Note: Descriptions are shown in the official language in which they were submitted.
CA 02333526 2000-11-27
Description
Corrosion Resistant Steel Materials
Technical Field
This invention concerns weathering resistant steel
materials and, it: relates to a flow rust reducing
weathering resistant steel materials capable of
effectively reducing occurrence of flow rust in relatively
less salty circumstances such as mountain districts, rural
districts and industrial districts, as wel l as steel
materials excellent in earthquake proofness and coast
weathering resistance applicable as steel structures such
as bridges used i.n salty circumstances such as coast
districts. The weathering resistance referred to in the
invention means weathering resistance in a case of use in
atmospheric air of coast districts.
Background Art
1) Less salty circumstance
Weathering resistant steels with improved weathering
resistance in atmospheric air with addition of alloying
elements such as P, Cu, Cr and Ni in the steels have been
used generally for structures such as bridges. The
weathering resistant steels form, in several years, rust
1
CA 02333526 2000-11-27
referred to as stable rust less permeating oxygen and
water causing corrosion and suppress subsequent corrosion.
Accordingly, the weathering resistant steels require no
coating of anti-ru~;t paints and they are highly corrosion
resistant material which can be used in a so-called naked
state.
However, since as long as several years are required
till the stable rust is formed during which flow rust
occurs in the weathering resistant steels, they involve
problems of deteriorating scenes and causing environmental
contamination.
In view of t:he problems described above, Japanese
Patent Laid-Open No. 136557/1994, for example, proposes a
surface treating method for steel materials of coating an
aqueous solution of chromium sulfate or an aqueous
solution of copper sulfate and further applying organic
resin coating after drying the water content. Further,
Japanese Patent Laid-Open . No. 13158/1996 proposes a
surface treating meahod of steel materials of coating an
2d aqueous solution containing aluminum ions and further
forming an organic resin film after drying of the water
content.
However, in the techniques described in Japanese
Patent Laid-Open No.. 136557/1994 and Japanese Patent Laid-
Open No. 13158/1996" while stable rust is grown in a short
2
CA 02333526 2000-11-27
period of time, they still leave problems such that steps
are complicated and the surface treating agents used are
expensive, and development of weathering resistant steel
materials not requiring surface treatment have been
demanded.
In view of tree above, for coping with such a demand,
the invention intends to provide flow rust reducing
weathering resistant steel materials capable of reducing
occurrence of flow rust in the course of forming stable
rust in weathering resistant steels used in a naked state.
2? Salty circumstance such as coast districts
Steel struci~ures such as bridge girders are
generally applied with corrosion preventive means such as
coating since their= service life is long. However, the
coating films are degraded to gradually reduce the
corrosion preventive effect by chalking due to UV-rays, or
expansion of rust by the corrosion under coating films.
Accordingly, re-coating has been obliged on every certain
periods. However, shortening of coating operators and
increase in the personal expense in recent years makes the
re-coating operation difficult. In view of the situations,
weathering resistant steels requiring no coating of anti-
rust paints and usable in a naked state have been applied
more and more in steel structures.
3
CA 02333526 2000-11-27
The weathering resistant steels are those steel
materials with addition of P, Cu, Ni and Cr in which
stable rusts as protective films are formed in several
years on the surface of steels in an atmospheric
circumstance. Since the stable rust suppresses further
development of corrosion, corrosion of the steel materials
can be minimized. .Accordingly, most of them are used with
no coating.
However, in salty circumstances such as coast
districts, no stable salt is formed after lapse of several
years even in weathering resistant steels and steel
materials are attacked violently.
In recent years, application guideline for
weathering resistant steels have been issued from Minister
of Construction (Joint Research Report Regarding
Application of Weather Resistant Steel Material to Bridges
(XX), March 1993" published from Civil Engineering
Institute of Minister of Construction, KOZAI CLUB Co. and
Nippon Kyoryo Kenseasu Kyokai), in which it is specified
that existent weathering resistant steels (JIS G 3114:
weathering resistant hot rolled steel materials for
welding structure) can not be used with no coating in the
district where atmospheric salt content is 0.05 mg/dm2/day
or more, that is, in coast districts.
Accordingly, in salty circumstances such as coast
4
CA 02333526 2000-11-27
districts, countermeasure has been adopted by applying
coating such as of phthalic acid resin, chlorinated rubber
or tar epoxy resin to ordinary steel materials. However,
since bridges con:~tructed in coast districts near the
estuaries are often long and large and the corrosion is
violent because of the use in the coast districts, the re-
coating operation is extremely difficult and, accordingly,
there is a strong demand for the steel materials that can
be used with no coating.
Regarding this Japanese Patent Laid-Open No.
136557/1994, for example, leaves problems as described
above.
Further, Japanese Patent No. 2572447, Japanese
Patent Laid-Open N0. 51668/1993 and Japanese Patent Laid-
Open No. 134587/1996 propose methods of improving the
coast weathering rea istance by adding a great amount of
alloying elements such as P, Cu, Ni and Mo to steel
materials.
However, referring to the bridge, the corrosive
circumstance for steel materials are not always identical
depending on the places to be used. Considering, for
example, four main beam bridge, while outside of the beams
are exposed to rainfall, water of condensation and
sunshine, inside of the beam are exposed only to water of
condensation but not suffer from rainfall. Generally, in
5
CA 02333526 2000-11-27
a clean circumstance with no atmospheric salt content, it
is said that the exaent of corrosion is less in the inside
of the beams when compared between the inside and the
outside of the beams. On the other hand, in the
circumstance with high atmospheric salt content, it is
said that the extent of corrosion is rather greater in the
inside of the beam than the outside of the beam. This
reversal phenomenon occurs at a certain content of the
atmospheric salt content as a boundary but the content can
not be specified.
However, since outer beams, main beams and webs are
exposed to two circumstances (with or without exposure to
rainfalls) simultaneously (rear face and surface of
plates), it is necessary for the steel materials to be
used in steel strucaures such as bridges to maintain high
weathering resistan~~e in both of the circumstances.
However, in t:he existent techniques , evaluation was
applied only under one circumstance (with rainfall or
without rainfall), and development for steel materials
having excellent; coast weathering resistance
simultaneously under two circumstances has been demanded:
3) Earthquake proofness
On the other hand, the structural steel materials of
this type utilized, for example, in bridge beams, have
6
CA 02333526 2000-11-27
been demanded to have an absorption energy of 47J or more
at -5°C in a Chalp:y impact test in the rolling direction
(L direction) and ~a cross direction (C direction) to the
rolling direction of the steel materials in view of the
safety. However, it has been found that high stresses may
possibly exert in t:he direction of the plate thickness of
the material to be: used (Z direction) depending on the
structure and the portions of the structures in large
scale earthquakes such as Hanshin-Awaji disaster, so that
it has been demanded for the steel materials for use in
structures to improve the toughness in the direction of
the plate thickness (Z direction) including the weld heat
affect zone in order to further increase the earthquake
proofness of steel materials after the Hanshin-Awaji
disaster.
From the view points (1) - (3) above, the invention
intends to provide a steel material capable of forming
stable rust with good protective performance in relatively
less salty districts and salty circumstance such as coast
districts, regardless of rainfalls, excellent in weather
proofness and excellent in earthquake proofness with
improved toughness in the direction of Z also including
the weld heat affeci:ive zone.
Disclosure of the Invention
7
CA 02333526 2000-11-27
1) Flow rust reducing weathering resistant steel material
The present ~_nventors have made an earnest study for
the thickness capable of reducing flow rust in weathering
resistant steels and, as a result, have found that a
weathering resist=ant steel material capable of
outstandingly reducing the amount of flow rust by adding B
and, further, by controlling the content of B and the
content of one or more of P, Cu, Ni, Cr and Mo based on a
certain relationship to each other.
The invention. has been achieved on the basis of this
finding and the feature resides in a flow rust reducing
weathering resistant stee7L material having a composition
containing, on the weight ~ basis,
C: from 0.001% to 0.050%, Si: 0.60% or less, Mn: from
0.50% to 3.00%, S: 0.01% or less, A1: 0.10% or less and B:
from 0.0003% to 0.0050% and, further, one or more of
elements selected from P: from 0.005% to 0.15%, Cu; from
0.1% to 2.0%, Ni: from 0.1% to 6.0%, Cr: from 0.005% to
1.0% and Mo: from 0.005% to 1.0%, and satisfying the
following equation (1):
{20P + 3Cu + 3Ni + ~SCr +.Mo) / (1-0.2 (10000B) °'4) >_ 18 ... (1)
in which P, Cu, Ni, Cr, Mo, B: content for each element
(wt%)), and the balance of Fe and inevitable impurities.
Further, in this invention, one or more of elements
selected from Nb: f:rom 0.005% to 0.20%, Ti: from 0.005% to
8
CA 02333526 2000-11-27
0.20%, V: from 0.005% to 0.20%, on the,weight % basis, may
be contained in addition. to the composition described
above.
Further, in 'the invention, one or more of elements
selected from Ca: 0.02% or less and REM: 0.02% or less may
be contained, on the weight % basis, in addition to the
composition described above.
2) Coast weathering resistant steel material
The present inventors have made an earnest study for
improving the coast weathering resistance and, as a result,
have obtained a knowledge that Cr degrade the weathering
resistance in circumstance containing much salt. Further,
the present inventors have found that steel materials of
excellent weathering resistance even in salty
circumstances such as coast districts can be obtained by
controlling the content of B and the content of one or
more of P, Cu, Ni and Mo in relation the atmospheric salt
content.
3) Compatibility wit=h earthquake proofness
Further, the inventars have found that the sum of
inclusions, particularly, the amount of A series and B
series inclusions gives a significant effect on the
toughness in the Z direction and the toughness in the Z
9
CA 02333526 2000-11-27
direction can be :improved remarkably by restricting the
sum (dA + dB) value for the A series inclusion amount and
the B series inclusion amount according to JIS G 0555 to
0.030 or less.
At first, the result of experiment conductive by the
present inventors regarding the relation between the
toughness in the Z direction and the amount of inclusions
is to be explained.
Steels were prepared by melting while variously
changing the forms and the amount of inclusions into steel
plates of 60 mm thickness by hot rolling. Test pieces for
microscopic observation and test pieces for Chalpy impact
shock in the Z direction (JIS No. 4 test specimen) were
sampled from the steel plates, and the form and the amount
of inclusions and the toughness in the Z direction
(adsorption energy) were measured.
Fig. 1 shows a relation between the sum (dA + dB)
value of the A type inclusions and the amount of B type
inclusions according to JIS G 0555 and the Chalpy
absorption energy ("E_5) in the Z direction at -5°C. In the
Chalpy impact test, ten specimens were used for each of
the steel plates. Mean values and the minimum values for
ten specimens are p:Lotted respectively in the drawing.
As shown in Fig. 1,, when the (dA + dB) value is
0.030% or less, ab:>orption energy of 47J or more at -5°C
CA 02333526 2000-11-27
and high toughness in the Z direction are shown including
minimum values. C)n the other hand when the (dA + dB)
value exceeds 0.30%, low values appear for the minimum
value and also the mean value decreases below 47J.
Fig. 2 shows a relation between the dC value for the
amount of C type inclusions according to JIS G 0555 and
the Chalpy absorption energy in the Z direction at -5°C
(~E_5) . In Fig. 2, the relation between the dC value and
~,E-5 is shown for t:he steel plates having the (dA + dB)
value within range from 0.021% to 0.028%, which show high
toughness in the Z direction.
It was not re=cognized from Fig. 2 that the dC value
for the amount of C type inclusions give particular effect
on the toughness in the Z direction.
In view of t:he above, the inventors have obtained
the knowledge that control of the sum (dA + dB) value for
the A type inclusions and the B type inclusions is
important for improving the toughness in the direction of
the plate thickness. Particularly, it has been found that
the toughness in the direction of the plate thickness is
improved remarkably by defining the (dA + dB) value to
0.030% or less.
Fig. 1 and F:ig. 2 show the knowledge obtained from
the coast weathering resisl~ant steel materials and similar
results have also b~aen obtained for the flow rust reducing
11
CA 02333526 2000-11-27
weathering resistant steel materials (Fig. 3 and Fig. 4).
This invention has been accomplished based on the
findings described above.
Brief Explanation for the :Drawings
Fig. 1 is a graph showing a relation between the
toughness in the Z direction and the sum for the amounts
of A type inclusions and B type inclusions in coast
weathering resistant steel materials.
dC = 0% - 0.020%
mean value, ~1 . minimum value
Fig. 2 is a graph showing a relation between he
toughness in the Z direction and the amount of C type
inclusions in coast weathering resistant steel materials.
dA + dB = 0.020% - 0.028%
mean value, ~~ . minimum value
Fig. 3 is graph showing a relation between the
toughness in the Z direction and the sum for the amounts
of A type inclusions and B type inclusions weathering
resistant steel materials for less salty circumstance.
dC <_ 0.020%
mean value, ~ . minimum value
Fig.. 4 is a graph showing a relation between the
toughness in the Z direci~ion and the amount of C type
inclusions in weathering resistant steel materials for
12
CA 02333526 2000-11-27
less salty circumst:ance.
dA + dB = 0.020% - 0.028%
mean value, ~ . minimum value
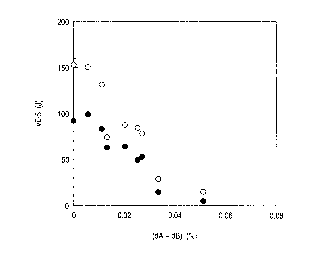
Fig. 5 is a graph showing a relation between the
amount of flow ru:~t and the A value (value in the left
side of the formula (1)) weathering resistant steel
materials for less salty circumstance.
Best Mode for Practicing the ,Invention
At first, reasons for defining the ingredients in
the steel materials according to the invention are to be
explained.
I) C: from 0.001% i~o 0.050%
C is an element for increasing the strength and a
content of 0.001% or more is necessary in order to obtain
a desired strength but the toughness is degraded when it
is contained by a great amount of exceeding 0.050%, so
that it is defined as from 0.001% to 0.050% in the
invention.
Preferably, ~~_t is from 0.005% to 0.030%. Further
preferably, it is from 0.005% to 0.025%.
2) Si: 0.60% or leas
Si is an element acting as a deoxidizer and
increasing the strength of the steel but, since the
13
CA 02333526 2000-11-27
toughness and the weldability are degraded if it is
contained by a greater amount, it is defined to 0.60% or
less. Preferably, :it is from 0.15% to 0.50%.
3) Mn: from 0.50% to 3.00%
Mn is an .element greatly contributing to the
increase of the strength and the toughness of the steel
and it is necessary to be contained by 0.50% or more in
order to ensure the desired strength in the invention.
However, when it is contained by a greater amount
exceeding 3.00%, ~t gives an undesired effect on the
toughness and the weldability, so that it is defined
within a range from 0.50% to 3.00%. Preferably, it is
0.50% to 1.80%:
4) S: 0.01% or les:~
Since S degrades the weathering resistance and
further degrades the weldability and the toughness , it is
defined to 0:01% or less.
Particularly, since it increases the amount of A
type inclusions and, particularly, lowers the toughness in
the direction of -the plate thickness and degrades the
weathering resistance, it is defined as 0.005% or less and,
it is preferably 0.003% or less with a view point of the
toughness.
14
CA 02333526 2000-11-27
5) A1: 0.10% or le;ss
A1 acts as a deoxidizer but since it gives an
undesired effect on the weldability when contained in
excess of 0.10%, the upper limit is defined to 0.10%.
Further, A1 i.s added as a deoxidizer but, when it is
contained in excess of 0.10%, the B type inclusions
increase to lower the toughness in the direction of the
plate thickness due: to the formation of alumina clusters.
Accordingly, A1 is defined to 0.10% or less and it is
preferably, 0.05% or less with a view point of the
toughness.
6) B: from 0.0003% to 0.0050%
B is an element for improving the hardenability and
also improving th.e weathering resistance and is an
important element in the invention. Such an effect is
recognized by the content of 0.0003% or~ more but no
corresponding effect to the content can be expected even
if it is contained in excess of 0.0050%. Accordingly, B
is defined within a range from 0.0003% to 0.0050%.
Preferably, it is within a range from 0.0003% to 0.0030%.
While the details for the mechanism in which B
improves the weathering resistance are not apparent, they
are generally considered as below.
CA 02333526 2000-11-27
Generally, for reducing flow rust, it is necessary
to form rust fronn the matrix in an early stage and,
further make the rust dense. The purpose of densification
is to improve the norrosion preventive effect by the rust
layer and to improve the adhesion of the rust layer to the
matrix. Adhesion of rust grains to the matrix is
considered to be attributable to the anchoring effect.
Accordingly, as t:he rust grains are more dense, the
anchoring effect i~: greater. By the way, the rust grains
formed from iron by anodic dissolution due to rainfall and
water of condensat:Lon are grown with water and densified
as pH value increases. In view of the above, it is
considered that B increases pH in the water immersed rust
layer to promote the densification of the rust grains.
7) P: from 0.005% t:o 0.15%
P is an element for promoting the anodic dissolution
of the matrix in the early stage of corrosion and making
the rust grains more dense and it is preferably
incorporated positively in this invention. Such an effect
is not recognized when the P content is less than 0.005%.
However, when it exceeds 0.15%, the effect of improving
the weathering resistance is saturated and, further, the
weldability is degraded. Accordingly, it is preferred to
define P within a range from 0.005% to 0:15%. Preferably
16
CA 02333526 2000-11-27
it is from 0.010% t:o 0.120%.
8) Cu: from 0.1% to 2.0%
Cu has an effect like P. That is, this is an
element for promoting the anodic dissolution of the matrix
in the early stage of corrosion and making the rust grains
more dense. However, the effect is insignificant if the
Cu content is less than 0.1% and, on the other hand, if it
exceeds 2.0%, it minders hot workability, the effect of
improving the weathering resistance is saturated to result
in economical disadvantage. Therefore, the content of Cu
is preferably within a range from 0.1% to 2.0%. It .is
preferably within a range from 0.1% to 1.5%.
9) Ni: from 0.1% to 6.0%
Ni densifies the rust grains to improve the
weathering resistance but the effect is insignificant if
it is less than 0.1%. On the other hand, even if it is
incorporated in excess of 6.0%, the effect is saturated
and the effect corresponding to the content can not be
recognized to result in economical disadvantage.
Therefore, Ni is preferably within a range from 0.1% to
6.0%. With a view point of the weathering resistance, a
range from 0.1% to :3.5% is desirable.
17
CA 02333526 2000-11-27
10) Cr: from 0.005% to 1.0%
Cr is an element for improving the weathering
resistance as far as less salty circumstance is concerned.
The effect is insufficient at the content of less than
0.005%. On the oi~her hand, even if it is contained in
excess of 1.0%, the effect of improving the weathering
resistance is saturated to result in economical
disadvantage. Thf~refore, the Cr content is suitably
within a range from 0.005% to 1.0%.
As described in the disclosure of the invention,
since Cr degrades the weathering resistance in a salty
circumstance it is not positively added.
11) Mo: frorn 0.005% to 1.0%
Mo improves i~he weathering resistance and, further,
increases the strength but the effect is insufficient at
the content less than 0.005%. On the other hand, even
when it is contained in excess of 1.0%, the effect is
saturated and no corresponding effect to the content is
recognized, to ~_esult in economical disadvantage.
Accordingly, Mo is preferably within a range from 0.005%
to 1.0%. With a view point of the toughness, it is
preferably within a range from 0.005% to 0.5%.
12) Ingredient defining formula (1)
18
CA 02333526 2000-11-27
[1] Relatively les;s salty circumstance
In the invention, the foregoing effects can be
provided by select_Lng one or more of five elements of P,
Cu, Ni, Cr and Mo and incorporating them respectively
within the ranges described above. However, the content
for each of the five elements has to be controlled in
relation with B so as to satisfy the following equation
(1)
(20P + 3Cu + 3Ni + 6Cr + Mo) / (1-0.2 (10000B) °'4) >_ 18 ... (1)
(where P, Cu, Ni, Cr, Mo, B: content for each element
(wt%) ) . This can outstandingly reduce the amount of flow
rust formed.
For example, Fig. 5 is a graph for the result
obtained by an atmospheric exposure test for weathering
resistant steel plates having various compositions for one
year in rural districts, taking the value in the leftside
of the equation (1) (referred to as A value) on the
abscissa and the amount of flow rust (Fe2+) from the test
specimens on the ordinate. As can be seen from the graph,
the amount of flow :rust is drastically reduced by defining
the A value to l8 or more.
[2] Salty circumstance such as coast district
In the invention, the P content and the content of
19
CA 02333526 2000-11-27
one or more of P, Cu, Ni and Mo are controlled, in
relation with the atmospheric salt content, so as to
satisfy the following equation (1) ;
(11P + 4 . OCu + 3. 1Ni + 2. ~Mo) / (1-0 . 1 (100008) °.3s) > 1 + 13X
... ( 1 )
(where P, Cu, Ni, Cr, Mo, B: content for each element
(wt%) , X: atmospheric salt content (mg/dm2/day) ) .
The weathering resistance in coast districts with
high atmospheric salt content is improved remarkably by
controlling the content for B and the content for one or
more of P, Cu, Ni and Mo so as to satisfy the equation (1).
Further, steel materials coping with corrosive
circumstance (atmospheric salt content X) are obtained by
controlling the content for B. P, Cu, Ni and Mo in
accordance with the atmospheric salt content X, which can
prevent incorporation of unnecessary alloying elements to
provide economical advantage.
In a case where the left side in the equation (1)
A _ (11P + 4. OCu + ~t.lNi + 2.6Mo) / (1-0.1 (100008) °~3s)
is smaller than the right side in the equation (1):
B = 1 + 13X,
that is, A < B, the corrosion resistant degrading effect
by the atmospheric salt content is greater than the
corrosion resistance improving effect by the alloying
CA 02333526 2000-11-27
elements. In order to improve the weathering resistance
by overcoming the c;orrosion resistance degrading effect by
the atmospheric salt content, it is necessary to control
the content for B, P, Cu, Ni and Mo so as to satisfy A < B.
In this invention, when there is an element not added
among the alloying elements in the equation (1), it is
assumed that the quotient of the elements is calculated as
0. X is defined as an atmospheric salt content measured
according to JIS Z 2381 gauge method.
13) One or more of elements selected from Nb: from 0.005%
to 0.20%, Ti: from 0.005% to 0.20% and V: from 0.005% to
0.20%.
Nb, V and Ti are elements increasing the strength of
steel and one or more of them can be added as required.
For any of Nb, V and Ti, the effect is recognized by the
incorporation of 0.005% or more but the effect is
saturated even when it is contained in excess of 0.20%
respectively. Accordingly, it is desirable that each of
Nb, V and Ti is from 0.005% to 0.20%.
14) One or more :>elected from Ca: 0.02% or less, REM
0.02% or less.
REM and Ca have an effect of improving the
weldability and can be added as required. The effect is
21
CA 02333526 2000-11-27
recognized by the addition of 0.0005% or more for any of
REM and Ca but the upper limit is defined as 0.02% since
addition of a greater amount degrades the cleanliness of
the steel material.
15) Other balance I?e and inevitable impurities
[1] Relatively less salty circumstance
In addition, the steel material according to this
invention comprises the balance Fe and inevitable
impurities. As the inevitable impurities, L: 0.010% or
less and 0: 0.010% or less are allowable.
[2] Salty circumstance such as coast districts
In the same manner, as the inevitable impurities,
Cr: 0.1% or less, n~: 0.010% or less and O: 0.010% or less
are allowable. Cr is added to weathering resistant steels
marketed at present as an element for improving the
corrosion resistance. However, this is a case in a less
salty circumstances and in those districts with high
atmospheric salt content, particularly, in coast districts,
the element rather deteriorates the weathering resistance
and, accordingly, 'this is not positively added in this
invention but it is allowable up to 0.1% as inevitable
impurities.
16) (dA + dB) value: 0.030% or less
22
CA 02333526 2000-11-27
In the invention, in addition to the definition for
the chemical ingredients described above, the sum (dA +
dB) value for the amount of A type inclusions and the
amount of B type inclusion according to JIS G 0555 is
defined as 0.030% or less considering the earthquake
proofness and with a view point of ensuring the toughness
in the Z direction (absorption energy in a Chalpy impact
test) of 47J or more at -5"C.
In this case,, the A type impurities are plastically
deformed by proce:>sing and B type impurities comprise
granular inclusions arranged discontinuously grouped in
the processing direction. In addition, C type impurities
(inclusions dispersing irregularly with no plastic
deformation) can be mentioned as one of classes.
The toughnescs in the Z direction is improved
remarkably by defining the (dA + dB) value to 0.030% or
less. It is considered that the A type or B type
inclusions have sensitive effect on the toughness in the Z
direction as stress concentration sources. It is
considered that decrease in the amount of the A type or B
type inclusions (dA + dB) decreases the stress
concentration sources, and, particularly, reduces the (dA
+ dB) value to 0.030% to thereby decrease the size of the
inclusions, so that: the toughness in the Z direction is
improved remarkably. Further, the corrosion resistance is
23
CA 02333526 2000-11-27
also improved by reducing the (dA + dB) value. This is
considered that local corrosion resulting from the matrix
and the inclusion boundary is suppressed by the decrease
in the amount of the impurities.
17) Manufacturing method
A manufactur5.ng method of steel materials' according
to the invention is to be explained.
The steel mE~lting according to the invention is
prepared by welding with a ordinary known melting method
such as a converter method or an electric furnace method
and prepared into steel materials by continuous casting
method or casting method. Further, in the melting step, a
vacuum degasing refining may be practiced. Then, the
steel materials ace after being heated in a heating
furnace or the like' and rolled to a desired shape by hot
rolling or directly not by way of heating. Further, the
steel materials according to this invention includes, for
example, steel plates, steel sheets, bar steel s and
profiled steels.
Example 1
Steels of chemical ingredient shown in Table 1 were
melted in a converter furnace and prepared into slabs by a
continuous casting process and the slabs were heated and
24
CA 02333526 2000-11-27
then hot rolled into steel plates of 25 mm thickness x
2500 mm width. Tensile property or characteristics and
impact shock characteristics of the steel plates were
investigated. Further, for the weldability, reproducing
heat cycles corresponding to 1 mm weld heat affect zone at
input heat of 100 kJ/cm were applied to determine the
absorption energy ~I~_5 at -5°C of the Chalpy impact test.
The result i:a shown in Table 2. Further, corrosion
test specimens of ~~ mm x 50 mm x 100 mm were sampled from
the steel plates. The specimens were shot blasted and
then served for atmosphere exposure test. In the
atmosphere exposure test, a rural district at an
atmospheric salt content of 0.02 mg/dm2/day was selected
and each of the test specimens was placed being directed
to a south direction and at an angle of 30° relative to
the ground surface and exposed for one year.
Simultaneously, flow rust from the specimens was received
in a plastic tank loo measure the amount of the flow rust
(Fe+2) . After the exposure test, a rust layer formed on
the surface of the matrix was removed and the weight
reduction of the test specimens was measured, which was
converted into the reduction of plate thickness. The
result is shown in Table 2.
Examples of t:he invention (steel types Nos. 1 to l1)
are excellent both in the toughness and the weldability.
CA 02333526 2000-11-27
On the other hand, comparative examples (steel type: Nos.
12 - 21) and an existent example (steel type: No. 22) have
comparable characteristics with those in the examples of
this invention excepting that they were degraded in those
in which the content for S, Cu and P are out of the upper
limit for the range of the invention (steel type: Nos. 13,
17, 18) .
The amount of flow rust in the examples of this
invention (steel type: Nos. 1 - 11) is as less as 29
~,g/cm3 to 67 ~.g/cm3, which is remarkably lowered compared
with 420 ~,g/crn2 of the existent example (steel type No.
22) with no addition of B and with lower A value, and the
reduction of the plate thickness is 8 (,gym to 23 Nm in the
example of the invention, which is smaller compared with
38 /am in the existent example, so that it can be seen that
the steel material according to the invention has
excellent weathering resistance.
On the other hand, the amount of flow rust in the
comparative examples (steel type: Nos. 12 - 16, 20, 21)
out of the range of the invention is increased as 300
~.~.g/cm2 to 390 ~,g/crn2 compared with the examples of this
invention.' The amount of the flow rust is large in each
of cases, that is, :in No. 12 since the P content and the A
value are excessively low, in No. 13 since the S content
is excessively high and the A value is excessively low, in
26
CA 02333526 2000-11-27
No. 14 since the Cu content and the A value are
excessively low, in No. 15 since the B content and the A
value are excessively low and in Nos. 20, 21 since the A
value is excessively law. Further, the comparative
example with excessively high P content (steel type: No.
17) and the comparative example with excessively high Cu
content (steel type: No. 18) are comparable with the
examples of the invention in view of the weathering
resistance (amount. of flow rust, reduction of plate
thickness) but the toughness and the weldability are
degraded. The comparative example of excessively high Ni
content (steel type No. 19) is comparable with the
examples of this invention in view of the weathering
resistance, the toughness and the weldability but the
elongation is poor since the strength is excessively high.
Example 2
Steels of chemical ingredients shown in Table 3
(Table 3-1 and Table 3-2) were melted in a converter
furnace and prepared into slabs by the continuous ousting
process. The slabs were heated and then hot rolled to
steel plates of 25 mm thickness x 2500 mm width. Further,
for a portion of th.e steels, H-steels of 800 x 400 x 16 x
36 size were also manufactured by hot rolling in addition
to the steel plates.
27
CA 02333526 2000-11-27
For the steel plates and the H steels, tensile
characteristics and the impact characteristics were
investigated.
Further, the test specimens were sampled at the
positions in the L direction and the Z direction at the
central portion of the plate thickness (1/2t part) for the
steel plates, and .in the L direction and the Z direction
at the central part of the plate thickness of a flange 1/4
part (1/2t part) for the H steels. The Chalpy impact test
pieces for the direction of the plate thickness (Z
direction) were sampled such that steel plates were
pressure welded to the surface and the rear face of steel
plates to increase the plate thickness up to 55 mm and the
notch part was at 1/2t part. The pressure welding was
applied under the condition considering so as not to
change the tissue a:nd the nature for the 1/2t part.
Further, for test specimens (in the Z direction)
applied with reproducing heat cycles corresponding to 1 mm
weld heat affect zone at input heat of 100 kJ/cm.
absorption energy in the Chalpy impact test -~E-5 was
determined to evaluate the weldability.
Further, corrosion test pieces each of 5 mm x 50 mm
x 100 mm were sampled from the steel plates and H steels,
shot blasted and served to an atmosphere exposure test to
evaluate the weathering resistance. In the atmosphere
28
CA 02333526 2000-11-27
exposure test, a rural district at an atmospheric salt
content of 0.01 mc~/dm2/day was selected and each of the
test specimens was placed being directed to a south
direction and at a.n angle of 30° relative to the ground
surface and exposed for one year. Simultaneously, the
amount of the flow rust (Fe2+) from the specimens was
measured. After the exposure test, a rust layer formed on
the surface of the matrix was removed and the weight
reduction of the test specimens was measured, which was
converted into the reduction of weight thickness.
The test results are shown in Table 4 (Table 4-1 and
Table 4-2).
Examples of the invention (steel materials Nos. 1 to
17) have high toughness of ~E_5: 6lJ or more including also
the toughness in the Z direction. Further, the examples
of this invention are excellent in the weathering
resistance evaluated based on the reduction of the plate
thickness and the amount of flow rust. The amount of the
flow rust in the examples of this invention (steel
material No. 1 to steel material No. 17) is as small as 25
~g/cm2 to 68 ~,g/cm2, which was remarkably decreased
compared with 420 Eig/cm2 for the amount of the flow rust
in the existent example (steel material No. 26), and it
can be seen that i~he steel materials according to this
invention have excellent weathering resistance.
29
CA 02333526 2000-11-27
On the other hand; in the comparative examples out
of the range of the: this invention (steel materials . Nos.
18 - 26), characteristics in one of the toughness in the Z
direction, the HAZ toughness (weldability) and the
weathering resistance are low and they are not suitable to
structural steel materials.
Example 3
Steels of the. chemical ingredients shown in Table 5
were melted in a converter furnace and prepared into slabs
by the continuous casting process. The slabs were heated
and then hot rolled into steel plates each of 25 mm
thickness x 2500 mm width, and H steels each of 800 x 400
x 16 x 36 size.
For the steel plates and the H steels, the amount of
inclusions, tensi7Le characteristics and the impact
characteristics were investigated according JIS G 0555.
The test specimen:> were sampled at a position for a
central part of the plate thickness (1/2t part) (L
direction) in the steel plates and for a flange 1/4B part
(1/2t part) (L direction) in the H steels.
Further, Chalpy impact test in the direction of the
plate thickness (2. direction) was also applied. The
Chalpy impact test pieces for the direction of the plate
thickness (Z direcaion) were sampled such that steel
CA 02333526 2000-11-27
plates were pressure welded to the surface and the rear
face of steel plates to increase the plate thickness up to
55 mm and the notch part was at 1/2t part. The pressure
welding was applied under the condition considering so ws
not to change the tissue and the nature for the 1/2t part.
Further, for the test specimens (Z direction)
applied with reproducing heat cycles corresponding to 1 mm
weld heat affect None at input heat of 100 kJ/cm, the
absorption energy ~H_5 at -5°C of the Chalpy impact test was
determined to evaluate weldability.
Further, the amount of inclusions was investigated
to determine the (dA + dB) according to JIS G 0555.
Corrosion teat pieces each of 5 mm x 50 mm x 100 mm
were sampled from t:he steel plates and the H steels, shot
blasted and then served to an atmospheric exposure test to
evaluate the weathering resistance.
In the atmosphere exposure test, a rural district at
an atmospheric salt: content of 0.8 mg/dm2/day measured by
JIS Z 2381 gauze method was selected and each of the test
specimens was placed with the matrix surface being
directed to a sough direr_tion under the condition free
from rainfall and exposed for one year. After the end of
the exposure test, a rust layer formed by exposure was
removed and the reduction of the plate thickness was
measured based on the reduction of weight.
31
CA 02333526 2000-11-27
The result is shown in Table 6.
The reduction of plate thickness in the examples of
the invention is from 6 Nm to 32 Nm, which is remarkably
smaller than the reduction of plate thickness (143 ~tm) of
comparative example (marketed weathering resistant steel,
steel material No. 19) showing excellent coast weathering
resistance.. The toughness in the Z direction in the
examples of this invention shows excellent earthquake
proofness as ~E_5 of 59J or more.
Any of the e~:amples of the invention shows excellent
earthquake proofless including the weld portion having ~E_~
at the weld heat affect zone of 169 J or more. Further,
the yield ratio was. as low as 76% in the examples of this
invention, which are excellent in the earthquake proofness.
On the other hand, all of the comparative examples
out of the range: of the invention show remarkable
reduction of plate thickness, lowering of the coast
weathering resistance or deterioration of the toughness in
the Z reduction.
In the steel No. 11, No. 13, No. 14, No. 15, No. 17,
the reduction of plate thickness is larger compared with
the reduction of plate thickness and the weathering
resistance is degraded.
The reduction of plate thickness of steel No. 12 is
comparable with that of the examples of this invention,
32
CA 02333526 2000-11-27
but the value (dA -~- dB) for the amount of inclusions is as
high as 0.074% and the toughness in the Z direction is as
low as "E_S . lOJ to lower the earthquake proofness
Further, the reduction of the plate thickness of the
steel No. 16 with high P content is comparable with the
examples of this invention and the coast weathering
resistance is excellent, but the toughness in the Z
direction is as low as ~E-5 . 33J to lower the earthquake
proofness and, further the toughness in the HAZ zone is as
low as ~E_5: 31J to .lower the weldability.
Further, in the steel No. 18 out of the range of
this invention with respect to Ni, the reduction of plate
thickness is small but the strength is excessively high as
TS: 92& MPa.
Example 4
Steels of chemical ingredients show in Table 7 were
melted in a converter furnace and prepared in the slabs by
the continuous casting process, the slabs were heated and
then hot rolled into steel plates of 25 mm thickness x
2500 mm width, and into H steels of 800 x 400 x 16 x 36
size.
Far the steel plates and the H steels, the amount of
inclusions, tensile characteristics and the Chalpy impact
characteristics were investigated according to JIS G 0555.
33
CA 02333526 2000-11-27
The test specimens were sampled at a position for a
central part of the plate thickness (1/2t part) (C
direction) in the ;>teel plates and for a flange 1/4B part
(1/2t part) (L direction) in the H steels.
Further, Chalpy impact test in the direction of the
plate thickness (;~ direction) was also applied. The
Chalpy impact test pieces for the direction of the plate
thickness (Z direction) were sampled such that steel
plates were pressure welded to the surface and the rear
face of steel plates to increase the plate thickness up to
55 mm and the notch part was at 1/2t part. The pressure
welding was applied under the condition considering so as
not to change the tissue arid the nature for the 1/2t part.
Further, for the test specimens (Z direction)
applied with reproducing heat cycles corresponding to 1 mm
weld heat affect zone at input heat of 100 kJ/cm, the
absorption energy ~E-5 at -5°C of the Chalpy impact test was
determined to evaluate weldability.
Further, the amount of inclusions was investigated
to determine the (dA + dB) according to JIS G 0555.
Further, corrosion test pieces each of 5 mm x 50 mm
x 100 mm were sampled from the steel plates and the H
steels, shot blasted and then served to an atmospheric
exposure test to evaluate the weathering resistance.
In the atmosphere exposure test, a rural district at
34
CA 02333526 2000-11-27
an atmospheric salt, content of 0.45 mg/dm2/day measured by
JIS Z 2381 gauze mE=thod w~.s selected and each of the test
specimens was placed with the matrix surface being upward
horizontally under the condition free from rainfall and
exposed for one year. After the end of the exposure test,
a rust layer fornned by exposure was removed and the
reduction of the plate thickness was measured based on the
reduction of weight.
The result is shown in Table 8.
The reduction of plate thickness in the examples of
this invention is from 14 ~1m to 40 ~zn, which is remarkably
smaller than the reduction of plate thickness (105 ~!m) of
comparative example (marketed weathering resistant steel,
steel material No:>. 2 to 16) showing excellent coast
weathering resistance. Th.e toughness in the Z direction
in the examples of the invention shows excellent
earthquake proofnes;s as ~E_S of 7OJ or more.
Any of the examples of this invention shows
excellent earthquake proofless including the weld portion
having ~E_5 at the weld heat affect zone of 292 J or more.
Further, the yield :ratio was as low as 80% in the examples
of this invention, which are excellent in the earthquake
proofness.
On the other hand, all of the comparative examples
out of the range of the invention show remarkable
CA 02333526 2000-11-27
reduction of plat:e thickness, lowering of the coast
weathering resistance or deterioration of the toughness in
the Z reduction.
deteriorates the toughness in the Z direction.
Steel materials Nos. 2-11, Nos. 2-13, Nos. 2-14, Nos.
2-15 of comparative: examples show more reduction of plate
thickness and deterioration in the weathering resistance
compared with examples of the invention since control for
the content of alloys is insufficient and the A value is
out of range of this invention and the corrosion resistant
deterioration due to the atmospheric salt content is
predominant.
In steel material Nos. 2-12 of the comparative
example, the reduction of plate thickness shows
substantially the same value as that of the invented
steels but since the amount of inclusions is more and the
(dA + dB) value is higher than 0.030, the toughness in
the Z directions is lowered to result in a problem in view
of the earthquake pi: oofnes s .
As described above, the steel material according to
this invention is a steel material excellent in weathering
resistance for coast districts with high atmospheric salt
content (coast weathering resistance) and further
excellent in the toughness in the Z direction also
including the weld portion and excellent in earthquake
36
CA 02333526 2000-11-27
proofness, which can be seen suitable as the steel
materials for use i.w steel structures.
Industrial Applicability
According to the .invention, weathering resistant
steel materials excellent in the earthquake proofness and
reduced flow rust can be provided. When the steel
materials are used for structural materials such as bridge
beams, the coating, surface treatment or the like can be
saved to give an e:~pectation for the economical effect of
reducing the maintenance cost to provide an outstandingly
excellent industrial effect.
Further, steel materials capable of forming stable
rust with good protective performance, excellent in the
coast weathering resistance and excellent earthquake
proofness also including the weld heat affect zone can be
manufactured at inexpensively. The steel materials
according to the invention can save the, painting or
surface treatment even in salty circumstances such as
coast districts, which can also expect an economical
effect of saving the maintenance cost and also can provide
a remarkable industrial effect.
37
CA 02333526 2000-11-27
P O~O- 1~P;COCO1~d;~ 07170P,N pptyI~~CO'c1,'OD
Q ~ N ~~ ~ N~ ~ N~ PM~I~I ~Ir"M ~ ~M o ~
~I
p
p ~
T
d
~_ O
O
C C C
4 O
C
W CO00I~COI~COO d7CO00P Ln V
r~-P ~-r-~-r ra-e- e'
I
\ ~ O Od O ~~ ~ ~~ ~ ~ ~~ ~ OO O ~tOO ~
O OO O OO O O C7O CO C
v
_!~
n
O O OO O OO O OO O O OO O OO O OO O O
S ~ ~ ~ ~ ~ ~
O,~~ OO In~ d' OId; L-
c o V
oo .-~-~-N Nc~io o cid o oo o cco cio
tC
~f~~ ~~ N t~~ ~ ~ m~ ~ ~f~~ N(~
c c co c co c c io o oc I
oo o c oo o o
t
a~ ~ ~~ ~ ~~ ~ ~~ ~ ~ g8 ~ ~~ ~ ~~ ~ ~'~
c d do c o0 o co c o cio citio o co c c M
t
c o cc o oc c ~oc c ciod o dd c cc c c ....
II
~'o ~ oo ~ ~~ ~ ~ c~~~~~ o ~~~o ~o ~ o
0 0 00 0 00 0 ~oo o o cic o.oc o oc c o
a
~~ ~3~~ ~ ~~ ~ ~~~ s g ~~ ~ ~~ ~1~~ ~ ~
r-!-P~-P PP O IOO P P ~'~-P !-P P O!'!'e-
f~M ~ ~~ ~ ~~ ~ ih~ ~ M
0 o do 0 00 0 to0 0 o cid o 00 0 00 0 0
vt ~tacsM coo ca~ r o ~- cor-
V ~ ~! ~ P!~~ I~ c T TP P~ ~ P r'T
~ ~ o o0 ~ P 0 00 ~ 0 ~o o Q
0
0 0 oo o ,o col~d o o oo o co ocoo o
o
~ teO C
O
P N Md'f3~Of~.CO~~ ~ ~ ~e ~ ~'-P r1~ N
'~ I - a
CA 02333526 2000-11-27
N
~1
C
i
~I
,
N N ~ ~ ~ s~~ ~ ~ ~ ~ M ~ ~ '~~ f~'tn~
M M N ~ r-
~
~ t~C~ c'~h~ e~~ d~'~ ~ c~
N ~ M ~ ~ ~ r"M
.-~ Cfl00 N Cf ~ N
~ M M ~ ~ M ~ ~ ~ ~ ~ ~ M ~ ~
H
I . Op Cfl C, 00~ r I~00
o N ~ ~ N ~ r9~ N M M ~ ~ ~ ~ ~ ~ N N
~~
W
a i ~ ~ ~~~ ~~~ ~ ~ ~ ~ ~ ~ ~ '~'~~d~ ~ ~ ~
a_~ u c c c c
f- o
5..
N
N
_
~ N M d'~ CC)I~.opM ~ ~ N M d't.~"acpI~00C5~ r-
F- ~ ,r~ ~ r ~ ~-~ ~-~ e-~ N
Z
CA 02333526 2000-11-27
CO Gfl ~- (~- N CC ~ 1' M C1 M r O 00 r CQ M N r CD CD
Op O ~ r Lri d' ~-' 00 00 CG1 CC r'I r C') C~7~ ~ OI
Q r N ~ r ~ ~ N N N M ~ ~ r N rI rI O ~ N N r
s O
0 O
O
r $s~
0 oco
r'°--' ~ ~ z
r
~~~ y
o ~ O ° ° ° ° m
>Z E= Z~z~z
00 O O 00 0~ c~0 O Co t~ 00 00 N C7 O 00 O et tp
r r r 1, r r r r r r r r
mg~~s~s~~sssss~sss.~~~~~ ~ N
ooodoo~ooococ~ooocooooo0 0
,
N O r N N 1~~ r r N r
~ U' ~ t!7 ~ ~ ~ ~ ~~ ~ ~ d: ~ 00 ~ ~ ~ ~ O ~ ~ ~ ~ ~ ~ ~
CO O O O O Ci « O O O CO GO O O cO C'O O O O O O O O
In Cfl r t r r r r
~z r r ~ ~ O ~ C~ s O O O M ~ M r N , ~ ~ , r MI r U
O O O O ~ r ~- N CV C'~ r O O O O O O O O CC CO O
i'
~~°~M~l i i i i i y '~'~ci o~!°~3 , ~3~ , ° ,
o co 0 o ci o o ~ o co ci o 0 0 +
U
N N M et
Uaoo~g~~~3~~~~~~a~~g~do~~~ M
oooococicoooodociooooo0000o a
C~ r ~ Ln 00 00 00 ~ Iln f~ P~~ Op l~ 4~ 00 Op
~~~~~~~~~~~~~~~~~~~~~g~~~ ~~
0ooooocooooooo~oooocoooc~
0oooooc~oooo0000000001000
~~~1~3~~~~~~~~d$~~I~~~~~~~M~~
r r r r r r e- CO O O r O r r r ~ r r r r r r r
d' Ln I~~ C N N lf~ N r r Op
(n ~ N r N ~ ~ ~ M ~ ~ M N r M M ~ ~ N
O C C O O O C7 O O C O O O O ~ O O C7 O O O O O
~r, c~, t~ a0 C7 ~c f~~ O CD 00 a0 r c~ c0 ~h ~ N ~- M o
r r r r r r r r r r r r
V~~~oo~c~~soo,o~NOOOONb''~~~oor
ociooco00000000000odococo00
M
a~ ~ZarnUO~~co=-~Y-Wzoa.a~um-»?~
CA 02333526 2000-11-27
Q
I N I N ~E ~ I ~ ~ N N ~ N
Cr0 ~ ~ ~ ~ ~ ~ ~ Mr ~ nj
N
s ~~~~~~3~~~~~~3~~~~~~~~~~~~~~
J
r [wIr rM tpl,~r M ~ ~ ~N
N ~ Nr ~ ~ r r r r r ~ r ~ N
M ~ ~ ~ ~ tN ~ ~ ~ ~ ~ ~ h ~ ~ dN' ~ N
U
~~~~~S~o~o~~~~~~og~~~~~~~g~
oococdoocooccoocoociococdco
~~~~~~~~on~~s~~s~~~~~~~~~~
o c o 0 0 0 0 0 0 0 o c c o c o c o 0 o c o o d d c
~~~~~~a~s~~~oo~~s~~~~~~~~~
d d o 0 0 0 0 0 0 0 0 o d c d o 0 0 ~ o of 0 0 o dl of
Q o0 U ~ I1I ~ O Z - ~ Y ~ ~ Z OI 0-I UI ~'.I fnl t-I ~I >I ~~
N
r N M d' tn to h~ 00 (~ a ~ N M d' ~ CO 1'~ 00 Oa ~ N ~ ~ N
r r r r r r r r r r
~f
CA 02333526 2000-11-27
vt;'vt;'stst;d;~i;d;~st'd; ~st; 'd;'~ d; d;
r rtP 1~1~~!~PP P1~'~t~ t~P !~!' P 1~
t~ P !~
!~!~P !~P Tr PP !~!'P!' !~!" PP !" !~
M No No ~cnr~rncyo wt aq y
rf~.r>N M~ c~iN ~n~fwo INo IMH ~~M ~ ~,jI
e-~-P e-!-a-P !-e-r-P P!- e-O ~-a- M
Q
tlJ
Q
~
Q Q ~ Q
~ Z E- Z
a_0~-_00r-_t~ _a00_0 _W - _4f7
8
O OO CC OO OC7OC OC OO OC O O
Z ~ ~3~ ~~ N~ g~~gS gS ~,o ~~ ~ oN
l
o
c
+
_
~ ~o ~~ ~~c~~~c~,o ~g ~~~ ~c~, ~ c~z
.-~-.-~-o oo oc~oo oo .-o .-~- o o~-
~
a ~ g 8~ ~8 ~~ ~~ ~g ~~ ~8 ~ ~~
o oo co oo oo 00 oo oo oc c oQ
~,:
U S H,
O OO OO OO OO OO OO OO OO O O'r .~.
!~
r
a ~ ~ ~~ So ~~~~~ ~~ ~o ~Ig ~ o~ ~
0 00 00 0o oo oo co oti oo o o~
MS ~~ S~~~ ~n/ ~~ ~M ~ ~~ m
P Pt~P!~1~' ~ ~ O ~~
! P! !r PP P! 1 O !"
OO O O C OO O OO ~ O
O
U ~ ~o ~~ ooo~~ ~o ~~ ~o ~~ g ~
C CC OC CO Ci OO OO OC OO C O
G
! - NM ~!O~ 0 O- M ~ M CO f~ a0 01
d' C ta ?.i ~N !- r P ~- P !- P
P
a-
4z
CA 02333526 2000-11-27
M M r r
. C7~ 1
Q
N
- ~ ~m
v
J
o N dO ~:~M ti'CpCDTN e- ~- N e- et'
v I~1~I~~Iwt~~ 1~!'~!1I'h 1~ (~ h~ W 1~
92 ~ ~ ~oc~o~~',~'Oc'~
d~W '
~ ~-O~ ~;rN ~ Or ~
~ ~a ~~~~ ~ ~o ~ ~o ~ ~ ~~ ~ o~
y ~ o o 0 0 0 00 o d oo o
c0 0 0 0 0 c 00
-Vo ~ ~~ ~~~~ o ~~ o ~~ ~ ~ ~~ ~ ~~
o do oc~o o cao o co 0 o cco d o0
C~Nt N ~ P. N
O O~~~ ~ ~O ~ ~OI~ ~ ~~ ~ O~~
C CC CO O O CC C OO O O OO O OGO
1C
CO~
N y - Nc~dW? O ~ OO O 'N C~ ~t ~ CO I~. 00 C~
C t C ' TT T r r' !~ T r T
T
CA 02333526 2000-11-27
m O OG~O C~07C~O(~O OO CnOO Q3
CG7CGCCCCCOCCCOCClCCCOCC~:i7CO!OCOCC
Q CpOIn~ O CO,P.tI7M ~ CO00tt7MM 0
~ I~00f~:f~~ f~-t~~~ r.COP:~I'7II I
,~~, C'CCtVI
iii
C
CC
O
O
E=
d'
= = ~'5
I I
_ODt_n ~ C_O ~_t~_t~'_~t O O_~-
I
\
O CC C O OC7OO COOO C CC
hl~iM~.~ ~~ ~0 ~ ~
C OO O ~ ~~ ~N ~ C7~'-~-Oa-O
t~~ ~~ ~ due,'~ ~ ~dN,'e~
O CO O COOO OO O OQ O OO G1
a ~ ~~ ~ ~ ~5 ~~ ~ ~~ ~ ~~ ~
c co c c oc cc c co c od o
~ ~ ~~ ~ 8 ~~ ~~ ~ ~8 ~ ~~ ~
0 0o o o oo ao o oa o oa o
~: o oo o ~ ~~ ~~ o o~ ~ ~~ s
0 00 0 0 0o oo o oa o oo o
~ c~'~~~ ~ c~c~c~'yg~ ~ ~c~~~ c~~ ~
, ,-~-~-, ~r-~o ~ ~-, ~ ,.-.,-
~ r. r- ~-
~f ~7~N ~ ~ ~~ I~G~M ~ ~~ ~ ~M ~
c oo o c oo ~oo c ocio oo o
U ~ o~ ~ o ~o ~~~ ~ o~ o oo ~I
o do o o oo ~oo o oo o do d
NM ~tun~~''CP~ O ~N M ttt~to
ei~~ c I~ a~~r-~ ~e~r-
N NN N N ~NN
N N
N NN N NN N
H
CA 02333526 2000-11-27
Q a
R
N
v
~s ~~~~~~~~~~~~~3~~~ ~
~~~~ ~~~~E:~~~~~~~~~~~
. ~~ ~3M~~R3~~~~~~~~3~~~
0
0 0 ° c c~ ° c o o c o o c o c o
°ccico~~ocrao°ccco°c
~~~8~~~~,'~~~~~~~~'~~
ticccidcccooccccc~c
~~ s~~
r-NCh~~~cp~°Pq'°'-NCnm.r~co
N N N N C~J N N N N ~ ~ ~ ~
N N N N N N N
øJ