optionally substituted
with 1-3 Ra groups. Examples of preferred 5-6 membered isolated
monocyclic heteroaryl groups include pyrrole, imidazole, triazole,
pyridine, pyrimidine, pyrazine, furan, thiophene, oxazole and thiazole.
Another subset of compounds that is of particular interest is
described with reference to formula I wherein the moiety:
(Ra)o 4
X (Ra)o-2
has 1-4 Ra groups attached, said Ra groups being selected from the
group consisting of:
halo, -C1_12 alkyl(Rb)3, -NH2, -NHC1_g alkyl(Rb)g, -N(C1-6
alkyl(Rb)3)2, -N3, -OC1_g alkyl(Rb)g, -S(O)mH, -S(O)mCl_g alkyl(Rb)g,
-C(O)C1_g alkyl(Rb)3, -C02H, -C(O)OC1_g alkyl(Rb)3, -C(O)NH2,
-C(O)NHC1_s alkyl(Rb)g, -NHC(O)C1_g alkyl(Rb)g, -S(O)mNH2,
-NHS(O)mCl_g alkyl(Rb)g, -S(O)mNHCl_g alkyl(Rb)g and
-11-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
-S(O)mN(C1_g alkyl(Rb)3)2, and
each Rb is independently selected from: H, OH, halo,
-CF3, -OCF3, -N02, -N3, -OC1_g alkyl, -S(O)mCl_g alkyl, -NH2,
-NHC1_g alkyl, -N(C1_g alkyl)2, -C(O)C1_g alkyl, -C02H, -C02C1_g alkyl,
-C(O)NH2, -C(O)NHC1_g alkyl, -C(O)N(C1_g alkyl)2, -NHC(O)C1_g alkyl,
-S(O)mNH2, -S(O)mNHCl_g alkyl, -S(O)m(C1_g alkyl)2, aryl, heteroaryl
and heterocyclyl. Within this subset of compounds, all other variables
are as originally defined.
Another subset of compounds that is of particular interest
relates to compounds of formula I wherein A represents a phenyl ring,
unsubstituted or substituted with 1-3 Ra moieties selected from the group
consisting of
halo, -C1_12 alkyl(Rb)3, -NH2, -NHC1_g alkyl(Rb)3,
-N(C1_g alkyl(Rb)3)2, -N3, -OC1_g alkyl(Rb)3, -S(O)mH, -S(O)mCl_6
alkyl(Rb)3,-C(O)C1_g alkyl(Rb)3, -C02H, -C(O)OC1_g alkyl(Rb)3,
-C(O)NH2, -C(O)NHC1_g alkyl(Rb)3, -NHC(O)C1_6 alkyl(Rb)3,
-S(O)mNH2, -NHS(O)mCl_g alkyl(Rb)3, -S(O)mNHCl_g alkyl(Rb)3 and
-S(O)mN(C1_g alkyl(Rb)3)2~
and each Rb is independently selected from: H, OH, halo,
-CF3, -OCF3, -N02, -N3, -OC1_s alkyl, -S(O)mCl_s alkyl, -NH2,
-NHC1_g alkyl, -N(C1_g alkyl)2, -C(O)C1_g alkyl, -C02H, -C02C1_g alkyl,
-C(O)NH2, -C(O)NHC1_s alkyl, -C(O)N(C1_g alkyl)2, -NHC(O)C1_g alkyl,
-S(O)mNH2, -S(O)mNHCI_g alkyl, -S(O)m(C1_g alkyl)2, aryl, heteroaryl
and heterocyclyl. Within this subset of compounds, all other variables
are as originally defined.
More particularly, a subset of compounds that is of
particular interest relates to compounds of formula Ia:
-12-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
- ~Ra)o-3
~Ra)o a N (Ra)0.1
H
la
with Ra as originally defined.
Another subset of compounds that is of particular interest
relates to compounds of formula Ib:
I a
HO, ~ W ~~ ~J ~R )o-a
OH
O
~Ra)o- ~ Ra
( )o-~
Ib
with Ra as originally defined.
More preferably, the compounds of formula Ia and Ib above
are described wherein:
0-3 Ra groups are present in the molecule and are selected
from the group consisting of halo, -C1_12 alkyl(Rb)3, -NH2, -NHC1_6
alkyl(Rb)3, -N(C1_6 alkyl(Rb)3)2, -N3, -OC1_g alkyl(Rb)3, -S(O)mH,
-S(O)mCl_g alkyl(Rb)3,-C(O)C1_g alkyl(Rb)3, -C02H, -C(O)OC1_g
alkyl(Rb)3, -C(O)NH2, -C(O)NHC1_g alkyl(Rb)3, -NHC(O)C1_g alkyl(Rb)3,
-S(O)mNH2, -NHS(O)mCl_g alkyl(Rb)3, -S(O)mNHCI_g alkyl(Rb)3 and
-S(O)mN(C1_g alkyl(Rb)3)2~
and each Rb is independently selected from: H, OH, halo,
-CF3, -OCF3, -N02, -N3, -OC1_g alkyl, -S(O)mCl_g alkyl, -NH2,
-NHC1_g alkyl, -N(C1_g alkyl)2, -C(O)C1_g alkyl, -C02H, -C02C1_g alkyl,
-C(O)NH2, -C(O)NHC1_g alkyl, -C(O)N(C1_g alkyl)2, -NHC(O)C1_s alkyl,
-13-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
-S(O)mNH2, -S(O)mNHC1_g alkyl, -S(O)m(C1_g alkyl)2, aryl, heteroaryl
and heterocyclyl, and m is 0, 1 or 2.
Representative examples of the compounds of formula I are
shown below in Table I.
TABLE I
O
HO
~OH
~Ra)o.4 Y ~ O
X (Ra)o-z
I
(Ra)o 4 Y
X ~Ra)o-2 A
~ OCH3
H
CH3
Bn0
y
H
-14-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
I~
I~
N
H
I
H
Me0
I~
N
H
I~
ly
Me02C ~ N
H
I~
N I~
Bn
I~
F \ N/
H
-15-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
F , \
\
N /
H
\ H
Me
\
\ /'' Me
H /
\ N
Me
OCH3
N3 ~ I \ ~ /
\ N,
H
~ OCH3
OCH3
\ .~ OCH3
\
N /
H
-16-
CA 02328607 2000-09-27
WO 99/51225 PCTNS99/06767
OCH3
\ N ~ /
Me
F
\ I \~ I ~ OCFi3
H /
Throughout the instant
application, the
following
abbreviations are
used with the following
meanings:
Bu butyl
Bn benzyl
BOC, Boc t-butyloxycarbonyl
BOP Benzotriazol-1-yloxy tris/dimethylamino)-
phosphonium hexafluorophosphate
talc. calculated
CBZ, Cbz Benzyloxycarbonyl
CDI N,N'-carbonyl diimidazole
DCC Dicyclohexylcarbodiimide
DCM dichloromethane
DIEA diisopropylethylamine
DMF N,N-dimethylformamide
DMAP 4-Dimethylaminopyridine
DSC N,N'-disuccinimidyl carbonate
EDC 1-(3-dimethylaminopropyl)-3-ethylcarbodi-imide
hydrochloride
EI-MS Electron ion-mass spectroscopy
Et ethyl
EtOAc ethyl acetate
EtOH ethanol
eq. equivalent(s)
FAB-MS Fast atom bombardment-mass spectroscopy
-1'7 -
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
HOAc acetic acid
HOBT, HOBt Hydroxybenztriazole
HPLC High pressure liquid chromatography
KHMDS Potassium bis(trimethylsilyl)amide
LAH Lithium aluminum hydride
LHMDS Lithium bis(trimethylsilyl)amide
Me methyl
MeOH methanol
MF Molecular formula
MHz Megahertz
MPLC Medium pressure liquid chromatography
NMM N-Methylmorpholine
NMR Nuclear Magnetic Resonance
Ph phenyl
Pr propyl
prep. prepared
TFA Trifluoroacetic acid
THF Tetrahydrofuran
TLC Thin layer chromatography
TMS Trimethylsilane
Specific compounds may require the use of protecting
groups to enable their successful elaboration into the desired structure.
Protecting groups may be chosen with,e.g., reference to Greene, T.W.,
et al., Protective Grou,.ps in Orga i~ a Synthesis, John Wiley & Sons, Ine.,
1991. The blocking groups are readily removable, i.e., they can be
removed, if desired, by procedures which will not cause cleavage or
other disruption of the remaining portions of the molecule. Such
procedures include chemical and enzymatic hydrolysis, treatment with
chemical reducing or oxidizing agents under mild conditions, treatment
with fluoride ion, treatment with a transition metal catalyst and a
nucleophile, and catalytic hydrogenation.
Non-limiting examples of suitable hydroxyl protecting
groups are: trimethylsilyl, triethylsilyl, o-nitrobenzyloxycarbonyl, p-
-18-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
nitrobenzyloxycarbonyl, t-butyldiphenylsilyl, t-butyldimethylsilyl,
benzyloxycarbonyl, t-butyloxycarbonyl, 2,2,2-trichloroethyloxycarbonyl,
and allyloxycarbonyl. Non-limiting examples of suitable carboxyl
protecting groups are benzhydryl, o-nitrobenzyl, p-nitrobenzyI, 2-
naphthylmethyl, allyl, 2-chloroallyl, benzyl, 2,2,2-trichloroethyl,
trimethylsilyl, t-butyldimethylsilyl, t-butyldiphenylsilyl, 2-
(trimethylsilyl)ethyl, phenacyl, p-methoxybenzyl, acetonyl, p-
methoxyphenyl, 4-pyridylmethyl and t-butyl.
Salts encompassed within the term "pharmaceutically
acceptable salts" refer to non-toxic salts of the compounds of this
invention which are generally prepared by reacting the free base with a
suitable organic or inorganic acid. Representative salts and esters
include the following:
Acetate, Benzenesulfonate, Benzoate, Bicarbonate,
Bisulfate, Bitartrate, Borate, Camsylate, Carbonate, Citrate,
Dihydrochloride, Edetate, Edisylate, Estolate, Esylate, Fumarate,
Gluconate, Glutamate, Hydrobromide, Hydrochloride,
Hydroxynaphthoate, Lactate, Lactobionate, Laurate, Malate, Maleate,
Mandelate, Mesylate, Mucate, Napsylate, Nitrate, N-methylglucamine
ammonium salt, Oleate, Oxalate, Pamoate (Embonate), Palmitate,
Pantothenate, Phosphate/diphosphate, Polygalacturonate, Salicylate,
Stearate, Sulfate, Subacetate, Succinate, Tannate, Tartrate, Tosylate,
and Valerate.
The compounds of the present invention may contain one or
more asymmetric carbon atoms and may exist in racemic and optically
active forms. All of these compounds are contemplated to be within the
scope of the present invention. Therefore, where a compound is chiral,
the separate enantiomers, substantially free of the other, are included
within the scope of the invention; further included are all mixtures of
the two enantiomers. Also included within the scope of the invention are
polymorphs and hydrates of the compounds of the instant invention.
Asymmetric centers may be present in the compounds of
the instant invention depending upon the nature of the various
substituents on the molecule. Each such asymmetric center will
-19-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
independently produce two optical isomers and it is intended that all of
the possible optical isomers and diastereomers in mixture and as pure
or partially purified compounds are included within the ambit of this
invention. Tautomeric forms of the compounds of formula I are also
included herein. Tautomeric forms as used herein refer to structures
which differ by the shift of double bonds and concommitant displacement
of hydrogen atoms.
The present invention also provides a method for treating or
preventing the onset of diabetes mellitis in a mammalian patient which
comprises administering to said mammal a compound of formula I in
an amount which is effective for modulating insulin receptor tyrosine
kinase activity.
The present invention further provides a method for
reducing blood glucose levels in a mammalian patient in need thereof,
which comprises administering to said mammal a glucose reducing
effective amount of a compound of formula I or a pharmaceutically
acceptable salt, hydrate or tautomer thereof, in an amount which is
effective for modulating insulin receptor tyrosine kinase activity.
Yet another aspect of the present invention provides
pharmaceutical compositions containing a compound of formula I and a
pharmaceutically acceptable carrier.
The term "to modulate insulin receptor tyrosine kinase
activity" includes activating insulin receptor tyrosine kinase,
stimulating insulin receptor tyrosine phosphorylation, or enhancing the
effect of insulin to stimulate insulin receptor tyrosine kinase activity or
insulin signal transduction pathway. The ability of the compound to
modulate insulin receptor tyrosine activity may be determined using the
methods described herein. Briefly, Chinese Hamster Ovary (CHO) cells
expressing human insulin receptor are plated and treated with insulin
and/or test agents. CHO.T cells are one type of CHO cells that express
human insulin receptor. The treated cells are lysed, and the insulin
receptor is purified. The level of tyrosine phosphorylation of the receptor
is determined using an anti-phosphotyrosine antibody conjugated to
alkaline phosphatase and its chromogenic substrate. The insulin
-20-
CA 02328607 2000-09-27
WO 99/51225 PCTNS99/06767
receptor tyrosine kinase activity (IRTK) is determined using an
exogenous substrate and y 32P-ATP. Although the procedures described
in the Examples utilize CHO.T cells, cell lines similar to the CHO.T cells
described herein may be prepared by one skilled in the art. For example,
NIH3T3 cells, COS cells, Rat-1 cells and other appropriate fibroblasts
transfected with cDNA encoding human insulin receptor can also be
used in the assays.
The concept of altered levels of insulin, biological activity of
insulin, and levels of insulin sensitivity includes impaired insulin
production and/or activity, lower than normal levels of endogenous
insulin, resistance to normal or elevated level of insulin, which may be
due to insufficient insulin receptor expression, reduced insulin-binding
affinity, or any abnormality at any step along the insulin signaling
pathway.
The compounds of formula I modulate insulin receptor
tyrosine kinase activity and are thus useful in the treatment, prevention,
amelioration, suppression or control of diseases, disorders or conditions
that are characterized by altered insulin levels, biological activity of
insulin, insulin sensitivity, or a combination thereof. Such diseases or
disorders include diabetes mellitus (Type I and Type II), atherosclerosis,
hypertension, lipid disorders, obesity, polycystic ovarian syndrome, and
other conditions associated with insulin deficiency or insulin resistance.
These compounds are also useful in the treatment or prevention of
hyperglycemia or for controlling blood glucose levels in an animal
suffering from Type I or Type II diabetes mellitus.
Without being bound by a particular theory, it is believed
that the compounds stimulate insulin receptor tyrosine kinase activity.
In addition, these compounds stimulate tyrosine phosphorylation of
insulin receptor (3 subunit and insulin receptor substrate-1 as well as
activity of phosphoinositide-3-kinase. These compounds have the
properties of an insulin mimetic and insulin sensitizing agent.
-21-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
Dose Ra_~,es
The effective dosage of active ingredient employed may vary
depending on the particular compound employed, the mode of
administration, the condition being treated and the severity of the
condition being treated. Although the compounds may be administered
by any conventional mode of administration, including intravenous,
intramuscular, subcutaneous, oral, topical, etc.; oral administration is
preferred.
When treating or preventing diabetes mellitus and/or
hyperglycemia generally satisfactory results are obtained when the
compounds of the present invention are administered at a daily dosage of
from about 0.1 milligram to about 100 milligram per kilogram of animal
body weight, preferably given as a single daily dose or in divided doses
two to six times a day, or in sustained release form. For most large
mammals, the total daily dosage is from about 1.0 milligrams to about
1000 milligrams, preferably from about 1 milligrams to about 50
milligrams. In the case of a 70 kg adult human, the total daily dose will
generally be from about 7 milligrams to about 350 milligrams. This
dosage regimen may be adjusted to provide the optimal therapeutic
response.
Pharmaceutical Composition
Another aspect of the present invention provides
pharmaceutical compositions which comprise a compound of formula I
and a pharmaceutically acceptable carrier. The term "composition", as
in pharmaceutical composition, is intended to encompass a product
comprising the active ingredient(s), and the inert ingredients)
(pharmaceutically acceptable excipients) that make up the carrier, as
well as any product which results, directly or indirectly, from
combination, complexation or aggregation of any two or more of the
ingredients, or from dissociation of one or more of the ingredients, or
from other types of reactions or interactions of one or more of the
ingredients. Accordingly, the pharmaceutical compositions of the
present invention encompass any composition made by admixing a
-22-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
compound of formula I, additional active ingredients) and
pharmaceutically acceptable excipients.
The pharmaceutical compositions of the present invention
comprise a compound of formula I as an active ingredient, and may also
contain a pharmaceutically acceptable carrier and optionally other
therapeutic ingredients. The compositions include compositions
suitable for oral, rectal, topical, and parenteral (including
subcutaneous, intramuscular, and intravenous) administrations,
although the most suitable route in any given case will depend on the
particular host, and nature and severity of the conditions for which the
active ingredient is being administered. The pharmaceutical
compositions may be conveniently presented in unit dosage form and
prepared by any of the methods well-known in the art of pharmacy.
In practical use, a compound of the invention can be
combined with a pharmaceutical carrier according to conventional
pharmaceutical compounding techniques. The carrier may take a wide
variety of forms depending on the form of preparation desired for
administration, e.g., oral or parenteral (including intravenous).
In preparing the compositions for oral dosage form, any of
the usual pharmaceutical media may be employed. For example, in the
case of oral liquid preparations such as suspensions, elixirs and
solutions, water, glycols, oils, alcohols, flavoring agents, preservatives,
coloring agents and the like may be used; or in the case of oral solid
preparations such as powders, capsules and tablets, carriers such as
starches, sugars, microcrystalline cellulose, diluents, granulating
agents, lubricants, binders, disintegrating agents, and the like may be
included. Because of their ease of administration, tablets and capsules
represent the most advantageous oral dosage unit form in which case
solid pharmaceutical carriers are obviously employed. If desired, tablets
may be coated by standard aqueous or nonaqueous techniques. In
addition to the common dosage forms set out above, the active ingredient
may also be administered by controlled release means and/or delivery
devices.
-23-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
Pharmaceutical compositions of the present invention
suitable for oral administration may be presented as discrete units such
as capsules, cachets or tablets each containing a predetermined amount
of the active ingredient, as a powder or granules or as a solution or a
suspension in an aqueous liquid, a non-aqueous liquid, an oil-in-water
emulsion or a water-in-oil liquid emulsion. Such compositions may be
prepared by any conventional method. In general, the compositions are
prepared by admixing the active ingredient with a liquid or finely divided
solid or both, and then, if necessary, shaping the product into the desired
preparation. For example, a tablet may be prepared by compression or
molding, optionally with one or more accessory ingredients.
Compressed tablets may be prepared by compressing, in a suitable
machine, the active ingredient in a free-flowing form such as powder or
granules, optionally mixed with a binder, lubricant, inert diluent,
surface active or dispersing agent. Molded tablets may be made by
molding in a suitable machine, a mixture of the powdered compound
moistened with an inert liquid diluent. Desirably, each tablet contains
from about 1 mg to about 500 mg of the active ingredient and each cachet
or capsule contains from about 1 to about 500 mg of the active ingredient.
Pharmaceutical compositions of the present invention
suitable for parenteral administration may be prepared as solutions or
suspensions of these active compounds in water suitably mixed with a
surfactant such as hydroxypropylcellulose. Dispersions can also be
prepared in glycerol, liquid polyethylene glycols, and mixtures thereof in
oils. Under ordinary conditions of storage and use, these preparations
contain a preservative to prevent the growth of microorganisms.
The pharmaceutical forms suitable for injectable use
include sterile aqueous solutions or dispersions and sterile powders for
the extemporaneous preparation of sterile injectable solutions or
dispersions. In all cases, the form must be sterile and must be fluid to
the extent that easy syringability exists. It must be stable under the
conditions of manufacture and storage and must be preserved against
the contaminating action of microorganisms such as bacteria and fungi.
The carrier can be a solvent or dispersion medium containing, for
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
example, water, ethanol, polyol (e.g. glycerol, propylene glycol and liquid
polyethylene glycol), suitable mixtures thereof, and vegetable oils.
Suitable topical formulations include transdermal devices,
aerosols, creams, ointments, lotions, dusting powders, and the like.
These formulations may be prepared via conventional methods
containing the active ingredient. To illustrate, a cream or ointment is
prepared by mixing sufficient quantities of hydrophilic material and
water, containing from about 0.5-90% by weight of the compound, in
sufficient quantities to produce a cream or ointment having the desired
consistency.
Pharmaceutical compositions suitable for rectal
administration wherein the carrier is a solid are most preferably
presented as unit dose suppositories. Suitable carriers include cocoa
butter and other materials commonly used in the art, and the
suppositories may be conveniently formed by admixture of the
combination with the softened or melted carriers) followed by chilling
and shaping moulds.
It should be understood that in addition to the
aforementioned carrier ingredients the pharmaceutical formulations
described above may include, as appropriate, one or more additional
carrier ingredients such as diluents, buffers, flavoring agents, binders,
surface-active agents, thickeners, lubricants, preservatives (including
anti-oxidants) and the like, and substances included for the purpose of
rendering the formulation isotonic with the blood of the intended
recipient.
Combination Therapy
The compounds of the present invention may be used in
combination with other drugs. Such other drugs may be administered,
by a route and in an amount commonly used, contemporaneously or
sequentially. When a compound of formula I is used
contemporaneously with one or more other drugs, a pharmaceutical
composition containing such other drugs in addition to the compound of
the invention is preferred. Accordingly, the pharmaceutical
-25-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
compositions of the present invention include those that also contain one
or more other active ingredients, in addition to a compound of formula I.
Examples of other active ingredients that are administered separately or
in the same pharmaceutical compositions, include, but are not limited to
antidiabetic agents such as insulin, sulfonylureas, biguanides (such as
metformin) a-glucosidase inhibitors (such as acarbose), and peroxisome
proliferator-activater receptor Y agonists such as the glitazones
(thiazolidinediones such as pioglitazone, troglitazone, MCC-555, and
BRL49653); cholesterol lowering agents such as HMG-CoA reductase
inhibitors (lovastatin, simvastatin, pravastatin, fluvastatin, atorvastatin
and others), sequestrants (cholestyramine, colestipol and
dialkylaminoalkyl derivatives of a cross-linked dextran), nicotinyl
alcohol nicotinic acid or a salt thereof, proliferator-activater receptor a
agonists such as fenofibric acid derivatives (gemfibrozil, clofibrate,
fenofibrate and benzafibrate), and probucol.
The compounds of Formula I of the present invention can be
prepared according to the following schemes, or using routine
modifications thereof. The definitions of the variables are as originally
described unless otherwise stated.
ch a 1
O O O
A
~A ~~- ~A
HO CI OH
1 2
O O
A
R02CCOC1 amine base
O~C02R' O
~OH
O O
4 5
Intermediates of Formula 5 can be synthesized according to
Scheme 1. Acid chlorides 2 are commercially available or can be
-26-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
prepared from the corresponding acids 1 using oxalyl chloride or thionyl
chloride under standard reaction conditions. Transformation of acid
chlorides 2 to a-hydroxy ketones 3 can be carried out using the method
described in J. Org. Chem., 44, 4617-4622 ( 1979).
Introduction of the oxalate group can be achieved using
alkyl oxalyl chloride, such as ethyl oxalyl chloride. Ring closure can be
effected by DBU or its equivalent to afford intermediate 5, which can be
used for the synthesis of the compounds of Formula I as shown in
Scheme 3.
Scheme 2
OH CI
O
R Y O --.~. Rao-4 Y ~ R --
a0 4 ~ a0-2
Rao 2
6
O
OH ROCO J'''p
ROCOCOCI
O ~ O
Rao_a Y ~ Rao_2 Rao_a Y ~' Rao_2
g 9
HO
amine base ~ O
R~ 4 ~Y )--..~~Rao 2
15 As shown in the Scheme 2, the intermediates of Formula 10
can be prepared similarly to intermediates of Formula 5, starting from
acids 6.
-27-
CA 02328607 2000-09-27
WO 99/51225 PCT/iJS99/06767
Scheme 3 ,
H+
A
O
OH CHO Rao_
O Rao~ , .~o z
Rao-4 Y
11 12
alkoxide base
Rao-
Intermediates of Formula 5 can be coupled to
intermediates of Formula 11 to afford compounds of Formula 12
under acidic conditions (see Liebigs Ann. Chem. 177-194 (1986)). The
aldehydes of Formula 11 are commercially available, known in the
literature or can be prepared following literature methods.
Rearrangement of compounds 12 to the products of Formula I can be
effected using an alkoxide base such as sodium methoxide and
sodium ethoxide.
-28-
CA 02328607 2000-09-27
WO 99/51225 PCTNS99/06767
O heme O
HO HO / A
I o H+ I o
R~-4 Y ~ A-CHO Rao 4 Y O
Rao-2 13 Rao-2
14
alkoxide base
Z( OH
Rao_a Y ~~ O
Rao-2
5 t
Alternatively, compounds of Formula I can be prepared
starting from intermediates of Formula 10 and aldehydes of Formula 13.
Aldehyde 13 is commercially available, known in the literature or can be
prepared following literature methods described for analogous
10 compounds. Condensation of compounds 10 and 13 leads to products of
Formula 14, which can be rearranged using alkoxide bases.
Compounds of formula I can be prepared using the
synthetic route depicted in Scheme 3 or 4.
The invention can be further illustrated in connection with
the following non-limiting examples. All temperature are degrees
Celsius unless noted otherwise.
-29-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
PREPARATIVE EXAMPLE 1
O
Step A:
OH
To a mixture of phenylacetyl chloride (3.1 g, 20 mmol) and
tris(trimethylsilyloxy)ethylene (13.5 g, 44 mmol) at room temperature
was added three drops of neat SnCl4 via syringe. The reaction mixture
was stirred for 3 h before it was poured into a mixture of dioxane (25
mL) and 0.6 N HCl aqueous solution (10 mL). The mixture was stirred
at 90 °C for 10 min, cooled to room temperature, and extracted twice
with
Et20. The combined organic layers were washed with saturated solution
of NaHC03 and brine, dried over anhydrous MgS04, filtered, and
concentrated in vacuo. The residue was crystallized from hexanes to
give 2.47 g of the desired product as a white solid.
1H NMR (CDC13, 400 MHz) 8 ?.35..7.15 (m, 5H, CBHs), 4.26 (d,
2H, CH20), 3.70 (s, 2H, CHZCO), 3.00 (t, 1H, OH).
-30-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
Step B:
O
O~C02Et
'IO
To a solution of the intermediate from the previous step (2.47
g, 16.4 mmol) in THF (120 mL) at 0 °C was added Et3N (2.7 mL, 19 mmol),
followed by ethyl oxalyl chloride ( 1.9 mL, 17 mmol). The mixture was
stirred at 0 °C for 3 h, poured into EtOAc (200 mL), washed with water
and brine, dried over anhydrous MgS04, filtered, and concentrated in
vacuo to give 3.9 g of the crude product as a slightly yellow oil, which was
used in the next step without further purification.
1H NMR (CDCl3, 400 MHz) 8 7.35-7.15 (m, 5H, CsHs), 4.85 (s,
2H, CH20), 4.37 (q, 2H, COOCH2), 3.77 (s, 2H, PhCH2C0), 1.38 (t, 3H,
CHI).
S~p,~
O
O
~OH
O
To a solution of DBU (4.9 mL, 32.8 mL) in DMF (16 mL) at -
20 °C was added dropwise a solution of the crude intermediate from the
previous step (3.9 g, 16 mmol) in DMF (16 mL). The reaction mixture
was stirred at -15 °C for 2.5 h before it was poured slowly into an ice-
cold
1.0 N HCl solution (100 mL). The crystalline product (1.85 g) was
collected by filtration, washed thoroughly with water, and dried under
high vacuum. The mother liquid was extracted with EtOAc. The
extract was washed with water and brine, dried over MgS04, filtered and
concentrated. The residue was purified by recrystallization from
-31-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
CH2C12/hexanes to give another 0.57 g of the product as slightly yellow
solid. The total yield was 2.42 g (two steps).
1H NMR (Acetone-ds, 400 MHz) 8 7.50-7.30 (m, 5H, CsH5),
5.I1 (s, 2H, OCH2).
PREPARATIVE EXAMPLE 2
O / OMe
O
~OH
O
Using the procedure set forth in Preparative Example I,
and substituting 4-methoxyphenylacetyl chloride for phenylacetyl
chloride, the target compound was prepared.
'H NMR (Acetone-ds, 400 MHz) s 7.47 (d, J = 9.0 Hz, 2H),
6.95 (d, J = 9.0 Hz, 2H), 5.08 (s, 2H, OCH2C0), 3.82 (s, 3H, OCH3).
CI-MS calc. for Cl2HOb (M + H): 235; Found: 235.
-32-
CA 02328607 2000-09-27
WO 99/51225 PCTNS99/06767
PREPARATIVE EXAMPLE 3
H
m
O
Step A: indole-3-acetxl chloride:
H
N
O ~ ~ \
C~
To a suspension of indole-3-acetic acid (1.0 g, 5.7 mmol) in
CHZC12 at 0 °C was added DMF (20 p,L), followed by oxalyl chloride
(2.5 g,
20 mmol). The reaction mixture was stirred at 0 °C for 1.5 h. The
solvent was removed in vacuo to give 1.25 g of the crude product, which
was used in the next step without further purification.
Step B:
H
N
O ~ ~ \
O
-OH
O
Prepared as described in Preparative Example 1 starting
from indole-3-acetyl chloride obtained in the previous step.
1H NMR (Acetone-ds, 400 MHz) 8 7.75 (s, 1H), 7.58 (d, 1H),
7.43 (d, 1H), 7.12 (t, 1H), 7.03 (t, 1H), 5.15 (s, 2H).
-33-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
,REPARATIVE EXAMPLE 4
Using the procedure set forth in Preparative Example 1,
and substituting benzo[b]thiophene-3-acetyl chloride for phenylacetyl
chloride, the target compound was prepared.
1H NMR (CDC13, 400 MHz) 8 7.92 (m, 1H), 7.70 (s, 1H),
?.52 (m, 1H), 7.40 (m, overlapping signals, 2H), 5.20 (s, 2H).
-34-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
EXAMPLE 1
HO,
~OH
N O
H
Step A:
o
N ~OH
H O
A mixture of the compound of Preparative Example 1 (560
mg, 2.74 mmol) and indole-3-carboxaldehyde (435 mg, 3.0 mmol) in
acetic acid (?:5 mL) was heated at 60 °C until a clear solution was
formed. To this solution was added 4 drops of concentrated HCl. The
resultant reddish solution was heated at 90 °C for 3 h. After cooling
to
room temperature, the reaction was diluted with a 1:1 mixture of
ether/hexanes (10 mL), then stirred at 0 °C for 10 min. The reddish
crystalline product (890 mg) was collected by filtration.
1H NMR (Acetone-ds, 400 MHz) 8 8.27 (d, 1H), ?.98 (m, 1H),
7.56 (m, 3H), 7.52 (s, 1H), 7.44 (m, 2H), 7.38 (tt, 1H), 7.26 (ddd, 1H), 7.23
(ddd, 1H).
-35-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
Step B:
HO\T'
I
OH
NJ O
H
To a suspension of the intermediate from step A (710 mg,
2.14 mmol) in methanol at room temperature was added a solution of
NaOMe in MeOH (25 wt%, 20 mL). The mixture was stirred for 2.5 h
before it was poured slowly into an ice-cold 1.0 N HCl solution (120 mL).
The precipitate was collected by filtration, washed thoroughly with
water, and dried under high vacuum. Recrystallization from
THF/hexanes gave 580 mg of the product as a greenish solid.
1H NMR (Acetone-ds, 400 MHz) 8 7.65 (d, 1H), 7.59 (m, 1H),
7.55 (m, 2H), 7.43 (m, 3H), 7.35 (tt, 1H), 7.13 (ddd, 1H), ?.04 (ddd, 1H).
EXAMPLE 2
a
IV
Me
Using the procedure set forth in Example 1, and starting
from the compound of preparative example 2 and 1-methylindole-3-
carboxaldehyde the target compound was prepared.
-36-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
1H NMR (Acetone-ds, 400 MHz) S 7.58 (dt, 1H), 7.54 (s, 1H),
7.51 (d, 2H), 7.42 (dt, 1H), 7.19 (ddd, 1H), 7.05 (ddd, 1H), 6.98 (d, 2H),
3.91
(s, 3H), 3.83 (s, 3H).
EXAMPLE 3
OMe
HO ~ OMe
-OH
N O
H
Step A:
H
Me0
The compound of preparative example 3 (9.4 mg, 0.039
mmol) and 3,4-dimethoxybenzaldehyde (16 mg, 0.1 mmol) were dissolved
in acetic acid (1.0 mL) at 60 °C and 1 drop of concentrated HCl was
added. The reaction mixture was warmed to 90 °C where it was stirred
for 3 h. After cooling to room temperature, the reaction was partitioned
between EtOAc (20 mL) and water (10 mL). The organic layer was dried
over anhydrous MgS04, filtered and concentrated in vacuo. The residue
was purified by preparative TLC plates (Si02, EtOAc) to give 6.0 mg of the
product.
1H NMR (Acetone-ds, 400 MHz) 8 7.83 (d, 1H), 7.62 (m, 1H),
7.58 (m, 1H), 7.44 (t, 1H), 7.16-7.00 (m, 4H), 3.80 (s, 6H).
-37-
OMe O
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06?67
to
OMe
HO ~ OMe
~OH
N
H
To a solution of the intermediate obtained from the previous
step (5.5 mg) in methanol (1.0 mL) at room temperature was added a
solution of NaOMe in methanol (25 wt%, 1.0 mL). After 3 h, the reaction
mixture was poured into 1.0 N HCl solution (10 mL), extracted with
EtOAc (15 mL). The extract was washed with water and brine, dried
over anhydrous MgS04, filtered, and concentrated in vacuo. The residue
was purified by HPLC to give 2.6 mg of the product.
1H NMR (CDC13/Acetone-ds, 400 MHz) b 7.50 (d, J = 2.8 Hz,
1H), 7.46 (d, J = 8.0 Hz, 1H), 7.27 (d, J = 8.1 Hz, 1H), 7.08-6.95 (m, 4H),
6.81
(d, J = 8.4 Hz, 1H), 3.78 (s, 3H), 3.76 (s, 3H).
EXAMPLE 4
Me
Using the procedure set forth in Example 1, and starting
from the compound of Preparative Example 1 and 1-methylindole-3-
carboxaldehyde, the target compound was prepared.
-38-
CA 02328607 2000-09-27
WO 99/51225 PCTNS99/06767
1H NMR (Acetone-ds, 400 MHz) 8 7.6 - 7.5 (m, overlapping
signals, 4H), 7.43 (m, overlapping signals, 3H), 7.36 (m, 1H), 7.19 (t,
1H), ?.06 (t, 1H), 3.91 (s, 3H).
EXAMPLE 5
Using the procedure set forth in Example 1, and starting
from the compound of Preparative Example 4 and 8-
quinolinecarboxaldehyde, the target compound was prepared.
1H NMR (DMSO-ds, 400 MHz) S 8.89 (d, J = 1.5 Hz, 1H), 8.53
(d, J = ?.8 Hz, 1H), 8.05 (d, J = 7.8 Hz, 1H), 8.00 (m, 1H), ?.52 (m,
overlapping signals, 3H), 7.60 (m, overlapping signals, 2H), 7.36 (m,
overlapping signals, 2H).
The procedures described in the examples noted above were
used to prepare the compounds shown in Table I, using modified
starting materials.
Utility for the compounds of formula I is demonstrated
using the following assays.
Cell-based assav for insulin receptor ~y~g~,~gg~rvlation
CHO.T cells, which overexpress human insulin receptor
are cultured in Hams F12 medium supplemented with 10% fetal calf
serum, fungizone, penicillin and streptomycin at approximately 1.5 x
105 cells/well. The 96-well plates are incubated for approximately 24 h at
37°C, which is when the cells reached confluency. The cells are washed
-39-
CA 02328607 2000-09-27
WO 99/51225 PCTNS99/06767
with phosphate buffered saline (PBS) three times and then incubated in
serum-free medium for 3 h at 37°C. Insulin and/or test compounds are
added to the wells, and the cells are incubated for an additional 20 min at
37°C. The cells are washed three times with PBS and lysates are
prepared. The lysates are transferred to a second 96 well plate. The
wells of the second plate are precoated with monoclonal anti-insulin
receptor antibody. Antibody is diluted to a final concentration of
approximately 4 mcg/mL in 20 mM NaHC03, pH 9.6. Approximately 50
mcL of diluted antibody solution is added to each well. The lysates are
incubated for 16 h at 4°C to immunopurify the insulin receptor.
To detect the level of tyrosine phosphorylation of the insulin
receptor captured on the plates, the washed plates are incubated for 5 h
at 4°C with monoclonal antiphosphotyrosine antibody conjugated to
alkaline phosphatase (Transduction Laboratories). The unbound
antibody is removed and chromogenic substrate of alkaline phosphotase
is added to the wells. Signals are detected at 405 nm with a microtiter
plate reader.
The cell culture conditions, preparation of lysates, and
assays are essentially those described in B. Zhang et al., J. Biol. Chem.,
Vol. 266, pages 990-996 (1991) and Zhang and Roth, J. Biol. Chem., Vol.
267, pages 18320-18328, (1992).
Cell-based assay for insulin rec r tyrosine kinase activity
CHO.T cells (approximately 1.5 x 105 cells/well) were
cultured in Hams F12 medium supplemented with 10% fetal calf serum,
fungizone, penicillin and streptomycin. The 96-well plates are incubated
for approximately 24 h at 37°C, which is when the cells reached
confluency. The cells are washed with phosphate buffered saline (PBS)
three times and then incubated in serum-free medium for 3 h at 37°C.
Insulin and/or test compounds are added to the wells, and the cells are
incubated for an additional 20 min at 3?°C. The cells are washed three
times with PBS and lysates are prepared. The lysates are transferred to
a second 96 well plate. The wells of the second plate are precoated with
monoclonal anti-insulin receptor antibody. Antibody is diluted to a final
-40-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
concentration of approximately 4 mcg/mL in 20 mM NaHC03, pH 9.6.
Approximately 150 mcL of diluted antibody solution is added to each
well. The lysates are incubated for 16 h at 4°C to immunopurify the
insulin receptor.
To determine the insulin receptor tyrosine kinase activity,
twenty microliters of the kinase reaction mixture (50 mM Hepes, pH 7.6,
150 mM NaCl, 5 mM MgCl2, 5 mM MnCl2, 0.1% Triton X-100, 1 mg/ml
poly(GIu:Tyr}(4:1), 2 m Ci of carrier-free [g-32P]ATP) is added to each
well of the 96-well plates and the .incubation is continued at 25°C for
40
min. The reaction is terminated by addition of 50 ml 100 mM phosphoric
acid. The mixture is transferred to Multiscreen PH plates and washed.
The radioactivities associated with the wells are determined using a
Topcount. The insulin receptor tyrosine kinase activities stimulated by
test agents are compared to that stimulated by insulin.
In vitro assay for insulin rece tp or tyrosine kinase acti~~~itv
A glutathione S-transferase fusion protein containing
intracellular domain of the insulin receptor (GST-IRTK) was expressed
in Baculovirus and affinity purified using glutathione-conjugated
sepharose. To activated the insulin receptor tyrosine kinase, an aliquot
of the GST-IRTK (200 nM final concentration) was incubated at 25 C for
15 min in a buffer containing 50 mM Tris-HCl (pH 7.4), 8 mM MgCl2,
and varying concentrations of ATP (from 1 ~.M to 1 mM) in the absence
or presence of test compounds. A substrate protein (histone H2B) (0.35
~,g/~.1 final concentration) was then added and the incubation was
continued at 25 C for 15 min and terminated by the addition of 50 mM
EDTA. The reaction mixtures were separated by SDS-PAGE followed by
immunoblotting. The blots were probed with a monoclonal anti-
phosphotyrosine antibody and developed using the ECF reagents. The
level of tyrosine phosphorylation of GST-IRTK and histone H2B was
determined using image analyses. Alternatively, following activation of
GST-IRTK with ATP in the presence or absence of test compounds,
histone H2B and 32-'y ATP were added to the reaction mixtures. The
samples were analyzed by SDS-PAGE followed by autoradiography.
-41-
CA 02328607 2000-09-27
WO 99/51225 PCT/US99/06767
In vivo assay for oral anti-hvper~lvcemic activity
Genetically altered obese diabetic mice (db/db) (male, 7-9 weeks
old) are housed (7-9 mice/cage) under standard laboratory conditions at
22°C and 50% relative humidity, and maintained on a diet of Purina
rodent chow and water czd libitum. Prior to treatment, blood is collected
from the tail vein of each animal and blood glucose concentrations are
determined using One Touch BasicGlucose Monitor System (Lifescan).
Mice that have plasma glucose levels between 250 to 500 mg/dl are used.
Each treatment group consists of seven mice that are distributed so that
the mean glucose levels are equivalent in each group at the start of the
study. db/db mice are dosed orally by gavage with either vehicle
(containing 0.5% methylcellulose ) or test compound from 0.2 to 30
mg/kg in a volume of 10 ml/kg. Blood is sampled from the tail vein
hourly for 4 hours and at 24, 30 h post-dosing and analyzed for blood
glucose concentrations. Food is withdrawn from 0-4 h post dosing and
reintroduced thereafter. Individual body weights and mean food
consumption (each cage) are also measured after 24 h. Significant
differences between groups (comparing drug-treated to vehicle-treated)
are evaluated using Student t-test.
While certain preferred embodiments are described in
detail, numerous alternative embodiments are contemplated as falling
within the invention. Consequently, the claims are not to be limited to
the specific teachings herein.
-42-