Note: Descriptions are shown in the official language in which they were submitted.
CA 02330894 2001-O1-12
Optically Aligned and Network-Stabilized Ferroelectric Liquid Crystals Using
Azobenzene-containing Diacrylate monomers
Yue Zhao~, Nadine paiement
Dpartement de chimie, Universit de Sherbrooke, Sherbrooke, Qubec, Canada J1K
2R.1
(yzhao@courrier.usherb. ca)
Background
Ferroelectric liquid crystals (I'LCs) have a chiral smectic-C phase (S~~). In
this phase, the
chirality gives rise to a spontaneous polarization (electric dipole without
the need for an
electric field) for each layer, which is normal to the director and the layer
normal.
However, the chirality also leads to rotation of the directors of successive
layers about the
layers' normal, thus forming a helical structure whose pitch is the distance
needed for a
complete rotation of the director. In a bulk S'c phase, the helical structure
is free to
develop and, consequently, no spontaneous polarization can be observed because
it
averages to zero upon a pitch. Therefore, the condition to obtain and to make
use of the
spontaneous polarization of FLCs is to suppress the helical structure by
aligning the FLC
molecules uniaxially along a certain direction. Currently, the only successful
method to
do that, which results in commercialized applications, is the surface-
stabilized FLCs
(SSFLCs)~.
CA 02330894 2001-O1-12
In SSFLCs, a FLC compound is filled in an ITO (indium-tin-oxide)-coated glass
cell
whose inner surfaces are parallely rubbed. When the cell gap is less than the
helical pitch
while the rubbed surfaces tend to align the molecules in the rubbing
direction, no helix
can be developed, and a spontaneous polarization normal to the binding plates
is
obtained. As the polarity of an electric field applied across the cell
changes, the
polarization switches between two (up and down) states, which correspond to
two stable
orientation states of the FLC molecules around the layers' normal. This
switching is the
basis for many applications of FLCs such as displays and light modulators. The
FCL-
based devices have a number of important advantages over other types of liquid
crystal
devices, such as a much faster switching speed and a viewing-angle independent
contrast.
However, some drawbacks limit the wide applications of SSFLCs. Among them, it
is
difficult to make and sustain a uniform alignment, particularly over large
areas, because
of the very small cell gap (generally 2-4 um). Also, similar to other liquid
crystal devices
that use surface orientation layers, surface-rubbing process can generate dust
and
electrostatic charges that are serious problems for manufacturing of the
devices. Despite
those difficulties, the great potential of FLCs has been the driving force for
considerable,
and increasing, worldwide research efforts in developing FLCs since the
discovery of
SSFLCs in 1980. For the reasons explained above, the uniaxial alignment,
leading to the
unwinding of the helix, is the key step for every FL,C technology. Polymer-
dispersed
FLCs2, microphase-stabilized FLCs3 and ferroelectric elastomers4 are examples
of the
intensive research efforts that are underway. In these techniques, a
mechanical shear is
used to induce the alignment.
Recently, making use of the well-known photoisomerization-induced alignment of
azobenzene molecules when exposed to linearly polarized light~'g, we have
succeeded in
aligning FLCs by light and fixing the molecular orientation by a polymer
network,
without the use of rubbed surfaces. This novel technique has been developed in
our
laboratory, and some results for nematic liquid crystals have been reported5'6
. The idea is
to use a diacrylate monomer bearing azobenzene group in its structure to
accomplish both
the induction and the stabilization of the FLC orientation. The basic
mechanism is as
follows: when an azobenzene-containing monomer is mixed with a FLC host,
irradiation
of the mixture with linearly polarized light results in preferential
orientation of the
azobenzene monomer in the direction normal to the polarization of the
irradiation light,
and a subsequent polymerization of the monomer under irradiation leads to the
formation
of an oriented azobenzene polymer network that, in turn, is able to align and
stabilize the
FLC molecular orientation in various liquid crystalline phases including the
chiral
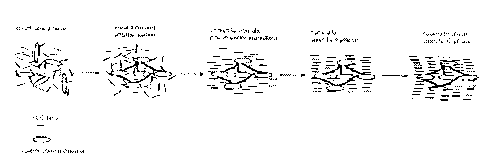
smectic-C phase. An example is given in Figure 1 that schematically
illustrates the action
of an oriented azobenzene network formed by irradiation and polymerization in
the
isotropic phase of the FLC host. When the mixture is cooled under irradiation,
the
anisotropy of the network induces a uniaxial molecular orientation of the FLC
in the
chiral nematic phase; once the orientation is built up, the irradiation can be
turned ofd; on
further cooling, this orientation is retained as the FL,C host goes through
phase transitions
into the smectic-A (Sri) and the S~~ phase.
As compared to SSFLCs, the optically aligned and network-stabilized FLCs may
have a
number of advantages. First, the photoalignrnent of the azobenzene monomer or
network
2
CA 02330894 2001-O1-12
is a bulk effect and can be uniform along the thickness direction;
consequently, the
preparation of thicker samples is feasible. Second, as no rubbed surfaces are
needed, the
rubbing-related problems are eliminated, and the cost of fabrication may also
be reducf:d.
The main disadvantage is the presence of a polymer network that can reduce the
spontaneous polarization and increase the switching time to some extent. This
optical
technique is completely original as it uses azobenzene-containing diacrylate
monomers to
induce uniaxial orientation in FLCs and to stabilize this orientation by the
azobenzene
network; no rubbed surfaces are required. To the best of our knowledge, a
number of
studies were reported utilizing a polymer network in FLCs. Among the most
representative and significant works, in one cases, the stabilization of FLC
alignment by
the network was demonstrated for thick films (cells); while in another case,
the polymer
network was used to improve the mechanical properties such as the shock
resistance of
SSFLCs9. In all cases, however, the orientation mechanism was different;
rubbed surfaces
were used to induce the FLC orientation, and the polymer networks contain no
azobenzene moieties.
Experimental Details for the Invention
The azobenzene-containing diacrylate monomer was synthesized in our
laboratorys and.
has the chemical structure shown below
=~~~0(C!-~)z~N/(C~)20~~==
,.
( ~~ ~:~ l
_,
N
N
[y
No~
It is nonmesogenic and has a crystal meting temperature at 80 °C and
the maximum
absorption around 442 nm. The FLC host used, CS-1031, was purchased from
CI~ISSO
Corporation (Japan). It is a ferroelectric smectic mixture having the
following phase
transition temperatures: Cr -12 °C Sc' 60 °C SA 85 °C N'
97 °C Iso. The helical pitch is
37 ~tm in the chiral nematic phase and 3 p,m in the Sc' phase. Typically, to
prepare a
mixture of CS-1031 with the azobenzene monomer, weighted amounts of both
components were dissolved in a common solvent, T'HF, together with an
initiator (~ 2
wt%) for polymerization; once a homogeneous solution was formed, the solvent
was
evaporated, and the mixture was dried in vacuum. For thermal polymerizations,
the
CA 02330894 2001-O1-12
initiator was azobisisobutyronitrile (AIBN) purchased from Aldrich, while for
photopolymerization it was Irgacure 907 provided by CIBA. The concentration of
the
azobenzene monomer that results in better alignment was found to be between 5
and 10
wt%. While warmed at about 50 °C, a freshly prepared mixture can then
be cast betwef;n
CaF2 windows to form thin films of about 4-5 Itm thick. For mixtures
containing the
photoinitiator, they can also be warmed at higher temperatures and flow-filled
in 5-pm
electrooptic cells. The cells used, purchased from E.H.C (Japan), have ITO-
coated but
non-rubbed surfaces. Various conditions for irradiation and polymerization
were
investigated. For thermal polymerization, it can be carried out either in the
isotropic or
the nematic phase of the FLC host; while temperatures in the SA and S'~ phases
are too
low to initiate thermal polymerization with AIBN. For photopolymerization, it
can be
performed in all phases of the FLC host. Using both polymerization methods,
better
alignment and homogeneity of the samples were obtained when the irradiation
was
applied at room temperature while heating the mixture to a specific phase for
polymerization. The two examples below describe the experimental details on
how to
prepare optically aligned and network-stabilized FLCs without the use of
rubbed
surfaces.
Example I Thermal polymerization
After warming a freshly prepared mixture, in a small bottle, containing 10 wt%
of the
azobenzene monomer at 50 °C for 10 min, a spatula was used to deposit a
small amount
of the mixture uniformly on a CaF2 window (12-mrn diameter), and a second CaF2
window was then placed to sandwich the mixture (a slight pressure was
necessary to
obtain a uniform film). No spacers were used in this case; the amount of the
mixture was
chosen to give rise to a film having a thickness of about 4 arm. Afterward,
the two
windows were fixed inside a temperature-controlled optical oven (microscope
hot stage:
purchased from lnstec) that was placed in front of a 1000-W Hg (Xe) lamp
(Oriel). The
distance separating the lamp and the sample was about 50 cm. The lamp was used
with a
polarizes for linearly polarized light and a filter allawing for the passage
of light at 440
nm (spectral resolution: 80 nm; transmittance: 45%). The irradiation was
turned on at
room temperature while the sample was heated to 120 °C for
polymerization in the
isotropic phase. It took about 5 min for the sample to reach 120 ° C
and the
polymerization at that temperature last 10 min. Finally the sample was cooled,
under
irradiation, to 90 °C (N' phase); then the irradiation was turned off
and the sample coolf:d
to room temperature.
To make sure that any obtained alignment in the sample was held by the
azobenzene
polymer network, following the preparation process the sample was reheated, in
the
absence of irradiation, to 120 °C for 10 min for equilibrium. It was
then cooled to the N',
SA and S'~ phases, and a number of techniques were employed to characterize
the
alignment. The example in Figure 2 shows a set of optical micrographs taken
under
crossed polarizers for this sample. Picture a) shows the texture of the
mixture before the:
irradiation and polymerization process; while pictures b), c) and d) reveal
the aligned
FLC host in its N', S~1 and S'c phases, respectively, when cooled from the
isotropic phase
after the preparation process. Bulk alignment is visible in both the S~ and
S'c phases, in
4
CA 02330894 2001-O1-12
the expected direction, i.e., normal to the polarization of the irradiation
light. The average
orientation of the FLC molecules was characterized by polarized infrared
spectroscopy
The spectra were recorded in the various phases during the cooling, with the
infrared
beam polarized parallel and perpendicular to the polarization direction of the
irradiations
light; the sampling area covered almost the entire film (about 10-mm
diameter). Figure 3
show the absorbance of the infrared band at 1430-crri 1, mainly arising from
the phenyl
groups of the FLC molecules, as a function of the angle between the
polarization of the
infrared beam and the normal of the polarization of the irradiation light. No
infrared
dichroism was observed in the isotropic phase, indicating the absence of a
molecular
orientation; once cooled into the Ns phase, the strong dichroism indicates the
induction of
a long-range molecular orientation; on further cooling this orientation is
retained in the
SA and S~c phases due to the network. Repeated heating-cooling cycles resulted
in no
change in the orientation. Moreover, polarized ultraviolet (UV) spectra (not
shown)
confirm that the azobenzene moieties on the network are oriented in the
expected
direction in all the phases.
Example 2 Photopolymerization
A freshly prepared mixture containing 10 wt% of the azobenzene monomer was
heated to
80 °C, and flow-filled in a S-trm, non-rubbed cell. The cell was then
fixed inside the
optical oven and placed in front of the lamp. The irradiation was turned on at
room
temperature, using the same polarizer and filter as indicated above. Before
photopolymerization, the mixture was heated to 120 °C for 5 min, and
then cooled to 90
°C for 10 min and to 50 °C for 30 min. As the linearly polarized
irradiation was set at 440
nm, which was far from the absorption maximum of the photoinitiator, no
polymerization
was initiated. After the 30-min-stay at 50 °C, both the polarizer and
the filter were
removed for 20 seconds, during which the non-polarized broadband light led to
the
photopolymerization. After the 20 seconds, the irradiation was turned off and
the sample
was cooled to room temperature.
In the absence of irradiation, the cell was reheated to 120 °C, in the
isotropic phase, for
min for equilibrium. On cooling, similar to samples prepared by thermal
polymerization, a long-range molecular orientation of the FLC host was
observed in the
N', SA and S~~ phases. Figure 4 shows an example of the optical micrographs
taken under
crossed polarizers for the cell. At room temperature, bulk alignment of the
S~~ phase is
obtained in the irradiated area while it is absent in non-irradiated areas.
Figure 4 also
shows another feature for the aligned S'~ phase. Its stable morphology is
characterized by
the formation of parallel lines of the azobenzene polymer network, in the
molecular
orientation direction of the FLC host. After the preparation process, it
generally takes
several hours to have those lines appeared, which are absent in the SA and N'
phases.
Fewer lines were observed for mixtures containing 5 wt% of the azobenzene
network.
In this example, the exposure with linearly polarized light as well as the
photopolymerization of the mixture was carried out using the same irradiation
source. It
seems that the very short time of photopolymerization using non-polarized
broadband
5
CA 02330894 2001-O1-12
light (e.g. 20 seconds) could not randomize the aligned azobenzene orientation
so that an
anisotropic polymer network could be formed. We have also examined the use of
two
different irradiation sources, one for the alignment and one for the
photopolymerization;
and found that alignment of FLCs could be achieved.