Note: Descriptions are shown in the official language in which they were submitted.
CA 02332795 2000-11-20
WO 99/61078 PCT/SE99/00904
FILM DRESSING OR A TAPE FOR ATTACHMENT TO SKIN.
The present invention relates to a plastic film dressing or an affixing tape
for skin
applications, comprising a plastic film layer coated with adhesive on one side
thereof.
Thin adhesive wound dressings made from a plastic film that is permeable to
water vapor,
for instance OpSite~ (Smith & Nephew, England) or Tegaderm~ (3M, USA), include
an
acrylate-type adhesive or an adhesive that has similar properties. When
repeatedly applied
and removed, such adhesives are apt to remove with them parts of the upper
skin layer (the
stratum corneum) and consequently dressings of this nature are liable to
result in skin
damage. These adhesives also fasten strongly to skin hairs, therewith causing
pain and
discomfort as the dressing is removed.
Also known to the art is a gauze dressing designated Mepitel~, which is
affixed to the
skin by means of a very soft adhesive silicone elastomer. Such a silicone
elastomer has
skin-friendly adhesive properties and is much more gentle to the skin than the
aforesaid
adhesives. Neither does it tend to strip away parts of the stratum corneum as
it is removed.
A person wearing such a dressing will not experience discomfort or pain as the
dressing is
removed. The adhesiveness of the silicone elastomer is not impaired by removal
of the
dressing, and the dressing can therefore be removed and replaced several
times.
Consequently it would be beneficial if the adhesives used with plastic film
dressings could
be replaced with an adhesive elastomer that has adhesive characteristics
similar to those of
the silicone elastomers used with Mepitel~, particularly when the dressing is
to be applied
to sensitive skin. One serious problem in this regard is that such elastomers
have been
found to adhere to the skin with the same strength as that to which they
adhere to the
plastic filin, and consequently there is a serious risk of the elastomer
layer, or large parts
thereof, remaining on the skin when attempting to remove the dressing.
One object of the present invention is to solve this problem and to provide a
fixnctional film
dressing which includes a layer of soft, adhesive elastomer.
This object is achieved in accordance with the invention by means of a
dressing or affixing
tape for skin applications which includes a plastic film layer that is coated
with adhesive
CA 02332795 2000-11-20
WO 99/61078 PCT/SE99/00904
2
on one side thereof and which is characterized in that anchoring elements in
particle form
are fastened to the plastic film layer over the whole of that side thereof
which is coated
with a fully or partially covering layer of adhesive; and in that the adhesive
is comprised of
a soft, adhesive elastomer which surrounds the anchoring elements and which
has a
smooth, unbroken surface on the side thereof distal from the plastic film.
Because the
adhesive elastomer is affixed to the plastic film via the anchoring elements,
the elastomer
will be anchored more strongly to the film than would otherwise be the case,
so as to avoid
the risk of the elastomer remaining on the skin when the dressing is removed
therefrom.
The anchoring elements affixed to the plastic film do not reduce the
flexibility and
stretchability of the plastic film to any appreciable extent, these properties
being important
in some types of dressing.
In one preferred embodiment of the invention, the anchoring elements are
comprised of
short fibres and the adhesive elastomer is a silicone elastomer.
In one variant, the anchoring elements are comprised of
particles.
The invention will now be described with reference to the accompanying
drawings, in
which
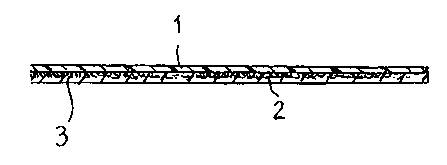
Figure 1 is a schematic cross-sectional view of a plastic film dressing or
affixing tape for
skin applications, according to one preferred embodiment of the invention; and
Figure 2 illustrates a softnesss measuring instrument.
The wound dressing illustrated in the figure is comprised of a plastic film
layer 1 whose
underside is coated with a layer of adhesive elastomer 2. Anchoring elements 3
in the form
of short, loose fibres are affixed to the plastic film over the whole of its
undersurface. The
anchoring elements 3 have been affixed to the underside of the layer 1 prior
to applying the
elastomer layer 2 and fimction to increase the surface area with which the
elastomer comes
into contact. As a result, the elastomer will adhere to the film layer 1
carrying the
anchoring elements 3 with a greater force than that with which it adheres to
the skin,
CA 02332795 2000-11-20
WO 99/61078 PCT/SE99/00904
3
therewith enabling a skin dressing of this kind to be removed safely from the
skin without
risk of the elastomer releasing its attachment with the film layer and
remaining on the skin.
In order to ensure that the flexibility and stretchability of the film layer
will not be reduced
appreciably as a result of applying the anchoring elements, said anchoring
elements will
preferably be affixed to the plastic film in a discrete fashion, i.e. mutually
unconnected.
The anchoring elements will also preferably be distributed relatively
uniformly over the
underside of the plastic film, so as to avoid local variations in the adhesion
of the elastomer
to the plastic film.
The anchoring elements 3 may comprise cellulose fibres measuring from 0.1-3 mm
in
length. Such fibres can be fastened to the film layer 1, by first providing
the layer with a
glue coating and then scattering or strewing the loose fibres over said glue
layer, e.g. by air
laying. Fibres other than cellulose fibres may, of course, be used, for
instance viscose
fibres, cotton fibres, polyester fibres, polyamide fibres and like fibres.
Alternatively, the anchoring elements may be affixed to the film by
flocculation or
extrusion for instance.
Although fibres are preferred as anchoring elements, there may be used, in
principle, all
types of particle material that can be fastened to plastic film. By particle
material is meant
in this document in addition to the aforesaid fibre material all material that
consists of
individual bodies having a size smaller than 10'3 mm3 regardless of the shape
of said
bodies. For instance, the particle material may comprise plastic granulate,
silicone dioxide,
silicone or the like. The elastomer layer shall have a thickness that exceeds
the largest
dimension of the particles, so as to ensure that all particles will be
enclosed regardless of
the position in which said particles have been affixed to the film layer.
The elastomer layer is preferably formed by an adhesive silicone elastomer
retailed under
the designation Silgel 612 by Wacker Chemie GmbH, Germany. It will be
understood,
however, that other soft, adhesive silicone elastomers, hydrogels or soft
adhesive hot melt
glue can be used.
CA 02332795 2000-11-20
WO 99/61078 PCT/SE99/00904
4
The wound dressing may, of course, be perforated, especially if it includes an
overlaying
absorbent pad.
It shall also be possible to sterilise the dressing by means of some
conventional sterilisation
method, e.g. b-sterilisation, steam sterilisation or sterilisation with
ethylene oxide.
The expression "skin-friendly adhesion" is used in this document to
characterise a
particular form of adhesion exhibited by the soft, adhesive eiastomers suited
for use with
the invention.
Different types of self adhering glues having relatively similar properties
are used
normally on adhesive dressings and surgical plasters of different kinds. A
common feature
of the glues normally used is that they adhere to the outermost layer of dead
skin cells
(stratum corneum) so firmly that a number of layers of these cells will be
stripped from the
skin by the glue as the adhesive dressing is removed. The glues used at
present are most
often acrylate-type glues, although hot melt glues and polyisobuthylene glues
are also
often used. In the case of skin-friendly glues, the penetration - which
constitutes a
measurement of softness - shall lie within the range of 7-20 mm, whereas
corresponding
values for those glues that are normally used with adhesive dressing are less
than 3 mm.
Penetration is measured by means of a method based on ASTM D 937 and D 51580.
Certain modifications are made. The equipment used is a penetrometer PNR 10,
Sommer
& Runge KG, Germany. A test body weighing 62.5 g and comprising a cone
weighing 15
g and bearing article number 18-0122 and a rod weighing 47.5 g and bearing the
article
number 18-0042 was placed in the penetrometer vertically above a cylindrical
cup
containing the material to be tested, with the apex of the cone touching the
surface of the
test material. The test body was then allowed to fall freely down into the
cylindrical cup.
The extent to which the test body had penetrated the test material was
measured after a
time lapse of 5 seconds. The cylindrical cup had a diameter of 50 mm and a
height of 30
mm. The cup was filled with test material to a level of 25 mm.
The soft elastomers having skin-friendly adhesive properties and used in
accordance with
the present invention had considerably weaker adhesive bonds to the skin than
the glues
normally used with adhesive dressings. Consequently, elastomers that have skin-
friendly
adhesive properties leave the stratum corneum essentially intact when
dressings containing
CA 02332795 2000-11-20
WO 99/61078 PCT/SE99/00904
such elastomers are peeled or pulled away. In spite of the weaker adhesive
bonds, the
elastomers nevertheless create secure and positive adhesion, i.e. there is
small risk of the
dressing loosening by itself, by virtue of the fact that the softness of the
elastomer causes it
to pass down into the skin and therewith provide a large effective contact
surface. The
5 softness of the elastomer also results in a large energy build-up in the
elastomer and its
carrier when removing the dressing, which also results in more positive
adhesion to the
skin.
An experiment was carried out on ten voluntary test persons with the intention
of
measuring the stripping effect, by which is meant the extent to which the
surface of the
dressing became covered with cells as a result of stripping-off the dressing.
Four different
types of plaster/dressings were used, these being Duoderm~, OpSite~,
Leukopore~ and
an elastomer-coated (200 g/m2) non-woven tape. The soft elastomer having skin-
friendly
adhesive properties was a silicone type elastomer. Three test samples of each
product type
were applied to each test person and left in place for 24 hours. The stripping
effect was
recorded, by coloring the stratum corneum cells present on the surface of the
removed
dressings selectively with toluidine, whereafter the percentage of the surface
covered by
cells was determined.
The results are evident from the table below:
Plaster/dressing number of dressings with stripping
within the range
<1% -10% 311%
Duoderm~ 0/30 0/30 30/30
OpSite~ 0/30 0/30 30/30
Leukopore~ 10/30 16/30 4/30
silicone tape 27/30 3/30 0/30
In order to be "skin-friendly adhesive", an adhesive dressing shall have a
stripping effect of
maximum 10% in the case of normal skin.
Because a dressing that includes a skin-friendly adhesive elastomer will only
carry with it a
very limited number of stratum comeum cells when the dressing is removed, the
surface of
CA 02332795 2000-11-20
WO 99/61078 PCT/SE99/00904
6
the elastomer layer will be relatively unchanged after removal of the
dressing. This enables
a dressing of this nature to be re-applied, since it adhesiveness has not been
impaired to
any appreciable extent. The adhesive surface of a dressing which pulls stratum
comeum
cells from the skin will be substantially covered with the cells subsequent to
its removal.
This means that dressings of this nature will fail to stick to the skin when
attempting to re-
apply the dressings. Duoderm~, OpSite~ and Leukoporev~ lose from 70 to 100% of
their
adhesiveness, whereas skin-friendly adhesive dressings lose less than 10%.
In order for an adhesive dressing or adhesive plaster to fimction effectively,
the force with
which it adheres to the skin must exceed the load to which the dressing or
plaster is
subjected during normal use. It has been found that there is generally
required in this
respect an adhesive force which exceeds 0,5 N measured when peeling or
stripping from
the skin a tape measuring 25 mm in width and angled at 135°, so that
the danger of the
dressing loosening by itself will not be unacceptably high. The adhesive force
will
preferably exceed 0.8 N/25 mm.
The adhesive force of Duoderm~, OpSite~ and Leukopore~ was measured at 1.2,
2.2 and
0.8 N/25 mm respectively with tape applied to the backs of healthy test
persons and left in
place for 24 hours. The silicone tape used in the stripping test above had a
skin adhesion
strength of 1.5 N/25 mm.
The plastic film will suitably comprise polyurethane, although other plastics,
such as
silicone plastic, may be used.