Note: Descriptions are shown in the official language in which they were submitted.
CA 02332842 2000-11-17
WO 99/60577 PCT/AU99/00376
HIGH LEVEL NUCLEAR WASTE DISPOSAL
This invention relates to the treatment and disposal of high level radioactive
wastes (HLW)
from nuclear reactors, and in particular relates to a mineral assemblage
incorporating HLW,
to a process for producing such .a mineral assemblage and to a process for
immobilisation of
HLW in a mineral assemblage which will retain dangerously radioactive isotopes
in the waste
for periods sufficient to ensure that they do not re-enter the biosphere prior
to their effective
decay.
HLW such as spent fuel from nuclear reactors, as are used in commercial power
plants,
contains a wide ralge of highly radioactive isotopes. These radioactive
isotopes emit
radiation which is dangerous to living organisms and must be disposed of in
such a manner
that they do not re-enter the biosphere during their effective decay periods.
Ore group of
these isotopes is formed by the fission of uranium (and plutonium). From the
disposal point
of view the most important components formed by such fission are 137Cs, 90Sr
and the
radio-active isotopes of the actinide elements. The fission products 137Cs and
90Sr have half
lives of about 30 years and must be contained for a period of about 600 years
before they
decay to safe levels. After 600 years, the dominant radioactive species in the
waste are the
actinide elements, principally isotopes of Pu, Am, Cm and Np which decay by
the emission
of alpha particles. After about a million years, the activity of the waste
becomes comparable
to that of the original uranium which was mined to produce the nuclear fuel.
This is usually
taken to be a suitable time for e:ontainment.
Spent fuel rods are generally reprocessed to recover plutonium and unused
uranium. During
this reprocessing the spent fuel rods are placed in cooling ponds for several
years to permit
the decay of several highly radioactive, short-lived fission products.
Subsequently, the rods
are chopped into sections and dissolved in nitric acid. Plutonium and uranium
are recovered
from the solution, the remainder of which is a HLW solution.
CA 02332842 2000-11-17
WO 99/60577 PCT/AU99/00376
_ '7 _
In most cases these HLW solutions are transformed initially into a solid,
insoluble form. This
is accomplished in the first instance by evaporating the HLW solution to
dryness and calcining
the material to produce a fine-grained mixture of radioactive oxides-called
"calcine". Calcine
is an unsatisfactory form for disposal because of its low density, low thermal
conductivity and
high solubility. Thus, further processing of this material is necessary for
its safe disposal.
One approach has been to incorporate the HLW calcine into a borosilicate
glass. The glass
is contained in thick stainless steel cylinders for burial in suitable
geological environments.
The shortcomings of this technique are well recognised and include the
thermodynamic
instability of glasses which is likely to lead to teaching of the HLW elements
over time.
Another approach to the problem has involved the incorporation of HLW caicines
into
ceramic materials composed of crystalline phases. Whilst this approach offers
some
advantages over the glass technique there are still a number of recognised
disadvantages
which result in the ceramic materials not being ideal for long term
containment of HLW
components.
A number of the problems inherent in the above approaches have been addressed
by the use
of a synthetic rock to retain the HLW elements. A synthetic rock known as
;iYNROC is
described for example in United States Patent No. 4,274,976. The SYNROC'.
materials are
a mineral assemblage containing well formed crystals capable of providing
lattice sites in
which the elements of the HLW are securely bound. The crystals belong to or
possess crystal
structures closely related to at le~rst two of the titanate mineral classes
selected from the group
consisting of perovskite (CaTiO,), zirconolite (CaZrTi20,) and hollandite-type
(I3aA12T16O16)
mineral classes. The SYNROC materials have been extensively investigated and
are predicted
to provide stable immobilisation of HLW elements, allowing the assemblage to
be safely
buried in an appropriate geological environment. Consequently, SYNROC
materials are in
the process of commercial development for the storage of HLW wastes.
CA 02332842 2000-11-17
WO 99/60577 PCT/AU99/00376
The extensive investigations into the SYNROC materials has led to the
incorporation of ruble,
and often with a small percentage of titanium metal. It has been reported that
the preferred
formulations of SYNROC have been designed to avoid destabilisation and
incorporate an
excess of rutile TiO~ such that rutile is the major phase of the SYNROC
assemblage.
Whilst SYNROC assemblages ai-e preferably formulated with substantial amounts
of ruble in
order to improve stability, ruble plays little or no part in taking up the
HLW. The present
invention is directed to an alternate assemblage which does not contain
signific;a~~t quantities
of rutile. Experiments associated with the present invention have identified
that mineral
assemblages which include cal:zirtite provide at least a useful alternative to
the' previously
disclosed SYNROC compositions.
Accordingly, in one aspect this invention provides a mineral assemblage
comprising crystals
belonging to or possessing cnrstal structures closely related to both the
perovskite and
zirconolite mineral classes and h~rther comprising crystals belonging to or
possessing crystal
structures closely related to th,e calzirtite mineral class wherein the
mineral assemblage
incorporates high-level radioactive wastes immobilized therein.
In the present specification, including the claims, the description of a
mineral complex which
forms part of a mineral assemblage is generally qualified by the term
"crystals belonging to
or possessing crystal structures closely related to ". This term will be
understood by those
skilled in the art to refer not only to the mineral complex having an ideal
crystal structure but
also to mineral complexes inc:arporating additional elements therein, such ;~s
elements
replacing one or more of the elements of the ideal crystal structure or
additional elements
retained interstitially within the ideal crystal structure. The additional
elements may give rise
to a departure of the crystal stmcture from the ideal.
Perovskite is a complex of calcium and titanium oxides having an ideal
formulation CaTi03.
Perovskite-type structures are adopted by ABO, compounds such as CaTi03
perovskite. The
ideal CaTi03 component is of orthorhombic symmetry.
CA 02332842 2000-11-17
WO 99/60577 PCTI.~LJ99/00376
_.
Zirconolite is a complex of calcium, titanium and zirconium oxides having an
ideal formula
CaZrTi,O,. Zirconolite may be amore generally described as CaZr~Ti2_x0, and is
a generic term
to encompass a group of closely related structural polyt;ypes which occur in
monoclinic, trigonal
and orthorhombic polytypes.
Calzirtite is a complex of calcium, titanium and zirconium oxides having an
ideal formula
Ca2Zr5Ti201~. Calzirtite may be more generally described as Ca2Zr5_XTix0r6 and
is a generic term
to encompass a group of closely related structural polytypes. Both calzirtite
and zirconolite are
anion deficient fluorite-related superstructure phases. In calzirtite, of
ideal composition
I0 Ca,ZrSTrzO,6, the canons occupy fluorite-type positions in the tetragonal
cell.
The mineral assemblages of thf: present invention preferably comprise crystals
of mineral
complexes which are relatively small in size, in order to maximise diffusion
controlled
uniformity of HLW incorporation into desired crystalline structures.
Preferably thf: crystals of
I 5 the mineral assemblages are generally of up to two hundred microns in
size.
We have found that the presence of titanium in the crystal structures of the
crystals of the
mineral assemblages of the present invention where the titanium is of at least
two different
co-existing valency states permits a more stable immobilization of the
radioactive isotopes of
20 the HLW within the mineral assemblage. The presence of Ti3+ and Ti 4+ is
particularly
preferred. In the present invention, particularly where it is desired to
incorporate Ti in a
number of different valency states, Ti203 may be present in solid solution in
the crystals of
the mineral assemblage.
25 Mineral complexes incorporating other elements may advantageously be
incorporated into the
mineral assemblages of the prest:nt invention. It is preferred that mineral
complexes which
incorporate barium and/or aluminium be incorporated into the mineral
assemblages.
Hollandite of general composition A,~BYCg_yO,b, ideal end member (BaAl2TisOr6)
is particularly
preferred as a host phase for HLW' elements together with perovskite,
zirconolite and calzirtite.
30 Under certain conditions of production, other Ba, Al phases may also occur.
The presence of
these elements and crystal structures is particularly preferred for hosting
certain HI:.W elements,
CA 02332842 2000-11-17
WO 99/60577 PCT/AU99/00376
_5_
which otherwise do nat readily ~aartition into the other phases. Other
hollandite-type mineral
complexes which may be included within the mineral assemblages include K and
Sr re;placing Ba.
Preferably, the assemblage includes at least some of each of the caizirtite,
zirconolite,
perovskite and hollandite. More preferably, the assemblage includes at least
10 weight
percent of each of calzirtite, z.irconolite, perovskite and hollandite.
We have found that the mineral assemblages may include at least 20 weight
percent HLW.
The present invention also provides a process for immobilizing high level
radio-active waste
comprising the steps of:
a) mixing a high level radio-active waste calcine with selected oxides;
b) heating and cooling said mixture to form a mineral assemblage comprising
crystals
belonging to or possessing crystal structures closely related to both the
peravskite and
zirconolite mineral classes and further comprising crystals belonging to or
possessing crystal
structures closely related to the calzirtite mineral class wherein the mineral
assemblage
incorporates high-level radio-active wastes immobilized therein.
The high level radio-active waster calcine is generally formed from a solution
of HLW such
as may be produced from commercial nuclear power plants. The calcine may
typically be
formed by evaporating the solution of HLW to dryness and calcining the
material to form a
fine-grained mixture of radio-a.etive oxides. The composition of a typical HLW
calcine
resulting from the fission of uranium (and plutonium) is set our in table 1
below:
CA 02332842 2000-11-17
WO 99/60577 PCT/AU99/00376
_(~_
TABLE 1
Typical composition
of calcin~ed high
level nuclear reactor
wastes
Mole per cent
Rare earths (REE elements) 26.4
Zr
13.f.
Mo 12.2
Ru 7. 6
Cs Fission
7.0
Products -
Pd 4.1
Sr 3.5
Ba 3.5
Rb 1.3
U + Th 1.4
Actinides
Am+Cm+Pu=Np 0.2
Fe 6. 4
(pp ) Processing contaminants3.2
4
Na 1.0
HLW elements may be incorporated into this mineral assembly by adding an 1-LLW
calcine
mixture prior to heating. The H~,W calcine may make up, up to about 20% by
weight of the
mixture of oxides.
The oxides may be mixed by any convenient means and heated by processes as are
known to
those skilled in the art..
CA 02332842 2000-11-17
WO 99/60577 PCT/AU99/00376
_ 'j _
The oxides are selected, having regard to their composition and their relative
proportions so
as to form the desired mineral a<,semblage. The oxides and amounts will be in
part dependent
upon the processing conditions and it will be apparent to those skilled in the
art how to make
such selections in order to obtan the mineral assemblages hereinabove
described. Oxides
which may be used in the process of the present invention include CaO, Zr02,
TiO~, Ti02-
Ti20, solution, AI203 and BaO, oxides, carbonates, gels or glasses.
The heating can be to either subsolidus or above solidus conditions. Heating
to above solidus
conditions may allow the mineral assemblage to be produced in less time.
In one form of the invention a mineral assemblage is preferably formed by
heating the
mixture of oxides to subsolidus conditions. The formation of the mineral
assernbiage under
subsolidus conditions requires they mixture be maintained at an elevated
temperature, say 1000
to 1600°C for a period sufficient for the mineral assemblage to achieve
phase equilibrium and
have the crystals of the desired particle size. The preferred time of heating;
varies with
temperature. It can be at a temperature of 1000°C for 36 hours or up to
a temperature of
1600°C for one four as well as intermediate temperatures and heating
times to produce
desirable results. It is then allowed to cool to ambient temperature.
The assemblage of this invention can be formed under pressure of one
atmosphere or by using
hot isostatic pressing techniques.
A reducing environment is preferably used for the incorporation Ti in a number
of different
valency states. This can be achieved by several methods under appropriate
reducing
atmospheric conditions. One approach is to take Ti02 and under appropriate
atmosphere
convert it into a Mag;nelli phase with the desired Ti4'-T'i~' solid solution.
This is then added to
the other oxides prior to synthesis. Another approach is to add Ti4+ directly
to the other oxides
and convert some of it into Ti3' prior to heating {with or without Ti metal).
CA 02332842 2000-11-17
WO 99/60577 PCT/AU99/00376
_g_
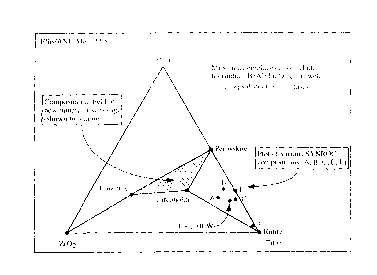
Figure 1 shows a molecular proportion phase diagram which illustrates the
mineral
assemblages of the present invention. Mineral assemblages according to this
invention are
within the area bordered by ithe lines respectively joining points marked as
calzirtite,
zirconolite and perovskite. The assemblages may also include hollandite (Ba
AIZTi60,6j.
Previous SYNROC compositions have fallen into the area bounded by the lines
connecting
zirconolite, ruble and perovskite.
Several reported SYNROC compositions are labelled A, B, C, E, F and fall
widzin the area
bounded by the lines joining the perovskite, zirconolite and rutile. The
mineral: assemblages
of the present invention at or alo~u~ phase equilibrium contain little or no
ruble. lJnder ideal
conditions at phase equilibrium t..ile mineral assemblages can contain no
ruble as ;rutile is not
stable at these proportions of Ca, Ti and Zr.
Figure 2 shows a molecular proportion phase diagram which incorporates Ti. As
can be seen
in Figure 2 the mineral assemblages which include perovskite, zirconolite and
calzirtite may
also include Ti203 in solid solution, in the absence of rutile.
In the drawings the calzirtite and zirconolite proportions are shown in
accordance with an
ideal consideration. As will be appreciated by those skilled in the art these
phases can show
significant solid solution formulation which has the practical effect of
varying the molecular
proportion co-ordinates.
The mineral assemblages according to the present invention contain a number of
coexisting
titanate and hollandite-type phases, in which calzirtite, zirconolite,
perovskite and preferably a
Ba-phase such as hollandite are prevalent. Other Ba-phases as well as
baddeleyite, srilankite may
occur under certain conditions. These phases, when synthesised under
appropriate conditions,
substitute HLW elements into their crystalline structures by a variety of
substitution mechanisms.
This results in significant departure from ideal end member compositions.
CA 02332842 2000-11-17
WO 99/60577 PCT/AU99/003?6
Without wishing to be bound by theory, it is believed that she HLW elements
are immobilized
in a number of coexisting minerals which depart from ideal compositions due to
their abilities to
accommodate HLW elements into their crystalline structures. The mineral
assemblages of the
present invention is believed to incorporate significant HLW components
through a variety of
S substitutions for Ga, Zr, Ti, Ba, Al in the various crystal structure sites.
Certain HLW elements
partition differently into the dif3erent phases, based on crystal chemical
principles. These
substitutions range from simple replacements (one element for another) through
to coupled
substitutions such that several HLW elements replace several element on
different
crystallographic sites in the ideal crystal structures.
In the perovskite-type crystals, it i s believed that both C:a and Ti may be
substituted to varying
amounts by certain HLW additive elements. This may be by either element-
element .replacements
(for example Ca replaced by Sr) or else more coupled substitutions involving
rare earth elements,
U, Na) as well as Ti3+. These sul'~stitutions can result in symmetries other
than orthorhombic
1 S (such as cubic, rhombohedral).
In the calzirtite and zirconolite type crystals, it is believed that certain
HLW elements such as
rare earth and actinide elements may be accommodated within the structure.
The present invention will now be described with reference to the following
non-limiting
examples, which vary depending; on solid solution within product phases as a
function of
synthesis conditions.
EXAMPLES 1
The oxides listed below were mixf~i in the specified amounts by physical
stirring .and grinding
until an essentially homogeneous. mixture was produced
Ca0 7. 9
ZrOz 26.1
Ti02 48.0
3 0 A1203 7.
2
Ba0 10.8
CA 02332842 2000-11-17
WO 99/60577 PCT/~U99/00376
- 10-
The mixture was heated in a meaal vessel at a temperature of 1300°C for
a period of 6 hours and
allowed to cool to ambient temperature. The resultant mineral assemblage had
the following
crystals present in the proportions listed below: ,
S perovskite 4.8
zirconolite 12
calzirtite 31.2
hollandite 52
EXA1VIPLE 2
The oxides listed below were mixed in the specified amounts by physical
stirrin~; and grinding
until an essentially homogeneous mixture was produced
Ca0 10.7
Zr02 35.2
TiO~ 41.9
A1203 4.9
Ba0 7.3
The mixture was heated in a metal vessel at a temperature of 1300°C for
6 hour's and allowed
to cool to ambient temperature. The resultant mineral assemblage had the
following crystals
present in the proportions listed below:
perovskite 6.5
zirconolite 16.2
calzirlite 42.3
hollandite 35
CA 02332842 2000-11-17
WO 99/60577 PCT/A~J99/00376
EXAMPLE 3
The oxides listed below were miixed in the specified amounts by physical
stirring and grinding
until an essentially homogeneous mixture was produced
S Ca0 10.0
ZrO~ 25.6
Ti02 40.2
A1203 6.0
Ba0 18.2
The mixture was heated in a metal vessel at a temperature of 1300°C for
6 hours and allowed
to cool to ambient temperature. The resultant mineral assemblage had the
following crystals
present in the proportions listed below:
perovskite 8.2
zirconolite 20.5
calzirlite 26.8
hollandite 44.5
The foregoing examples illustrate describes only some of the possible
variations in the present
invention and modifications can be made without departing from the scope of
the invention.