Note: Descriptions are shown in the official language in which they were submitted.
CA 02333697 2000-12-O1
WO 00/00139 PCT/US99/i4641
DEVICE FOR MANAGING BODY FLUIDS COMPRISING A FAST ACQUIRING
LIQUID HANDLING MEMBER THAT EXPANDS UPON LIQUID ACQUISITION
AND CONTRACTS UPON LIQUID RELEASE
FIELD OF THE INVENTION
The present invention relates to devices for managing body fluids such as
urine, sweat, saliva, blood, menses, purulence, or fecal material, and in
particular
to their ability to acquire and retain aqueous based materials. The invention
further relates to disposable absorbent articles such as baby diapers or
training
pants, adult incontinence products, and feminine hygiene products and other
body liquid handling articles such as catheters, urinals, and the like.
Devices for managing body fluids are well known in the art and are
frequently used for a wide variety of purposes. For example, the devices may
serve hygienic purposes such as diapers, sanitary napkins, adult incontinence
products, underarm sweat pads, and the like. There is another class of such
devices which serve medical purposes such as wound dressings or drainages,
catheters, and the like. Accordingly such devices have been designed to cope
with a large variety of different body liquids such as for example urine,
sweat,
saliva, blood, menses, purulence, fecal material, and the like.
CA 02333697 2004-05-21
2
It has been recognized in the prior art that it is desirable to have large
open pores
in the acquisition region of the device to be able to readily accept body
liquids into
those voids even at high delivery rates. In addition, it has been recognized
that,
depending on the use, a large void volume for acquisition and intermediate
storage of
the liquid gushes is required close to the loading point. Large void volume,
however,
creates unwanted bulk for the device. The bulk thus created is particularly
undesirable
close to the body exits from which the body liquids are discharged such as the
urethra.
In order to limit the unwanted bulk at least before the first acquisition of
body liquid, it
has been suggested in the prior art to compress the acquisition material.
Examples for
this are acquisition patches comprising chemically stiffened cellulose which
have been
compressed in the moist condition in order to create hydrogen bonding to hold
the
material thin before discharge is acquired such as those disclosed for example
in
European patent publication No. 0 512 010 (Cook et al). Upon the first wetting
of such
a material, however, these material tend to irreversibly expand. Hence, all
such
materials still create unwanted bulk for the remainder of the usage period.
Other
acquisition material its which are thin until wetted for the first time have
been disclosed
in PCT patent publication No. W094/13704 which teaches a polymeric foam
material
commonly known as high internal phase emulsions. Some of the latter materials
will re-
contract upon dewatering, but those materials acquire liquid too slow to be
useful for
acquiring large gushes sufficiently fast.
It is an object of an aspect of the present invention to provide a liquid
handling
member which overcomes the problems posed by the prior art.
It is a further object of an aspect of the present invention to provide a
liquid
handling member which expands upon liquid absorption and contracts upon liquid
desorption while having a short absorption time for the absorption of 80% of
its total
liquid capacity.
It is a further object of an aspect of the present invention to provide a
device for
managing body liquids which comprises a liquid handling member of the present
CA 02333697 2004-05-21
3
invention.
It is a further object of an aspect of the present invention to provide
disposable
absorbent articles such as a baby diaper which comprises a liquid handling
member
according to the present invention.
SUMMARY OF THE INVENTION
The present invention provides a liquid handling member wherein the liquid
handling member has a contraction factor of less than 0.8 after the first test
cycle and
an expansion factor of at least 1.25 after the first test cycle according to
the Reversible
volume expansion test. The liquid handling member of the present invention
further has
a 80% absorption time of less than 2 seconds according to the Demand
Absorbency
Test.
It is another aspect of the present invention to provide a device for handling
body
liquids which comprises a liquid handling member according to the present
invention.
It is a further aspect of the present invention to provide a liquid handling
member
having a volume contraction factor of less than 0.8 after the first test cycle
and a
volume expansion factor of at least 1.25 after the first test cycle according
to the
Reversible expansion test and the liquid handling member has a 80% absorption
time
of less than 2 seconds according to the Demand Absorbency Test.
In accordance to a further aspect of the present invention, there is provided
a
device for managing body liquids comprising a liquid handling member as
described
above.
BRIEF DESCRIPTION OF THE FIGURES
Figure 1 shows a schematic drawing of the experimental setup for capillary
sorption test.
CA 02333697 2004-05-21
3A
DETAILED DESCRIPTION OF THE INVENTION
The present invention is described in the following by means of a variety of
different embodiments and by means of a variety of different features. Further
embodiments of the present invention may be obtained by combining features of
one
embodiment with features of another embodiment disclosed herein and/or with
other
features disclosed herein. These further embodiments are considered
CA 02333697 2000-12-O1
WO 00/00139 PCT/US99/14641
4
to be implicitly disclosed herein and hence form part of the present
invention. It
will be apparent to the skilled person that combinations of certain features
may
lead to non-functional articles not forming part of this present invention.
It is one aspect of the present invention to provide a liquid handling member
which may be used for example in devices for handling body liquids such as
diapers, training pants, sanitary napkins, adult incontinence devices and the
like.
The term "liquid handling device" as used herein refers to devices which are
designed to handle body liquids such as urine, blood, menses, sweat, saliva,
feces, and the like. Handling body fluids includes but is not limited to
acquiring,
distributing, and storing the body liquids.
The liquid handling member of the present invention is designed to rapidly
acquire body liquids. This capability may be quantified by the demand
absorbency test defined hereinafter. The liquid handling member of the present
invention has a 80 percent absorption time of less than 2 seconds, preferably
a
80 percent absorption time of less than 1.5 second, more preferably a 80
percent
absorption time of less than 1.25 seconds, most preferably a 80 percent
absorption time of less than 1 second.
The liquid handling member of the present invention further exhibits an
expansion contraction behavior that allows the liquid handling member to only
have a high bulk when it is needed for body liquid acquisition and to have
substantially reduced bulk when it is not needed. In particular, the liquid
handling
member of the present invention expands when liquid is absorbed such that the
liquid handling member provides about as much bulk as is needed to acquire the
body liquid. Furthermore, the liquid handling member of the present invention
contracts again upon desorption of the previously acquired body liquid. In
other
words, the bulk of the liquid handling member of the present invention is
reduced
when the body liquid is removed again from the liquid handling member for
example to be stored in a different region of the device for handling body
liquids
CA 02333697 2000-12-O1
WO 00/00139 PCTNS99/14641
of the present invention. This expansion contraction behavior of the liquid
handling member of the present invention is quantified by the reversible
volume
expansion test defined hereinafter. The liquid handling member of the present
invention has a volume contraction factor of less than 0.8 after the first
cycle,
5 preferably of less than 0.7 after the first cycle, more preferably of less
than 0.6
after the first cycle, most preferably of less than 0.5 after the first cycle.
In
addition the liquid handling member has a volume expansion factor of at least
1.25 after the first cycle, preferably of at least 1.43 after the first cycle,
more
preferably of at least 1.67 after the first cycle, and most preferably of at
least 2.0
after the first cycle.
It is further desirable for the liquid handling member of the present
invention
to exhibit a similar expansion contraction behavior also for subsequent body
liquid loadings. This capability can be quantified by running two absorption
desorption cycles of the reversible volume expansion test defined hereinafter.
Preferably, the liquid handling member of the present invention has a volume
contraction factor of less than 0.9 of the second cycle more preferably of
less
than 0.8 after the first cycle, most preferably of less than 0.7 after the
first cycle.
Preferably, the liquid handling member of the present invention also has a
volume expansion factor of at least 1.11 after the second cycle more
preferably
of at least 1.25 after the second cycle, and most preferably of at least 1.43
after
the second cycle.
It is further desirable for the liquid handling member of the present
invention
in view of subsequent body liquid loadings, to not lose absorbent capacity on
subsequent absorption desorption cycles. This capability can also be
quantified
by the reversible volume expansion test defined hereinafter. Preferably, the
liquid handling member of the present invention has a capacity decrease factor
of more than 0.7 after the first cycle more preferably of at least 0.8 after
the first
cycle, and most preferably of at least 0.9 after the first cycle. In addition,
the
liquid handling member of the present invention preferably has a capacity
decrease factor of more than 0.5 after the second cycle, more preferably of at
CA 02333697 2000-12-O1
WO 00/00139 PCT/US99/14641
6
least 0.6 of the second cycle, and most preferably of at least 0.7 after the
second
cycle.
It is further desirable for the liquid handling member of the present
invention
to provide sufficient absorbent capacity to be able to completely acquire a
gush
of body liquid. Obviously, the size of a gush depends on the intended use of
the
liquid handling member or the respective device for handling body liquids. For
example, the average gush size for toddlers in the weight range between nine
and 18 kilograms is 75 milliliter, the 95 percentile of the gush size for this
user
group is at 110 milliliters. In contrast, the average gush size of adult
incontinent
persons is about 180m1. For the purposes of this invention, the absorbent
capacity of the liquid handling member of the present invention is be
quantified
by the demand absorbency test defined hereinafter. Preferably, the liquid
handling member has an absorbent capacity of at least 80 percent of an average
gush volume of the intended user group, more preferably an absorbent capacity
of at least 100 percent of an average gush volume of the intended user group,
most preferably an absorbent capacity equivalent to at least the 95 percentile
of
liquid gushes of intended user group.
In some embodiments of the present invention, the liquid handling member
is intended to only temporarily store the acquired liquid. Accordingly, the
liquid
handling member must allow for liquid release sometime after the liquid
acquisition into a liquid storage member. In this context, it is desirable for
the
liquid handling member of the present invention that only a low capillary
pressure
is needed for dewatering the liquid handling member. On the other hand, the
liquid handling member must hold the acquired liquid in order to avoid
immediate
flow-back of the acquired liquid. Inventors have found a optimum desorption
pressure range which is representative for this capability. The optimum medium
desorption pressure is determined by the capillary sorption test defined
hereinafter. Preferably, the liquid handling member of the present invention
has
a medium desorption pressure between 5cm and 20cm, more preferably
between 5 cm and 15 cm, most preferably between 5 cm and 10 cm.
CA 02333697 2004-05-21
7
Optionally, the liquid handling member of the present invention has a
high permeability in order to be able to efficiently transport the acquired
liquid.
For the purpose of this invention. For the purpose of this invention, this
capability is quantified by the permeability test defined in PCT publication
No.
WO 00/00129, published January 6, 2000. Preferably, the liquid handling
member of the present invention has a permeability of at least 10 Darcy, more
preferably of at least 50 Darcy, most preferably of at least 100 Darcy.
In the following, a suitable embodiment of the liquid handling member will
be described. The liquid handling member is assembled from an open celled
foam material which is completely envelope by a membrane. A suitable
membrane material is available from SEFAR of Riischlikon, Switzerland, under
the designation SEFART"" 03-20/14. A suitable foam material is available from
Recticel of Brussels, Belgium, under the designation Bulpren SIO black. A
suitable technique to completely envelope the foam material with the
membrane material is to wrap the membrane material around the foam
material and to subsequently heat seal all open edges of the membrane
material. It will be readily apparent to the skilled practitioner to choose
other
similarly suitable materials. Depending on the specific intended application
of
the liquid handling member, it may also be required to choose similar
materials
with slightly different properties. After assembly, the liquid handling member
is
activated by immersing the liquid handling member in water or in synthetic
urine until the liquid handling member is completely filled with liquid and
until
the membranes are completely wetted with liquid. After activation, a part of
the
liquid inside the liquid handling member may be squeezed out by applying an
external pressure to the liquid handling member. If the activation of the
liquid
handling member was successful, the liquid handling member should not suck
air through the membranes.
Other liquid handling members suitable for the purposes of the present
invention are described for example in the PCT publication No.
CA 02333697 2004-05-21
8
WO 00/00129 entitled "Liquid transport member for high flux rates between two
port regions" filed in the name of Ehrnsperger et al., published on June 6,
2000,
and in the following PCT patent applications co-filed with the present
application entitled "High flux liquid transport members comprising two
different
permeability regions" (WO 00/00146) filed in the name of Ehrnsperger et al.,
"Liquid transport member for high flux rates between two port regions" (WO
00/00143) filed in the name of Ehrnsperger et al., "Liquid transport member
for
high flux rates against gravity" (WO 00/00138) filed in the name of
Ehrnsperger
et al., "Liquid transport member having high permeability bulk regions and
high
bubble point pressure point regions" (WO 00/00136) filed in the name of
Ehrnsperger et al.
The particular geometry of the liquid handling member of the present
invention can be varied according to the specific requirements of the intended
application. If, for example, the liquid handling member is intended to be
used
in an absorbent article the liquid handling member may be defined such that
its zone of intended liquid acquisition fits between the legs of the wearer
and
further that its intended liquid discharge zone matches the form of the
storage
member associated to it. Accordingly, the outer dimensions of the liquid
handling member such as length, width, or thickness may also be adapted to
the specific needs of the intended application. In this context, it has to be
understood , however, that the design of the outer form of the liquid handling
member may have an impact on its performance. For example, the cross
section of the liquid handling member directly impacts on its permeability.
For application of the liquid handling member in a device for handling
body liquids according to the present invention, the liquid handling member
may be combined with a storage member. The term "liquid storage member"
refers to a device which is capable of acquiring and storing liquid. The
volume
of the liquid storage member may vary with the amount of stored liquid such
as by swelling. Typically, the storage member wilt imbibe the liquid by means
of capillary suction
CA 02333697 2004-05-21
9
and/or osmotic pressure. Other storage members may-also use vacuum as a
means to store the liquid. The liquid storage member is further capable of
holding at least a portion of the stored Liquid under pressure. Suitable
storage
members are well known in the art and may comprise for example a super
absorbent polymeric material such as polyacrylate. The storage member may
further comprise a fibrous structure, such as a pad of cellulosic fibers, in
which
the particulate superabsorbent material is dispersed. A suitable storage
member is for example a superabsorbent polymer such as available from
CHEMDAL, United Kingdom, under the designation ASAP400T"". Further
examples of suitable superabsorbent polymers, often also referred to as
"hydrogel forming polymer" or "absorbent getting material", are described in
U.S. Patent 5,562,646 (Goldman et al.), issued Oct. 8, 1996 and U.S. Patent
5,599,335 (Goldman et al.), issued Feb. 4, 1997.
In order to pick up the liquid discharged from the liquid handling
member, the storage member may be placed in direct liquid communication
with the intended liquid discharge zone of the liquid handling member.
In one embodiment of the present invention, the liquid handling member
of the present invention is geometrically saturated or substantially
geometrically saturated with free liquid. The term "free liquid" as used
herein
refers to liquid which is not bound to a specific surface or other entity.
Free
liquid can be distinguished from bound liquid by measuring the proton spin
relaxation time T2 of the liquid molecules a according to NMR (nuclear
magnetic resonance) spectroscopy methods well known in the art.
The term "geometrically saturated" as used herein refers to a region of a
porous material in which the liquid accessible void spaces have been filled
with a liquid. The void spaces referred to in this definition are those which
are
present in the current geometric configuration of the porous material. In
other
words, a geometrically saturated device may still be able to accept additional
liquid by and
CA 02333697 2000-12-O1
WO 00/00139 PCTNS99/14641
only by changing its geometric configuration for example by swelling, although
all
voids of the device are filled with liquid in the current geometric
configuration. A
device for handling liquids is called geometrically saturated, if all porous
materials
that are part of the device and intended for liquid handling are geometrically
5 saturated.
The term "porous material" as used herein refers to materials that comprise
at least two phases a solid material and a gas or void phase - and optionally
a
third liquid phase that may be partially or completely filling said void
spaces. The
10 porosity of a material is defined as the ratio between the void volume and
the
total volume of the material, measured when the material is not filled with
liquid.
Non-limiting examples for porous materials. are foams such as polyurethane,
HIPS (see for example PCT patent application W094/13704), superabsorbent
foams and the like, fiber assemblies such as meltblown, spunbond, carded,
cellulose webs, fiber beds and the like, porous particles such as clay,
zeoiites,
and the like, geometrically stnrctured materials such as tubes, balloons,
channel
structures etc. Porous materials might absorb liquids even if they are not
hydrophilic. The porosity of the materials is therefore not linked to their
affinity for
the liquid that might be absorbed.
The term "substantially geometrically saturated" as used herein refers to a
member in which at least 90% of the macroscopic void volume of the member
are geometrically saturated, preferably at least 95% of the macroscopic void
volume of the device are geometrically saturated, more preferably 97% of the
macroscopic void volume of the device are geometrically saturated, most
preferably 99% of the macroscopic void volume of the device are geometrically
saturated.
Device for handling body,~~ ~ir~
It is one aspect of the present invention to provide a device for handling
body liquids which comprises a liquid transport member according to the
present
CA 02333697 2000-12-O1
WO 00/00139 PCTNS99/14641
11
invention. Such devices include but are not limited to disposable absorbent
articles such as baby diapers or training pants, adult incontinence products,
and
feminine hygiene products and other body liquid handling articles such as
catheters, urinals, and the like.
In one embodiment of the present invention, the device for handling body
liquids is a disposable absorbent article such as a diaper, a training pant, a
sanitary napkin, an adult incontinence device, or the like. Such an absorbent
article may further comprise a liquid pervious topsheet, a liquid impervious
backsheet at least partially peripherally joined to the topsheet. The
absorbent
article may further comprise an absorbent core which may serve as a storage
member for the body liquid. Topsheets, backsheet, and absorbent cores suitable
for the present invention are well known in the art. In addition, there are
numerous additional features known in the art which can be used in combination
with the absorbent article of the present invention such as for example
closure
mechanisms to attach the absorbent article around the lower torso of the
wearer.
Unless stated otherwise, all tests are carried out at about 32°C +/-
2°C and
at 35+/- 15% relative humidity.
Unless stated otherwise, the synthetic urine used in the test methods is
commonly known as Jayco SynUrine and is available from Jayco
Pharmaceuticals Company of Camp Hill, Pennsylvania. The formula for the
synthetic urine is: 2.0 g1: of KCI; 2.0 g/1 of Na2S04; 0.85 gll of (NH4)H2P04;
0.15
g/1 (NH4)H2P04; 0.19 g/1 of CaCl2; ad 0.23 g/1 of MgCl2. All of the chemicals
are
of reagent grade. The pH of the synthetic Urine is in the range of 6.0 to 6.4.
Demand Absorbency Test
The demand absorbency test is intended to measure the liquid capacity of
liquid handling member and to measure the absorption speed of liquid handling
CA 02333697 2000-12-O1
WO 00/00139 PCTNS99/14641
12
member against zero hydrostatic pressure. The test may also be carried out for
devices for managing body liquids containing a liquid handling member.
The apparatus used to conduct this test consists of a square basket of a
sufficient size to hold the liquid handling member suspended on a frame. At
least the lower plane of the square basket consists of an open mesh that
allows
liquid penetration into the basket without substantial flow resistance for the
liquid
uptake. For example, an open wire mesh made of stainless steel having an open
area of at least 70 percent and having a wire diameter of 1 mm, and an open
mesh size of at about 6mm is suitable for the setup of the present test. In
addition, the open mesh should exhibit sufficient stability such that it
substantially
does not deform under load of the test specimen when the test specimen is
filled
up to its full capacity.
Below the basket, a liquid reservoir is provided. The height of the basket
can be adjusted so that a test specimen which is placed inside the basket may
be brought into contact with the surface of the liquid in the liquid
reservoir. The
liquid reservoir is placed on the electronic balance connected to a computer
to
read out the weight of the liquid about every 0.01sec during the measurement.
The dimensions of the apparatus are chosen such that the liquid handling
member to be tested fits into the basket and such that the intended liquid
acquisition zone of the liquid handing member is in contact with the lower
plane
of the basket. The dimensions of the liquid reservoir are chosen such that the
level of the liquid surface in the reservoir does not substantially change
during
the measurement. A typical reservoir useful for testing liquid handling
members
has a size of at least 320 mm x 370 mm and can hold at least about 4500 g of
liquid.
Before the test, the liquid reservoir is filled with synthetic urine. The
amount
of synthetic urine and the size of the liquid reservoir should be sufficient
such that
the liquid level in the reservoir does not change when the liquid capacity of
the
liquid handing member to be tested is removed from the reservoir.
CA 02333697 2000-12-O1
WO 00/00139 PCT/US99/14641
13
The temperature of the liquid and the environment for the test should reflect
in-use conditions of the member. Typical temperature for use in baby diapers
are
32 degrees Celsius for the environment and 37 degrees Celsius for the
synthetic
urine. The test may be done at room temperature if the member tested has no
significant dependence of its absorbent properties on temperature.
The test is setup by lowering the empty basket until the mesh is just
completely immersed in the synthetic urine in the reservoir. The basket is
then
raised again by about 0.5 to 1 mm in order to establish an almost zero
hydrostatic
suction, care should be taken that the liquid stays in contact with the mesh.
If
necessary, the mesh needs to be brought back into contact with the liquid and
zero level be readjusted.
The test is started by:
1. starting the measurement of the electronic balance;
2. placing the liquid handling member on the mesh such that the acquisition
zone of the member is in contact with the liquid;
3. immediately adding a low weigh on top of the member in order to provide
a pressure of 165 Pa for better contact of the member to the mesh.
During the test, the liquid uptake by the liquid handing member is recorded
by measuring the weight decrease of the liquid in the liquid reservoir. The
test is
stopped after 30 minutes.
At the end of the test, the total liquid uptake of the liquid handing member
is
recorded. In addition, the time after which the liquid handling member had
absorbed 80 percent of its total liquid uptake is recorded. The zero time is
defined as the time where the absorption of the member starts. The initial
absorption speed of the liquid handling member is from the initial linear
slope of
the weight vs. time measurement curve.
CA 02333697 2005-O1-12
14
C~~j~lla~ Sor lion Test
P
The purpose of this test is to measure the capillary sorption absorbent
capacity, as a function of height, of liquid handling members of the present
invention. This test may also be used to measure the capillary sorption
absorbent capacity of devices for handling body liquids according to the
present invention. Capillary sorption is a fundamental property of any
absorbent that governs how liquid is absorbed into the absorbent structure. in
the Capillary Sorption experiment, capillary sorption absorbent capacity is
measured as a function of fluid pressure due to the height of the sample
relative to the test fluid reservoir.
The method for determining capillary sorption is well recognized. See
Burgeni, A.A, and Kapur, C., "Capillary Sorption Equilibria in Fiber Masses,"
Textile Research Journal, 37 (1967), 35fi-366; Chatterjee, P.K., Absorbency,
Textile Science and Technology 7, Chapter Il, pp 29-84, Elsevier Science
Publishers B.V, 1985; and U.S. Patent No. 4,610,678, issued September 9,
1986 to Weisman et al. for a discussion of the method for measuring capillary
sorption of absorbent structures.
Principle
A porous glass frit is connected via an uninterrupted column of fluid to a
fluid reservoir on a balance. The sample is maintained under a constant
confining weighs during the experiment. As the porous structure absorbs fluid
upon demand, the weight loss in the balance fluid reservoir is recorded as
fluid
uptake, adjusted for uptake of the glass frit as a function of height and
evaporation. The uptake or capacity at various capillary suctions (hydrostatic
tensions or heights) is measured. Incremental absorption occurs due to the
CA 02333697 2000-12-O1
WO 00/00139 PCT/US99/14641
incremental lowering of the frit (i.e., decreasing capillary suction).
Time is also monitored during the experiment to enable calculation of
initial effective uptake rate (g/g/h) at a 200 cm height.
5
Reagents
Test Liquid: Synthetic urine is prepared by completely dissolving the
following
materials in distilled water.
Compound F.W. Concentration (g/L)
10 KCI 74.6 2.0
Na2S04 142 2.0
(NH4)H2P04 115 ' 0.85
(NH4)2HP04 132 0.15
CaC12~2H20 147 0.25
15 MgC12.6H20 203 0.5
General Description of Apparatus Set Un
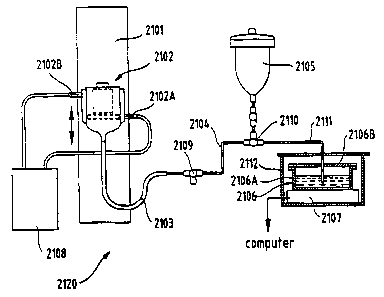
The Capillary Sorption equipment, depicted generally as 520 in Figure 1 ,
used for this test is operated under TAPPf conditions (50% RH, 25°C). A
test
sample is placed on a glass frit shown in Figure 1 as 502 that is connected
via
a continuous column of test liquid (synthetic urine) to a balance liquid
reservoir, shown as 506, containing test liquid. This reservoir 506 is placed
on
a balance 507 that is interfaced with a computer (not shown). The balance
should be capable of reading to 0.001 g; such a balance is available from
Mettler Toledo as PR1203 (Hightstown, NJ). The glass frit 502 is placed on a
vertical slide, shown generally in Figure 1 as 501, to allow vertical movement
of the test sample to expose the test sample to varying suction heights. The
vertical slide may be a rodless actuator which is attached to a computer to
record suction heights and corresponding times for measuring liquid uptake by
the test sample. A preferred rodless actuator is available from Industrial
Devices (Novato, CA) as item 202X4X34N-1 D4B-84-P-C-S-E, which may be
CA 02333697 2004-05-21
16
powered by motor drive ZETAT"" 6104-83-135, available from CompuMotor
(Rohnert, CA). Where data is measured and sent from actuator 501 and
balance 507, capillary sorption absorbent capacity data may be readily
generated for each test sample. Also, computer interface to actuator 501 may
allow for controlled vertical movement of the glass frit 502. For example, the
actuator may be directed to move the glass frit 502 vertically only after
"equilibrium" (as defined below) is reached at each suction height.
The bottom of glass frit 502 is connected to TygonO tubing 503 that 10
connects the frit 505 to three-way drain stopcock 509. Drain stopcock 509 is
connected to liquid reservoir 505 via glass tubing 504 and stopcock 510. (The
stopcock 509 is open to the drain only during cleaning of the apparatus or air
bubble removal.) Glass tubing 511 connects fluid reservoir 505 with balance
fluid reservoir 506, via stopcock 510. Balance liquid reservoir 506 may
consist
of a lightweight 12 cm diameter glass dish 506A and cover 5068. The cover
506B has a hole through which glass tubing 511 contacts the liquid in the
reservoir 506. The glass tubing 511 must not contact the cover 506B or an
unstable balance reading will result and the test sample measurement cannot
be used. In this context, it is to be understood that the volume of the liquid
reservoir needs to be compatible with the absorbent capacity of the liquid
handing member or the device to be tested. Hence, it may be necessary to
choose a different liquid reservoir.
The glass frit diameter must be sufficient to accommodate the
piston/cylinder apparatus, discussed below, for holding the test sample. The
glass frit 502 is jacketed to allow for a constant temperature control from a
heating bath. A suitable frit is a 350 ml fritted disc funnel specified as
having 4
to 5.5 mm pores, available from Corning Glass Co. (Corning, NY) as #36060-.
350F. The pores are fine enough to keep the frit surface wetted at capillary
suction heights specified (the glass frit does not allow air to enter the
continuous column of test liquid below the glass frit).
CA 02333697 2000-12-O1
WO 00/00139 PCT/US99/14641
17
As indicated, the frit 502 is connected via tubing to fluid reservoir 505 or
balance liquid reservoir 506, depending on the position of three-way stopcock
510.
Glass frit 502 is jacketed to accept water from a constant temperature
bath. This will ensure that the temperature of the glass frit is kept at a
constant temperature of 88°F {31 °C) during the testing
procedure. As is
depicted in Figure 1, the glass frit 502 is equipped with an inlet port 502A
and
outlet port 5028, which make a closed loop with a circulating heat bath shown
generally as 508. {The glass jacketing is not depicted in Figure 1. However,
the water introduced to the jacketed glass frit 502 from bath 508 does not
contact the test liquid and the test liquid is not circulated through the
constant
temperature bath. The water in the constant temperature bath circulates
through the jacketed walls of the glass frit 502.)
Reservoir 506 and balance 507 are enclosed in a box to minimize
evaporation of test liquid from the balance reservoir and to enhance balance
stability during performance of the experiment. This box, shown generally as
512, has a top and walls, where the top has a hole through which tubing 511 is
inserted.
The glass frit 502 is shown in more detail in Figure 2B. Figure 2B is a
cross-sectional view of the glass frit, shown without inlet port 502A and
outlet
port 5028. As indicated, the glass frit is a 350 ml fritted disc funnel having
specified 4 to 5.5 mm pores. Referring to Figure 2B, the glass frit 502
comprises a cylindrical jacketed funnel designated as 550 and a glass frit
disc
shown as 560. The glass frit 502 further comprises a cylinderlpiston assembly
shown generally as 565 (which comprises cylinder 566 and piston 568), which
confines the test sample, shown as 570, and provides a small confining
pressure to the test sample. To prevent excessive evaporation of test liquid
from the glass frit disc 560, a Teflon ring shown as 562 is placed on top of
the
CA 02333697 2004-05-21
18
glass frit disc 560. The TeflonOT"" ring 562 is 0.0127 cm thick (available as
sheet stock from McMasterCarr as # 8569K16 and is cut to size) and is used to
cover the frit disc surface outside of the cylinder 566, and thus minimizes
evaporation from the glass frit. The ring outer diameter and inner diameter is
7.6 and 6.3 cm, respectively. The inner diameter of the Teflon~ ring 562 is
about 2 mm less than the outer diameter of cylinder 566. A Viton~ 0-ring
(available from McMasterCarr as # AS568A-150 and AS568A-151 ) 564 is
placed on top of Teflon~ ring 562 to seal the space between the inner wall of
cylindrical jacketed funnel 550 and Teflon~ ring 562, to further assist in
prevention of evaporation. If the 0-ring outer diameter exceeds the inner
diameter of cylindrical jacketed funnel 550, the 0-ring diameter is reduced to
fit
the funnel as follows: the 0-ring is cut open, the necessary amount of 0-ring
material is cut off, and the 0-ring is glued back together such that the 0-
ring
contacts the inner wall of the cylindrical jacketed funnel 550 all around its
periphery. While the above described frit represents one suitable example of
frit, it may be necessary to use of frit having dimensions different from the
above dimensions in order to better fit the dimensions of the liquid handling
member or the device to be tested. The surface area of the frit should
resemble
as closely as possible the surface area of the acquisition zone of the liquid
handling member or the device in order to fully use the acquisition zone and
in
order to minimize the evaporation from the frit.
As indicated, a cylinder/piston assembly shown generally in Figure 2B as
565 confines the test sample and provides a small confining pressure to the
test sample 570. Referring to Figure 2C, assembly 565 consists of a cylinder
566, a cup-like TeflonO piston indicated by 568 and, when necessary, a weight
or weights (not shown) that fits inside piston 568. (Optional weight will be
used
when necessary to adjust the combined weight of the piston and the optional
weight so a confining pressure of 0.2 psi is attained depending on the test
sample's dry diameter. This is discussed below.) The cylinder 566 is LexanOTnn
bar stock and has the following dimensions: an outer diameter of 7.0 cm, an
inner diameter of 6.0 cm and a height of 6.0 cm. The TeflonO piston
CA 02333697 2000-12-O1
WO 00/00139 PCTNS99/14641
19
568 has the following dimensions: an outer diameter that is 0.02 cm less than
the inner diameter of cylinder 566. As shown in Figure 2D, the end of the
piston 568 that does not contact the test sample is bored to provide a 5.0 cm
diameter by about 1.8 cm deep chamber 590 to receive optional weights
(dictated by the test sample's actual dry diameter) required to attain a test
sample confining pressure of 0.2 psi (1.4 kPa). In other words, the total
weight
of the piston 568 and any optional weights (not shown in figures) divided by
the test sarnpie's actual diameter (when dry) should be such that a confining
pressure of 0.2 psi is attained. Cylinder 566 and piston 568 (and optional
weights) are equilibrated at 31 °C for at least 30 minutes prior to
conducting the
capillary sorption absorbent capacity measurement. Again, the above
described dimensions are chosen to fit the above described exemplary frit. Of
course, when a different frit is chosen the dimensions of the cylinderlpiston
assembly need to be adjusted accordingly.
A non-surfactant treated or incorporated apertured film (14 cm x 14 cm)
{not shown) is used to cover the glass frit 502 during Capillary Sorption
experiments to minimize air destablization around the sample. Apertures are
large enough to prevent condensation from forming on the underside of the
film during the experiment.
Test Sam~paration
For the present procedure, it is important, that the dimensions of the
sample and of the frit should not be too different. To achieve this, two
approaches can be taken:
a) For test samples, which can be readily adjusted to a suitable
size, such as by cutting these, both the size of this cutting as well
as of the frit are chosen to be a circular shaped structure of 5.4
cm diameter, such as can be done by using a conventional arc
punch.
b) When the test sample cannot readily be cut to this dimension,
the size and preferably also the shape of the frit has to be
CA 02333697 2004-05-21
adjusted to the size and shape of the test sample.
In both cases, the test sample can be a readily separable element of a
member or a device, it can be a particular component of any of these, or can
be a combination of components thereof. It might also be necessary to adjust
the size of the liquid reservoir to match the varying requirements.
The dry weight of the test sample (used below to calculate capillary
sorption absorbent capacity) is the weight of the test sample prepared as
above under ambient conditions.
Experimental Set Up
1. Place a clean, dry glass frit 502 in a funnel holder attached to the
vertical
slide 501. Move the funnel holder of the vertical slide such that the glass
frit is at the 0 cm height.
2. Set up the apparatus components as shown in Figure 1, as discussed
above.
3. Place 12 cm diameter balance liquid reservoir 506 on the balance 507.
Place plastic lid 506B over this balance liquid. reservoir 506 and a plastic
lid over the balance box 512 each with small holes to allow the glass
tubing 511 to fit through. Do not allow the glass tubing to touch the lid
506B of the balance liquid reservoir or an unstable balance reading will
result and the measurement cannot be used.
4. Stopcock 510 is closed to tubing 504 and opened to glass tubing 511.
Fluid reservoir 505, previously filled with test fluid, is opened to allow
test
fluid to enter tubing 511, to fill balance fluid reservoir 506.
5. The glass frit 502 is leveled and secured in place. Also, ensure that the
glass frit is dry.
6. Attach the TygonOT"" tubing 503 to stopcock 509. (The tubing should be
long enough to reach the glass frit 502 at its highest point of 200 cm with
no kinks.) Fill this TygonO tubing with test liquid from liquid reservoir 505.
CA 02333697 2000-12-O1
WO 00/00139 PCT/US99/14641
21
7. Attach the Tygon(~ tubing 503 to the level glass frit 502 and then open
stopcock 509 and stopcock 510 leading from fluid reservoir 505 to the
glass frit 502. (Stopcock 510 should be closed to glass tubing 511.) The
test liquid fills the glass frit 502 and removes all trapped air during
filling
of the level glass frit. Continue to fill until the fluid level exceeds the
top
of the glass frit disc 560. Empty the funnel and remove all air bubbles in
the tubing and inside the funnel. Air bubbles may be removed by
inverting glass frit 502 and allowing air bubbles to rise and escape
through the drain of stopcock 509. (Air bubbles typically collect on the
bottom of the glass frit disc 560.) Relevel the frit using a small enough
level that it will fit inside the jacketed funnel 550 and onto the surface of
glass frit disc 560.
8. Zero the glass frit with the balance liquid reservoir 506. To do this, take
a
piece of Tygond tubing of sufficient length and fill it with the test liquid.
Place one end in the balance liquid reservoir 506 and use the other end
to position the glass frit 502. The test liquid level indicated by the tubing
(which is equivalent to the balance liquid reservoir level) is 10 mm below
the top of the glass frit disc 560. If this is not the case, either adjust the
amount of liquid in the reservoir or reset the zero position on the vertical
slide 501.
9. Attach the outlet and inlet ports from the temperature bath 508 via tubing
to the inlet and outlet ports 502A and 5028, respectively, of the glass frit.
Allow the temperature of the glass frit disc 560 to come to 31 °C.
This
can be measured by partially filling the glass frit with test liquid and
measuring its temperature after it has reached equilibrium temperature.
The bath will need to be set a bit higher than 31 °C to allow for
the
dissipation of heat during the travel of water from the bath to the glass
frit.
10. The glass frit is equilibrated for 30 minutes.
CA 02333697 2000-12-O1
WO 00/00139 pC'T/US99/14641
22
The following describes a computer program that will determine how long
the glass frit remains at each height.
In the capillary sorption software program, a test sample is at some
specified height from the reservoir of fluid. As indicated above, the fluid
reservoir is on a balance, such that a computer can read the balance at the
end of a known time interval and calculate the flow rate (Delta reading/time
interval) between the test sample and reservoir. For purposes of this method,
the test sample is considered to be at equilibrium when the flow rate is less
than a specified flow rate for a specified number of consecutive time
intervals.
It is recognized, that for certain material, actual equilibrium may not be
reached when the specified "EQUILIBRIUM CONSTANT' is reached. The time
interval between readings is 5 seconds.
The number of readings in the delta table is specified in the capillary
sorption menu as "EQUILIBRIUM SAMPLES". The maximum number of
deltas is 500. The flow rate constant is specked in the capillary sorption
menu as "EQUILIBRIUM CONSTANT'.
The Equilibrium Constant is entered in units of grams/sec, ranging from
0.0001 to 100.000.
The following is a simplified example of the logic. The table ,shows the
balance reading and Oelta Flow calculated for each Time Interval.
Equilibrium Samples = 3
CA 02333697 2000-12-O1
WO 00/00139 PCTNS99/14641
23
Equilibrium
Constant
= 0.0015
Time Balance Delta
Interval Value Flow
(g) (g/sec)
0 0
1 0.090 0.0180
2 0.165 0.0150
3 0.225 0.0120
4 0.270 0.0090
5 0.295 0.0050
6 0.305 0.0020
7 0.312 0.0014
8 0.316 0.0008
9 0.318 0.0004
Delta Table:
Time 0 1 2 3 4 5 6 7 g g
Deltal 99990.0180 0.0180 0.0180 0.00900.00900.00140.00140.0014
0.0090
Delta2 99999999 0.0150 0.0150 0.0150 0.00500.00500.00080.0008
0.0050
Delta399999999 9999 0.0120 0.0120 0.00200.00200.00200.0004
0.0120
The equilibrium uptake for the above simplified example is 0.318 gram.
The following is the code in C language used to determine equilibrium
uptake:'
takedata.c '/
int take data(int equil samples,double equilibrium constant)
double delta;
static double deltas[500]; r table to store up to 500 deltas'I
double value;
CA 02333697 2000-12-O1
WO 00/00139 PCT/US99/14641
24
double prev_vaiue;
clock t next_time;
int i;
for (i=0; i<equil samples; i++)
deltas[i] = 9999.; /' initialize all values in the delta table to 9999.
gms/sec '/
delta table index = 0; /' initialize where in the table to store the next
delta 'I
equilibrium reached = 0; /' initialize flag to indicate equilibrium has not
been
reached '/
next time = clock(); I' initialize when to take the next reading 'I
prey reading = 0.; /' initialize the value of the previous reading from the
balance
./
while (!equilibrium reached) { I' start of loop for checking for equilibrium
'I
next_time += 5000L; I' calculate when to take next reading 'I
while (clock() < next time); /' wait until 5 seconds has elasped from prey
reading '/
value = get balance reading(); /' read the balance in grams'!
delta = fabs(prev value - value) I 5.0; l' calculate absolute value of flow in
last 5 seconds 'I
prey value = value; I' store current value for next loop 'I
deltas[delta_table_index] = delta; /' store current delta value in the table
of deltas '!
delta table index++; /' increment pointer to next position in table'/
if (delta table index == equii samples) /' when the number of deltas = the
number of 'I
delta_tabie index = 0; /' equilibrium samples specified, r
I' reset the pointer to the start of the table. This way 'I
r the table always contains the last xx current samples. 'I
equilibrium reached = 1; I' set the flag to indicate equilibrium is reached'/
for (i=0; i < equit samples; i++) r check all the values in the delta table 'I
if (deltas[i] >= equilibrium constant)r if any value is > or = to the
equilibrium constant'!
equilibrium reached = 0; /' set the equlibrium flag to 0 (not at equilibrium)
'1
} r go back to the start of the loop '/
Cai ilfaryr Sorr~tion Parameters
Load Description (Confining Pressure): 0.2 psi load
Equilibrium Samples (n): 50
Equilibrium Constant: 0.0005 g/sec
Setup Height Value: 100 cm
CA 02333697 2000-12-O1
WO 00/00139 PCT/US99/14641
Finish Height Value: 0 cm
Hydrostatic Head Parameters: 200, 180, 160, 140, 120, 100, 90, 80, 70, 60,
50, 45, 40, 35, 30, 25, 20, 15, 10, 5 and 0 cm.
5 The capillary sorption procedure is conducted using all the heights
specified above, in the order stated, for the measurement of capillary
sorption
absorbent capacity. Even if it is desired to determine capillary somtion
absorbent capacity at a particular height (e.g., 35 cm), the entire series of
hydrostatic head parameters must be completed in the order specified.
10 Although all these heights are used in performance of the capillary
sorption
test to generate capillary sorption isotherms for a test sample, the present
disclosure describes the storage absorbent members in terms of their
absorbent properties at specified heights of 200, 140, 100, 50, 35 and 0 cm.
15 Caoil~ lanr Sorlation Procedure
1 ) Follow the experimental setup procedure.
2) Make sure the temperature bath 508 is on and water is circulating through
the glass frit 502 and that the glass frit disc 560 temperature is
31°C.
3) Position glass frit 502 at 200 cm suction height. Open stopcocks 509 and
20 510 to connect glass frit 502 with the balance liquid reservoir 506.
(Stopcock 510 is closed to liquid reservoir 505.) Glass frit 502 is
equilibrated for 30 minutes.
4) Input the above capillary sorption parameters into the computer.
5) Close stopcocks 509 and 510.
25 6) Move glass frit 502 to the set up height, 100 cm.
7) Place Teflon~ ring 562 on surface of glass frit disc 560. Put O-ring 564 on
Teflon~ ring. Place pre-heated cylinder 566 concentrically on the Teflon~
ring. Place test sample 570 concentrically in cylinder 566 on glass frit disc
560. Place piston 568 into cylinder 566. Additional confining weights are
placed into piston chamber 590, if required.
8) Cover the glass frit 502 with apertured film.
CA 02333697 2000-12-O1
WO 00/00139 PCT/US99/14641
26
9) The balance reading at this point establishes the zero or tare reading.
10) Move the glass frit 502 to 200 cm.
11 ) Open stopcocks 509 and 510 (stopcock 510 is closed to fluid reservoir
505) and begin balance and time readings.
Mass Frit Correction (blank correct uptake)
Since the glass frit disc 560 is a porous structure, the glass frit (502)
capillary sorption absorption uptake (blank correct uptake) must be determined
and subtracted to get the true test sample capillary sorption absorption
uptake.
The glass frit correction is performed for each new glass frit used. Run the
capillary sorption procedure as described above, except without test sample,
to obtain the Blank Uptake (g). The elapsed time at each specified height
equals the Blank Time (s).
Evaporation Loss Correctsnn
1 ) Move the glass frit 502 to 2 cm above zero and let it equilibrate at this
height for 30 minutes with open stopcocks 509 and 510 (closed to
reservoir 505).
2) Close stopcocks 509 and 510.
3) Place Teflon~ ring 562 on surface of glass frit disc 560. Put O-ring 564 on
Teflon~ ring. Place pre-heated cylinder 566 concentrically on the Teflon~
ring. Place piston 568 into cylinder 566. Place apertured film on .glass frit
502.
4) Open stopcocks 509 and 510 (closed to reservoir 505) and record balance
reading and time for 3.5 hours. Calculate Sample Evaporation (g/hr) as
follows:
[balance reading at 1 hr - balance reading at 3.5 hr] / 2.5 hr
Even after taking all the above precautions, some evaporative toss will
occur, typically around 0.10 gm/hr for both the test sample and the frit
correction. Ideally, the sample evaporation is measured for each newly
installed glass frit 502.
CA 02333697 2000-12-O1
WO 00/00139 PCT/US99/14641
27
Cleaning the E,g,~nment
New Tygon~ tubing 503 is used when a glass frit 502 is newly installed.
Glass tubing 504 and 511, fluid reservoir 505, and balance liquid reservoir
506
are cleaned with 50% Clorox Bleach~ in distilled water, followed by distilled
water rinse, if microbial contamination is visible.
a. Cleaning after each experiment
At the end of each experiment (after the test sample has been removed),
the glass frit is forward flushed (i.e., test liquid is introduced into the
bottom of
the glass frit) with 250 ml test liquid from liquid reservoir 505 to remove
residual test sample from the glass frit disc pores. With stopcocks 509 and
510 open to liquid reservoir 505 and closed to balance liquid reservoir 506,
the
glass frit is removed from its holder, turned upside down and is rinsed out
first
with test liquid, followed by rinses with acetone and test liquid (synthetic
urine).
During rinsing, the glass frit must be tilted upside down and rinse fluid is
squirted onto the test sample contacting surface of the glass frit disc. After
rinsing, the glass frit is forward flushed a second time with 250 ml test
liquid
(synthetic urine). Finally, the glass frit is reinstalled in its holder and
the frit
surface is leveled.
b. Monitoring glass frit performance
Glass frit performance must be monitored after each cleaning procedure
and for each newly installed glass frit, with the glass frit set up at 0 cm
position. 50 ml of test liquid are poured onto the leveled glass frit disc
surface
(without Teflon~ ring, O-ring and the cylinderlpiston components). The time it
takes for the test fluid level to drop to 5 mm above the glass frit disc
surface is
recorded. A periodic cleaning must be performed if this time exceeds 4.5
minutes.
c. Periodic cleaning
CA 02333697 2000-12-O1
WO 00/00139 PCT/US99/14641
28
Periodically, (see monitoring frit performance, above) the glass frits are
cleaned thoroughly to prevent clogging. Rinsing fluids are distilled water,
acetone, 50% Clorox Bleach~ in distilled water (to remove bacterial growth)
and test liquid. Cleaning involves removing the glass frit from the holder and
disconnecting all tubing. The glass frit is forward flushed (i.e., rinse
liquid is
introduced into the bottom of the glass frit) with the frit upside down with
the
appropriate fluids and amounts in the following order:
1. 250 ml distilled water.
2. 100 ml acetone.
3. 250 ml distilled water.
4. 100 ml 50:50 Clorox~/distilled water solution.
5. 250 ml distilled water.
6. 250 ml test fluid.
The cleaning, procedure is satisfactory when glass frit performance is
within the set criteria of fluid flow (see above) and when no residue is
observable on the glass frit disc surface. If cleaning can not be performed
successfully, the frit must be replaced.
Calculations
The computer is set up to provide a report consisting of the capillary
suction height in cm, time, and the uptake in grams at each specified height.
From this data, the capillary suction absorbent capacity, which is corrected
for
both the frit uptake and the evaporation loss, can be calculated. Also, based
on the capillary suction absorbent capacity at 0 cm, the capillary absorption
efficiency can be calculated at the specified heights. in addition, the
initial
effective uptake rate at 200 cm is calculated.
Blank Time(s)~ Blank Evap.(g / hr)
Blank Correct Uptake (g) = Blank Uptake(g) - 3600(s / hr)
CA 02333697 2000-12-O1
WO 00/00139 PCT/US99/14641
29
Capillary Suction Absorbent Capacity ("CSAC")
~e_ T~(S) * ~ cg/~)_ ~c~a
360~s/ Ir
~' ~~ ~~d8~
Initial Effective Uptake Rate at 200 cm ("IEUR")
IEUR (g/g/hr) - CSAC at 200 cm (g/g)
Sample Time at 200 cm (s)
A minimum of two measurements should be taken for each sample and
the uptake averaged at each height to calculate Capillary Sorption Absorbent
Capacity (CSAC) for a given absorbent member or a given high surface area
material.
With these data, the respective values can be calculated:
- The Capillary Sorption Desorption Height at which the material has
released x% of its capacity at 0 cm (i.e. of CSAC 0), (CSDH x)
expressed in cm;
- The Capillary Sorption Absorption Height at which the material has
absorbed y % of its capacity at 0 cm (i.e. of CSAC 0), (CSAH y)
expressed in cm;
- The Capillary Sorption Absorbent Capacity at a certain height z
(CSAC z) expressed in units of g {of fluid} / g { of material}; especially
at the height zero (CSAC 0), and at heights of 35cm, 40cm, etc
- The Capillary Sorption Absorption Efficiency at a certain height z
CA 02333697 2000-12-O1
WO 00/00139 PCT/US99/14641
(CSAE Z) expressed in %, which is the ratio of the values for CSAC 0
and CSAC z.
If two materials are combined (such as the first being used as acquisition
5 / distribution material, and the second being used as liquid storage
material),
the CSAC value (and hence the respective CSAE value) of the second
material can be determined for the CSDH x value of the first material .
Reversible expansion test
10 The intention of this test is to measure the expansion of a liquid handling
member and the subsequent contraction of the liquid handling member over a
series of liquid acquisition and release cycles. This test this suitable for
liquid
handling members according to the present invention. This test maybe
equivalently applied to devices for handling body liquids according to the
present
15 invention.
The test specimen is a liquid handling member according to the present
invention. The liquid handling member should be configured to resemble as
closely as possible its in use configuration. If the liquid handling member is
part
20 of the device for handling body liquids, those parts of the device which do
not
contribute to the performance of the liquid handling member may be removed
prior to testing the liquid handling member. It is, however, also possible to
test a
device for handling body liquids in its entirety.
25 At the beginning of the test, the total absorbent capacity of the specimen
is
determined via the demand absorbency test defined herein. The specimen which
is now filled with liquid up to its total absorbent capacity is now placed on
the
glass frit of the capillary sorption test defined herein which has been set at
0 cm
hydrohead.
The experimental set up for this test comprises the set up for the capillary
sorption test defined herein in combination with a volume measurement device
CA 02333697 2000-12-O1
WO 00/00139 PCT/US99/14641
31
which is installed such that it is capable of measuring the dimensions of the
specimen when the specimen is placed on the glass frit of the capillary
sorption
experimental set up.
For the purpose of this test, a Cartesian coordinate system is defined as
follows. The z - direction is direction perpendicular to the upper major
surface of
the glass frit also termed caliper direction about. Accordingly, x -, and y -
direction are parallel to the upper major surface of the glass frit. The x -
direction is defined by the direction of most effcient liquid transportation
within
the test specimen. For example, if the test specimen has a first region for
liquid
acquisition and the second region for liquid discharge or storage the x -
direction
would point from the first region to the second region.
For example, the volume measurement device may consist of a caliper (z -
direction) measurement device, in combination with two devices which measure
the expansion of the test specimen in the two dimensions (x -, and y -
direction)
parallel to the surface of the glass frit. Since the two major surfaces of the
glass
frit in the capillary sorption experimental setup are oriented horizontally,
the
caliper measurement device in this test measures the vertical expansion of the
test specimen whereas the other two measurement devices measure the
horizontal expansion of the test specimen. If, for example, the test specimen
is
substantially rectangular simple mechanic devices for manual determination of
length may be used to determine the dimensions of the ,test specimen. If the
geometry of the test specimen is more complex, contraction and expansion of
the test specimen may be recorded for example on video tape which allows for
exact analysis of expansion and contraction of the test specimen during the
test.
Suitable methods for the determination of each of the dimensions are well
known
in the art. If such method requires that the test specimen is put under a
confining
pressure, the confining pressure should be chosen low enough such that the
respective dimension of the test specimen remains substantially unchanged.
Furthermore, it is important that a dimension of the test specimen is measured
over a surface area which is at least 20 percent of the respective surface
area of
CA 02333697 2000-12-O1
WO 00/00139 PCT/US99/14641
32
the test specimen such that the measurement is representative of the
dimension.
During the first step of this test, the total absorbent capacity of the test
specimen is determined via the demand absorbency test defined herein. The
test specimen which is now fit with liquid up to its total absorbent capacity
is now
placed on the glass frit of the capillary sorption test defined herein which
has
been set at 0 cm hydrohead. On the glass frit, the test specimen is oriented
such
that its region which is intended for liquid acquisition is facing towards the
upper
surface of the glass frit.
During the second step of this test, the capillary suction is continuously
increased until half of the liquid initially stored in the liquid handling
member is
removed from the liquid handling member or device respectively. At the
beginning of this step and at the end of this step, the dimensions of the
liquid
handling member or the device are recorded. The contraction factor for each
dimension is determined by dividing the respective dimension of the test
specimen at the end of this test phase by its respective dimension at the
beginning of this test phase. Accordingly, the value of each contraction
factor will
be between 0 and 1. The volume contraction factor is determined by dividing
the
volume at the end of this step by the volume at ttie beginning of this step.
For a
substantially rectangular test specimen for example, the volume may be
obtained
by multiplying the x -, y -, and the z - dimension of the test specimen.
During the third step of this test the capillary suction is decreased to zero
pressure such that the test specimen will take up liquid again until the
specimen
is filled up to its total capacity. At the beginning of this test phase and at
the end
of this test phase, the dimensions of the liquid handling member or the device
are recorded. The expansion factor for each dimension is determined by
dividing
the respective dimension of the test specimen at the end of this test phase by
its
respective dimension at the beginning of this test phase. Often, the value of
each
expansion factor will be at least 1. The volume expansion factor is determined
by
dividing the volume at the end of this test phase by the volume at the
beginning
CA 02333697 2000-12-O1
WO 00/00139 PCT/US99/14641
33
of this test phase. In addition, the liquid capacity at the end of the cycle
is
divided by the total absorbent capacity of the test specimen as determined by
the
demand absorbency test prior to this test to obtain the capacity decrease
factor.
The above measurement cycle of second step and third step may be
repeated in order to examine the longtime behavior of the test specimen. The
respective contraction factors, expansion factors, and capacity decrease
factors
are then denoted together with the number of their respective test cycle.