Note: Descriptions are shown in the official language in which they were submitted.
CA 02330468 2000-10-26
WO 99/55244 PCTNS99/09294
METHOD AND SYSTEM FOR TRANS-LUMENAL
RADIO-FREQUENCY ABLATION
THROUGH AN ENDOSCOPE
FIELD OF THE INVENTION
This invention relates generally to advances in medical systems and procedures
for
prolonging or improving human life. More particularly, this invention relates
to an improved
method and system for ablating clinical abnormalities such as tumors through
the use of a high
frequency electrode or a laser fiber that is passed within an endoscope in a
bodily passageway and
that trans-lumenally pierces the wall of the passageway.
BACKGROUND OF THE INVENTION
It is of increasing importance to treat diseases with minimally invasive
techniques. For
example, use of minimally invasive cannulae or endoscopes within the body
reduces the trauma
from surgery and enables access and visualization of internal structures
without major surgical
wounds. This is especially important in highly inaccessible areas such as in
the gut, pancreas,
abdomen, genitourinary tract, and so on. Access through a natural bodily
opening or lumen such
as the throat, rectum, urethra, or vessels saves further trauma.
There exist today advanced endoscopic systems, some with rigid and others with
flexible,
elongated shafts, to gain visual access and mechanical access through natural
bodily lumens.
Certain flexible endoscopes also have built-in ultrasonic scanning heads so
that they can visualize
tissue proximate to the distal end of the endoscope's tip by ultrasound
scanning. By reference, see
the gastroenterological endoscopes of the Panasonic and Olympus companies.
High energy or electrical current probes have been passed through an endoscope
to
coagulate structures on the surface of bodily lumens. For example,
hemorrhaging surfaces of the
stomach have been treated by inserting an endoscope into the stomach through
the throat. An
electrical coagulation probe is passed through the endoscope and put in
contact with the tissue
CA 02330468 2000-10-26
WO 99/55244 PCT/US99/09294
2
that is bleeding. Electrical current can be passed through the electrode to
thereby coagulate the
bleeding tissue and to stop the hemorrhage.
Endoscopes together with radio-frequency electrodes have been used to treat
benign
prostatic hyperplasia, which is an enlargement of the prostate that causes
urethral obstruction.
One such procedure, called "trans-urethral needle ablation" or "TUNA,"
involves passing a radio-
frequency (RF) instrument through a cystoscope (a rigid endoscopic device used
for viewing in
the urethra) into the urethra. The cystoscope is first placed in the urethra
for visualization of the
urethral wall in the region of the prostate. Once in place, a radio-frequency
electrode is passed
inside the cystoscope to the position of the open end of the cystoscope near
the urethral wall. A
tip of the radio-frequency electrode is pushed out along an off axis path to
pierce the urethral wall
so that it passes into the prostatic tissue outside of the urethra. Radio-
frequency energy from an
external generator system is then applied to the radio-frequency electrode tip
in the prostatic
tissue to ablate the tissue outside the urethral wall. For further explanation
of such a system and
procedure, see the paper by Goldwasser, et al., entitled "Trans-Urethral
Needle Ablation (TUNA)
of the Prostate Using Low-Level Radio-Frequency Energy: An Animal Experiment
Study;" Eur.
Urol., Vol. 24, pp. 400-405, (1993). Also, product literature on the TUNA
system available from
a company named Vitamed, Inc. Menlo Park, California, carries some description
of the
procedure.
The TUNA cystoscope is a rigid tube. It carries a straight fiber optic
visualization channel
so that the surgeon can view the scene directly ahead and slightly to the side
of the opening at the
distal end of the cystoscope. It is through that opening that the radio-
frequency electrode passes
and then pierces the urethral wall to enter the prostatic tissue. Although
there is some degree of
visualization intra-lumenally, that is, before the electrode pierces the
urethral wail, there is no
trans-lumenal visualization of the electrode tip in its placement after the
piercing of the urethral
wall. Thus, the TUNA procedure is a relatively blind procedure in the sense
that the end of the
RF electrode, once having penetrated the target tissue, cannot be seen.
Furthermore, a straight,
rigid endoscope, such as the urethral cystoscope, can not be used in many
clinical settings. For
example, to access the stomach, throat, or portions of the colon through the
rectal opening, a
straight endoscope is inadequate.
CA 02330468 2000-10-26
WO 99/SSZ44 PCT/US99/09294
3
It would be desirable to be able to perform radio-frequency ablation
procedures in many
organs throughout the body. However, many of these organs, such as the
pancreas, are very
difficult to access with an RF electrode in a minimally invasive way. For
instance, a tumor which
is 2 centimeters deep within the pancreas cannot be seen with a conventional
endoscope and
S therefore cannot be treated with a blind electrode approach. Percutaneous
techniques involving
passing electrodes through the skin are technically difficult, and associated
visualization and
navigation methods are elaborate and technically challenging. In other cases,
such as with
ailments of the gut or the abdomen, it may be desirable to ablate tissue and
internal organs that lie
adjacent to or several centimeters away from a natural bodily lumen.
Consequently, the techniques described above are limited in that they are not
well-adapted
to performing RF ablation in deep-lying tumors. Among the limitations of these
techniques are
the restrictions of using a straight endoscope and the lack of extra-lumenal
imaging to control
positioning of a radio-frequency electrode. Accordingly, an effective
technique for performing
minimally invasive, trans-lumenal radio-frequency ablation with image guidance
through a natural
bodily lumen is desirable for the purposes of treating cancerous tumors and
other clinical diseases
associated with bodily organs.
SUMMARY OF THE INVENTION
The present invention is directed to a system and procedure for trans-lumenal
radio-
frequency (RF) heat ablation of bodily tissue through and by the use of an RF
electrode or a laser
fiber that is passed through an endoscope. The system and procedure of the
present invention are
different than any of the systems and procedures discussed in the Background
section above. The
advantages of the present system and method reside, in part, in their superior
ability to access
non-superficial tumors and to provide image guidance. Image guidance
mechanisms may be
provided within the intra-lumenal endoscope itself or from an external image
guidance apparatus
to visualize the position of the RF electrode or laser fiber tip in the target
tissue.
As one example, a tumor of the pancreas can be effectively treated using the
present
minimally invasive system and technique. The technique of the present
invention involves
inserting a flexible endoscope through the throat to reach the region of the
stomach wall. One
CA 02330468 2000-10-26
WO 99/55244 PCT/US99/09294
4
portion of the stomach wall is in close proximity to the pancreas, which in
this example contains a
cancerous tumor identified by previous CT or MRI scanning. A long radio-
frequency electrode is
passed through the flexible endoscope. The electrode has a pointed tip which
emerges through an
opening in the distal end of the endoscope thereby enabling piercing of the
stomach wall to
penetrate the pancreas. An ultrasonic imaging head is built into the distal
end of the flexible
endoscope to visualize the pancreatic tissue near the distal end. The position
of the tip of the
radio-frequency electrode can then be adjusted under direct ultrasonic visual
guidance, so that it
can be placed into the pancreatic tumor. The RF electrode is then connected to
an RF generator
external to the body, thereby producing a heat ablation of the pancreatic
tumor. According to
clinical needs, other visualization methods such as MRI, CT, or external X-ray
or external
ultrasound could be used to assist visualization of the electrode tip as its
emerges from the
endoscope.
In contrast to the endoscope-directed intra-lumenal coagulation discussed in
the
Background section above, the RF electrode of the present invention has the
advantage that it can
be used to pierce the natural lumen wall; that is, it is used traps-lumenally.
It thus enables
treatment and ablation of tumors which lie deep within tissue in the region of
a portion of a
natural lumenal passage in the body.
The present invention procedure has the further advantage of being able to
control the
positioning of the ItF electrode through intra-lumenal image guidance via
ultrasound, or through
external image guidance using ultrasound or other image modalities. This
reduces the risks
associated with blind procedures such as the TUNA procedure cited above.
Also, the present technique, system, and method has the advantage that it
enables use of
flexible endoscopes, not just the straight cystoscope employed in the TUNA
procedure. This
makes possible access to a much wider range of target sites and cancerous
tumors. For example,
tumors in the liver, kidney, spleen, and pancreas may be accessed through a
flexible endotrachial
endoscope. Such access may further be enhanced by endoscopic ultrasonography
built into the
endoscope itself.
In other examples of the present invention, other forms of endoscopes can be
used to meet
clinical needs in other part of the body. For example, a bronchoscope enables
access to the lung
CA 02330468 2000-10-26
WO 99/55244 PCT/US99/09294
to perform RF ablation of tumors of the lung and mediastinum. A
choledochoscope can be used
to access the bile ducts for ablating tumors in the vicinity of the hepatic
portal and biliary tree.
Angioscopes can be used with RF electrodes according to the present system and
invention to
access organs through the vessels or arteries of the patient's body. Cranial
endoscopes or flexible
cranial endoscopes may access portions of the brain or endocranial cavity for
this purpose.
Ureteroscopes can be used for treating the upper genitourinary tract.
These features and advantages as well as others of the present method and
system will
become apparent in the detailed description that follows.
BRIEF DESCRIPTION OF THE DRAWINGS
In the drawings which constitute a part of the specification, embodiments
exhibiting
various forms and features hereof are set forth, specifically:
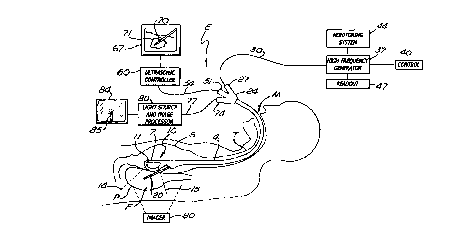
FIGURE 1 is a schematic diagram showing a portion of a patient along with a
system
according to the invention for performing trans-lumenal radio-frequency (RF)
ablation of the
pancreas using a flexible endoscope passed into the stomach through the
throat;
FIGURE 2 illustrates a portion of an intra-lumenal ultrasonic imaging
endoscope head
with an optical viewing channel and a trans-lumenal radio-frequency electrode
piercing a bodily
lumen wall to penetrate a target volume outside the lumen; and
FIGURE 3 is a flowchart of the process employed in operating a system in
accordance
with the present invention.
DETAILED DESCRIPTION OF THE INVENTION
Referring initially to Figure 1, in a system in accordance with the present
invention, a
flexible endoscope E is inserted into the stomach S of a patient through the
patient's mouth M and
throat T. The endoscope E has a flexible, elongated body 4 that can be
manipulated to direct a
distal end 7 of the endoscope E into the stomach S. The operating field of the
endoscope may
include organs within, near, or around the stomach S in this illustration.
Inside the distal end 7 of
the endoscope E is an ultrasonic scanner 10 having a scanning head 11 capable
of scanning a field
of view F demarcated by dashed lines 14 and 1 S. This field of view F may
include an organ such
CA 02330468 2000-10-26
WO 99/55244 PCTNS99/09294
6
as the patient's pancreas P, which lies near the stomach. Thus, in the
illustrated embodiment, the
ultrasonic scanner 10 is capable of scanning a portion of the wall of the
stomach S and the nearby
pancreas P.
To give a specific illustration in accordance with the system of Figures 1 and
2, an RF
electrode 106 having a stainless steel shaft 111 approximately one to several
millimeters in
diameter is partially covered with an insulating coating (illustrated by
hatched lines 114 in Figure
2). In one embodiment of the invention, the coating is one of many standard
plastic insulative
materials. The shaft 111 has a tip 121 that in various embodiments may be a
sharpened cone,
trocar, bevel, or other tissue-piercing structure. For example, in one
embodiment, 18-gauge
stainless steel tubing is used for the elongated shaft 111 of the electrode
106 in Figure 2. An
exposed tip portion 117 of the electrode shaft 111 has a length between one
millimeter and
several millimeters or several centimeters, depending on clinical needs. The
entire length of the
RF electrode 106 may be several centimeters to as long as 200 or 300
centimeters or more,
depending on which orifice and lumen the endoscope is designed for in the
patient's body.
Other materials may be used for the RF electrode shaft 111 and exposed tip
117, including
MRI-compatible materials with low magnetic susceptibility, such as high-cobalt
nickel, copper, or
Inconel. In one embodiment, the electrode shaft 111 is fabricated in a spiral
configuration for
greater flexibility, such as in a Seldinger wire. In another possible
embodiment, the shaft 111
comprises a wire construction coated by a catheter-like sheath, or
alternatively, a catheter with a
ring or helical coil external surface as part of its tip end. Examples of
various electrode
configurations can be seen in the article by E. R. Cosman entitled, "Radio-
Frequency Lesions,"
from Gildenberg and Tasker (eds.), Textbook of Stereotactic and Functional
Neurosurgery, New
York, NY: McGraw-Hill (1996), and also in the article, "Physical Aspects
ofRadio-Frequency
Energy Applications," by E. R. Cosman and W. J. Rittman, from Huang SKS,
(ed.), Radio-
Frequency Catheter Ablation of Cardiac Arrhythmias: Basic Concepts and
Clinical
Applications, Armonk, NY: Futura Publishing Company Inc. (1994).
The endoscope E itself may take any of various possible forms. In one
embodiment of the
invention, the endoscope E is a flexible device used for upper
gastrointestinal (GI) endoscopy.
Such devices are typically up to 1 meter long, and have a snake-like, flexible
or steerable body 4
CA 02330468 2000-10-26
WO 99/55244 PCT/US99/09294
7
(Figures 1 and 2). In an alternative embodiment, the endoscope E is a
gastroenterological
endoscope for lower endoscopy in the rectum or bowel. In another alternative
embodiment, the
endoscope is a cystoscope for urological applications; these are typically
much shorter in length.
In further embodiments, the endoscope may be a bronchoscope or a
choledochoscope for
applications in the lung, mediastinum, and upper thorax; or an endoscope which
is capable of
being inserted into a vessel in the vascular system, such as a vein or artery;
or an endoscope
which is capable of being inserted into the bile ducts, renal collecting
system, and upper urinary
tract. For known examples of such endoscopes, see the product lines of the
Panasonic and
Olympus companies.
In the example of Figures 1 and 2, the RF electrode 106 is inserted into a
tumor 108 (the
outline of which is indicated in a sectional view in Figure 2). Once the
exposed tip 1 I7 has been
positioned in the tumor 108, as identified via the ultrasonic scanner 10, the
electrode 106 is
connected externally to a high-frequency generator 37 (Figure 1), and radio-
frequency ablation
can begin.
. To give illustrative RF parameters that may be used in such a procedure, the
frequency of
the radio-frequency energy used for ablation can range from a few to many
hundreds or even
thousands of kilohertz. In a preferred embodiment of the invention, as with
other known ablation
systems and procedures, the high-frequency generator 37 is set to a frequency
in the range of 500
kHz (see, e.g., some of the generators sold by Radionics, Inc., Burlington,
Massachusetts). In
one embodiment of the invention, the RF electrode 106 has at least one
temperature sensor 118
(such as a thermocouple or a thermistor) attached to the exposed tip 117. The
temperature of the
tissue surrounding the exposed tip 117 can then be monitored during the heat
ablation process via
a monitoring system 44 used in association with the generator 37, as
illustrated in Figure 1.
Typically, the power output from the generator 37 that is delivered to the
tumor 108
through the RF electrode 106 is raised to an appropriate level to heat ablate
the tumor. If
temperature monitoring is performed, this can mean that the RF output is
raised sufficiently to
heat the tissue near the exposed tip 117 to greater than approximately 45
° C. Depending on
clinical needs, tissue temperatures as high as 90 to 100° C may be
necessary. Multiple passes of
the electrode into the tumor in different positions can further enlarge the
heat ablation volume. In
CA 02330468 2000-10-26
WO 99/55244 PCT/US99/09294
one embodiment of the invention, a cooling system is employed to circulate
coolant through the
electrode to produce even larger-sized lesions without overheating the tissue
near the exposed tip
117. By reference, see the paper entitled "Hepatic Metastases: Percutaneous
Radio-Frequency
Ablation with Cooled-Tip Electrodes," L. Solbiati, S. N. Goldberg, T. Ierace,
et al.,
Interventional Radiology, 205:367-373, 1997.
Typically, lesions of up to several centimeters in diameter can be
accomplished by using an
approximately 18-gauge radio-frequency electrode tip 117 having a length of
approximately 1 to 2
cm, raised to a temperature of around 90 ° C and kept at that
temperature for 3 0 seconds to
several minutes. As discussed above, the electrode 106 can range in diameter
from 0.1 to several
millimeters, and its length can range from 3" to 30" (approximately 8 to 80
cm) or more,
depending on the kind and size of endoscope used and the clinical application.
The temperature, amount of power, and other system parameters (such as the
length of
the exposed tip 117) are related to the size of the lesion desired. Desired
heat lesion sizes may
vary from several millimeters to several centimeters, depending on clinical
considerations. RF
generators with RF power output ranging from several hundred watts may be
needed, depending
on the power requirements to ablate a particular tumor volume. Monitoring of
lesion parameters
during the heating process is typical, and in one embodiment of the invention,
includes monitoring
tip temperature and RF power, current, volume, impedance, and time.
As shown in the embodiment of Figure 1, an external imaging apparatus, shown
as an
imager 90, may be employed to guide or control the position of the RF
electrode. In various
alternative embodiments, a CT, MRI, X-ray, or ultrasonic scanner may be used
to monitor the
position of the distal end 7 of the endoscope E and the exposed RF electrode
tip 117 in its
placement within the target region. An MRI imager is capable of visualizing
temperature
isotherms, and ultrasound can identify cavitating boiling during such
procedures. Thus,
monitoring with these two modalities as well as other available imaging
techniques can provide an
indication of the size of the heat ablation volume which is being produced to
treat the tumor
volume.
Referring further to Figure 2, the flexible endoscope body 4 has a distal end
7 which
includes within it an ultrasonic imaging head 11. The imaging head 11 has an
ultrasonic
CA 02330468 2000-10-26
WO 99/55244 PCT/US99/09294
9
transmission and detection element 104 pointing toward one surface of the
distal end 7. The
detection element 104 is capable of imaging ultrasonic signals in an angular
slice between the
dashed lines 14 and 1 S (as in Figure 1 ). This field of view of the
ultrasonic scanner 10 preferably
includes the tumor 108. The field of view also typically includes a portion of
the lumenal wall
100, as well as the tissue near it between the dashed lines 14 and 15.
Information from the ultrasonic scanner head 11 is communicated by connection
54 to
ultrasonic controller element 60 (Figure I). Further in Figure 1, a graphics
display 67 presents
representations of the ultrasonic scanning image. For instance, in one
embodiment of the
invention, the graphics display 67 is a CRT display, on which a rendering 70
of a radio-frequency
tip 20 within the tissue is shown together with a rendering 71 of the tumor
108 (Figure 2). A hub
24 of the endoscope has an adaption port S l which accepts the connection 54
for the ultrasonic
display.
As an alternative to the exemplary embodiments of Figures 1 and 2, the distal
end 7 may
include a portion of the components of an MRI scanner to produce MRI images of
tissue near the
distal end. This portion of components could comprise, for example, a sensing
coil as the
detection element 104 operating in cooperation with an external imaging
apparatus 90 to produce
MRI images through the controller 60 and the display 67.
Also shown in Figure 2 is an optical viewing element 130, which in a preferred
embodiment is a fiber optic illumination and viewing channel. At its distal
end is a viewing port
133, typically a lens. The optical viewing element 130 provides visual
information on the passage
of the exposed electrode tip 117 through the wall of the stomach S during its
passage to the
tumor 108. The hub 24 of the endoscope E (Figure 1 ) also has a port 74
through which the fiber
optic channel 77, which transmits a visual image from the viewing element 130
to the outside
world, is passed. A fiber optic light source and image processor 80 enables
visual representations
to be displayed on fiber optic display 84. In one embodiment of the invention,
the fiber optic
display 84 is a CRT display capable of showing a rendering 85 of the electrode
20. Thus, the
display 84 may show a representation of the actual electrode 106 as it passes
out through a port
124 at the distal end 7 of the endoscopic head, as shown in Figure 2.
As discussed above, the embodiment of Figure 1 includes a high-frequency
generator 37.
CA 02330468 2000-10-26
WO 99/55244 PCTNS99/09294
In embodiments of the invention in which the electrode 20 is a radio-frequency
electrode, the
generator 37 is a radio-frequency generator providing an electrical output. In
an alternative
embodiment in which the endoscope E provides, for example, a fiber optic fiber
or channel as an
ablative element, then the generator 37 is a power source for the generation
of laser signals and an
S accompanying power output.
A set of controls 40 for the high-frequency generator 37 (Figure 1) may
comprise knobs,
levers, or other control facilities enabled to control, for example, the power
output from the
generator 37. In one embodiment of the invention, the controls 40 allow the
power output to be
raised or lowered, started or stopped, or automatically or manually regulated.
A readout 47 is
10 also provided; in various embodiments it may comprise signal readouts or
representations of the
output parameters and other parameters associated with the generator 37. For
example, in an
embodiment of the invention in which the generator 37 is a radio-frequency
generator, the
associated parameters displayed on the readout 47 would preferably include
indications of power,
current, voltage, time, impedance, or other parameters associated with the
radio-frequency output
to the electrode 20. In the embodiment in which the generator 37 is associated
with a fiber optic
power source, then the readout 47 would preferably include indications of
laser energy,
frequency, and so on.
Also shown in Figure 2 is a satellite temperature monitor 150 with an
associated readout
and control system 154. In one embodiment, this apparatus includes a secondary
temperature
probe which can be inserted into, for example, the pancreas P, to monitor the
temperature of
tissue in the vicinity of the heat ablation region. For example, when a radio-
frequency current is
applied to the exposed electrode tip 117, then an ablative temperature zone
may be indicated by a
dashed line 110. In a preferred embodiment, the ablative temperature zone 110
is the isotherm
corresponding to approximately 45°C. Any tissue within that isotherm
may be permanently
destroyed or ablated if the temperature is sustained for several seconds or
minutes. The
temperature monitor 150, which in one embodiment is a thermocouple temperature
probe, is
placed in the pancreas P (as an example) at a point adjacent to very critical
structures such as
nerves, vessels, or adjacent organs. The temperature sensor can be used to
thereby ensure that
the temperature of that region does not exceed a dangerous temperature during
the course of heat
CA 02330468 2000-10-26
WO 99/55244 PCT/US99/09294
11
ablation of the target. The control and readout element 154 may be associated
with the control
and readout elements 40 and 47 (Figure 1 ), and in a preferred embodiment is
integrated with
them.
Referring now to Figure 3, a flow chart is shown to illustrate the process of
extra-lumenal
S RF ablation by means of an intra-iumenal endoscopic system according to the
invention. The
procedure starts by inserting the desired endoscope into the appropriate body
lumen (step 200).
As discussed above, the endoscope may be either flexible or rigid, and is of a
correct size and
length to accommodate the clinical need. Depending on the application, the
endoscope may be
inserted into the appropriate body lumen such as the throat, bronchi, bile
ducts, rectum, lung
cavity, upper urinary or lower urinary system, vagina, heart, or arterial or
venous vessels, etc.
This positioning step 200 of the endoscope may include the use of external or
internal imaging.
For example, the endoscope may incorporate an internal ultrasonic head, as
illustrated in Figures 1
and 2, and this can be used to achieve a desired position relative to the wall
of the lumen in which
it is inserted.
Once in the appropriate position, a high frequency electrode is passed through
the
endoscope channel (step 204). In one embodiment, the electrode has a tissue-
piercing point; it
punctures the lumenal wall for its trans-lumenal course into a target volume.
This process is
visualized (step 207) by way of an internal ultrasonic head within the
endoscope, as illustrated
above. External visualization using ultrasound, X-ray, MR, or CT may also be
employed in
addition to, or as an alternative to, intra-lumenal ultrasonic applications.
These internal and/or
external imaging methods may be continued during and after making the heat
lesion to help in
determining the adequacy of the ablation size and when to stop the heating
process.
The positioning of the RF electrode tip to the desired target volume (step
211) is
performed based on the intra-lumenal imaging apparatus or the external imaging
apparatus. For
example, the position of the exposed electrode tip 117 (Figure 2) can be
adjusted based on the
intra-lumenal scanning image on the display 67 (Figure 1 ) to achieve the
appropriate positioning
of the electrode tip within a tumor.
When the exposed RF electrode tip 117 (Figure 2) is in its proper position
within the
tumor, it is connected to the external generator 37, and high frequency power
is delivered through
CA 02330468 2000-10-26
WO 99/55244 PCT/US99/09294
12
the electrode tip 117 to the tissue to ablate the tumor (step 214). This step
can involve elevating
the voltage, current, or power applied by the high frequency generator 37 to
the electrode and
therefore to the tumor tissue. The generator 37 may have manual controls such
as knobs or
levers, or other elements to control its output levels that can be actuated at
this point. As an
S alternative, the process may be automated with an initial power setting or
temperature level
predetermined by the operator. The generator can then, under automatic or semi-
automatic
control, achieve the pre-selected parameter (such as temperature) and lock
onto it by a feedback
or control system within the generator 37. These elements are well-known in
the art, and could
be built into the control system 40 (Figure 1 ).
The step of adjusting and setting the output values (step 217) may include
setting the RF
power, voltage, or current levels, among other parameters. In one embodiment
of the invention,
this step includes achieving a desired temperature as recorded at the RF
electrode tip 117 or at the
satellite temperature monitor 150 placed elsewhere within the target volume or
neighboring the
operative field as illustrated in Figure 2. The time of RF power application
may also be
monitored. A pre-determined exposure time for high-frequency power to the
electrode may also
be desirable, depending on clinical needs, or may depend on the reading of
temperature sensors in
the RF ablation electrode at various positions. In one embodiment, multiple
temperature sensors
(e.g., 118) are placed along the RF electrode tip 117 to monitor the
temperature at various
positions within the target volume, and these temperatures can be read out on
a temperature
monitoring system 44 (Figure 1 ).
As an illustration, it may be known from clinical experience that a desired
ablation volume
can be achieved with a certain electrode geometry by applying a known value of
RF power,
current, or voltage, or alternatively by achieving a known temperature as
recorded in one or more
temperature monitors within the RF electrode or in surrounding tissue. These
parameters may be
monitored during the ablation process to influence the decision of the
clinician to terminate or
continue the process according to experience and parameter values. By
reference, measurement
of such parameters is illustrated by the RFG-3C lesion generator systems of
Radionics, Inc.
(Burlington, Massachusetts).
As discussed above, the duration and parameter settings used to achieve the
desired RF
CA 02330468 2000-10-26
WO 99/55244 PCT/US99/09294
13
heat ablation volume or effect may, depending on the position and type of
target to be ablated,
determine the time at which the heating process is stopped (step 221). The
decision to stop the
procedure when it is believed that the tumor volume or other target structure
of interest is
adequately ablated is made at this time. As previously stated, the use of
internal and/or external
imaging or diagnostic detection methods may be involved in this step. For
example, the use of
ultrasonic scanning or MRI imaging may enable visualization of the heat
ablation volume as it is
being made or after it has been made. An ultrasound scan can identify gas
bubble formation in the
heated region and MRI can visualize thermal distributions, both of which may
be an indication of
actual lesion volume.
In accordance with one embodiment of the present invention, the clinician may
choose an
RF electrode tip geometry of a certain size, diameter, and length. He may know
from experience
that the insertion of an electrode trans-lumenally in a particular clinical
site and delivering RF
power to raise the tissue temperature near the electrode tip to a certain
level will produce a
known and adequate ablation volume. These criteria may be used by the
clinician to induce
sui~cient ablation sizes. In accordance with another embodiment of the present
invention, the RF
electrode may not have a temperature sensor. The correlation of ablation size
desired for a given
electrode tip geometry may be determined by considering RF parameters such as
power, output,
voltage, and current. Generally, it may be known that RF power or current
levels greater than
certain values for a known electrode geometry will produce a desired size of
ablation volume. In
that case, the clinician may select the criteria of power and time to
determine a desired ablation
effect. Variations of the use of such parameters are embodied in the process
set forth in Figure 3.
In accordance with another embodiment of the present invention, if CT, MR, X-
ray, or
ultrasound images are used to monitor the ablation size, then they may be used
to decide an
adequate ablation size. For example, certain MR images can represent thermal
distribution
around the electrode and thus indicate the ablation zone. This may be used as
exemplary criteria
in determining when to terminate the ablation process, as in step 221.
Using extra-lumenal RF electrodes in combination with endoscopes and
endoscopic
ultrasonography has the advantages of making accessible target volumes such as
tumors in organs
that are near body lumens and cavities. The present system and method thereby
enable image-
CA 02330468 2000-10-26
WO 99/55244 PCT/US99/09294
14
guided control of radio-frequency ablation with minimal invasion. Tumors of
the pancreas, liver,
intestines, lungs, spleen, kidney, and upper and lower digestive system may be
reached in this
way, and accurate placement of ablation volumes can be made without the need
for major
surgery. Under image-guided control, such as endoscopic ultrasonography as
described above,
monitoring of the process and electrode placement becomes more accurate. These
advantages
will reduce the trauma to the patient, thereby permitting minimally invasive
ablation to be
administered to patients in frail health who cannot withstand open surgery,
and reduce hospital
expenses by minimizing hospital stay.
A further advantage of the present invention is that it enables precise
control of the
placement of electrodes in organs which otherwise may not be amenable to open
surgery. For
example, with ultrasound combined in the endoscope, visualization of the heat
ablation of the
tumor can be gauged in a visual and quantitative way. Because most bodily
tissues remain intact,
the visualization of the tumor by imaging remains largely undisturbed by the
minimal invasion of
the RF electrode. This is in contrast to open surgery, in which large position
shifts can take place
during surgical incision and retraction.
A further advantage of the present system and method over previous use of
coagulation
through endoscopes is that it is not limited to coagulation or burning of the
lining of the lumenal
structure. The present invention greatly expands the scope of, for example,
radio-frequency
ablative coagulation to much deeper structures within the organs near to the
lumenal structure.
Yet a further advantage of the present system and method is that it minimizes
risk to the
membranes or the mucosal structures of the lumen through which the RF
electrode is passed. In a
preferred embodiment of the invention, the RF electrode or other delivery
means is sufficiently
small-gauged so that it will not cause hemorrhage or permanent damage to the
lumenal surface.
By ultrasonography, the placement of the exposed RF electrode tip, for
example, can be deep
enough and away from the lumenal lining to prevent destruction of the lumenal
lining from the RF
heat itself.
Because the minimally invasive nature of the present invention is better
tolerated by
patients who may be otherwise too weak to withstand surgery, a wider
population of patients will
be suitable for this method. The minimally invasive character of the treatment
will also reduce
CA 02330468 2000-10-26
WO 99/55244 PCTNS99/09294
1$
side effects such as bleeding, the need for heavy anesthetics, prolonged
hospitalization and
recuperation, and convalescent care. All of these advantages have the
potential to reduce hospital
and medical reimbursement expenses.
While various forms and embodiments of the trans-lumenal radio-frequency
ablation
system and method involving various electrode designs and various temperature
sensors and
monitoring systems have been described in detail above, it should be
recognized that other forms
may be used. A wide variation of parameters for the electrode size, shape,
geometries, curvature,
and materials may be used without departing from the scope of the invention.
For example, an
electrode may be pre-shaped in a curved configuration to permit aiming the
electrode in a desired
direction once it has projected beyond the opening of the endoscope. Different
geometries of
electrode may be suitable for different clinical needs or target sites, and
these can be developed by
those skilled in the art without departing from the scope of the invention.
Electrode structures
having multiple electrode tips to fan out into the tumor volume may also be
used. In an
alternative embodiment, bipolar electrodes can be used, in which one or more
separate electrical
conductive surface areas are present on or along the elongated electrode
shaft. The different
electrode areas can be raised to different high frequency voltages at the same
or different times to
alter or grade the shape of the heat ablation region. Furthermore, the high
frequency electrode
can be cooled internally or by ejection of a fluid out of the tip region. In
one embodiment, cooled
saline injected into the proximal hub 24 (Figure 1 ) runs through a channel
inside the endoscope E
and flows out of the distal end 7 near the electrical contact so as to cool
the tissue near the tip 117
(Figure 2).
It is also important to note that various frequency ranges may be employed by
the RF
generator 37. For example, low radio-frequency signals in the range of 10 to
50 kHz,
intermediate radio-frequency signals between 50 and 1,000 kHz (1 MHz), or high
radio-frequency
into the microwave range of tens or hundreds of megahertz may be used, all
without departing
from the scope of the invention. Moreover, other elements analogous to the
electrode 20 in
Figure 1 may be used within the flexible endoscope, using endoscopic
ultrasonography to produce
extra-iumenal ablation. For example, the radio-frequency electrode could be
replaced by a laser
fiber. This may transmit optical energy from a laser generator (replacing the
high-frequency
CA 02330468 2000-10-26
WO 99/55244 PCT/US99/09294
16
generator 37 ofFigure 1) through a carrier 30, which may include fiber optic
bundles to deposit
energy in the region of the tumor 108 (Figure 2). Thus in Figures 1, 2, and 3,
the ablator or
ablative element used with the flexible endoscope and in combination with
image-guided ultra-
sonography and other imaging means may be considered generally as one of
several known
ablation systems. The device may include, therefore, electrical current and
power, or optical
current and power, or microwave antennas to produce the heat ablation of the
target volume.
In view of these considerations, as would be apparent by persons skilled in
the art,
implementations and systems should be considered broadly and with reference to
the claims set
forth below.