Note: Descriptions are shown in the official language in which they were submitted.
CA 02328867 2000-10-13
w0 99153993 PCT/US99~083aa
5 Technical Field
The present invention relates generally to medical devices and in
particular to implantable endocardial catheters for use with medical devices.
Background o~[~e Invention
Ventricular fibrillation of the heart is characterized by fine, rapid,
10 fibrillatory movements of the ventricular muscle that replace the normal
cardiac
contraction. Since very little pumping action occurs during ventricular
fibrillation, the situation is fatal unless quickly corrected by cardiac
conversion.
During conversion, defibrillation level electrical energy is applied to the
heart in
an attempt to depolarize the myocardial tissue of the heart and allow a normal
15 sinus rhythm to be reestablished.
One theory that has been proposed to explain the mechanism of
conversion by the application of defibrillation electrical current is the
critical
mass hypothesis. The critical mass hypothesis suggests that it is not
necessary to
halt all fibrillation activity in order to have defibrillation occur, but that
it is
20 sufficient to halt only a "critical mass" (perhaps 75%) of the myocardium
in the
ventricles. In this theory, the assumption is made that if all fibrillation
activity is
localized to a region smaller than the critical mass of myocardium, the
remaining
fibrillation activity is not capable of maintaining fibrillation and will die
out after
one or two cycles, resulting in normal sinus rhythm.
25 Impiantable cardioverter/defibrillators (ICDs) have been
successfully used to treat patients who have experienced one or more
documented episodes of hemodynamically significant ventricular tachycardia or
ventricular fibrillation. The basic ICD consists of a primary battery,
electronic
circuitry to control both the sensing of the patient's cardiac signals and the
30 delivery of electrical shocks to the patient's heart, and a high-voltage
capacitor
bank housed within a hermetically sealed titanium case. One or more catheter
leads having defibrillation electrodes are implanted within the heart of the
patient or on the epicardial surface of the patient's heart. The catheter
leads are
kC' ~'Oi~l : EPrl-~1~~E'VCHI~!V 0'~ : 1.5- 5- ~'~' 02328867 2000-10-13 ~'~C ~
~~~~~ EChI-i +4~ ~~ ~;j~;j$.il.ti~ : r'F1 1
~i ~-05-2000 US 009908344
2
then coupled to the implantabl~ housing and the electronic circuitn~ of the
ICD
and are used to deliver defibrillation level electrical energy io the heart.
U_S.
fztent numbers 5,683,447, 5,534,022 and 4,497,326; and European Patent EP 0
$12 886 arc examples of some catheter leads.
It has been suggested that a mi,nnmum and even ( i_e., similar in
all parts of the v;.n'~ricles) potential gradient generated by a
def~brillativn. level
shock is necessary Cor eff~tive cardiac defibrillation. TI~zS potential
gradient is
afi:ected; and thus determined, by the voltage of the shock and the electrode
configurarion employed. It has also bee~o suggested that a maximum potential
gradient also exists that, beyond this value, deletezious electrophysiological
and
mechanical effects may c~ecur, such as new arrlxythmias, myocardial necrosis,
or
contractile dysfunction. Therefore, how and wh~:re defibrillation electrode;
are
placed on andlor within the heart has a major effect on whether or not ~
critical
mass of cardiac tissue is captured during a defiurillation attempt.
Endocardial de~F.ibrillation catheters; those not requiring a
thoracotomy to be place on the heart, have a maJor advantage over the
epicardial
lead ,ystezns by red'acing the morbidity, ntortality, and cast of tlioracotomy
procedures. However, a major problem with these systems is the potential far
high defibrillation thresholds as compared to system employing epicardial
ZO defibrillation electrodes. Changes to the waveform ofthe defibrillation
shack
and t:o the combinations of endocardiat leads implanted into a patient and the
cr~-rent pathways used can result in efficacious deFbrillatian therapy being
delivered to the patient.
~'he easiest and most conrenient ~uvay to perform the implantaf~on
of a fully trausvenous system is to use only one endocardial lead with bcth
sensing and pacing and defibrillation capabilities. One such endocardial lead
is
sold under the tra.desnark El'~TDOTA~ C (cardiac Pacemaker, Inc.l Guidant
Co:poration, St_ Paul,1~, which is a tripolar, tined, end~ocardiai lead
featuring
a porous tip elecfirode (placed in the apex of the right ventricular) that
serves as
t_he cathode for intracardiac ri ,,,~ht ventricular electrogram rate sensing
and pacing,
and two defibrillation coil eiech'odes, with the distal one serving as the
anode for
rate sensing and as the cathode For morphology s~r~sing and defibrillation
which
AMENDED SHEET
CV. t:ON:EPA-btl.'EtJCi-(EN <)'~ : lu- 5- O ~ 02328867 2000-10-13 CCf1'T EC~i-
~ X49 89 23994465:#12
15-05-2000 U S 009908344
2a
the proximal coil electrode positioned wittfifo. the superior via cava
functions as
the anode for deh3rillation.
AMENDED SHEET
?C1%. VU!\=EPA-A9U)=..~CHE1 U? : 1J- 5- U ' ~'~' ~'~ ' CC:I rT ECV1-. +4:~ tip
'W3~J~44'vS: ,~ l
CA 02328867 2000-10-13
15-05-2000 US 009908344
3
Hov~~cver, single body cndocardial leads used for both
defibrillation and rate smsing have been reported to suffer technical
inadequacies that xnay pose significant risks to the patient_ Endoca.Tdial
el~ctrogt~.ms obtained from integrated senselpace-defibrillation leads haws
bean
shown to be affected after shock delivery, with their amplitude decreasing to
such a significant degree that arrhythmia redetectiort is dangerously
compromised. As already meni~ioned 'hove, obtaining adequate defibrillation
threslaoids has been a major problem v;~th the nonthoracotomy eadocardial lead
systems. Therefore, a need exists to design an endocardial lead system that
effectively reduces defibrillation thresholds and allow for reliable post-
defibri Ilation shock sensing and pacing.
~ti~n
The present invention provides a single body endocardial lead
that reduces defibrillation thresholds anal improves post-defibrillation shock
therapy redetection. One aspect of these improvements is the placement of the
elec~od~;s on the endocardial lead. '.fhe electFOde configuration on the
cndecardial lead improves the potential gradient generated by a defibriLation
Ievcl shoel~, u;hieh increases thz effectiveness of the cardiac defibrillation
shock
and reduces the deF.brillation threshold as compared to conventional
endocardial
leads. Also, the position of the pacing electrode relative to the
defibrillation
electrodes provides for a more reliable and accurate post-defibrillation shock
electrogam. Furthermore, the reduction in dcfbrillation thresholds allows far
reduced battery oonsumptivn of the implantable device, potential ly
prUlvngiz~g
fee life of the device and/or allowing for an overall reduction in the size of
the
dez~ice.
The eudocardial lc~d oFthe present invention has an elongate
body with a peripheral surface, a proxirnai end, a distal end, and a first
defibrillation coil electrode and a first pacinglsensing electrode on the
peripheral
surface. T"na first defibrillation coil electrode is pasztioned on the
endocardial
lead at or near the distal end of the elongate body. The first pacinglsensing
electrode is spaced longitudinally aloag the peripheral surT~ace from the
first
defibrillation coil electrode to afford positioning both the first
dc~orillation coil
and the first paoinflsensing electrode in a right vexrtricle of a heaat. hx
one
AMENDED SHEET
CA 02328867 2000-10-13
WO 99/53993 PCT/US99/083s4
4
embodiment, the endocardial lead is positioned within the right ventricle of
the
heart with the first defibrillation coil electrode positioned longitudinally
adjacent
the right ventricular septal wall. In an additional embodiment, the
endocardial
lead is positioned within the right ventricle of the heart with the first
5 defibrillation coil electrode positioned directly within the ventricular
apex, where
the first defibrillation coil is longitudinally adjacent to the apex of the
right
ventricle of the heart.
In an additional embodiment of the invention, the endocardial
lead further includes a second defibrillation coil electrode on the peripheral
10 surface. The second defibrillation coil electrode is spaced longitudinally
along
the peripheral surface from the first pacing/sensing electrode to afford
positioning the first defibrillation coil and the first pacing/sensing
electrode
within the right ventricle and the second defibrillation coil within the
supraventricular region of the heart. In one embodiment, the second
15 defibrillation coil electrode is positioned within a right atrial chamber
or a major
vein leading to the right atrial chamber of the heart.
Different types and configurations of first pacingisensing
electrodes can be used with the endocardial lead of the present invention. In
one
embodiment, the first pacing/sensing electrode includes a retaining element
20 integrated into or positioned adjacent the first pacingisensing electrode.
The
retaining element is adapted to be embedded in the tissue of the right
ventricle of
the heart to secure the first pacing/sensing electrode, and the elongate body
of
the endocardial lead, to the right ventricle of the heart. In one embodiment,
the
retaining element is a helical wire which used to secure the first
pacing/sensing
25 electrode to the cardiac tissue of the ventricular septum.
In an additional embodiment, the peripheral surface of the
elongate body defines an electrode housing having an opening, the housing
being
adapted to sheathe the first pacingisensing electrode and the retaining
element
and through which the first pacing/sensing electrode and/or the retaining
element
30 extends from the peripheral surface to engage the right ventricular chamber
of
the heart. A stylet lumen extends through the elongate body of the endocardial
lead to the first pacing/sensing electrode and is adapted to receive a stylet
that is
used for extending and rotating the first pacing/sensing electrode and the
CA 02328867 2000-10-13
WO 99/53993 PCT/US99/083.1d
retaining element to embed the retention element of the first pacing/sensing
electrode into the right ventricle of the heart.
In an alternative embodiment, the elongate body of the
endocardial lead further has a curved portion spaced between the proximal end
5 and the distal end. The curved portion has an outer radial surface and an
inner
radial surface, where the outer radial surface generally has a larger radius
of
curvature then the inner radial surface. The electrode housing of the first
pacing/sensing electrode is positioned generally on the outer radial surface
of the
curved portion such that when the first pacingisensing electrode is extended
10 beyond the peripheral surface of the elongate body to engage the tissues of
the
heart it is along an axis that is essentially parallel with a longitudinal
axis of the
proximal end of the elongate body. In one embodiment, the curved portion
creates an angle of between approximately 45 to 60 degrees relative to a
longitudinal axis of the distal end and a longitudinal axis of the proximal
end of
15 the elongate body.
In an alternative embodiment, the curved elongate body has a first
pacing/sensing electrode that is a porous woven mesh having a semi-spherical
shape located on the peripheral surface of the elongate body. The porous woven
mesh semi-spherically shaped first pacing/sensing electrode is generally
20 positioned on the outer radial surface of the curved portion such that when
the
endocardial lead is implanted in the body, the first defibrillation coil
electrode is
positioned in the right ventricular apex and the first pacing/sensing
electrode is
in physical contact with the tissues of the right ventricle chamber of the
heart. In
one embodiment, the first pacing/sensing electrode is positioned on the septal
25 wall of the right ventricle of the heart.
In an alternative embodiment, the first pacing/sensing electrode is
an annular or semi-annular ring electrode, as are known in the art, generally
positioned on the outer radial surface of the curved portion such that when
the
endocardial lead is implanted in the body, the first defibrillation coil
electrode is
30 positioned in the right ventricular apex and the first pacing/sensing
electrode is
in physical contact with the tissues of the right ventricle chamber of the
heart.
;CV. 'i0~! : EPA-V1l~ENCHE~~ O2 : 15- 5- 0 ~ o232aas~ 2ooo-io-is CC i 1'T
EC~1~ +49 8:~ '?3994-i-65 : ~ 14
15-05-2000 U S 009908344
In the drawings, where Iike numerals describe Like components
throug'nout the several views:
Figure 1 is a schematic view of an implantable
cardiovcrter/defrbriliator ~Nith one embodiment of an endocardial lead
implanie~i
in a heart from which segments have bean removed io show details;
Figu:e ~ is a schematic view of one embodiment of an
endocardial lead according to the present iracention;
Figure 3 is a crass-sectional view of the embodiment of an
1G endotrardial lead according to Fi~ure 2 taken along the lines 3-3;
Figclre 4 is a schematic view of one embodiment of an
endoeardial lead according to the present invention;
Figare ~ is a schematic view of an implantable
cardioverterldefibrilIator with one embodiment of an endocardial lead
implanted
IS in a heart from which segments have been removed to show details;
Figure 5 (A-C) are enlarged segrnentary views of one
embodiment of an electrode housing on an endocarTdiaI Iead according to the
present invention;
Figure ? is a block diagram of an implantable
20 cardioverter,~deisbrillataa,
Figure $ is a schematic view of an embodiment of an endocardial
lead according to the present invention;
Figvs'e 9 is a schematic vicv~ of an embodiment of an enciocardial
lead according to the present invention;
Figure lfl is a schematic viev~ of an embodiment or an
endocardial lead according to the present invention;
Figure 11 is a cross-sectional view of the embodiment of an
endocardial lead according to Fi~ure I O taken along the Iines 11-11; and
Figure 12 is a sclaernatic view of an implantable
30 c~d:overter/defibrillatar'twith one embodiment of an endocardial lead
implantal
in a heart from w~~ich segments have been removed to show detail s.
AMENDED SHEET
i~1r ~'i~~''t=7J°-~1UE\CHE1 02 :17- 5- ~ ~ 02328867 2000-10-13 CCITT
Et:v1-~ +øg $s '~:i4C74.1R:~1.-~',
15-05-2000 US 009908344
7
In the following detailed description, reference is made to the
accompanying drawings which farm a part 1~.ereof and in v~~hich is shown by
way
of illustration specific embodiments in which the ~rrentian raay be practiced.
These embodiments are described in sufficient detail to enable those skilled
in
the art to practice and use the invention, and it is to be uneerstood that
other
embodiments may be utilized and that ele~i.cal, logioal, and struc:ural
chaaoes
may be made without departing from the scope of the preset invention,. 'Th~~
followuig detailed description is, therefore, not to be taken in a limiting
sense
and the scope of the present in~~ention is de~r!ed by the appended claims.
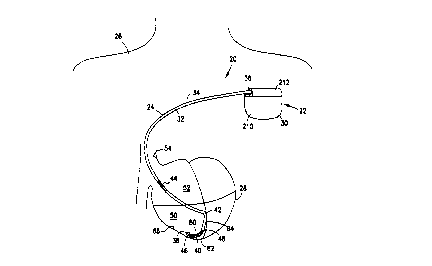
Referring now to Figure i of tl~e drawings, there is shown one
embodiment of an apparatus 20 including a cardioverterldefibrillator 22
physically and electrically coupled to an endocardial lzad 24. The apparatus
20
is implanted in a human bo dy 26 with portions of the endocardial lead 24
1 ~ inserts into a heart 28 to detect a~~d analyze electric cardiac signals
produced by
the heart 28 and to provide electrical energy to the heart 28 under certain
pTedeterniined conditions to treat ventricular arrhythmias, including
ventricular
tachyarrhythmias and ventricular F~briIlation, of the heart 28.
The endocardial lead 24 corz~prises an elongate body 32 having a
peripherdI surface 34, a proximal end 36 and a distal end 38. The endocardial
lead 24 also includes one or mare defibrillation coil electrodes and one or
more
pacir~glsensing electrodes. In one embodiment, the endocaTdial Lead ~ has a
first defibrillation coil electrode 40, a first pacinglsensing electrode ~2
and a
second defibrillation coil electrode 4.4 attached to the pezpher»I surface 34
of the
elongate body 32.
In one embodiment the first defibrillaiivn coat electrode 40 and
tide second defibrillation coil electrode 44 are helieally womd sprang
electrodes
as are known in the art. The first defibrillation coil electrode 40 and the
second
defibrillation coil electrode 44 have surface areas that sre between 200 to
1000
square millimeter, where a surface area of 504 square millimeters for the
first
defibrillation coil eiecfiode 40 2nd a surface area of 800 square millimeters
for
Lhe second defibrillation coil electrode 44 are acceptable ~ralue5. In as
2dditional
AMENDED SHEET
CA 02328867 2000-10-13
WO 99153993 PCTIUS99i083ss
embodiment, the first defibrillation coil electrode 40 and the second
defibrillation coil electrode 44 have a helical coil diameter of between 2.5
to 4.0
millimeters and a length in the range of 2 to 6 cm, 3 to 6 cm, 4 to 6 cm, 2 to
4 cm
where 3 to 4 cm is an acceptable range.
5 The first defibrillation coil electrode 40 further includes a first
end 46 and a second end 48, where the first end 46 is at or near the distal
end 38
of the elongate body 32 and the second end 48 is spaced longitudinally along
the
peripheral surface from the first end 46 of the first defibrillation coil
electrode 40
and the distal end 38 of the elongate body 32. In one embodiment the first end
10 46 of the first coil electrode 40 forms a portion of the distal end 38 of
the
elongate body 32. In an alternative embodiment, the first end 46 of the first
coil
electrode 40 is spaced longitudinally along the peripheral surface 34 from the
distal end 38 by a distance in the range of 0 to 7 millimeters.
The first pacing/sensing electrode 42 is spaced longitudinally
15 along the peripheral surface 34 from the second end 48 of the first
defibrillation
coil electrode 40 by a distance in the range of 1 to 10 centimeters, where an
acceptable range is between 1 to 3 centimeters. In one embodiment, the spacing
of the first defibrillation coil electrode 40 and the first pacing/sensing
electrode
42 is to afford positioning the first defibrillation coil 40 and the first
20 pacing/sensing electrode 42 in the right ventricle 50 of the heart 28. In
one
embodiment, the first defibrillation coil electrode 40 is implanted into the
apical
location of the right ventricle 50 such that the first defibrillation coil
electrode 40
is positioned longitudinally adjacent the septal location of the right
ventricle 50
of the heart 28 and the first pacing/sensing electrode 42 is in physical
contact
25 with the septal wall of the right ventricle 50.
The second defibrillation coil electrode 44 is spaced
longitudinally along the peripheral surface 34 from the first pacing/sensing
electrode 42 by a distance in the range of 8 to 1 ~ centimeters. In one
embodiment, the spacing of the second defibrillation coil electrode 44 and the
30 first defibrillation coil electrode 40 is to afford positioning the second
defibrillation coil electrode 44 within a right atrial chamber ~? or a maior
vein
54 leading to the right atrial chamber 52 when the first defibrillation coil
electrode 40 and the first pacing/sensing electrode 42 are positioned within
the
CA 02328867 2000-10-13
WO 99/53993 PCT/11S99/08344
9
right ventricular chamber 50. In one embodiment, the major vein 54 leading to
the heart right atrial chamber 52 is the superior vena cava.
Referring now to Figures 2 and 3 there is shown one embodiment
of the endocardial lead 24 according to the present invention. A first
electrical
5 conductor 56 is shown extending longitudinally within the elongate body 32
from a first contact end 58 at the proximal end 36 and is electrically
connected to
the first defibrillation coil electrode 40. A second electrical conductor 60
is also
shown extending longitudinally within the elongate body 32 from a second
contact end 62 at the proximal end 36 and is electrically connected to the
first
10 pacing/sensing electrode 42. Finally, a third electrical conductor 64 is
shown
extending longitudinally within the elongate body 32 from a third contact end
66
at the proximal end 36 and is electrically connected to the second
defibrillation
coil electrode 44. In one embodiment, the first contact end 58, the second
contact end 62 and the third contact end 66 are tubular or solid metallic pins
15 which are constructed of titanium, stainless steel, or MP35N.
The endocardial lead has at least one stylet lumen extending
longitudinally in the elongate body 32. In one embodiment, the elongate body
32 has a first stylet lumen 68 and a second stylet lumen 70, where the first
stylet
lumen 68 extends from a first inlet end 72 at the proximal end 36 to the
distal
20 end 38. The first stylet lumen 68 is adapted to receive a guide stylet for
stiffening and shaping the endocardial lead 24 during the insertion of the
endocardial lead 24 into the heart 28. The second stylet lumen 70 extends from
a
second inlet end 74 at the proximal end 36 to the first pacing/sensing
electrode
42. In one embodiment, the second stylet lumen 70 is formed by the second
25 electrical conductor 60, which has an elongate helical coil configuration
as is
known in the art.
In an additional embodiment, the first pacinglsensing electrode 42
includes a retaining element 76, where the retaining element 76 is adapted to
be
embedded in the right ventricle SO of the heart 28. The retaining element 76
is
30 designed to secure the first pacing/sensing electrode 42 and the elongate
body 32
of the endocardial lead 24 to the heart 28. In one embodiment, the retaining
element 76 is intended to secure the first pacing/sensing electrode 42 and the
CA 02328867 2000-10-13
WO 99/53993 PCT/US99108344
10
elongate body 32 of the endocardial lead 24 at an endocardial position within
the
right ventricle 50 of the heart 28.
In one embodiment, the retaining element 76 is a straight segment
of wire. The straight segment of wire has a proximal and a distal end, where
the
5 distal end is sharpened to a point and further includes a retaining barb.
The
retaining barb at the distal end projects away from the peripheral surface of
the
straight wire and toward the proximal end of the straight wire and is intended
to
engage and embed into the tissue of the heart. In an additional embodiment,
the
retaining element 76 is a wire shaped into a helical cork-screw like
projection,
10 where the wire has a proximal end and a distal end. In one embodiment, the
distal end is sharpened to a point which is adapted to engage and embed into
the
ventricular tissue of the heart. In an additional embodiment, the proximal end
of
the retaining element 76 is secured within the first pacing/sensing electrode
42
by welding the proximal end to the first pacing/sensing electrode. In an
15 alternative embodiment, the proximal end of the retaining element 76 is
physically secured to the first pacing/sensing electrode 42 by engaging the
proximal end and the first pacing/sensing electrode 42 so as to create a
friction
fit between the two elements.
In a further embodiment, the retaining element 76 forms a portion
20 of the first pacing/sensing electrode, where the wire retaining element
emanates
from and extends away from an outer surface of the first pacing/sensing
electrode 42. In an additional embodiment, the helical wire of the retaining
element 76 extends around the peripheral surface of the first pacing/sensing
electrode, extending away from the outer surface of the first pacing/sensing
25 electrode. In an alternative embodiment, the retaining element 76 is a
hooked
projection having a sharped distal end which is used to engage the tissues of
the
right ventricle of the heart and to secure the first pacing/sensing electrode
42 and
the elongate body 32 to the heart 28.
Referring now to Figures 4 and 5, there is shown an additional
30 embodiment of an endocardial lead 24, in which the elongate body 32 of the
endocardial lead 24 further includes an arc-shaped end portion 80. In one
embodiment, the arc-shaped end portion 80 curves away from the long-axis of
the elongate body 32 to create a "J-tip" at the distal end 38 of the
endocardial
CA 02328867 2000-10-13
WO 99/53993 PCT/L1S99J08344
11
lead 24. In an alternative embodiment, the arc-shaped end portion 80 curves
away from the long-axis of the elongate body 32 to create a "L-tip" at the
distal
end 38 of the endocardial lead 24, where the distal end 38 of the elongate
body
32 is positioned perpendicularly to the long-axis of the elongate body 32. The
5 arc-shaped end portion 80 is adapted to be positioned within and adjacent to
the
apex 82 of the right ventricle 50.
In one embodiment, the arc-shaped end portion 80 curves away
from the longitudinal axis of the proximal end 36 of the elongate body 32 in a
direction that is opposite the side on which the first pacingisensing
electrode 42
10 is positioned. In one embodiment, this configuration of the endocardial
lead 24
allows the first pacingisensing electrode 42 to be implanted or positioned
along
the septal wall 84 of the right ventricle 50. As the elongate body 32 extends
down and adjacent the septal wall 84 the arc-shaped end portion 80 begins to
curve or deflect away from the septal wall 84 as the elongate body 32 extends
15 into the apex 82 of the right ventricle 50. The arc-shaped end portion 80
is
adapted to be positioned in the apex 82 of the right ventricle 50. As a
result, the
first defibrillation coil electrode 40 is located in the apex 82 and along the
endocardial wall 86 of the right ventricle 50. Depending upon the length of
the
first defibrillation coil electrode 40, a portion of the electrode extends
along the
20 endocardial wall 86 of the right ventricle 50 from the region of the apex
82 of the
right ventricle 50.
In an alternative embodiment, the arc-shaped end portion 80
curves away from the longitudinal axis of the proximal end 36 of the elongate
body 32 in a direction that is perpendicular to the side on which the first
25 pacing/sensing electrode 42 is positioned. In an additional embodiment, the
arc-
shaped end portion 80 curves away from the longitudinal axis of the proximal
end 36 of the elongate body 32 in any direction that is between being opposite
or
perpendicular to the side on which the first pacing/sensing electrode 42 is
positioned on the peripheral surface 34 of the elongate body 32. Generally,
this
30 configuration of the endocardial lead 24 allows the first pacing/sensing
electrode
42 to be implanted or positioned along the septal wall 84 of the right
ventricle
50. As the elongate body 32 extends down and adjacent the septal wall 84 the
arc-shaped end portion 80 begins to curve or deflect away from the
longitudinal
CA 02328867 2000-10-13
WO 99153993 PCT/US99/0834.1
12
axis of the elongate body 32 along the septal wall as the elongate body 32
extends into the apex 82 of the right ventricle 50. The arc-shaped end portion
80
is adapted to be positioned in the apex 82 of the right ventricle 50 so that
the first
defibrillation coil electrode 40 is located along both the endocardial wall 86
and
5 the septal wall 84 in the region of the apex 82 of the right ventricle 50.
Depending upon the length of the first defibrillation coil electrode 40, a
portion
of the electrode extends along the endocardial wall 86 of the right ventricle
SO
from the region of the apex 82 of the right ventricle 50.
In one embodiment of creating the arc-shaped end portion 80 of
10 the endocardial lead 24, the first defibrillation coil electrode 40 is
formed with a
mechanical bias in the electrode structure. In one embodiment, the mechanical
bias in the first defibrillation coil electrode 40 is imparted into the
electrode
during the winding of the electrode. In an alternative embodiment, the
mechanical bias is created by mechanically deforming the electrode after it
has
1 S been wound. In an alternative embodiment, the polymer structure of the
elongate body 32 is modified to create the arc-shaped end portion 80. In one
embodiment, the arc-shaped end portion 80 is constructed of a polymer having
an enhanced stiffness relative to the remainder of the elongate body 32. In an
alternative embodiment, the arc-shaped end portion 80 is molded into the
20 elongate body 32 during the construction of the elongate body 32.
In one embodiment, the curvature of the arc-shaped end portion
80 generally conforms to the curvature of the apex 82 region. This radius of
curvature maximizes direct contact between the first defibrillation coil
electrode
40 and the endocardial tissue of the right ventricle 50. Because the shape of
25 diseased hearts varies considerably, an optimized radius of curvature will
be
determined on a patient by patient basis.
In one embodiment, the arc-shaped end portion 80 has a
semicircular shape. In an alternative embodiment, the arc-shaped end portion
80
has a parabolic shape. In an additional embodiment, the arc-shaped end portion
30 80 has a small radius of curvature which creates an abrupt angular
deflection in
the elongate body of the endocardial lead 24. In one embodiment, the radius of
curvature creates an angle of between approximately 10 to 70 degrees relative
to
a longitudinal axis of the distal end 38 and a longitudinal axis of the
proximal
CA 02328867 2000-10-13
WO 99!53993 PCTNS99/0834J
13
end 36 of the elongate body 32. In an alternative embodiment, the radius of
curvature is in the range of 0.25 to 1 cm, 0.5 to 1 cm, 1 to 2 cm, 1 to 3 cm
when
a radius of curvature of approximately 1 cm is an acceptable value.
Referring now to Figure 6 (A-C), there is shown an additional
5 embodiment of the endocardial lead 24 in which the peripheral surface 34 of
the
elongate body 32 further defines an electrode housing 100 having walls
defining
an opening therethrough, and where the electrode housing 100 is adapted to
sheathe the first pacing/sensing electrode 42 and through which the first
pacing/sensing electrode 42 extends to engage the right ventricular chamber 50
10 of the heart 28.
In one embodiment, the electrode housing 100 is attached to and
projects away from the peripheral surface 34 of the elongate body 32. The
electrode housing 100 has a first wall portion 102 that partially encircles
and
projects away from the peripheral surface 34 in an arcuate fashion until it
reaches
15 an upper limit 104 at which point the first wall portion 102 becomes
parallel
with the longitudinal axis of the elongate body 32. In one embodiment, the
cross-sectional shape of the electrode housing 100 at the upper limit 104 of
the
first wall portion 102 is that of a partial ellipse.
The electrode housing 100 also includes a second wall portion
20 106, where the second wall portion 106 is positioned essentially
perpendicular to
the first wall portion 102 so that the second wall portion 106 projects from
the
upper limit 104 of the first wall portion 102 to a portion of the peripheral
surface
34 of the elongate body 32. The second wall portion 106 also defines the
opening 108 through the electrode housing 100, where the opening 108 through
25 the electrode housing 100 is coupled to an opening through the peripheral
surface 34 of the elongate body 32. The second electrical conductor 60 extends
through the opening in the elongate body 32 and into the opening 108 defined
by
the second wall portion 106 of the electrode housing 100. In one embodiment,
the second wall portion 106 defines a tubular shaped opening 108 through the
30 electrode housing 100.
In one embodiment, the second electrical conductor 60 is coupled
to and makes an electrical connection with a moveable element 110 which is
housed within the opening 108 of the electrode housing 100. The moveable
CA 02328867 2000-10-13
WO 99!53993 PCT/US99/0834-t
14
element 110 has an outer surface 112, an inner surface 114 and a
circumferential
surface 116, where the circumferential surface 116 is sealed against the
second
wall portion 106 of the opening 108.
In one embodiment, the moveable element 110 is intended to
move longitudinally within the opening 108 from a first or recessed position
118
to a second or extended position 120 and also to rotate on the circumferential
surface 116 due to force applied to the inner surface of the sleeve by a guide
stylet inserted through the second stylet lumen 70, where the second stylet
lumen
70 is adapted to receive a stylet for extending and rotating the first
10 pacingisensing electrode 42 to embed the retaining element 76 of the first
pacing/sensing electrode 24 into the right ventricle 50 of the heart 28.
The second electrical conductor 60 is secured to the elongate
body 32 at the location where it emerges from the opening through the
peripheral
surface 34 of the elongate body 32 into the opening 108 through the electrode
15 housing 100. The helical coil construction of the second electrical
conductor 60
then allows the conductor to extend in a spring like fashion as the moveable
element 110 moves between the first position 118 and the second position 120.
In one embodiment, the first pacing/sensing electrode 42 is
coupled to the outer surface 112 of the moveanle element 110. In the first
20 position 118 of the moveable element 110, t; ~ first pacing/sensing
electrode 42
and the retaining element 76 are housed within the opening 108 in the
electrode
housing 100. After the moveable element 110 is advanced to the second position
120, both the retaining element 76 and the first pacing/sensing electrode 42
extend a predetermined distance beyond the second wall portion 106 of the
25 electrode housing 100. In one embodiment, up to 3 centimeters is an
acceptable
predetermined distance. In one embodiment, the retaining element 76 and the
first pacinglsensing electrode 42 extend beyond the second wall portion 106 in
plane that is essentially parallel to the longitudinal axis of the elongate
body 32.
In an alternative embodiment, the retaining element 76 and the first
30 pacinglsensing electrode 42 extends beyond the second wall portion 106 in
plane
having an acute angle (less than 90 degrees) relative to the longitudinal axis
of
the eloneate body 32.
CA 02328867 2000-10-13
WO 99/53993 PCT/US99I08344
l~
In an additional embodiment, the elongate body fiirther has a
plurality of tines 78 at or adjacent the distal end 38, the plurality of tines
78
being circumferentially spaced and projecting both radially away from the
peripheral surface 34 and toward the proximal end 36 of the elongate body 32.
5 In one embodiment, the plurality of tines is constructed of the same
material
used to make the elongate body 32 of the endocardial lead 24.
In one embodiment, the elongate body 32 of the endocardial lead
24 is made by extrusion of an implantable polyurethane, silicone rubber or
other
implantable flexible biocompatible polymer. The length of the elongate body 32
10 of the endocardial lead 24 between the proximal end 36 and the distal end
38 is
in the range of between 60 to 120 centimeters. In an additional embodiment,
the
elongate body 32 has a diameter of less than or equal to 4 millimeters. The
electrical conductors 56, 60 and 64 are made of a MP35N nickel-cobalt alloy,
or
other electrical conductor metal as are known in the art. The first
defibrillation
15 coil electrode 40, the second defibrillation coil electrode 44, the first
pacing/sensing electrode 42, the moveable element 110, and the retaining
element 76 are made of an implantable metal such as platinum/iridium alloys,
titanium or other implantable metals as are known in the art.
Experimental data indicate that the use of the endocardial lead 24
20 has the potential of reducing a patient's defibrillation strength
requirements.
Experimental tests on defibrillation energy requirements using both the
endocardial lead 24 of the present invention and an endocardial lead sold
under
the trademark ENDOTAK (Cardiac Pacemaker, Inc.l Guidant Corporation, St.
Paul, MN), in porcine and canine models indicate that the use of the
endocardial
25 lead 24 reduced defibrillation delivered energy requirements by 26% as
compared to the use of the ENDOTAK lead. Also, the use of the endocardial
lead 24 and the ENDOTAK lead in the same animal models showed that the use
of the endocardial lead 24 reduced the average peak current requirements of
22%
as compared to the use of the ENDOTAK lead.
30 The experimental endocardial defibrillation systems incorporated
either the single-pass ENDOTAK or the endocardial lead 24 of the present
invention with an impiantable cardioverter/defibrillator sold under the
trademark
MINI II (Cardiac Pacemaker, Inc.l Guidant Corporation, St. Paul, MN), shell
CA 02328867 2000-10-13
WO 99153993 PCT/US99/08344
16
electrode to create a defibrillation electrode system sold under the trademark
TRIAD, (Cardiac Pacemaker, Inc./ Guidant Corporation, St. Paul, MIA'). Both
leads consisted of an approximately 3.4 cm long, 0.110 inch diameter distal
and
a 6.8 cm long, 0.110 inch diameter proximal tri-filar DBS spring shocking
S electrodes. The ENDOTAK lead had a standard porous tip pace/sense electrode
with a tip-to-shocking electrode length of approximately 1.2 cm. Conversely,
the endocardial lead 24 had the shocking electrode positioned at the end of
the
elongate body 32 with the first pacing/sensing electrode 42 approximately 1.2
cm proximal to the shocking electrode. In one embodiment, the first
i 0 pacing/sensing electrode 42 consisted of a miniaturized electrode position
within
the helical coil wound around the outside diameter of the electrode. For
purposes of the experimental procedure, the elongate body 32 further includes
a
curved portion, where the curved portion is positioned between the proximal
end
36 and the distal end 38 of the elongate body 32. The curved portion has an
15 outer radial surface and an inner radial surface, where the outer radial
surface
generally has a larger radius of curvature then the inner radial surface. The
first
pacing/sensing electrode 42 was positioned on the outer radial surface of the
curved portion so that the first pacing/sensing electrode 42
extended beyond the peripheral surface 34 of the elongate body 32 along an
axis
20 that is essentially parallel with a longitudinal axis of the proximal end
36 of the
elongate body 32 to engage the tissue of the heart 28. The curved portion of
the
endocardial lead 24 created an approximately 60 degree arc relative to a
longitudinal axis of the distal end 38 and a longitudinal axis of the proximal
end
36 of the elongate body 32 to facilitate septal positioning and to serve as a
2S platform for the first pacing/sensing electrode 42.
Pacing and sensing characteristics and defibrillation strength
requirements for each lead system were determined in six swine. Under
fluoroscopic guidance, both lead systems were positioned into the right
ventricular apex through a left external jugular venotomy. Once apically
placed,
30 each lead was advanced until the right ventricular shocking electrode was
positioned into the anterior groove of the right ventricular out-flow' tract
against
the septum. The MINI II shell electrode was subcutaneously implanted in the
CA 02328867 2000-10-13
WO 99/53993 PCT/US99/08349
17
left pectoral region. For defibrillation trials, the right ventricular
shocking
electrode served as the cathode.
Pacing thresholds (0.5 ms pulse widths), impedances and sensing
characteristics (R-wave amplitudes) were determined prior to the
defibrillation
5 trials using a SEAMED external stimulator (Redmond, WA). Defibrillation
strength requirements (delivered energy, peak voltage and peak current) and
system impedances for each lead system were determined using 80% fixed-tilt
biphasic shocks generated from a LABVIEW directed current amplifier
(National Instrument, Austin, TX). The defibrillation requirements, pacing
10 thresholds and sensing characteristics of the two lead systems were
compared
using paired t tests.
Defibrillation strength requirements for the endocardial lead 24
was lower than with the ENDOTAK system. Delivered energy, peak voltage
and peak current requirements were 32%, 17% and 25%, (p<0.01 ) respectively,
1 ~ lower with the endocardial lead 24 when applied to the TRIAD system as
compared to the ENDOTAK TRIAD system. Although not statistically
significant with the paired t-test, pacing and sensing characteristics of the
endocardial lead 24 were different from the ENDOTAK system. Sensed R-wave
amplitudes were 14% (p>0.05) lower with the endocardial lead 24 system than
20 with the ENDOTAK system. Pacing thresholds were 38% lower (0.3V, p>0.05)
with the ENDOTAK passive electrode than with the retractable miniaturized
positive fixation electrode of the endocardial lead 24 system. The endocardial
lead 24 test lead system impedance was 12% higher than the ENDOTAK
system.
25 Referring now to Figure 7, there is shown one embodiment of an
electronics block diagram of the cardioverter/defibrillator 22. The
cardioverter/defibrillator 22 includes electronic control circuitry 200 for
receiving cardiac signals from the heart 28 and delivering electrical energy
to the
heart 28. In one embodiment, the electronic control circuitry 200 includes
30 terminals, labeled with reference numbers 202, 204, 206, and 208 for
connection
to the first defibrillation coil electrode 40, the first pacing/sensing
electrode 42,
and the second defibrillation coil electrode 44 attached to the surface of the
endocardial lead 24.
CA 02328867 2000-10-13
WO 99/53993 PCT/US99/08344
18
The electronic control circuitry 200 of the
cardioverter/defibrillator 22 is encased and hermetically sealed in a housing
210
(Figures 1 and 5) suitable for implanting in a human body 26. In one
embodiment, titanium is used for the housing 210, however, other biocompatible
5 housing materials as are known in the art may be used. A connector block 212
(Figures 1 and 5) is additionally attached to the housing 210 of the
cardioverter/defibrillator 22 to allow for the physical and the electrical
attachment of the endocardial lead 24 and the electrodes to the
cardioverter/defibrillator 22 and the encased electronic control circuitry
200.
10 The electronic control circuitry 200 of the
cardioverter/defibrillator 22 is a programmable microprocessor-based system,
with a microprocessor 214 a memory circuit 216, which contains parameters for
various pacing and sensing modes, and stores data indicative of cardiac
signals
received by the electronic control circuitry 200. A transmitter circuit 218 is
15 additionally coupled to the electronic control circuitry 200 and the memory
circuit 214 to allow the cardioverter/defibrillator 22 to communicate with an
external controller unit 220. In one embodiment, the transmitter circuit 218
and
the external controller unit 220 use a wire loop antenna 222 and a radio
frequency telemetric link, as is known in the art, to receive and transmit
signals
20 and data to and from the external controller unit 220 and the electronic
control
circuitry 200. In this manner, programming commands or instructions are
transferred to the microprocessor 214 of the cardioverter/defibrillator 22
after
implant, and stored cardiac data pertaining to sensed arrhythmic events within
the heart 28 and subsequent therapy, or therapies, applied to correct the
sensed
25 arrhythmic event are transferred to the external controller unit 220 from
the
cardioverter/defibrillator 22.
In the cardioverter/defibrillator 22 of Figure 7, the first
defibrillation coil electrode 40 and the first pacing/sensing electrode 42 are
coupled to a sense amplifier 224, whose output is shown connected to an R-wave
30 detector 226. These components serve to sense and amplify the QRS waves of
the heart, and apply signals indicative thereof to the microprocessor 214.
Among other things, microprocessor 214 responds to the R-wave detector 226 by
providing pacing signals to a pace output circuit 228, as needed according to
the
CA 02328867 2000-10-13
WO 99/53993 PCTNS99/08344
19
programmed pacing mode. Pace output circuit 228 provides output pacing
signals to terminals 202 and 204, which connect to the first pacing/sensing
electrode 42 and the first defibrillation coil electrode 40, for bipolar
cardiac
pacin;. In an alternative embodiment, the pace output circuit 228 provides
5 output pacing signals to terminal 202 and to the housing 210 of the
cardioverter/defibrillator 22 to provide both unipolar sensing of the heart 28
and
unipolar pacing of the heart 28.
The first defibrillation coil electrode 40 and the second
defibrillation coil electrode 44 are coupled to a sense amplifier 230, whose
10 output is connected to a cardiac morphology detector 232. These components
serve to sense and amplify the QRS-waves of the cardiac cycle from the
ventricular region of the heart 28, and apply signals indicative thereof to
the
microprocessor 214. In one embodiment, the cardiac morphology detector 232
includes an analog filter for filtering cardiac signal noise sensed by the
15 electrodes. The cardiac signals are then A/D converted into a digital
signal and
subsequently received by the microprocessor 214.
Among other things, microprocessor 214 responds to the sensed
QRS-waves of the cardiac cycle from the sense amplifier 230 applied to the
morphology detector 232 by providing pacing signals to the pace output circuit
20 228, as needed according to the programmed pacing mode. Pace output circuit
228 provides output pacing signals to terminals 202 and 204, which connect to
the first pacing/sensing electrode 42 and the first defibrillation electrode
40, for
bipolar pacing or to the first pacing/sensing electrode 42 and the housing 210
for
unipolar pacing as previously described.
25 The microprocessor 214 also responds to the cardiac signals
sensed within the heart 28 using the endocardial lead 24 by providing signals
to
cardioversion/defibrillation output circuitry 234 to provide either
cardioversion
or defibrillation electrical energy to the heart 28 depending upon nature of
the
arrhythmia sensed by the cardioverter/defibrillator 22. Power to the
30 cardioverter/defibrillator 22 is supplied by an electrochemical battery 236
that is
housed within the cardioverter/defibrillator 22.
In one embodiment, the cardioversion or defibrillation electrical
energy pulses delivered to the heart 28 are either a monophasic, biphasic or
CA 02328867 2000-10-13
WO 99/53993 PCT/US99/08344
20
multiphasic pulses of electrical energy, as are known in the art. In an
additional
embodiment, more than one of the cardioversion or defibrillation electrical
energy pulses are delivered to the heart, where the pulses are delivered
either
simultaneously or sequentially. In one embodiment, the defibrillation
electrical
S energy is delivered between first defibrillation coil electrode 40 and the
second
defibrillation coil electrode 44 and the housing 210 of the
cardioverter/defibrillator 22. In a further embodiment, the first
defibrillation coil
electrode 40 is a cathode terminal and the second defibrillation coil
electrode 44
and the housing 210 are anode terminals. In an alternative embodiment,
10 cardioversion or defibrillation electrical energy is delivered between the
first
defibrillation coil electrode 40 and the second defibrillation coil electrode
44,
where, in one embodiment, the first defibrillation coil electrode 40 is a
cathode
terminal and the second defibrillation coil electrode 44 is an anode terminal.
In
another embodiment, additional defibrillation electrodes, such as subcutaneous
15 patch electrode, epicardial defibrillation electrodes and the like can be
incorporated into and added to the cardioverter/defibrillator 22 to allow for
further defibrillation electrical energy pathways.
Referring now to Figure 8, there is shown an additional
embodiment of an endocardial lead 24, in which the elongate body 32 of the
20 endocardial lead 24 includes a curved portion 300. The curved portion 300
is
positioned between the proximal end 36 and the distal end 38 of the elongate
body 32. In one embodiment, the curved portion 300 has an outer radial surface
310 and an inner radial surface 320, where the outer radial surface 310
generally
has a larger radius of curvature then the inner radial surface 320. In an
additional
25 embodiment, the electrode housing 100 is positioned generally on the outer
radial surface 310 of the curved portion 300. This configuration allows the
first
pacing/sensing electrode 42 to extend beyond the peripheral surface 34 of the
elongate body 32 along an axis that is essentially parallel with a
longitudinal axis
of the proximal end 36 of the elongate body 32 to engage the tissue of the
heart
30 28. In an alternative embodiment, the first pacing,~sensing electrode 42 to
extend
beyond the peripheral surface 34 of the elongate body 32 along an axis that
has
an obtuse angle greater than 90 degrees) relative to the longitudinal axis of
the
proximal end 36 of the elongate body 32 to engage the tissue of the heart 28.
CA 02328867 2000-10-13
WO 99/53993 PCT/US99/08344
21
The curved portion 300 of the endocardial lead 24 creates an
angle of between approximately 45 to 60 degrees relative to a longitudinal
axis
of the distal end 38 and a longitudinal axis of the proximal end 36 of the
elongate
body 32. In one embodiment, the curved portion 300 of the elongate body is
5 created by a mechanical bias in one or more of the first electrical
conductor 56,
the second electrical conductor 60 or the third electrical conductor 64. In an
additional embodiment, the polymer structure of the elongate body 32 is
modified to create the curved portion 300. In one embodiment, the curved
portion 300 is constructed of a polymer having an enhanced stiffness relative
to
10 the remainder of the elongate body 32. In an alternative embodiment, the
curved
portion 300 is molded into the elongate body 32 during the construction of the
elongate body 32.
Referring now to Figure 9, there is shown an additional
embodiment of the endocardial lead 24 according to the present invention. The
15 endocardial lead 24 comprises an elongate body 32 having a peripheral
surface
34, a proximal end 36 and a distal end 38. The endocardial lead 24 also
includes
one or more defibrillation coil electrodes and one or more pacing/sensing
electrodes. In one embodiment, the endocardial lead 24 has a first
defibrillation
coil electrode 40, a first pacing/sensing electrode 400 and a second
defibrillation
20 coil electrode 44 attached to the peripheral surface 34 of the elongate
body 32.
In one embodiment the first defibrillation coil electrode 40 and
the second defibrillation coil electrode 44 are helically wound spring
electrodes
as are known in the art. The first defibrillation coil electrode 40 further
includes
a first end 46 and a second end 48, where the first end 46 is at or near the
distal
25 end 38 of the elongate body 32 and the second end 48 is spaced
longitudinally
along the peripheral surface from the first end 46 of the first defibrillation
coil
electrode 40 and the distal end 38 of the elongate body 32. In one embodiment
the first end 46 of the first coil electrode 40 forms a portion of the distal
end 38
of the elongate body 32. In an alternative embodiment, the first end 46 of the
30 first coil electrode 40 is spaced longitudinally along the peripheral
surface 34
from the distal end 38 by a distance in the range of 0 to 7 millimeters.
The first pacing/sensing electrode 400 is spaced longitudinally
along the peripheral surface 34 from the second end 48 of the first
defibrillation
CA 02328867 2000-10-13
WO 99/53993 PCT/L;S9910834d
coil electrode 40 by a distance in the range of 1 to 10 centimeters, where an
acceptable range is between 1 to 3 centimeters. In one embodiment, the spacing
of the first defibrillation coil electrode 40 and the first pacing/sensing
electrode
400 is to afford positioning the first defibrillation coil 40 and the first
5 pacing/sensing electrode 400 in the right ventricle 50 of the heart 28. In
one
embodiment, the first defibrillation coil electrode 40 is implanted directly
along
the septal wall of the right ventricle 50 such that the first defbrillation
coil
electrode 40 is positioned longitudinally adjacent the septum of the right
ventricle SO of the heart 28 and the first pacing/sensing electrode 400 is in
10 physical contact with a wall of the right ventricle. In one embodiment, the
pacing electrode is positioned such that it is in contact with the ventricular
septum of the heart. In an alternative embodiment, the first defibrillation
coil
electrode 40 is implanted directly along the apex location of the right
ventricle
50 such that the first defibrillation coil electrode 40 is positioned
longitudinally
15 adjacent the apex location of the right ventricle 50 of the heart 28 and
the first
pacing/sensing electrode 400 is in physical contact with a wall of the right
ventricle. In one embodiment, the pacing electrode is positioned such that it
is in
contact with the ventricular septum of the heart.
The second defibrillation coil electrode 44 is spaced
20 longitudinally along the peripheral surface 34 from the first
pacing/sensing
electrode 400 by a distance in the range of 8 to 15 centimeters. In one
embodiment, the spacing of the second defibrillation coil electrode 44 and the
first defibrillation coil electrode 40 is to afford positioning the second
defibrillation coil electrode 44 within a right atrial chamber 52 or a major
vein
25 leading to the right atrial chamber 52 when the first defibrillation coil
electrode
40 and the first pacing/sensing electrode 400 are positioned within the right
ventricle chamber 50. In one embodiment, the major vein leading to the heart
right atrial chamber 52 is the superior vena cava.
Figure 9 shows one embodiment in which the elongate body 32 of
30 the endocardial lead 24 includes a curved portion 300. The curved portion
300 is
positioned between the proximal end 36 and the distal end 38 of the elongate
body 32. The curved portion 300 allow the endocardial lead 24 to be implanted
within the heart 28 with the first pacing/sensing electrode 400 engaging the
CA 02328867 2000-10-13
WO 99/53993 PCT/US99/0834.i
23
tissue of the heart 28 while the remaining distal portion of the endocardial
lead
24, including the first defibrillation coil electrode 40, is positioned
adjacent the_
endocardial wall of the right ventricle, where the first defibrillation coil
electrode
40 is in the apex of the right ventricle.
The curved portion 300 of the endocardial lead 24 creates an
angle of between approximately 45 to 60 degrees relative to a longitudinal
axis
of the distal end 38 and a longitudinal axis of the proximal end 36 of the
elongate
body 32. In one embodiment, the curved portion 300 of the elongate body is
created by a mechanical bias in one or more of the first electrical conductor
56,
10 the second electrical conductor 60 or the third electrical conductor 64. In
an
additional embodiment, the polymer structure of the elongate body 32 is
modified to create the curved portion 300. In one embodiment, the curved
portion 300 is constructed of a polymer having an enhanced stiffness relative
to
the remainder of the elongate body 32. In an alternative embodiment, the
curved
15 portion 300 is molded into the elongate body 32 during the construction of
the
elongate body 32.
In one embodiment, the curved portion 300 has an outer radial
surface 310 and an inner radial surface 320, where the outer radial surface
310
generally has a larger radius of curvature then the inner radial surface 320.
The
20 first pacing/sensing electrode 400 is positioned generally on the outer
radial
surface 310 of the curved portion 300. This configuration allows the first
pacing/sensing electrode 400 to extend beyond the peripheral surface 34 of the
elongate body 32 to engage the tissue of the heart 28 when the endocardial
lead
24 is positioned within the heart 28. In one embodiment, the first
pacing/sensing
25 electrode 400 and the first defibrillation coil electrode 40 are implanted
within
the heart such that both the first pacing/sensing electrode 400 and the first
defibrillation coil electrode 40 are located within the right ventricle with
the first
defibrillation coil electrode 40 in the apex of the right ventricle and the
first
pacing/sensing electrode 400 on the ventricular septum of the heart.
30 In one embodiment, the first pacing/sensing electrode 400 is a
porous woven mesh on the peripheral surface of the elongate body as is shown
in
Figure 9. The porous woven mesh is created from implantable metal wire such
as platinum/iridium alloys, titanium or other implantable metals as are known
in
CA 02328867 2000-10-13
WO 99/53993 PCT/US99/0834.t
24
the art. In one embodiment, the porous woven mesh has a semi-spherical shape
and is positioned on the peripheral surface of the elongate body. In an
alternative embodiment, the first pacing/sensing electrode is annular and
encircles the peripheral surface of the elongate body. In an additional
5 embodiment, the first pacing/sensing electrode is semi-annular and partially
encircles the peripheral surface of the elongate body.
In an additional embodiment, the elongate body further has a
plurality of tines 78 at or adjacent the distal end 38, the plurality of tines
78
being circumferentially spaced and projecting both radially away from the
10 peripheral surface 34 and toward the proximal end 36 of the elongate body
32.
In one embodiment, the plurality of tines is constructed of the same material
used to make the elongate body 32 of the endocardial lead 24. In an
alternative
embodiment, the elongate body 32 of the endocardial lead 24 is physically or
chemically treated to promote tissue in-growth. Tissue in-growth allows for
the
15 increased stabilization and retention of the endocardial lead 24 after
being
implanted in the heart 28. In one embodiment, a micro-texturing is created on
the surface of the elongate body 32 from chemical or mechanical processing to
allow for tissue in-growth.
Referring now to Figures 10 and 11, there is shown an additional
20 embodiment of the endocardial lead 24 according to the present invention. A
first electrical conductor 56 is shown extending longitudinally within the
elongate body 32 from a first contact end 58 at the proximal end 36 and is
electrically connected to the first defibrillation coil electrode 40. A second
electrical conductor 60 is also shown extending longitudinally within the
25 elongate body 32 from a second contact end 62 at the proximal end 36 and is
electrically connected to the first pacing/sensing electrode 42. A third
electrical
conductor 64 is shown extending longitudinally within the elongate body 32
from a third contact end 66 at the proximal end 36 and is electrically
connected
to the second defibrillation coil electrode 44. Finally, a fourth electrical
30 conductor 420 is also shown extending longitudinally within the elongate
body
32 from a fourth contact end 422 at the proximal end 36 and is electrically
connected to a second pacing electrode 424. In one embodiment, the first
contact end 58, the second contact end 62, the third contact end 66, and the
CA 02328867 2000-10-13
WO 99153993 PC'T/US99/08344
25
fourth contact end 422 are tubular or solid metallic pins which are
constructed of
titanium, stainless steel, or MP35N.
The endocardial lead 24 has at least one stylet lumen extending
longitudinally in the elongate body 32. In one embodiment, the elongate body
S 32 has a first stylet lumen 68 and a second stylet lumen 70, where the first
stylet
lumen 68 extends from a first inlet end 72 at the proximal end 36 to the
distal
end 38. The first stylet lumen 68 is adapted to receive a guide stylet for
stiffening and shaping the endocardial lead 24 during the insertion of the
endocardial lead 24 into the heart 28. In one embodiment, a portion of the
first
10 stylet lumen 68 is formed by the fourth electrical conductor 420, which has
an
elongate helical coil configuration as is known in the art. In one_embodiment,
the elongate helical coil configuration extends longitudinally through the
elongate body 32 to a point that is just proximal or adjacent to the second
pacing
electrode 424. The fourth electrical conductor 420 is then coupled to the
second
1 S pacing electrode 424. The second stylet lumen 70 extends from a second
inlet
end 74 at the proximal end 36 to the first pacing/sensing electrode 42. The
second stylet lumen 70 is formed by the second electrical conductor 60, which
has an elongate helical coil configuration as is known in the art.
In an additional embodiment, the first pacing/sensing electrode 42
20 and the second pacing electrode 424 provide for bipolar sensing and pacing
of
the heart 28. In one embodiment, the second pacing electrode 424 is an annular
ring electrode that extends completely around the peripheral surface 34 of the
elongate body 32. In an alternative embodiment, the second pacing electrode
424 is a semi-annular ring that extends only partially around the peripheral
25 surface 34 of the elongate body 32.
Referring now to Figure 12 of the drawings, there is shown an
additional embodiment of the apparatus 20 including the
cardioverter/defibrillator 22 physically and electrically coupled to the
endocardial lead 24 and to a second endocardial lead 450. The apparatus 20 is
30 implanted in the human body 26 with portions of the endocardial lead 24 and
the
second endocardial lead 450 inserted into the heart 28 to detect and analyze
electrical cardiac signals produced by the heart 28 and to provide electrical
energy to the heart 28 under certain predetermined conditions to treat
ventricular
CA 02328867 2000-10-13
WO 99/53993 PCrIUS99/083d~
26
arrhythmias, including ventricular tachyarrhythmias and ventricular
fibrillation,
of the heart 28.
The second endocardial lead 450 comprises an elongate body 452
having a peripheral surface 454, a proximal end 456 and a distal end 458. The
S second endocardial lead 450 also includes one or more pacing and sensing
electrodes. The second endocardial lead 450 is adapted to be releasably
attached
to the connector block 212 to allow pacing and sensing electrodes attached to
the
peripheral surface of the second endocardial lead 450 to be physically and
electrically coupled to the housing 210 and the electronic control circuitry
200 of
10 the cardioverter/defibrillator 22.
In one embodiment, the second endocardial lead 450 has a distal
pacing/sensing electrode 460 attached to the peripheral surface 454 of the
elongate body 452. In one embodiment, the distal pacinglsensing electrode 460
is spaced longitudinally along the peripheral surface 454 from the distal end
458
15 of the elongate body 452 by a distance in the range of 0 to 2 centimeters,
where
an acceptable range is between 0 to 1 centimeters. In one embodiment, the
distal
pacing/sensing electrode 460 is an annular, or a semi-annular ring electrode
positioned on the elongate body 452 of the second endocardial lead 450. In an
alternative embodiment, the distal pacing/sensing electrode 460 is a tip
electrode
20 positioned on the distal end 458 of the second endocardial lead 450. The
distal
pacing/sensing electrode 460 is electrically connected to the electronic
control
circuitry 200 through a contact end located at the proximal end 456 which is
coupled to a first distal electrode electrical conductor extending
longitudinally
within the elongate body 452 of the second endocardial lead 450.
25 In one embodiment, the second endocardial lead 450 is positioned
on or adjacent a left ventricular epicardial surface 462. In one embodiment,
the
second endocardial lead 450 is introduced through the coronary sinus vein 464
to
an apical branch of the great coronary vein 466 anti advanced to a position
within the great coronary vein 466, or a tributary vein to the great coronary
vein
30 466, so that the distal pacing/sensing electrode 460 is positioned on or
adjacent
the left ventricular epicardial surface 462.
In one embodiment, the distal pacing/sensing electrode 460
positioned on or adjacent to the left ventricular epicardial surface 462 and
the
CA 02328867 2000-10-13
WO 99/53993 PCT/US9910834d
27
housing 210 are used to provide unipolar pacing and sensing of the ventricles
of
the heart. In an alternative embodiment, the distal pacing/sensing electrode
460
and the first pacing/sensing electrode 42 provide bipolar pacing and sensing
of
the ventricles of the heart. In an additional embodiment, a second distal
5 pacing/sensing electrode is added to the peripheral surface of the second
endocardial lead 450 to provide for bipolar sensing and pacing of the
ventricles
of the heart.
In an additional embodiment, the elongate body 452 further has a
plurality of tines 468 at or adjacent the distal end 458, the plurality of
tines 468
10 being circumferentially spaced and projecting both radially away from the
peripheral surface 454 and toward the proximal end 456 of the elongate body
452. In one embodiment, the plurality of tines is constructed of the same
material used to make the elongate body 452 of the second endocardial lead
450.
The aspects of the invention illustrated herein have been
15 described as having applications for implantable
cardioverter/defibrillators,
which may include numerous pacing modes as are known in the art. However,
the endocardial lead of the present invention is also used in any number of
implantable or external medical devices, including external
defibrillator/monitor
devices. Additionally, the endocardial lead of the present invention
alternatively
20 can include additional or fewer defibrillation coil electrodes and/or
pacing/sensing electrodes. For example, the endocardial lead can include only
the first defibrillation coil electrode 40 and the first pacing/sensing
electrode 42,
where cardiac sensing is accomplished with unipolar sensing between the first
pacing/sensing electrode 42 and housing 210 of the cardioverter/defibrillator
22.
25 Additionally, unipolar defibrillation electrical energy is supplied to the
heart
between the first defibrillation coil electrode 40 and the housing 210 of the
cardioverter/defibrillator 22.