Note: Descriptions are shown in the official language in which they were submitted.
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
DELTA CLEAVAGE PRODUCTS AND METHODS BASED THEREON
The application claims priority benefits of Lf.S.
Provisional Application Serial No. 60/104,834, filed October
19, 1998 and U.S. Provisional Application Serial No.
60/092,513 filed July 13, 1998, each of which is incorporated
by reference herein i.n its entirety.
1. FIELD OF THE INVENTION
The present. invention is directed to a peptide, and
its encoding nucleic acids, of the toporythmic protein Delta
that contains a sequence which is cleaved by the
metalloprotease-disintegrin Kuzbanian (Kuz) ("Delta cleavage
peptide"), as well a~; derivatives and analogs thereof. The
present invention is also directed to an extracellular
soluble peptide, and its encoding nucleic acids, of the:
toporythmic protein Delta ("soluble Delta peptide" or "Disc"
as well as derivatives and analogs thereof. Production of
the Delta cleavage peptide or DlEC, and derivatives, and
antibodies thereto are also provided. The present invention
is also directed to methods for detecting or measuring Delta
activation by observing or measuring Delta cleavage products
that are indicative of Delta activation. The present
invention is also directed to methods for detecting a
molecule that modulates Delta activation by observing ar
measuring a change in the amount or pattern of Delta cleavage
products. The present invention is further directed tc>
methods for detecting or measuring Kuz function by observing
or measuring Delta cleavage products that are indicative of
Kuz function. The present invention is also directed to
methods for detecting a molecule that modulates Kuz function
by observing or measuring a change in the amount or pattern
of Delta cleavage products. The present invention is also
directed to certain ~>rotein complexes of Delta and Kuz and of
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
D1E~ and Notch, and methods for their use in screening,
diagnosis and therapy.
2. BACKGROUND OF THE INVENTION
Genetic and molecular studies have led to the
identification of a gx-oup of genes which define distinct
elements of the Notch signaling pathway. While the
identification of the:>e various elements has come exclusively
from Drosophila using genetic tools as the initial guide,
subsequent analyses have lead to the identification of
homologous proteins in vertebrate species including humans.
Figure 1 depicts the nnolecular relationships between the'
known Notch pathway elements as well as their subcellular
localization (Artavanis-Tsakonas et al., 1995, Science
268:225-232).
The Drosoph:ila Notch gene encodes an -300 kD
transmembrane protein that acts as a receptor in a cell-cell
signaling mechanism controlling cell fate decisions
throughout development: (reviewed, e.g., in Artavanis-Tsakonas
et al., 1995, Science 268:225-232). Closely related homologs
of Drosophila Notch h<~ve been isolated from a number of
vertebrate species, including humans, with multiple paralogs
representing the single Drosophila gene in vertebrate
genomes. The isolation of cDNA clones encoding the C-
terminus of a human Notch paralog, originally termed hN, has
been reported (Stifani et al., 1992, Nature Genetics 2:119-
127). The encoded protein is designated human Notch2 because
of its close relationship to the Notch2 proteins found in
other species (Weinmaster et al., 1992, Development 116:931-
941). The hallmark Notch2 structures are common to all the
Notch-related proteins, including, in the extracellular
domain, a stretch of 34 to 36 tandem Epidermal Growth Factor-
like (EGF) repeats and three Lin-12/Notch repeats (LN
repeats), and, in the intracellular domain, 6 Ankyrin repeats
- 2 -
CA 02333893 2001-O1-11
WO 00/02897 PC'f/US99/15817
and a PEST-containing region. Like Drosophila Notch and the
related C. elegans genes tin-12 and glp-1 (Sternberg, 1993,
Current Biology 3:763-765; Greenwald, 1994, Current Opinion
in Genetics and Development 4:556-562), the vertebrate Notch
homologs play a role in a variety of developmental processes
by controlling cell fate decisions (reviewed, e.g., in
Blaumueller and Artavanis-Tsakonas, 1997, Persp. on Dev.
Neurobiol. 4:325-343). (For further human Notch sequences,
see International Publication WO 92/19734.)
The extracellular domain of Notch carries 36
Epidermal Growth Factor-like (EGF) repeats, two of which
(repeats 11 and 12) have been implicated in interactions with
the Notch ligands Serrate and Delta. Delta and Serrate are
membrane bound ligands with EGF homologous extracellular
domains, which interact physically with Notch on adjacent
cells to trigger signaling.
Functional analyses involving the expression of
truncated forms of the Notch receptor have indicated that
receptor activation depends on the six cdcl0/ankyrin repeats
in the intracellular domain. Deltex and Suppressor of
Hairless, whose over-expression results in an apparent
activation of the pathway, associate with those repeats.
Deltex is a cytoplasmic protein which contains a
ring zinc finger. Suppressor of Hairless on the other hand,
is the Drosophila homologue of CBF1, a mammalian DNA binding
protein involved in the Epstein-Harr virus-induced
immortalization of B cells. It has been demonstrated that,
at least in cultured cells, Suppressor of Hairless assaciates
with the cdcl0/ankyrin repeats in the cytoplasm and
translocates into the nucleus upon the interaction of the
Notch receptor with its ligand Delta on adjacent cells
(Fortini and Artavanis, 1994, Cell 79:273-282). The
association of Hairless, a novel nuclear protein, with
Suppressor of Hairless has been documented using the yeast
- 3 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
two hybrid system; therefore, it is believed that the
involvement of Suppre:ssor of Hairless in transcription is
modulated by Hairles~~ (Brou et al., 1994, Genes Dev. 8:2491;
Knust et al. 1992, Genetics 129:803).
Finally, it is known that Notch signaling results
in the activation of at least certain basic helix-loop-helix
(bHLH) genes within the Enhancer of Split complex (Delidakis
et al ., 1991, Genetics 129:803). Mastermind encodes a novel
ubiquitous nuclear protein whose relationship to Notch
signaling remains unclear but is involved in the Notch
pathway as shown by genetic analysis (Smoller et al., 1990,
Genes Dev. 4:1688).
The generality of the Notch pathway manifests
itself at different levels. At the genetic level, many
mutations exist which affect the development of a very broad
spectrum of cell types in Drosophila. Knockout mutations in
mice are embryonic lethals consistent with a fundamental role
for Notch function (Swiatek et al., 1994, Genes Dev. 8:707).
Mutations in the Notch pathway in the hematopoietic system in
humans are associated with lymphoblastic leukemia (Ellison et
al., 1991, Cell 66:649-661). Finally the expression of
mutant forms of Notch in developing Xenopus embryos
interferes profoundly with normal development (Coffman et
al., 1993, Cell 73:659). Increased level of Notch expression
is found in some malignant tissue in humans (International
Publication WO 94/07474).
The expression patterns of Notch in the Drosophila
embryo are complex and dynamic. The Notch protein is broadly
expressed in the early embryo, and subsequently becomes
restricted to uncommitted or proliferative groups of cells as
development proceeds. In the adult, expression persists in
the regenerating tissues of the ovaries and testes (reviewed
in Fortini et al., 1993, Cell 75:1245-1247; Jan et al., 1993,
Proc. Natl. Acad. Sci. USA 90:8305-8307; Sternberg, 1993,
- 4 -
CA 02333893 2001-O1-11
WO 00/02897 PCT1US99/15817
Curr. Biol. 3:763-765; Greenwald, 1994, Curr. Opin. Genet.
Dev. 4:556-562; Artavanis-Tsakonas et al., 1995, Science
268:225-232). Studies of the expression of Notchl, one of
three known vertebrate homologs of Notch, in zebrafish and
Xenopus, have shown that the general patterns are similar;
with Notch expression associated in general with non-
terminally differentiated, proliferative cell populations.
Tissues with high expression levels include the developing
brain, eye and neural tube (Coffman et al., 1990, Science
249:1438-1441; Bierkamp et al., 1993, Mech. Dev. 43:87-:L00).
While studies in mammals have shown the expression of the
corresponding Notch homologues to begin later in development,
the proteins are expressed in dynamic patterns in tissues
undergoing cell fate determination or rapid proliferation
(Weinmaster et al., 1991, Development 113:199-205; Reaume et
al., 1992, Dev. Biol. 154:377-387; Stifani et al., 1992,
Nature Genet. 2:119-127; Weinmaster et al., 1992, Development
116:931-941; Kopan et al., 1993, J. Cell Biol. 121:631-641;
Lardelli et al., 1993, Exp. Cell Res. 204:364-372; Lardelli
et al., 1994, Mech. Dfsv. 46:123-136; Henrique et al., 1995,
Nature 375:787-790; Horvitz et al., 1991, Nature 351:535-541;
Franco del Amo et al.,, 1992, Development 115:737-744). Among
the tissues in which mammalian Notch homologues are first
expressed are the pre-somitic mesoderm and the developing
neuroepithelium of then embryo. In the pre-somitic mesoderm,
expression of Notchl is seen in all of the migrated mesoderm,
and a particularly dense band is seen at the anterior edge of
pre-somitic mesoderm. This expression has been shown to
decrease once the som_Ltes have formed, indicating a role for
Notch in the differentiation of somatic precursor cells
(Reaume et al., 1992, Dev. Biol. 154:377-387; Horvitz et al.,
1991, Nature 351:535-541). Similar expression patterns are
seen for mouse Delta (Simske et al., 1995, Nature
375:142-145).
- 5 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99115817
Within the developing mammalian nervous system,
expression patterns of Notch homologue have been shown to be
prominent in particular regions of the ventricular zone of
the spinal cord, as well as in components of the peripheral
nervous system, in an overlapping but non-identical pattern.
Notch expression in the nervous system appears to be limited
to regions of cellular proliferation, and is absent from
nearby populations of recently differentiated cells
(Weinmaster et al., 1991, Development 113:199-205; Reaume et
al., 1992, Dev. Biol. 154:377-387; Weinmaster et al., 1992,
Development 116:931-941; Kopan et al., 1993, J. Cell Biol.
121:631-641; Lardelli et al., 1993, Exp. Cell Res.
204:364-372; Lardelli et al., 1994, Mech. Dev. 46:123-136;
Henrique et al., 1995, Nature 375:787-790; Horvitz et al..,
1991, Nature 351:535-5.41). A rat Notch ligand is also
expressed within the developing spinal cord, in distinct.
bands of the ventricular zone that overlap with the
expression domains of the Notch genes. The spatio-temporal
expression pattern of this ligand correlates well with t;he
patterns of cells committing to spinal cord neuronal fates,
which demonstrates the: usefulness of Notch as a marker of
populations of cells f:or neuronal fates (Henrique et al..,
1995, Nature 375:787-',~90). This has also been suggested for
vertebrate Delta homologues, whose expression domains also
overlap with those of Notchl (Larsson et al., 1994, Genomics
24:253-258; Fortini et~ al., 1993, Nature 365:555-557; Simske
et al., 1995, Nature :375:142-145). In the cases of the
Xenopus and chicken homologues, Delta is actually expressed
only in scattered cel:Ls within the Notchl expression domain,
as would be expected :From the lateral specification model,
and these patterns "foreshadow" future patterns of neuronal
differentiation (Lars~son et al., 1994, Genomics 24:253-258;
Fortini et al., 1993, Nature 365:555-557).
- 6 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
Other vertebrate studies of particular interest
have focused on the expression of Notch homologues in
developing sensory structures, including the retina, hair
follicles and tooth buds. In the case of the Xenopus retina,
Notchl is expressed i:a the undifferentiated cells of the
central marginal zone and central retina (Coffman et al.,
1990, Science 249:1439-1441; Mango et al., 1991, Nature
352:811-815). Studies in the rat have also demonstrated an
association of Notchl with differentiating cells in the
developing retina have been interpreted to suggest that
Notchl plays a role in successive cell fate choices in this
tissue (Lyman et al., 1993, Proc. Natl. Acad. Sci. USA
90:10395-10399).
A detailed <~nalysis of mouse Notchl expression in
the regenerating matrix cells of hair follicles was
undertaken to examine the potential participation of Notch
proteins in epithelial/mesenchymal inductive interactions
(Franco del Amo et al.,, 1992, Development 115:737-744). Such
a role had originally been suggested for Notchl based on the
its expression in rat whiskers and tooth buds (Weinmaster et
al., 1991, Development: 113:199-205). Notchl expression was
instead found to be limited to subsets of non-mitotic,
differentiating cells that are not subject to
epithelial/mesenchymal. interactions, a finding that is
consistent with Notch expression elsewhere.
Expression :studies of Notch proteins in human
tissue and cell lines have also been reported. The aberrant
expression of a truncated Notchl RNA in human T-cell leukemia
results from a translocation with a breakpoint in Notchl
(Ellisen et al., 1991, Cell 66:649-661). A study of human
Notchl expression during hematopoiesis has suggested a role
for Notchl in the early differentiation of T-cell precursors
(Mango et al., 1994, Development 120:2305-2315). Additional
studies of human Notchl and Notch2 expression have been
-
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
performed on adult tissue sections including both normal and
neoplastic cervical .and colon tissue. Notchl and Notch2
appear to be expressed in overlapping patterns in
differentiating populations of cells within squamous
epithelia of normal tissues that have been examined and are
clearly not expressed in normal columnar epithelia, except in
some of the precursor cells. Bath proteins are expressed in
neoplasias, in cases ranging from relatively benign squamous
metaplasias to cancerous invasive adenocarcinomas in which
columnar epithelia are replaced by these tumors (hello et
al., 1994, Cell 77:95-106).
Insight into the developmental role and the general
nature of Notch signaling has emerged from studies with
truncated, constitut_Lvely activated forms of Notch in several
species. These recornbinantly engineered Notch forms, which
lack extracellular ligand-binding domains, resemble the
naturally occurring oncogenic variants of mammalian Notch
proteins and are constitutively activated using phenotypic
criteria (Greenwald, 1994, Curr. Opin. Genet. Dev. 4:556;
Fortini et al., 1993, Nature 365:555-557; Coffman et al.,
1993, Cell 73:659-677.; Struhl et al., 1993, Cell 69:1073;
Rebay et al., 1993, Genes Dev. 7:1949; Kopan et al., 1994,
Development 120:2385; Roehl~et al., 1993, Nature 364:632).
- Ubiquitous expression of activated Notch in the
Drosophila embryo suppresses neuroblast segregation without
impairing epidermal differentiation (Struhl et al., 1993,
Cell 69:331; Rebay et. al., 1993, Genes Dev. 7:1949).
- Persistent expression of activated Notch in
developing imaginal epithelia likewise results in an
overproduction of epidermis at the expense of neural
structures (Struhl et. al., 1993, Cell 69:331).
- Neuroblast segregation occurs in temporal waves
that are delayed but not prevented by transient expression of
_ g _
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
activated Notch in the embryo (Struhl et al., 1993, Cell
69:331) .
- Transient expression in well-defined cells of the
Drosophila eye imaginal disc causes the cells to ignore their
normal inductive cues and to adopt alternative cell fates
(Fortini et al., 199'.3, Nature 365:555-557).
- Studies ut_Llizing transient expression of activated
Notch in either the Drosophila embryo or the eye disc
indicate that once Notch signaling activity has subsided,
cells may recover and differentiate properly or respond to
later developmental cues (Fortini et al., 1993, Nature
365:555-557; Struhl e:t al., 1993, Cell 69:331).
For a general review on the Notch pathway and Notch
signaling, see Artavanis-Tsakonas et al., 1995, Science
268:225-232.
Ligands, cytoplasmic effectors and nuclear elements
of Notch signaling have been identified in Drosophila, and
vertebrate counterparts have also been cloned (reviewed in
Artavanis-Tsakonas et al., 1995, Science 268:225-232). While
protein interactions between the various elements have been
documented, the biochemical nature of Notch signaling remains
elusive. Expression of truncated forms of Notch reveal that
Notch proteins without transmembrane and extracellular
domains are translocated to the nucleus both in transgenic
flies and in transfected mammalian or Drosophila cells
(Lieber et al., 1993, Genes and Development 7:1949-1965;
Fortini et al., 1993, Nature 365:555-557; Ahmad et al., 1995,
Mechanisms of Development 53:78-85; Zagouras et al., 1995,
Proc. Natl. Acad. Sci. USA 92:6414-6418). Sequence
comparisons between mammalian and Drosophila Notch molecules,
along with deletion analysis, have found two nuclear
localization sequences that reside on either side of the
Ankyrin repeats (Stifani et al., 1992, Nature Genetics 2:119-
127; Lieber et al., 1993, Genes and Development 7:1949-1965;
- g _
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
Kopan et al., 1994, Development 120:2385-2396). These
findings prompted the .speculation that Notch may be directly
participating in nuclear events by means of a proteolytic
cleavage and subsequent translocation of the intracellular
fragment into the nucleus. However, conclusive functional
evidence for such a hypothesis remains elusive (Artavanis-
Tsakonas et al., 1995, Science 268:225-232).
Citation or identification of any reference in
Section 2 or any other section of this application shall not
be construed as an admission that such reference is available
as prior art to the present invention.
3. SUI~iARY OF THE INVENTION
The inventors have discovered that Delta is cleaved
by the metalloprotease-disintegrin Kuzbanian (Kuz) into two
fragments, a soluble amino-terminal fragment consisting
essentially of the extracellular domain, and a membrane-bound
fragment consisting essentially of the transmembrane domain
and the intracellular domain. The soluble fragment of Delta,
like the full length, membrane-bound Delta, is able to bind
to Notch. Although not intending to be limited to any
particular mechanism, Applicants believe that even though
full length Delta is able to bind to Notch, it is the soluble
fragment of Delta that is the actual ligand for Notch in
vivo.
The detection or measurement of Delta activation,
i.e., cleavage, is important in the study and manipulation of
differentiation processes, since Delta plays a key role in
cell fate (differentiation) determination, and since Delta is
a ligand of Notch, Notch also playing a key role in cell fate
(differentiation} determination. Molecules that modulate
Delta and Notch function are important tools for studying and
manipulating differentiation processes, e.g., in expanding
cell populations without substantial differentiation
- 10 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
(International Publication WO 97/11716), in cancer studies
and therapy (International Publication WO 94/07474), and
differentiation studies on normal tissue. Molecules that
allow the detection or measurement of Notch or Delta mRI~TA or
protein levels or acvtivity also have use in studying and
manipulating differentiation processes. Accordingly,
molecules that can be used to generate or detect anti-Delta
antibodies or Delta nucleic acids have use in such detection
or measurement.
One embodiment of the present invention is directed
to a peptide of approximately 30 amino acids, and its
encoding nucleic acids, of the toporythmic protein Delta that
contains a sequence which is cleaved by the metalloprot~ease-
disintegrin Kuzbanian (Kuz), (herein termed "cleavage
peptide") as well as derivatives (e.g., fragments) and
analogs thereof. For example, the Delta cleavage peptide
consists of the sequence of amino acid Cyssl6 to amino acid
Phesaa in human Delta (SEQ ID NO:10) , of amino acid Cyssls to
amino acid Phes43 in mouse Delta (SEQ ID N0:6) , of aminc> acid
Cyssz3 to amino acid F>hessl in chick Delta (SEQ ID N0:7) , of
amino acid Cyssle to amino acid Phes44 in Xenopus Delta (SEQ ID
N0:8) , and the sequence of amino acid Cysss4 to amino acid
Alas93 or Glns99 in Drcrsophila Delta (SEQ ID N0:9) . Nucleic
acids hybridizable to or complementary to the cleavage
peptide encoding nuc:Leic acids are also provided. In a
specific embodiment, the Delta cleavage peptide is a partion
of a mammalian Delta,, preferably a human Delta. Such a
peptide is believed too have the ability to modulate Kuz
cleavage of Delta, and thus, Delta and Notch activation.
In a specific embodiment, the present invention is
directed to a peptides comprising a fragment of a Delta
protein, the amino a<:id sequence of the peptide consisting of
the amino acid sequence CySsl6 to amino acid Phesa3 in human
Delta (SEQ ID NO:10) ,, Cyssls to amino acid Phesas in mouse
- 11 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
Delta (SEQ ID NO: 6) , Cyssz3 to amino acid Phessl in chick Delta
(SEQ ID N0:7) , Cyssl$ to amino acid Phes44 in Xenopus Delta
(SEQ ID N0:8) , or Cy~~ss4 to amino acid Alas93 or Glns94 in
Drosophila Delta (SEQ ID N0:9). In another embodiment, a
fragment of a Delta protein of not more than 150 or 50 or 30
amino acids comprising a Delta sequence selected from t:he
group consisting of amino acid sequence Cyssls to amino acid
Phes43 in human Delta (SEQ ID N0:10) , Cyssls to amino acid Phes43
in mouse Delta (SEQ ID N0:6) , Cyssz3 to amino acid Phessl in
chick Delta (SEQ ID N0:7) , CysslB to amino acid Phes44 in
Xenopus Delta (SEQ ID N0:8) , and Cyss64 to amino acid Alas93 or
Glns94 in Drosophila Delta (SEQ ID N0:9). In yet another
embodiment, the invention is directed to a peptide the amino
acid sequence of which consists of amino acid sequence Cyssls
to amino acid Phes43 in human Delta (SEQ ID NO:10) , Cyssls to
amino acid Phes4s in mouse Delta (SEQ ID N0:6) , Cyssza to amino
acid Phessi in chick Delta (SEQ ID N0:7) , CysslB to amino acid
Phes44 in Xenopus Delta (SEQ ID N0:8) , or Cysss4 to amino acid
Alas9s or Glns94 in Drosophila Delta (SEQ ID N0:9) .
The invention is also directed to a derivative or
analog of the cleavage peptide which is functionally active,
i.e., capable of displaying one or more known functional
activities associated with the "wild type" cleavage peptide.
Such functional activities include but are not limited to
antigenicity [ability to bind (or compete with the cleavage
peptide for binding) to an anti-Delta cleavage peptide
antibody], immunogenicity (ability to generate antibody which
binds to the cleavage peptide), ability to bind (or compete
with the cleavage peptide for binding) to Kuz. The invention
is further directed to a fragment (and derivatives or analogs
thereof) of the Delta cleavage peptide which is able to bind
to Kuz.
Antibodies t:o the Delta cleavage peptide, its
derivatives and analogs, are additionally provided.
- 12 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
Delta fragments that comprise the cleavage peptide
sequence are also provided, as are fusion proteins comprising
a Delta fragment containing a sequence of Delta that includes
at least the cleavage peptide sequence, fused to a non-Delta
sequence at the amino- and/or carboxy-terminal end of the
Delta sequence. Concatamers of Delta fragments containing at
least the cleavage peptide sequence (e.g., two, three, ar
more copies of a portion of the Delta sequence consisting of
at least the cleavage peptide sequence) are also provided.
In particular embodiments, the Delta fragments comprising the
cleavage peptide sequence are not greater than 35, 50, ;~5,
100, 150, or 200 amino acids in length. In a specific
embodiment, the present invention is directed to a chimeric
protein comprising a Delta protein sequence fused to a non-
Delta protein sequence:, wherein the Delta protein sequence is
a sequence of not more: than 100 or 50 or 30 amino acids that
comprises the amino acid sequence Cyssls to amino acid Phes43
in human Delta (SEQ ID NO:10) , Cyssls to amino acid Phe54~ in
mouse Delta (SEQ ID NC>:6) , Cyssza 'to amino acid Phessl in chick
Delta (SEQ ID N0:7) , C:yssl8 to amino acid Phes44 in Xenopus
Delta (SEQ ID N0:8) , or Cysss4 to ami''no acid Alas93 or Glns94 in
Drosophila Delta (SEQ ID N0:9).
In another Embodiment, the present invention is
directed to a peptide comprising an amino-terminal fragment
of a full length Delta protein, which fragment is cleaved
from the full length Delta protein by two proteolytic
processing events, thE; cleavage of the signal peptide and the
cleavage by Kuz, (herein termed "soluble Delta peptide" or
"D1E~") as well as derivatives and analogs thereof. For
example, the soluble Delta peptide amino acid sequence begins
at amino acid Serzz and terminates between amino acid Cys516
and amino acid Phes43 in human Delta (SEQ ID N0:10); begins at
amino acid Serzz and terminates between amino acid Cyssls and
amino acid Phes93 in mouse Delta (SEQ ID N0:6); begins at
- 13 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
amino acid Ser24 and germinates between amino acid Cyssz3 and
amino acid Phessl in chick Delta (SEQ ID N0:7), begins at
amino acid Ser2z and t:erminates between amino acid Cyssle and
amino acid Phe544 in Xenopus Delta (SEQ ID N0:8), or begins at
amino acid Ser23 and germinates between amino acid Cysss4 and
amino acid A1a593 or G1n594 in Drosophila Delta (SEQ ID N0:9).
Such a peptide is believed to have the ability to bind Notch,
and thus modulate Delta and Notch activation.
The invention is also directed to a derivative or
analog of the soluble Delta peptide which is functionally
active, i.e., capable of displaying one or more known
functional activities associated with the "wild type" soluble
peptide. Such functional activities include but are not
limited to antigenicity [ability to bind (or compete with the
soluble peptide for binding) to an anti-Delta soluble peptide
antibody], immunogenicity (ability to generate antibody which
binds to the soluble peptide), ability to bind (or compete
with the soluble peptide for binding) to Notch.
Antibodies i;.o the Delta soluble peptide, its
derivatives and analo<3s, are additionally provided.
Methods of production of the Delta cleavage
peptide, derivatives and analogs, e.g., by recombinant means,
are also provided. Methods of production of the soluble
Delta peptide, derivatives and analogs, e.g., by recombinant
means, are also provided.
The present invention is also directed to certain
compositions comprising and methods for production of protein
complexes of Delta and Kuz. Specifically, in this
embodiment, the invention is directed to complexes of Delta,
and derivatives, fragments and analogs of Delta, with Kuz,
and its derivatives, fragments and analogs (a complex of
Delta and Kuz is designated as "Delta:Kuz" herein). Methods
of production of a Del.ta:Kuz complex, and a derivative or
- 14 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
analog thereof, e.g., by recombinant means, are also
provided.
The present invention is also directed to certain
compositions comprising and methods for production of protein
complexes of Notch anct a soluble fragment of Delta consisting
essentially of the ext.racellular domain that is liberated by
the proteolytic proceeesing of Delta by Kuz ("soluble Delta
peptide" or "D1E~"). Specifically, in this embodiment, the
invention is directed to complexes of the soluble Delta
peptide, and derivatives, fragments and analogs of the
soluble Delta peptide, with Notch, and its derivatives,
fragments and analogs (a complex of the soluble fragment of
Delta and Notch is designated as "D1E~:Notch" herein).
Methods of production of a D1E~:Notch complex, and a
derivative or analog thereof, e.g., by recombinant means, are
also provided.
The invention is further directed to methods for
modulating (i.e., inhi.biting or enhancing) the activity of
Notch or Delta or Kuz by contacting a cell expressing Notch
or Delta o. Kuz, or an organism comprising a cell expressing
Notch or Delta or Kuz, a peptide comprising a fragment of
Delta having the amino acid sequence of about amino acid
Cysslb to about amino acid Phe543 in human Delta (SEQ ID
NO:10), of about amino acid Cyssls to about amino acid Phe54a
in mouse Delta (SEQ ID N0:6), of about amino acid Cyss23 to
about amino acid Phessl in chick Delta (SEQ ID N0:7), of about
amino acid CysslB to about amino acid Phe544 in Xenopus Delta
(SEQ ID N0: S) , and the: sequence of about amino acid Cys~64 to
about amino acid A1a593 or G1n594 in Drosophila Delta (SEQ ID
N0:9). in specific embodiments, the peptide comprises 25,
30, 35, 40, 50, 100, 1.50, 200 or 250 amino acids of Delta.
The invention is further directed to methods for
modulating (i.e., inhi.biting or enhancing) the activity of
Notch or Delta or Kuz or at least one of their signalling
- 15 -
CA 02333893 2001-O1-11
WO 00/OZ897 PCT/US99/15817
pathways
by
contacting
a cell
or
organism
expressing
Notch
or
Delta
or
Kuz
with
a ;peptide
comprising
a fragment
of
a Delta
protein
having
the
amino
acid
sequence
beginning
at
amino
acid Ser22 terminating between amino acid Cyssls and amino
and
acid Phes43 human Delta (SEQ ID NO:10); beginning at amino
in
acid Ser2Z terminating between amino acid Cyssls and amino
and
acid Phe543 mouse Delta (SEQ ID N0:6); beginning at amino
in
acid Ser24 terminating between amino acid Cys523 and amino
and
acid Phessl chick Delta (SEQ ID N0:7); beginning at amino
in
acid Serz2 terminating between amino acid Cyssle and amino
and
acid Phe544 Xenopu:> Delta (SEQ ID N0:8) ; and the sequence
in
beginning
at
amino
acid
Serz3
and
terminating
between
amino
acid Cysss4 amino acid A1a593 or G1n594 in Drosophila Delta
and
(SEQ ID N0:9)
.
The invention is further directed to methods for
modulating (i.e., inhibiting or enhancing) the activity of a
Delta:Kuz complex or a D1E~~Notch complex. The protein
components of a Delta~:Kuz complex or a DlEC:Notch complex have
been implicated in cell fate and differentiation.
Accordingly, the present invention is directed to methods for
screening a Delta:Kuz complex, as well as a derivative or
analog of the complex:, for the ability to alter cell fate or
differentiation. The present invention is also directed to
methods for screening a D1E~:Notch complex, as well as a
derivative or analog of the complex, for the ability to alter
Cell fate or differentiation.
The present invention is also directed to
therapeutic and diagnostic methods and compositions based on
the Delta cleavage peptide and encoding nucleic acids, as
well as on soluble Delta peptide and encoding nucleic acids.
The invention provides for the treatment of disorders of cell
fate and differentiation by administration of a therapeutic
compound of the invention. Such therapeutic compounds
(termed herein "Therapeutics") include: Delta cleavage
- 16 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99115817
peptides and derivative and analogs (including fragments)
thereof, antibodies thereto, nucleic acids encoding the Delta
cleavage peptide, derivatives, or analogs, Delta cleavage
peptide antisense nucleic acids, Delta:Kuz complexes and
antibodies thereto, and D1E~:Notch complexes and antibodies
thereto. In addition, such Therapeutics include soluble
Delta peptides and derivatives and analogs thereof,
antibodies thereto, nucleic acids encoding the soluble Delta
peptides, derivatives, or analogs, and soluble Delta peptide
antisense nucleic acids. In a preferred embodiment, a
Therapeutic of the invention is administered to treat a
cancerous condition, o:r to prevent progression from a pre-
neoplastic or non-malic3nant state into a neoplastic or a
malignant state. In ol~her specific embodiments, a
Therapeutic of the invc=_ntion is administered to treat a
nervous system disorder or to promote tissue regeneration and
repair.
In one embodiment, Therapeutics which antagonize,
or inhibit, Notch, Delta cleavage peptide and/or Kuz function
(hereinafter "Antagoni;st Therapeutics") are administered for
therapeutic effect. In another embodiment, Therapeutics
which promote Notch, D~=lta cleavage peptide and/or Kuz
function (hereinafter ":Agonist Therapeutics") are
administered for therapeutic effect.
Disorders of cell fate, in particular
hyperproliferative (e.~~., cancer) or hypoproliferative
disorders, involving aberrant or undesirable levels of
expression or activity or localization of Notch, Delta
cleavage peptide and o:r Kuz protein can be diagnosed by
detecting such levels, as described more fully infra.
Yet another embodiment of the present invention is
directed to methods fo:r detecting or measuring Delta
activation by observing or measuring Delta cleavage products
that are indicative of Delta activation. In one aspect of
- 17 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
this embodiment of the: invention, the method for detecting or
measuring Delta activation in a cell comprises detecting or
measuring the expression of one or more Delta cleavage
products selected from the group consisting of D1E~ and D1TM.
In yet another aspect, the method comprises detecting or
measuring an amino-terminal fragment of full-length Delta
terminating between amino acid Cyss fi4 and amino acid Alas93 or
Glns94 in Drosophila Delta (SEQ ID N0:9), between amino acid
CYssls and amino acid Phesaa in human Delta (SEQ ID N0:10) ,
between amino acid Cys~s~s and amino acid Phes4a in mouse Delta
(SEQ ID N0:6), between amino acid Cyss23 and amino acid Phessl
in chick Delta (SEQ ILr N0:7), or terminating between amino
acid CysslB and amino acid Phes44 in Xenopus Delta (SEQ ID
N0:8). In yet another aspect, the method comprises detecting
or measuring under reducing conditions, a soluble Delta
fragment of approximately 67 kilodaltons (D1E~) . In yet
another aspect, the method comprises detecting or measuring a
soluble Delta peptide having the amino acid sequence
beginning at amino acid Serzz and terminating between amino
acid Cyssls and amino acid Phes43 in human Delta (SEQ ID
NO:10); beginning at amino acid Ser2z and terminating between
amino acid Cyssls and amino acid Phes43 in mouse Delta (SEQ ID
N0:6); beginning at amino acid Ser24 and terminating between
amino acid Cyssaa and amino acid Phessl in chick Delta (SEQ ID
N0:7); beginning at amino acid Ser~Z and terminating between
amino acid Cyssle and amino acid Phes44 in Xenopus Delta (SEQ
ID N0:8); and the sequence beginning at amino acid Ser23 and
terminating between amino acid Cyss64 and amino acid Alas9, or
Glns94 in Drosophila Delta (SEQ ID N0:9) .
The present invention is also directed to methods
for detecting or measuring Kuz function by observing or
measuring Delta cleavage products that are indicative of Kuz
function. In one aspE;ct of this embodiment of the invention,
the method for detecting or measuring Kuz function in a cell
- 18 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
comprises detecting or measuring the expression of one or
more Delta cleavage products selected from the group
consisting of D1E~ and D1T'''. In yet another aspect, the method
comprises detecting or measuring an amino-terminal fragment
of full-length Delta which terminates between amino acid
Cys564 and amino acid A1a593 or G1n594 in Drosophila Delta,
between amino acid CysS,s and amino acid Phesaa in human Delta,
between amino acid Cyssls and amino acid Phe543 in mouse Delta,
between amino acid Cyss23 and amino acid Phessl in chick Delta,
or terminates between amino acid Cyssle and amino acid Phe5,4
in Xenopus Delta. In yet another aspect, the method
comprises detecting or measuring under reducing conditions, a
soluble Delta fragment of approximately 67 kilodaltons. In
yet another aspect, t;he method comprises detecting or
measuring a soluble Delta peptide having the amino acid
sequence beginning at amino acid Ser22 and terminating between
amino acid Cyssls and amino acid Phes43 in human Delta (SEQ ID
NO:10); beginning at amino acid Ser22 and terminating between
amino acid CYS515 and amino acid Phe543 in mouse Delta (SEQ ID
N0:6); beginning at amino acid Serz4 and terminating between
amino acid Cys5z3 and amino acid Phessl in chick Delta (SEQ ID
N0:7); beginning at amino acid Serz2 and terminating between
amino acid CysslB and amino acid Phe5,4 in Xenopus Delta (SEQ
ID N0:8); and the sequence beginning at amino acid Serz3 and
terminating between amino acid Cysss, and amino acid Aia593 or
G1n5s4 in Drvsophila Delta (SEQ ID N0:9) .
In another embodiment, the present invention is
also directed to methods for identifying a molecule that
modulates Delta activation by detecting or measuring a change
in the amount or pattern of Delta cleavage products. In one
aspect of this embodiment of the invention, the method for
identifying a modulator of Delta activation comprises
providing a cell with a candidate modulator molecule and
detecting or measuring the expression by the cell of one or
- 19 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
more Delta cleavage products selected from the group
consisting of D1E~ and D1TM, in which a difference in the
presence or amount of said one or more cleavage products
compared to a Delta cell not contacted with the candidate
molecule indicates that the molecule modulates Delta
activity.
In an alternative aspect, the method for
identifying a modulator of Delta activation comprises
contacting a candidate modulator molecule with a full length
Delta in the presence of a composition comprising Kuz and
optionally other cellular proteins, under conditions
conducive to cleavage of the full-length Delta by Kuz and
optionally one or more components of the composition and
detecting or measuring' the amount of Delta cleavage products
D1E~ and Dlz" that result, in which a difference in the
presence or amount of said Delta cleavage products compared
to a full-length Delta. in presence of said composition not
contacted with the candidate molecule indicates that the
molecule modulates Delta activity.
Ir_ yet another embodiment, the present invention is
also directed to methods for identifying a molecule that
modulates Kuz function by detecting or measuring a change in
the amount of Delta cleavage products that are necessary for
Kuz function. In one aspect of this embodiment of the
invention, the method for identifying a modulator of Kuz
function comprises providing a cell with a candidate
modulator molecule anc~ detecting or measuring the expression
by the cell of one or more Delta cleavage products selected
from the group consisting of D1E~ and D1T"', in which a
difference in the presence or amount of said one or more
cleavage products compared to a Delta cell not contacted with
the candidate molecule indicates that the molecule modulates
Notch function.
- 20 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
In yet another embodiment, the present invention is
also directed to methods for identifying a molecule that
modulates Kuz function by detecting or measuring a change in
the amount of Delta c7.eavage products that are indicative of
Kuz function. In one aspect of this embodiment of the
invention, the method for identifying a modulator of Kuz
function comprises providing a cell with a candidate
modulator molecule and detecting or measuring the expression
by the cell of one or more Delta cleavage products selected
from the group consisting of DlE~ and D1'~'', in which a
difference in the presence or amount of said one or more
cleavage products compared to a Delta cell not contacted with
the candidate molecule. indicates that the molecule modulates
Kuz function.
The present invention is also directed to
therapeutic and prophylactic, as well as diagnostic,
prognostic, and screening methods and compositions based upon
the Delta:Kuz complex or the Dl~~:Notoh complex (and the
nucleic acids encoding the individual proteins that
participate in the complex). Therapeutic compounds of the
invention include, but are not limited to, a Delta:Kuz
complex, and a comple:K where one or both members of the
complex is a derivative, fragment, homolog or analog of Delta
or Kuz; antibodies to and nucleic acids encoding the
foregoing; and antisense nucleic acids to the nucleotide
sequences encoding the complex components. Diagnostic,
prognostic and screening kits are also provided.
Animal models and methods of screening for
modulators (i.e., agonists, and antagonists) of the activity
of a Delta:Kuz complex or of a D1E~:Notch complex are also
provided.
Methods of identifying molecules that inhibit, or
alternatively, that increase formation of a Delta:Kuz complex
or of a Dlr':Notch complex are also provided.
- 21 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99J15817
4. BRIEF DESCRIPTION OF THE FIGURES
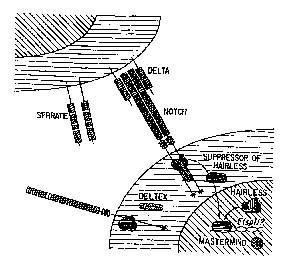
Figure 1 is a schematic diagram of the Notch
signaling pathway. The Notch receptor can bind to either
Delta or Serrate through its extracellular domain. Ligand
binding can result in receptor multimerization that is
stabilized by interactions between the intracellular ankyrin
repeats of Notch and t:he cytoplasmic protein Deltex. These
events can control the nuclear translocation of the DNA-
binding protein Suppressor of Hairless and its known
association with the Hairless protein. The transcriptianal
induction of the Enhancer of Split basic helix-loop-helix
(bHLH) genes appears t:o depend on Notch signaling.
Figure 2 is a Notch homolog sequence comparison.
The human Notch2 (humDl2) (SEQ ID NO:1), human Notchl (humNl)
(SEQ ID N0:2), Xenopu~~ Notch/Xotch (XenN) {SEQ ID N0:3), and
Drosophila Notch (DrasN) (SEQ ID N0:4) protein sequences are
aligned, with names indicated to the left and numbering to
the right (Wharton et al., 1985, Cell 43:567-581; Coffman et
al., 1990, Science 249:1438-1441; Ellisen et al., 1991, Cell
66:649-661; Stifani et al., 1992, Nature Genetics 2:119-127).
Major Notch protein me>tifs are enclosed in boxes. Starting
from the N-terminal, the boxed regions indicate: EGF repeats,
Lin-12/Notch (LN) repeats, transmembrane domain (TM), Ankyrin
repeats, and PEST-containing region. Also indicated are the
putative CcN motif components (Stifani et ai., 1992, Nature
Genetics 2:119-127) nuclear localization signal (NLS, BNTS)
and putative CKII and cdc2 phosphorylation sites. The
calculated signal cleavage site is indicated with an arrow.
Figure 3 is a Delta homolog sequence comparison.
The human Delta (HDL) (SEQ ID N0:5), mouse Delta {MDL) (SEQ
ID N0:6), chick Delta (CDL) (SEQ ID N0:7), Xenopus Delta
{XDL) (SEQ ID N0:8), amd Drosophila Delta (DDL) (SEQ ID N0:9)
protein sequences are aligned, with names indicated to t:he
- 22 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
left and numbering to the right. Major Delta protein motifs
are labeled.
Figure 4A and 4B is the amino acid sequence (SEQ iD
NO:10) and the nucleic acid sequence (SEQ ID NO:11),
respectfully, of human Delta.
Figure 5A and 5B is the amino acid sequence (SEQ ID
N0:12) and the nucleic acid sequence (SEQ ID N0:13),
respectfully, of the human Kuz homolog.
Figures 6A-6F shows results of a genetic modifier
screen that was carried out to identify genes that
genetically interact with kuz. A strain constitutively
expressing a KuzDN construct in developing imaginal discs was
used in the screen (expression of a KuzDN construct lacking
the proprotein and metalloprotease domains was driven by a
GAL4 line 32B) which causes adult mutant phenotypes,
including extra wing vein materials, mostly notably deltas at
the ends of the longitudinal veins (denoted by arrowheads in
Figure 6A), small and rough eyes, and extra bristles on the
notum (denoted by arrowheads in Figure 6E). More than 2400
lethal P-element insertions were screened for phenotypic
modification effects on KuzDN. Seven P-insertions were found
to cause significant reduction of the viability (semi-lethal)
of the KuzDN flies when they are also heterozygous for each
of the P-insertion. F~reliminary characterization of these P-
insertions revealed that two of them are Kuz alleles and one
is a loss-of-function Delta allele while the nature of t:he
other insertions are unknown. Flies that carry an extra copy
of the Delta gene (+/+~%+) with the KuzDN background (Figures
6B, 6F) show an almost. complete suppression of the KuzDN
phenotypes. (Figure E~C) An extra copy of Notch (+/+/+)
(Ramos et al., 1989, CTenetics 123:337-348) alone has an
essentially normal phenotype (Figure 6C). Notch (+/+/+)
gives negligible suppression of the KuzDN phenotype in KuzDN
flies (Figure 6D).
- 23 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/x58I7
Figures 7A-7E show that a soluble fragment of Delta
(D1E~) is released cons;titutively in S2 cells in vivo. Figure
7A: Expression of Delta (Dl) antigen in stably transfected
S2 cells (Rebay, et al., 1991, Cell 67:687-699) is detected
by SDS-PAGE and western blotting with monoclonal antibody 9B
of reduced {+Bme) and non-reduced (-Bme) cell extracts (c)
and culture media (m). A product consistent with full length
Delta is clearly detectable in the cell extract (MW- 83,000
Daltons non-reduced and 90,000 Daltons reduced). A
significant amount of a product of greater mobility is seen
in the media (MW~ 62,000 Daltons non-reduced and 67,000
Daltons reduced) that is consistent in size with the
extracellular domain of Delta (estimated MW-- 65,000 Daltons)
and is referred to as D1E~. A 40-fold higher affinity of the
antibody was observed for the non-reduced versus reduced
I5 Delta and was compensated for by increased protein load (4X)
and exposure times (10X) in the reduced samples. Figure 7B:
Bands of the same mobility are seen in extracts of wild type
Drosophila embryos (l6hr). Note that 1, 3, 5 and 10 embryos
loaded on the gel demonstrate that the antigen is barely
detectable in a single embryo ("1") but becomes clearer with
the greater number of embryos loaded ("10"). Figure 7C:
Affinity purified D1E~ migrates at MW- 62,000 Daltons under
reducing conditions anal at MW-. 67,000 Daltons under non-
reducing conditions on. a coomassie blue-stained SDS-PAGE gel.
Figure 7D: Schematic of the Drosophila Delta protein
demonstrates the DSL domain (DSL), the epidermal growth
factor like repeats (E'GF) and the transmembrane domain (TM).
Amino acid numbering of N-terminus, the beginning of the TM
domain and the C-terminus is shown. Figure 7E: Thirteen
cycles of N-terminal amino acid sequence analysis of DlE'= is
shown with alignment to the Drosophila (dDl), Xenopus (xDl)
and human (hDl) Delta amino acid sequences. The arrow
indicates the conserved serine residue in the position of the
- 24 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
N-terminus of DlEC and the potential site of signal peptide
processing for D1.
Figures 8A-8l7 shows that Kuz plays a direct role in
Delta processing in vivo and in vitro. Figure 8A: The first
two panels (-): Expression of Delta and DlEC are apparent by
western blotting with the 9B antibody in the cell pellet (c)
and the medium (m) in ;32 cells transiently transfected with
full length Delta (Fehon, et al., 1990, Cell 61:523-534).
The second two panels (Kuz): Cotransfection of S2 cells with
Kuz and Delta results :in an increase in the DlEC fragment in
the cell culture media (m) which correlates with an apparent
decrease in Delta in the cell pellet (c). The third two
panels (KuzDN): Cotransfection with dominant negative Kuz
dramatically decreases the DlEC observed in the media (m) and
corresponds with greater amounts of full length Delta in the
cell pellet (c). Figure 8B: Cotransfection of Kuz and KuzDN
with Notch was done under identical experimental conditions
as for Delta and western blotted with the 9C6 Notch
intracellular domain antibody (Fehon, et al., 1990, Cell
61:523-534) demonstrates a negligible effect on the
Processing of Notch as seen by the invariant levels of N'''"',
the constitutively processed form of Notch (Blaumueller et
al., 1997, Cell 90:281-291). Figure 8C: The metalloprotease
inhibitors EDTA and 1,10-phenanthroline inhibit the
endogenous S2 cell proteolytic activity yielding DlEC. The
left panel demonstrates the accumulation of DlEC at various
time points up to 60 minutes in the medium of S2 cells stably
expressing full length Delta (Rebay, et al., 1991, Cell
67:687-699). The right panel shows the accumulation of DlEc
at 60 minutes in the presence of EDTA (5, 10, 15 mM) and
1,10-phenanthroline (5, 10 mM). Relatively high
concentrations of the chelators were required to overcome the
concentrations Ca2' (~8.6 mM) and other metal ions in the
media and serum. Higher concentrations of 1,10-
- 25 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
phenanthroline proved to alter cell morphology. Hoth of these
reagents, which are well documented metalloprotease
inhibitors, inhibit accumulation of DlEC in the media. Figure
8D: Delta processing is inhibited in Kuz -/- embryos. Nine
Kuz +/- and Kuz -/- embryos were identified by morphology and
the extracts analyzed by SDS-PAGE and western blotting with
antibody 9B. DlEC is absent in Kuz -/- embryos and
demonstrates a higher level of full length Delta compared to
Kuz +/- embryos.
Figures 9A-9C shows that DlEC binds to Notch,
competes for Notch-Delta interaction and acts as an agonist
of the Notch pathway. Figure 9A: The DlEC fragment
specifically binds to Notch expressing S2 cells and does not
bind to S2 cells alone. Notch expressing S2 cells (lane 1,
2) incubated in the absence (lane 1) or presence (lane 2) of
DlEC (lane 6) were sedimented through a sucrose cushion and
the extract was western blotted with antibody 9B. DlEC was
prepared as a 5X concentrate of 16 hour culture media (Sang~s
M3) of 0.7mM CuS04 induced Delta-S2 cells. Notch-S2 and
nontransfected S2 cells were induced with 0.7 mM CuS04 for
l6hrs in media with 5zc serum. The cells were collected by
centrifugation and washed once in serum free media with 1~
bovine serum albumin (BSA) and resuspended at 2x106 cells/mL
in M3, 1~ BSA. 250 JCL of cells were added to 100 ~tL of D1~c
concentrate, raised to 500 ~.L with M3, 1~ BSA and incubated
one hour at room temperature on a rocking table at five
oscillations per minute. The mixture was layered over a
cushion of 20~ sucrose, 20mM TRIS-HC1, 150mM NaCl, 2mM CaCl2,
1~ BSA, pH 7.4, in microfuge tubes that had previously been
blocked with 1~ BSA. The tubes were centrifuged at 14,000
rpm for 3 minutes and the supernatant aspirated. The cell
pellets were washed two times with cold serum free media
without resuspension of the pellet. The pellet was then
lysed and dissolved in SDS-PAGE sample buffer without f3-
- 26 -
CA 02333893 2001-O1-11
WO OO/OZ897 PCT/US99/15817
mercaptoethanol and boiled for five minutes. The proteins
were resolved by SDS-PAGE and western blotting with the 9B
antibody. Lane 3 and 4 show parallel incubations with S2
cells in the absence (lane 3) or presence (lane 4) of DlEC.
Figure 9B: Preincubation of Notch-S2 cells with DlEc
concentrate reduces their subsequent rate of aggregation with
Delta-S2 cells as measured turbidimetrically with transmitted
light at 320nm. At the concentration shown (1X DlEC, clased
circles), a 60~ inhibition in the initial rate of aggregation
was seen compared to control media concentrate (1X ~ECN,
closed squares). The error bars show the standard deviation
of the mean of triplicate determinations. Figure 9C shows
the effect of DlEC on primary cultured cortical neurons i.n the
representative images as labeled: (I) seven to ten days in
vitro cortical neurons before treatment, (II) cultured in the,
presence of ~ECN media, (III) cultured in the presence of DlEc
media, (IV) affinity purified DlEC, and (V) buffer control for
purified D1E'. The graph represents the mean length of
neurites per neuron. Each bar represents the mean ~ SEM of
three separate experimental trials. Primary cortical neurons
exhibit multipolar morphology and the extensive neurite
network in control cultures (I), cultures in the presence of
~ECN media (II) and buffer control of purified DlEC (V) . Note
the decrease in the mean neurite length per neuron and
limited neurite branching in cultures treated with DlEC media
(III) and purified Dlec (IV) . Scale bar = 50 Vim.
Figure 10 is a schematic diagram comparing the
soluble fragment of Delta (DlEC) that is clipped by Kuz with
D1S.
Figure 11 shows the amino acid sequence of the
Delta cleavage peptide of Drosophila Delta (SEQ ID N0:9).
Bold arrows indicate potential cleavage sites identified by
data from both C-terminal sequence analysis and LC/MS; dashed
arrows indicate potential cleavage sites identified by only
- 27 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
one of the analyses. (A) indicates the alanine instead of
the threonine reported by Vassin et a1.,1987, EMBO J. 6:3431-
3440.
5. DETAILED DESCRIPTION OF THE INVENTION
The inventors have discovered that Delta is cleaved
by the metalloprotease-disintegrin Kuzbanian (Kuz) into two
fragments, a soluble amino-terminal fragment consisting
essentially of the extracellular domain, and a membrane-bound
fragment consisting essentially of the transmembrane domain
and the intracellular domain. The soluble fragment of Delta,
like the full length, membrane-bound Delta, is able to mnct
to Notch. Although not intending to be limited to any
particular mechanism, Applicants believe that even though
full length Delta is able to bind to Notch, it is the saluble
fragment of Delta that is the actual ligand for Notch in
vi vo .
The detection or measurement of Delta activation,
i.e., cleavage, is important in the study and manipulation of
differentiation processes, since Delta plays a key role in
cell fate (differentiation) determination, and since Delta is
a ligand of Notch, Notch also playing a key role in cell fate
(differentiation) determination. Molecules that modulate
Delta and Notch function are important tools for studying and
manipulating differentiation processes, e.g., in expanding
cell populations without substantial differentiation
(International Publication WO 97/11716), in cancer studies
and therapy (International Publication WO 94/07474), and
differentiation studies on normal tissue. Molecules that
allow the detection or measurement of Notch or Delta mRNA or
protein levels or activity also have use in studying and
manipulating differentiation processes. Accordingly,
molecules that can be used to generate or detect anti-Delta
- 28 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
antibodies or Delta nucleic acids have use in such detection
or measurement.
One embodiment of the present invention is directed
to a peptide of approximately 30 amino acids, and its
encoding nucleic acids, of the toporythmic protein Delta that
contains a sequence which is cleaved by the metalloprotease-
disintegrin Kuzbanian (Kuz), (herein termed "cleavage
peptide") as well as derivatives (e.g., fragments) and
analogs thereof. For example, the Delta cleavage peptide
consists of the sequence of amino acid Cyssls to amino acid
Phe54, in human Delta, of amino acid CysSls to amino acid Phes4a
in mouse Delta, of amino acid Cys523 to amino acid Phe$S1 in
chick Delta, of amino acid CysSl~ to amino acid Phe544 in
Xenopus Delta, and the sequence of ammo acia c:ysss4 Lo amino
acid A1a593 or G1n594 in Drosophila Delta. Nucleic acids
hybridizable to or complementary to the cleavage peptide
encoding nucleic acids are also provided. In a specific
embodiment, the Delta cleavage peptide is a portion of a
mammalian Delta, preferably a human Delta. Such a peptide is
believed to have the .ability to modulate Kuz cleavage of
Delta, and thus, Delta and Notch activation.
The invention is also directed to a derivative or
analog of the cleavage peptide which is functionally active,
i.e., capable of displaying one or more known functional
activities associated with the "wild type" cleavage peptide.
Such functional activities include but are not limited to
antigenicity [ability to bind (or compete with the cleavage
peptide for binding) to an anti-Delta cleavage peptide
antibody], immunogenicity (ability to generate antibody which
binds to the cleavage peptide), ability to bind (or compete
with the cleavage peptide for binding) to Kuz. The invention
is further directed to a fragment (and derivatives or analogs
thereof) or the Delta cleavage peptide which is able to bind
to Kuz.
- 29 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99115817
Antibodies t:o the Delta cleavage peptide, its
derivatives and analogs, are additionally provided.
Delta fragments that comprise the cleavage peptide
sequence are also provided, as are fusion proteins comprising
a Delta fragment containing a sequence of Delta that includes
at least the cleavage peptide sequence, fused to a non-Delta
sequence at the amino-~ and/or carboxy-terminal end of the
Delta sequence. Concatamers of Delta fragments containing at
least the cleavage peptide sequence (e.g., two, three, or
more copies of a portion of the Delta sequence consisting of
at least the cleavage peptide sequence) are also provided.
In particular embodimE:nts, the Delta fragments comprising the
cleavage peptide sequence are not greater than 35, 50, '75,
100, 150, or 200 amino acids in length.
Methods of production of the Delta cleavage
peptide, derivatives and analogs, e.g., by recombinant means,
are also provided.
In another embodiment, the present invention is
directed to a peptide comprising an amino-terminal fragment
of a full length Delta protein, which fragment is cleaved
from the full length Delta protein by two proteolytic
processing events, the' cleavage of the signal peptide and the
cleavage by Kuz (herein termed "soluble Delta peptide" or
"D1E~" ) as well as derivatives and analogs thereof . Far
example, the soluble Delta peptide amino acid sequence begins
at amino acid Ser2z and terminates between amino acid Cyssls
and amino acid Phes4a z.n human Delta (SEQ ID NO:10); begins at
amino acid Ser2z and terminates between amino acid Cyss~s and
amino acid Phes43 in mouse Delta (SEQ ID N0:6); begins at
amino acid Ser24 and terminates between amino acid Cys5Z3 and
amino acid PheSS~ in chick Delta (SEQ ID N0:7), begins at
amino acid Ser22 and terminates between amino acid Cyssle and
amino acid Phes44 in Xenopus Delta (SEQ ID N0:8), or begins at
amino acid Serz3 and terminates between amino acid Cys564 and
- 30 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
amino acid A1a59, or 6171594 in Drosophila Delta (SEQ ID NO: 9) .
Such a peptide is believed to have the ability to bind Notch,
and thus modulate Delta and Notch activation.
The invention is also directed to a derivative or
analog of the soluble Delta peptide which is functionally
active, i.e., capable of displaying one or more known
functional activities associated with the "wild type" soluble
peptide. Such functional activities include but are not
limited to antigenicity [ability to bind (or compete with the
soluble peptide for binding) to an anti-Delta soluble peptide
antibody], immunogenicity (ability to generate antibody which
binds to the soluble peptide), ability to bind (or compete
with the soluble peptide for binding) to Notch.
Antibodies to the Delta soluble peptide, its
derivatives and analogs, are additionally provided.
Methods of production of the soluble Delta peptide,
derivatives and analogs, e.g., by recombinant means, are also
provided.
The present invention is also directed to certain
compositions comprising and methods for production of protein
complexes of Delta and. Kuz. Specifically, in this
embodiment, the invention is directed to complexes of Delta,
and derivatives, fragments and analogs of Delta, with Kuz,
and its derivatives, fragments and analogs (a complex of
Delta and Kuz is designated as "Delta:Kuz" herein). Methods
of production of a Del.ta:Kuz complex, and a derivative ar
analog thereof, e.g., by recombinant means, are also
provided.
The present invention is also directed to certain
compositions and methods for production of protein complexes
with Notch of the soluble fragment of Delta liberated by Kuz.
Specifically, in this embodiment, the invention is directed
to complexes of the soluble Delta peptide, and derivatives,
fragments and analogs of the soluble fragment, with Notch,
- 31 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99115817
and its derivatives, fragments and analogs (a complex of the
soluble fragment of Delta and Notch is designated as
"DlE~:Notch" herein) . Methods of production of a D1E~:Notch
complex, and a derivative or analog thereof, e.g., by
recombinant means, are: also provided.
The invention is further directed to methods for
modulating (i.e., inhibiting or enhancing) the activity of
Notch or Delta or Kuz by contacting a cell expressing Notch
or Delta or Kuz, or an organism comprising a cell expressing
Notch or Delta or Kuz, a peptide comprising a fragment of
Delta having the amino acid sequence of about amino acid
Cyssls to about amino acid Phesaa in human Delta (SEQ ID
NO:10) , of about amino acid CysSls to about amino acid Phe543
in mouse Delta (SEQ ID N0:6), of about amino acid Cys5z3 to
about amino acid Phessl in chick Delta (SEQ ID N0:7), of about
amino acid Cyssle to about amino acid Phe544 in Xenopus Delta
(SEQ ID N0:8), and the sequence of about amino acid Cys564 to
about amino acid Alas9, or G1n594 in Drosophila Delta (SEQ ID
N0:9). In specific embodiments, the peptide comprises 25,
30, 35, 40, 50, 100, 150, 200 or 250 amino acids of Delta.
The invention is further directed to methods for
modulating (i.e., inhibiting or enhancing) the activity of
Notch or Delta or Kuz or at least one of their signalling
pathways by contacting a cell or organism expressing Notch or
Delta or Kuz with a peptide comprising a fragment of a Delta
protein having the amino acid sequence beginning at amino
acid Ser22 and terminating between amino acid Cyssls and amino
acid Phe54a in human Delta (SEQ ID N0:10); beginning at amino
acid Ser22 and terminating between amino acid Cyssls and amino
acid Phe543 in mouse Delta (SEQ ID N0:6); beginning at amino
acid Ser24 and terminating between amino acid Cyssz3 and amino
acid Phessl in chick Delta (SEQ ID N0:7); beginning at amino
acid Ser22 and terminating between amino acid Cyssle and amino
acid Phe54< in Xenopus Delta (SEQ ID N0:8); and the sequence
- 32 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
beginning at amino aced Ser23 and terminating between amino
acid Cys56a and amino acid A1a593 or G1n594 in Drosophila Delta
(SEQ ID N0:9).
The invention is further directed to methods for
modulating (i.e., inhibiting or enhancing) the activity of a
Delta:Kuz complex or t:he activity of a D1E~:Notch complex.
The protein components of a Delta:Kuz complex and of a
D1E~~Notch complex have been implicated in cell fate and
differentiation. Accordingly, the present invention is
directed to methods for screening a Delta:Kuz complex, as
well as a derivative or analog of the complex, for the
ability to alter cell fate or differentiation. The present
invention is also directed to methods for screening a
D1E~:Notch complex, as well as a derivative or analog of the
complex, for the ability to alter cell fate or
differentiation. .
The present invention is also directed to
therapeutic and diagnostic methods and compositions based on
the Delta cleavage peptide and encoding nucleic acids, as
well as on soluble De:Lta peptides and encoding nucleic acids.
The invention provides for the treatment of disorders of cell
fate and differentiation by administration of a therapeutic
compound of the invent:ion. Such therapeutic compounds
(termed herein "Therapeutics") include: Delta cleavage
peptides and derivati~,re and analogs (including fragments)
thereof, antibodies thereto, nucleic acids encoding the Delta
cleavage peptide, derivatives, or analogs, Delta cleavage
peptide antisense nuc:Leic acids, Delta:Kuz complexes and
antibodies thereto, and D1E~:Notch complexes and antibodies
thereto. In addition, such Therapeutics include soluble
Delta peptides and derivatives and analogs thereof,
antibodies thereto, nucleic acids encoding the soluble Delta
peptides, derivatives, or analogs, and soluble Delta peptide
antisense nucleic acids. In a preferred embodiment, a
- 33 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
Therapeutic of the invention is administered to treat a
cancerous condition, or to prevent progression from a pre-
neoplastic or non-malignant state into a neoplastic or a
malignant state. In other specific embodiments, a
Therapeutic of the im;rention is administered to treat a
nervous system disorder or to promote tissue regeneration and
repair.
In one embodiment, Therapeutics which antagonize,
or inhibit, Notch, De:Lta cleavage peptide and/or Kuz function
(hereinafter "Antagon:ist Therapeutics") are administered for
therapeutic effect. :Ln another embodiment, Therapeutics
which promote Notch, l7elta cleavage peptide and/or Kuz
function (hereinafter "Agonist Therapeutics") are
administered for ther<~peutic effect.
Disorders o:E cell fate, in particular
hyperproliferative (e. g., cancer) or hypoproliferative
disorders, involving aberrant or undesirable levels of
expression or activity or localization of Notch, Delta
cleavage peptide and or Kuz protein can be diagnosed by
detecting such levels, as described more fully infra.
Yet another embodiment of the present invention is
directed to methods for detecting or measuring Delta
activation by observing or measuring Delta cleavage products
that are indicative o:E Delta activation. In one aspect of
this embodiment of the invention, the method for detecting or
measuring Delta activ<~tion in a cell comprises detecting or
measuring the expression of one or more Delta cleavage
products selected from the group consisting of D1E~ and D1T'".
In yet another aspect, the method comprises detecting or
measuring an amino-terminal fragment of full-length Delta
terminating between amino acid Cysss4 and amino acid Ala5s3 or
G1n594 in Drosophila Delta, between amino acid Cys516 and amino
acid Phe543 in human Delta, between amino acid Cyssls and amino
acid Phe543 in mouse I3e~lta, between amino acid Cys5z3 and amino
- 34 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/158I7
acid Phe551 in chick Delta, or terminating between amino acid
Cys518 and amino acid Phe544 in Xenopus Delta . In yet another
aspect, the method comprises detecting or measuring under
reducing conditions, a soluble Delta fragment of
approximately 67 kilod.altons. In yet another aspect, the
method comprises detecting or measuring a soluble Delta
peptide having the amino acid sequence beginning at amino
acid Serzz and terminating between amino acid Cys516 and amino
acid Phe543 in human Delta (SEQ ID N0:10); beginning at amino
acid Ser22 and terminating between amino acid Cys515 and amino
acid Phe54a in mouse Delta (SEQ ID N0:6); beginning at amino
acid Serz4 and terminating between amino acid Cys523 and amino
acid Phessi in chick Delta (SEQ ID N0:7); beginning at amino
acid Serz2 and terminating between amino acid CysS,e and amino
acid Phes44 in Xenopus Delta (SEQ ID N0:8); and the sequence
beginning at amino acid Serz3 and terminating between amino
acid Cys564 and amino acid A1a593 or G1n594 in Drosophila Delta
(SEQ ID N0:9).
The present invention is also directed to methods
for detecting or measuring Kuz function by observing or
measuring Delta cleavage products that are indicative of: Kuz
function. In one aspect of this embodiment of the invention,
the method for detecting or measuring Kuz function in a cell
comprises detecting or measuring the expression of one or
more Delta cleavage praducts selected from the group
consisting of D1E~ and DlTr'. In yet another aspect, the method
comprises detecting or measuring an amino-terminal fragment
of full-length Delta which terminates between amino acid
Cyss64 and amino acid A.1a593 or G1n594 in Drosophila Delta,
between amino acid Cys;516 and amino acid Phe543 in human Delta,
between amino acid Cy:o515 and amino acid Phes43 in mouse Delta,
between amino acid Cys>5z3 and amino acid Phe551 in chick Delta,
or terminates between amino acid CysSle and amino acid Phe544
in Xenopus Delta. In yet another aspect, the method
- 35 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
comprises detecting o~- measuring under reducing conditians, a
soluble Delta fragment: of approximately 67 kilodaltons. In
yet another aspect, the method comprises detecting or
measuring a soluble Delta peptide having the amino acid
sequence beginning at amino acid Ser22 and terminating between
amino acid Cys516 and amino acid Phe5,3 in human Delta (SEQ ID
NO:10); beginning at amino acid Ser2z and terminating between
amino acid Cyssls and amino acid Phe543 in mouse Delta (SEQ ID
N0:6); beginning at amino acid Ser24 and terminating between
amino acid Cys523 and amino acid Phessl in chick Delta (SEQ ID
N0:7); beginning at amino acid Serzz and terminating between
amino acid Cys5~8 and amino acid Phe544 in Xenopus Delta {SEQ
ID N0:8); and the sequence beginning at amino acid Serz3 and
terminating between amino acid Cys564 and amino acid Alasss or
G1n594 in Drosophila Delta (SEQ ID N0:9) .
In another embodiment, the present invention is
also directed to methods for identifying a molecule that
modulates Delta activation by detecting or measuring a change
in the amount or patte=rn of Delta cleavage products. In one
aspect of this embodiment of the invention, the method for
identifying a modulator of Delta activation comprises
providing a cell with a candidate modulator molecule and
detecting or measurinc3 the expression by the cell of one or
more Delta cleavage p=roducts selected from the group
consisting of D1E~ and D1~"', in which a difference in the
presence or amount of said one or more cleavage products
compared to a Delta cell not contacted with the candidate
molecule indicates that the molecule modulates Delta
activity.
In an alter=native aspect, the method for
identifying a modulator of Delta activation comprises
contacting a candidate modulator molecule with a full length
Delta in the presence of a composition comprising Kuz and
optionally other cellular proteins, under conditions
- 36 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
conducive to cleavage of the full-length Delta by Kuz and
optionally one or more components of the composition and
detecting or measuring the amount of Delta cleavage products
D1E~ and Dl's' that resu:l t , in which a di f f erence in the
presence or amount of said Delta cleavage products compared
to a full-length Delta. in presence of said composition not
contacted with the candidate molecule indicates that the
molecule modulates Delta activity.
In yet another embodiment, the present invention is
also directed to methods for identifying a molecule that:
modulates Notch function by detecting or measuring a change
in the amount of Delta. cleavage products that are necessary
for Notch function. In one aspect of this embodiment of the
invention, the method for identifying a modulator of Notch
function comprises providing a cell with a candidate
modulator molecule and. detecting or measuring the expression
by the cell of one or more Delta cleavage products selected
from the group consisting of D1E~ and D1'1'r', in which a
difference in the presence or amount of said one or more
cleavage products comF~ared to a Delta cell not contacted with
the candidate molecule indicates that the molecule modulates
Notch function.
In yet another embodiment, the present invention is
also directed to methods for identifying a molecule that
modulates Kuz function by detecting or measuring a change in
the amount of Delta cleavage products that are indicative of
Kuz function. In one aspect of this embodiment of the
invention, the method for identifying a modulator of Kuz
function comprises providing a cell with a candidate
modulator molecule and detecting or measuring the expression
by the cell of one or more Delta cleavage products selected
from the group consisting of DIES and D1T"', in which a
difference in the pre~oence or amount of said one or more
cleavage products compared to a Delta cell not contacted with
- 3'7 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
the candidate molecule indicates that the molecule modulates
Kuz function.
The present invention is also directed to
therapeutic and prophylactic, as well as diagnostic,
prognostic, and screening methods and compositions based upon
the Delta:Kuz complex or a D1E~:Notch complex (and the nucleic
acids encoding the individual proteins that participate .in
the complex). Therapeutic compounds of the invention
include, but are not limited to, a Delta:Kuz complex, and a
complex where one or both members of the complex is a
derivative; fragment, homolog or analog of Delta or Kuz;
antibodies to and nucleic acids encoding the foregoing; and
antisense nucleic acids to the nucleotide sequences encoding
the complex components. Diagnostic, prognostic and screening
kits are also provided.
Animal models and methods of screening for
modulators (i.e., agonists, and antagonists) of the activity
of a Delta:Kuz complex or the activity of a D1E~:Notch complex
are also provided.
Methods of identifying molecules that inhibit, or
alternatively, that increase formation of a Delta:Kuz complex
or of a D1E~~Notch complex are also provided.
For clarity of disclosure, and not by way of
limitation, the detailed description of the invention is
divided into the subsections that follow.
5.1 DELTA CLEAVAGE PEPTIDES, SOLUBLE DELTA
PEPTIDES .AND DELTA:KUZ PROTEIN COMPLEXES
5.1.1 DELTA CLEAVAGE PEPTIDES AND SOLUBLE
DELTA PEPTIDES
Delta encoding nucleic acids from both vertebrate
and non-vertebrate species have been cloned, see e.g.,
International Patent Publication WO 97/01571 for a
description of vertebrate, including human, Delta encoding
nucleic acids. Human Delta encoding sequences and the
- 38 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
encoded amino acid sequence is available in GenBank under
Accession No. AF003522 and are depicted in Figures 4A and 4H.
The nucleotide sequence coding for a Delta cleavage peptide,
or for a soluble Delta peptide, or a functionally active
fragment or other derivative thereof, can be inserted into an
appropriate expression vector, i.e., a vector which contains
the necessary elements for the transcription and translation
of the inserted protein-coding sequence. The necessary
transcriptional and translational signals can also be
supplied by the native Delta gene and/or its flanking
regions. A variety of host-vector systems may be utilized to
express the protein-coding sequence. These include but are
not limited to mammalian cell systems infected with virus
(e. g., vaccinia virus, adenovirus, etc.); insect cell systems
infected with virus (e'.g., baculovirus); microorganisms such
as yeast containing yeast vectors, or bacteria transformed
with bacteriophage, DNA, plasmid DNA, or cosmid DNA. The
expression elements of vectors vary in their strengths and
specificities. Depending on the host-vector system utilized,
any one of a number of suitable transcription and translation
elements may be used. In a specific embodiment, the human
Delta cleavage peptide is expressed. In another specific
embodiment, the human soluble Delta peptide is expressed.
Any of the methods previously described for the
insertion of DNA fragments into a vector may be used to
construct expression vectors containing a chimeric gene
consisting of appropriate transcriptional/translational
control signals and the protein coding sequences. These
methods may include ir.~ vitro recombinant DNA and synthetic
techniques and in viva recombinants (genetic recombination).
Expression of nucleic acid sequence encoding a Delta cleavage
peptide or peptide fragment thereof may be regulated by a
second nucleic acid sequence so that the Delta cleavage
peptide is expressed i.n a host transformed with the
- 39 -
CA 02333893 2001-O1-11
WD 00/02897 PCT/US99/15817
recombinant DNA molecule. For example, expression of a Delta
cleavage peptide may be controlled by any promoter/enhancer
element known in the art. Promoters which may be used to
control Delta cleavage peptide expression include, but are
not limited to, the SV40 early promoter region (Bernoist and
Chambon, 1981, Nature 290:304-310), the promoter contained in
the 3' long terminal repeat of Rous sarcoma virus (Yamamoto,
et al., 1980, Cell 22:787-797), the herpes thymidine kinase
promoter (Wagner et al., 1981, Proc. Natl. Acad. Sci. U.S.A.
78:1441-1445), the regulatory sequences of the
metallothionein gene (Brinster et al., 1982, Nature 296:39-
42); prokaryotic expression vectors such as the (3-lactamase
promoter (Villa-Kamaroff, et al., 1978, Proc. Natl. Acad.
Sci. U.S.A. 75:3727-3731), or the tac promoter (DeBoer, et
al., 1983, Proc. Natl. Acad. Sci. U.S.A. 80:21-25); see also
~~Useful proteins from recombinant bacteria" in Scientific
American, 1980, 242:74-94; plant expression vectors
comprising the nopalin.e synthetase promoter region (Herrera-
Estrella et al., Nature 303:209-213) or the cauliflower
mosaic virus 35S RNA promoter (Gardner, et al., 1981, Nucl.
Acids Res. 9:2871), anal the promoter of the photosynthetic
enzyme ribulose biphos,phate carboxylase (Herrera-Estrella et
al., 1984, Nature 310:115-120); promoter elements from yeast
or other fungi such as~ the Gal 4 promoter, the ADC (alcohol
dehydrogenase) promoter, PGK (phosphoglycerol kinase)
promoter, alkaline phosphatase promoter, and the following
animal transcriptional. control regions, which exhibit tissue
specificity and have x>een utilized in transgenic animals:
elastase I gene control region which is active in pancreatic
acinar cells (Swift et. al., 1984, Cell 38:639-646; Ornitz et
al., 1986, Cold Spring Harbor Symp. Quant. Biol. 50:399-409;
MacDonald, 1987, Hepat:ology 7:425-515); insulin gene control
region which is active' in pancreatic beta cells (Hanahan,
1985, Nature 315:115-7.22), immunoglobulin gene control region
- 40 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
which is active in lymphoid cells (Grosschedl et al., 1984,
Cell 38:647-658; Adamea et al., 1985, Nature 318:533-538;
Alexander et al., 198.', Mol. Cell. Biol. 7:1436-1444), mouse
mammary tumor virus control region which is active in
testicular, breast, lymphoid and mast cells (Leder et al.,
1986, Cell 45:485-495), albumin gene control region which is
active in liver (PinkE:rt et al., 1987, Genes and Devel.
1:268-276), alpha-fetoprotein gene control region which is
active in liver (Krum7_auf et al., 1985, Mol. Cell. Biol.
5:1639-1648; Hammer et: al., 1987, Science 235:53-58; alpha 1-
antitrypsin gene control region which is active in the liver
(Kelsey et al., 1987, Genes and Devel. 1:161-171), beta-
globin gene control region which is active in myeloid cells
(Mogram et al., 1985, Nature 315:338-340; Kollias et al.,
1986, Cell 46:89-94; nnyelin basic protein gene control region
which is active in oligodendrocyte cells in the brain
(Readhead et al., 198',x, Cell 48:703-712); myosin light chain-
2 gene control region which is active in skeletal muscle
(Sari, 1985, Nature 37L4:283-286), and gonadotropic releasing
hormone gene control region which is active in the
hypothalamus (Mason et: al., 1985, Science 234:1372-1378).
Expression vectors containing inserts of nucleic
acids encoding a Delta cleavage peptide or encoding a soluble
Delta peptide can be :identified by three general approaches:
(a) nucleic acid hybr:idization, (b) presence or absence of
"marker" gene functions, and (c) expression of inserted
sequences. In the first approach, the presence of a foreign
gene inserted in an e:~pression vector can be detected by
nucleic acid hybridization using probes comprising sequences
that are homologaus to the inserted Delta cleavage peptide
coding sequences. In the second approach, the recombinant
vector/host system can be identified and selected based upon
the presence or absence of certain "marker" gene functions
(e. g., thymidine kina;se activity, resistance to antibiotics,
- 41 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
transformation phenotype, occlusion body formation in
baculovirus, etc.) caused by the insertion of foreign genes
in the vector. For e~;ample, if the Delta cleavage peptide
encoding nucleic acid: are inserted within the marker gene
sequence of the vector, recombinants containing the insert
can be identified by t:he absence of the marker gene function.
In the third approach, recombinant expression vectors can be
identified by assaying the foreign gene product expressed by
the recombinant. Such assays can be based, for example,, on
the physical or functional properties of the encoded cleavage
peptide in irr vitro a:~say systems, e.g., binding to Kuz,
binding with antibody.
Once a particular recombinant DNA molecule is
identified and isolated, several methods known in the art may
be used to propagate i.t. Once a suitable host system and
growth conditions are established, recombinant expression
vectors can be propagated and prepared in quantity. As
previously explained, the expression vectors which can be
used include, but are not limited to, the following vectors
or their derivatives: human or animal viruses such as
vaccinia virus or adenovirus; insect viruses such as
baculovirus; yeast vecaors; bacteriophage vectors (e. g."
lambda), and plasmid and cosmid DNA vectors, to name but a
few.
In addition, a host cell strain may be chosen which
modulates the expression of the inserted sequences, or
modifies and processe:~ the gene product in the specific
fashion desired. Expression from certain promoters can be
elevated in the presence of certain inducers; thus,
expression of the geneaically engineered Delta cleavage
peptide may be controlled. Furthermore, different host cells
have characteristic and specific mechanisms for the
translational and post:-translational processing and
modification (e. g., gl.ycosylation, cleavage [e. g., of signal
- 42 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
sequence ) of proteins. Appropriate cell lines or host
systems can be chosen to ensure the desired modification and
processing of the foreign protein expressed. For example,
expression in a bacterial system can be used to produce an
unglycosylated core protein product. Expression in yeast
will produce a glycosylated product. Expression in mammalian
cells can be used to ensure "native" glycosylation of a
heterologous mammalian. Delta cleavage peptide, or to ensure
"native" glycosylation. of a heterologous mammalian soluble
Delta peptide. Furthermore, different vector/host expression
systems may effect processing reactions to different extents.
In other specific embodiments, the Delta cleavage
peptide, fragment, analog, or derivative may be expressed as
a fusion, or chimeric protein product (comprising the
peptide, fragment, analog, or derivative joined via a peptide
bond to a heterologous~ protein sequence (of a different
protein)). Such a chi.meric product can be made by ligating
the appropriate nucleic acid sequences encoding the desired
amino acid sequences t.o each other by methods known in the
art, in the proper coding frame, and expressing the chimeric
Product by methods commonly known in the art. Alternatively,
such a chimeric product may be made by protein synthetic
techniques, e.g., by use of a peptide synthesizer.
Both cDNA and genomic sequences can be cloned and
expressed.
One embodiment of the present invention is directed
to a peptide of appro};imately 30 amino acids, and its
encoding nucleic acids, of the toporythmic protein Delta that
contains a sequence which is cleaved by the metalloprotease-
disintegrin Kuzbanian (Kuz), (herein termed "cleavage
peptide") as well as derivatives (e.g., fragments) and
analogs thereof. For example, the Delta cleavage peptide
consists of the sequence of about amino acid Cyssls to about
amino acid Phe54s in human Delta (SEQ ID NO:10), of about.
- 43 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
amino acid Cyssls to about amino acid phe543 in mouse Delta
(SEQ ID N0:6), of about amino acid Cys5~3 to about amino acid
Phessl in chick Delta (ISEQ ID N0:7) , of about amino acid Cyssle
to about amino acid Phe549 in Xenopus Delta (SEQ ID N0:8), and
the sequence of about amino acid Cys5s4 to about amino acid
Ala59a or G1n594 in Drosophila Delta (SEQ ID N0:9) . Such a
peptide is believed to have the ability to modulate Kuz
cleavage of Delta, and thus, Delta and Notch activation. In
a specific embodiment, the Delta cleavage peptide is a
portion of a mammalian Delta, preferably a human Delta.
The invention further relates to Delta cleavage
peptides, and derivatives (including but not limited to
fragments) and analogs of Delta cleavage peptides. Nucleic
acids encoding Delta cleavage peptide derivatives and peptide
analogs are also provided. In particular aspects, the
peptides, derivatives, or analogs are of mouse, chicken,
frog, rat, pig, cow, d.og, monkey, or human Delta cleavage
peptides.
The production and use of derivatives and analogs
related to Delta cleavage peptides are within the scope of
the present invention. In a specific embodiment, the
derivative or analog is functionally active, i.e., capable of
exhibiting one or more functional activities associated with
wild-type Delta cleavage peptide. As one example, such
derivatives or analog; which have the desired immunogenicity
or antigenicity can be used, for example, in immunoassays,
for immunization, for inhibition of Delta activity, etc.
Such molecules which retain, or alternatively inhibit, a
desired Delta property, e.g., binding to kuz or other
toporythmic proteins, can be used as inducers, or inhibitors,
respectively, of such property and its physiological
correlates. Derivatives or analogs of a Delta cleavage
peptide can be tested for the desired activity by procedures
- 44 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
known in the art, including but not limited to the assays
described herein.
In particular, Delta cleavage peptide derivatives
can be made by altering Delta cleavage peptide encoding
sequences by substitutions, additions or deletions that
provide for functionally equivalent molecules. Due to the
degeneracy of nucleotide coding sequences, other DNA
sequences which encode substantially the same amino acid
sequence as a Delta cleavage peptide may be used in the
practice of the present invention. These include but are not
limited to nucleotide sequences comprising all or portions of
the encoding Delta cleavage peptide genes which are altered
by the substitution of different codons that encode a
functionally equivalent amino acid residue within the
sequence, thus producing a silent change. Likewise, the
1.5 Delta cleavage peptide derivatives of the invention include,
but are not limited to, those containing, as a primary amino
acid sequence, all or part of the amino acid sequence of a
Delta protein including altered sequences in which
functionally equivalent amino acid residues are substituted
for residues within the sequence resulting in a silent
change. For example, one or more amina acid residues within
the sequence can be substituted by another amino acid of a
similar polarity which acts as a functional equivalent,
resulting in a silent alteration. Substitutes for an amino
acid within the sequence may be selected from other members
2. 5
of the class to which the amino acid belongs. For example,
the nonpolar (hydrophobic) amino acids include alanine,
leucine, isoleucine, valine, proline, phenylalanine,
tryptophan and methionine. The polar neutral amino acids
include glycine, serine, threonine, cysteine, tyrosine,
?~0 asparagine, and glutamine. The positively charged (basic)
amino acids include arginine, lysine and histidine. The
- 45 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
negatively charged (acidic) amino acids include aspartic acid
and glutamic acid.
In a specific embodiment, fragments of Delta that
comprise the cleavage peptide sequence are also provided. In
particular embodiments, the Delta fragments comprising the
cleavage peptide are not greater than 35, 50, 75, 100, 150,
or 200 amino acids in length. For example, a Delta fragment
containing the cleavage peptide sequence comprises the
cleavage peptide sequence and 35 contiguous amino-terminal
amino acids. In another example, the fragment comprises the
cleavage peptide sequence and 100 contiguous amino-terminal
amino acids. In yet another example, the fragment comprises
the cleavage peptide sequence and 50 contiguous carboxy-
terminal amino acids. In yet another example, the fragment
comprises the cleavage peptide sequence and 50 contiguous
amino-terminal amino acids and 50 contiguous carboxy-terminal
amino acids. In yet another embodiment, oncatamers of Delta
fragments containing at least the cleavage peptide sequence
(e. g., two, three, or more copies of a portion of the Delta
sequence consisting of at least the cleavage peptide
sequence) are also provided.
The Delta cleavage peptide derivatives and analogs
of the invention can be produced by various methods known in
the art. The manipulations which result in their production
can occur at the gene or protein level. For example, the
cloned Delta gene sequence can be modified by any of numerous
strategies known in the art (Maniatis, T., 1990, Molecular.
Cloning, A Laboratory Manual, 2d ed., Cold Spring Harbor
Laboratory, Cold Spring Harbor, New York). The sequence c:an
be cleaved at appropriate sites with restriction
endonuclease(s), followed by further enzymatic modification
if desired, isolated, and ligated in vitro. In the
production of the gene encoding a derivative or analog of a
Delta cleavage peptide, care should be taken to ensure that
- 46 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/U599/15817
the modified gene remains within the same transiational
reading frame as Delta, uninterrupted by translational stop
signals.
Additionally, the Delta cleavage peptide-encoding
nucleic acid sequence can be mutated in vitro or in vi vo, to
create and/or destroy translation, initiation, and/or
termination sequences, or to create variations in coding
regions and/or form new restriction endonuclease sites or
destroy preexisting ones, to facilitate further in vitro
modification. Any technique for mutagenesis known in the art
can be used, including but not limited to, in vitro site-
directed mutagenesis (Hu.tchinson, C., et al., 1978, J. Biol.
Chem 253:6551), etc. PC'R primers containing sequence changes
can be used in PCR to introduce such changes into the
amplified fragments.
Manipulations of the Delta cleavage peptide
sequence may also be macLe at the protein level. Included
within the scope of the invention are Delta cleavage peptide
fragments or other derivatives or analogs which are
differentially modified during or after translation, e.g., by
2.0 glYcosylation, acetylati.on, phosphorylation, amidation,
derivatization by known protecting/blocking groups,
proteolytic cleavage, linkage to an antibody molecule or
other cellular ligand, etc. Any of numerous chemical
modifications may be carried out by known techniques,
including but not limited to specific chemical cleavage by
~~5 cyanogen bromide, trypsin, chymotrypsin, papain, V8 protease,
NaBH4; acetylation, form~ylation, oxidation, reduction;
metabolic synthesis in t:he presence of tunicamycin; etc.
In addition, analogs and derivatives of Delta
cleavage peptide can be chemically synthesized. Furthermore,
~30 if desired, nonclassical amino acids or chemical amino acid
analogs can be introduced as a substitution or addition into
the Delta seauence. Non-classical amino acids include but
- 47 -
CA 02333893 2001-O1-11
WO 00/02897 PCTIUS99115817
are not limited to the iD-isomers of the common amino acids,
a-amino isobutyric acid, 4-aminobutyric acid, hydroxyproline,
sarcosine, citrulline, ~~ysteic acid, t-butylglycine, t-
butylalanine, phenylgly~~ine, cyclohexylalanine, (3-alanine,
designer amino acids su~~h as (3-methyl amino acids, Ca-methyl
amino acids, and Na-met:hyl amino acids and amino acid analogs
in general.
In a specific embodiment, the Delta cleavage
peptide derivative is a chimeric, or fusion, peptide
comprising a Delta cleavage peptide or fragment thereof
joined at its amino- or carboxy-terminus via a peptide bond
to an amino acid sequence of a different protein. In one
embodiment, such a chim~eric protein is produced by
recombinant expression of a nucleic acid encoding the protein
(comprising a Delta cleavage peptide-coding sequence joined
in-frame to a coding sequence for a different protein). Such
a chimeric product can :be made by ligating the appropriate
nucleic acid sequences encoding the desired amino acid
sequences to each other by methods known in the art, in the
proper coding frame, and expressing the chimeric product by
methods commonly known in the art. Alternatively, such a
chimeric product may be made by protein synthetic techniques,
e.g., by use of a peptide synthesizer. In a specific
embodiment, a chimeric nucleic acid encoding a Delta cleavage
peptide with a heterologous signal sequence is expressed such
that the chimeric protein is expressed extracellularly by the
cell.
The invention is also directed to a derivative or
analog of the cleavage peptide which is functionally active,
i.e., capable of displaying one or more known functional
activities associated with the "wild type" cleavage peptide.
Such functional activities include but are not limited to
antigenicity [ability to bind (or compete with the cleavage
peptide for binding) to an anti-Delta cleavage peptide
- 48 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
antibody], immunogenicity (ability to generate antibody which
binds to the cleavage peptide), ability to bind (or compete
with the cleavage peptide for binding) to Kuz. The invention
is further directed to a fragment (and derivatives or ana:Logs
thereof) of the Delta cleavage peptide which is able to bind
to Kuz.
In another embodiment, the present invention is
directed to a peptide comprising an amino-terminal fragment
of a full length Delta protein, which fragment is cleaved
from the full length Delta protein by two proteolytic
:L 0
processing events, the cleavage of the signal peptide and the
cleavage by Kuz, (herein termed "soluble Delta peptide") as
well as derivatives and analogs thereof. For example, the
soluble Delta peptide amino acid sequence begins at amino
acid Ser22 and terminates between amino acid Cyssls and amino
-~5 acid Phes4a in human Delta (SEQ ID N0:1U) ; begins at amino
acid Ser22 and terminates between amino acid Cys515 and amino
acid Phe543 in mouse Delt:a (SEQ ID N0:6); begins at amino acid
Ser24 and terminates between amino acid Cyssza and amino acid
Phe551 in chick Delta (SE;Q ID N0:7) , begins at amino acid SerZZ
;0 and terminates between amino acid CysSl~ and amino acid Phes.a
in Xenopus Delta (SEQ II7 N0:8), or begins at amino acid Ser23
and terminates between amino acid Cys56,, and amino acid A1a593
or G1n599 in Drosophila Delta (SEQ ID N0:9). Such a peptide
is believed to have the ability to bind Notch, and thus
modulate Delta and Notch activation.
~; 5
The invention further relates to soluble Delta
peptides, and derivatives (including but not limited to
fragments) and analogs of soluble Delta peptides. Nucleic:
acids encoding soluble Delta peptide derivatives and peptide
analogs are also provide=d. In particular aspects, the
'-~0 peptides, derivatives, or analogs are of mouse, chicken,
frog, rat, pig, cow, dock monkey, or human soluble Delta
peptides.
- 49 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
The productic>n and use of derivatives and analogs
related to soluble Delta peptides are within the scope of the
present invention. In a specific embodiment, the derivative
or analog is functionally active, i.e., capable of exhibiting
one or more functional activities associated with wild-type
soluble Delta peptide. As one example, such derivatives or
analogs which have the desired immunogenicity or antigenicity
can be used, for example, in immunoassays, for immunization,
for promotion of Delta activity, etc. Such molecules which
retain, or alternatively inhibit, a desired Delta property,
e.g., binding to Notch or other toporythmic proteins, can be
used as inducers, or inhibitors, respectively, of such
property and its physiological correlates. Derivatives or
analogs of a soluble Delta peptide can be tested for the
desired activity by procedures known in the art, including
I5 but not limited to the assays described herein.
In particular, soluble Delta peptide derivatives
can be made by altering soluble Delta peptide encoding
sequences by substitutions, additions or deletions that
provide for functionally equivalent molecules. Due to the
degeneracy of nucleotide coding sequences, other DNA
sequences which encode substantially the same amino acid
sequence as a soluble Delta peptide may be used in the
practice of the present invention. These include but are not
limited to nucleotide sequences comprising all or portions of
the encoding soluble Delta peptide genes which are altered by
the substitution of different codons that encode a
functionally equivalent amino acid residue within the
sequence, thus producing a silent change. Likewise, the
soluble Delta peptide derivatives of the invention include,
but are not limited to, those containing, as a primary amino
acid sequence, all or part of the amino acid sequence of a
Delta protein including altered sequences in which
functionally equivalent amino acid residues are substituted
- 50 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
for residues within the sequence resulting in a silent
change. For example, one or more amino acid residues within
the sequence can be substituted by another amino acid of a
similar polarity which acts as a functional equivalent,
resulting in a silent alteration. Substitutes for an amino
acid within the sequence may be selected from other members
of the class to which t:he amino acid belongs. For example,
the nonpolar (hydrophobic) amino acids include alanine,
leucine, isoleucine, valine, proline, phenylalanine,
tryptophan and methioni.ne. The polar neutral amino acids
include glycine, serine, threonine, cysteine, tyrosine,
asparagine, and glutami.ne. The positively charged (basic)
amino acids include arginine, lysine and histidine. The
negatively charged (acidic) amino acids include aspartic acid
and glutamic acid.
The soluble Delta peptide derivatives and analogs
of the invention can be produced by various methods known in
the art. The manipulations which result in their production
can occur at the gene o~r protein level. For example, the
cloned Delta gene sequence can be modified by any of numerous
strategies known in the art (Maniatis, T., 1990, Molecular
Cloning, A Laboratory Manual, 2d ed., Cold Spring Harbor
Laboratory, Cold Spring Harbor, New York). The sequence can
be cleaved at appropriate sites with restriction
endonuclease(s), followed by further enzymatic modification
if desired, isolated, and ligated in vitro. In the
production of the gene encoding a derivative or analog of a
soluble Delta peptide, care should be taken to ensure that
the modified gene remains within the same translational
reading frame as Delta, uninterrupted by translational stop
signals.
Additionally, the soluble Delta peptide-encoding
nucleic acid sequence can be mutated in vi tro or in vivo, to
create and/or destroy translation, initiation, and/or
- 51 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
termination sequences, or to create variations in coding
regions and/or form new restriction endonuclease sites or
destroy preexisting onea, to facilitate further in vitro
modification. Any technique for mutagenesis known in the art
can be used, including but not limited to, in vitro site-
directed mutagenesis (Hutchinson, C., et al., 1978, J. B:iol.
Chem 253:6551), etc. F?CR primers containing sequence changes
can be used in PCR to ~_ntroduce such changes into the
amplified fragments.
Manipulations of the soluble Delta peptide sequence
may also be made at thE: protein level. Included within the
scope of the invention are soluble Delta peptide fragments or
other derivatives or analogs which are differentially
modified during or after translation, e.g., by glycosylation,
acetylation, phosphoryl.ation, amidation, derivatization by
known protecting/blocki.ng groups, proteolytic cleavage,
linkage to an antibody molecule or other cellular ligand,
etc. Any of numerous chemical modifications may be carried
out by known technique;, including but not limited to
specific chemical cleavage by cyanogen bromide, trypsin,
chYTT~otrypsin, papain, V'8 protease, NaBH4; acetylation,
formylation, oxidation, reduction; metabolic synthesis in the
presence of tunicamycin; etc. In a specific embodiment, N-
or C-terminal modifications are made, e.g., N-acetylation.
In addition, analogs and derivatives of soluble
Delta peptide can be chemically synthesized. Furthermore, if
desired, nonclassical amino acids or chemical amino acid
analogs can be introduced as a substitution or addition into
the Delta sequence. Non-classical amino acids include but
are not limited to the D-isomers of the common amino acids,
a-amino isobutyric acid., 4-aminobutyric acid, hydroxyproline,
sarcosine, citrulline, cysteic acid, t-butylglycine, t-
butylalanine, phenylglycine, cyclohexylalanine, (3-alanine,
designer amino acids such as a-methyl amino acids, Ca-methyl
- 52 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/158I7
amino acids, and Na-methyl amino acids and amino acid analogs
in general.
In a specific embodiment, the soluble Delta peptide
derivative is a chimeric, or fusion, peptide comprising a
soluble Delta peptide ~or fragment thereof joined at its
amino- or carboxy-terminus via a peptide bond to an amino
acid sequence of a different protein. In one embodiment,
such a chimeric protein is produced by recombinant expression
of a nucleic acid encoding the protein (comprising a soluble
Delta peptide-coding sequence joined in-frame to a coding
sequence for a different protein). Such a chimeric product
can be made by ligating the appropriate nucleic acid
sequences encoding the desired amino acid sequences to each
other by methods known in the art, in the proper coding
frame, and expressing the chimeric product by methods
commonly known in the art. Alternatively, such a chimeric
product may be made by protein synthetic techniques, e.g., by
use of a peptide synthesizer. In a specific embodiment, a
chimeric nucleic acid encoding a soluble Delta peptide with a
heterologous signal sequence is expressed such that the
chimeric protein is expressed extracellularly by the cell.
The invention is also directed to a derivative or
analog of the soluble peptide which is functionally active,
i.e., capable of displaying one or more known functional
activities associated urith the "wild type" soluble peptide.
Such functional activit:ies include but are not limited to
antigenicity [ability t:o bind (or compete with the soluble
peptide for binding) to an anti-soluble Delta peptide
antibody], immunogenici_ty (ability to generate antibody which
binds to the soluble peptide), ability to bind (or compete
with the soluble peptide for binding) to Notch. The
invention is further directed to a fragment (and derivatives
or analogs thereof) of the soluble Delta peptide which is
able to bind to Notch.
- 53 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
5.1.2 PROTEIN COMPLEXES OF DELTA AND KUZ
AND DELTA AND NOTCH
The present invention is directed to a Delta:Kuz
protein complex. The present invention is also directed to a
DlE~:Notch protein comp:Lex. Delta, Kuz and Notch have been
cloned, see e.g., WO 9.?/19734, WO 97/01571 and WO 98/08933.
Figure 2 depicts the annino acid sequences of several Notch
homologs (SEQ ID NOS:1, 2, 3 and 4), including human Notch
(SEQ ID NOS:1 and 2). Figure 3 depicts the amino acid
sequences of several Delta homologs (SEQ ID NOS:S, 6, 7, 8
and 9) and the nucleic acid sequence encoding human Delta is
depicted in Figure 4B (:SEQ ID N0:13). The amino acid
sequence (SEQ ID N0:12) of the human homolog of Kuz and its
encoding nucleic acid ~oequence {SEQ ID N0:13) is depicted in
Figures 5A and 5B, respectively. D1E~ is the amino-terminal
fragment of full length Delta consisting of essentially the
extracellular domain of wild-type Delta that is liberated
when Kuz cleaves Delta. The D1E~ fragment is soluble and
begins at amino acid Ser23 and terminates between amino acid
Cysss4 and amino acid Alasg3 or G1n594 in Drosophila Delta (SEQ
ID N0:9), begins at amino acid Serz2 and terminates between
amino acid Cyssls and amino acid Phe543 in human Delta (SEQ ID
N0:10), begins at amino acid Ser22 and terminates between
amino acid Cys515 and amino acid Phes43 in mouse Delta (SEQ ID
N0:6), begins at amino acid Ser24 and terminates between amino
acid Cyssz3 and amino acid Phessl in chick Delta {SEQ ID N0: 7) ,
.25 or begins at amino acid Ser22 and terminates between amino
acid Cyss,e and amino acid Phe544 in Xenopus Delta (SEQ ID
N0:8) .
In a preferred embodiment of the present invention,
the Delta:Kuz complex or the D1E~:Notch complex is a complex
:30 of human proteins. The invention is also directed to
complexes of derivatives (including fragments) and analogs of
Delta with Kuz, complexes of Delta with derivatives
- 54 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99115817
(including fragments) arid analogs of Kuz, and complexes of
derivatives (including fragments) and analogs of Delta and
Kuz (as used herein, fragment, derivative, homolog or analog
of a Delta:Kuz complex includes complexes where one or both
members of the complex are fragments, derivatives or analogs
of the wild-type Delta or Kuz protein). The present
invention is also directed to complexes of derivatives
(including fragments) and analogs of DlE~ with Notch,
complexes of D1E~ with derivatives (including fragments) and
analogs of Notch, and complexes of derivatives (including
:LO fragments) and analogs of D1E~ and Notch (as used herein,
fragment, derivative, homolog or analog of a D1E~:Notch
complex includes complexes where one or both members of the
complex are fragments, <ierivatives or analogs of the wild-
type D1E~ or Notch protein). In a preferred embodiment, the
D1E~.Notch complex in which one or both members of the complex
is a fragment, derivative, homolog or analog of the wild type
protein is a functional:Ly-active D1E~:Notch complex. In
particular aspects, the native proteins, or derivatives or
analogs of Delta, Notch and/or Kuz are obtained from an
animal, e.g., mouse, rat, pig, cow, dog, monkey, human, fly,
frog. In another aspect, the native proteins are obtained
from plants.
As used herein, a "functionally active Delta:Kuz
complex" refers to that material displaying one or more known
functional attributes of a complex of wild type Delta with
wild type Kuz, including protein-protein binding, binding to
a Delta-, a Kuz-, and/or a Delta:Kuz complex-specific
antibody, or has the functional attributes) of Delta, Kuz,
and/or a Delta:Kuz complex involved in cell fate and
differentiation.
As used herein, a "functionally active D1E~:Notch
complex" refers to that material displaying one or more known
functional attributes of a complex of wild type DlE~ with wild
- 55 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
type Notch, including protein-protein binding, binding to a
D1E~-, a Notch-, and/or a D1E~:Notch complex-specific antibody,
or has the functional attribute (s) of D1E~, Notch, and/or a
D1E~:Notch complex involved in cell fate and differentiation..
The present invention is also directed to a method
of screening a Delta:Kuz complex, particularly a complex of
Delta with Kuz for the ability to alter a cell function,
particularly those cell functions in which Delta and/or Kuz
has been implicated, including, e.g., physiological processes
such as cell fate determination and differentiation, binding
to an anti-Delta:Kuz complex antibody, etc., and other
activities as they are described in the art. The present
invention is also directed to a method of screening a
Dlfi~:Notch complex, particularly a complex of D1E~ with Notch
for the ability to alter a cell function, particularly those
~~5 cell functions in which D1E~ and/or Notch has been implicated,
including, e.g., physio:Logical processes such as cell fate
determination and differentiation, binding to an anti-
D1E~:Notch complex antibody, etc., and other activities as
they are described in the art.
>,0 The present invention is also directed to a method
for screening a complex of a derivative, fragment, or analog
of Delta and/or Kuz for the ability to alter a cell function
such as differentiation. For example, such derivatives or
analogs which have the desired immunogenicity or antigenicity
can be used in immunoassays, for immunization, for inhibition
:! 5
of Delta:Kuz complex act:ivity, etc. Derivatives or analogs
that retain, or alternatively lack or inhibit, a property of
interest (e.g., participation in a Delta:Kuz complex) can be
used as an inducer, or ~.nhibitor, respectively, of such a
property and its physio7.ogical correlate. The present
=~0 invention is also directed to a method for screening a
complex of a derivative, fragment, or analog of D1E~ and/or
Notch for the ability to alter a cell function such as
- 56 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
differentiation. For example, such derivatives or analogs
which have the desired i.mmunogenicity or antigenicity can be
used in immunoassays, for immunization, for inhibition of
D1E~:Notch complex activity, etc. Derivatives or analogs that
retain, or alternatively lack or inhibit, a property of
interest (e.g., participation in a D1E~:Notch complex) can be
used as an inducer, or inhibitor, respectively, of such a
property and its physiological correlate.
A specific embodiment of the present invention is
directed to a Delta:Kuz complex of a fragment of Delta and/or
7~ 0
a fragment of Kuz that c:an be bound by an anti-Delta antibody
and/or bound by an anti-~Kuz antibody, respectively, or bound
by an antibody specific for a Delta:Kuz complex. Another
specific embodiment of t:he present invention is directed to a
DlE~:Notch complex of a :Fragment of D1E~ and/or a fragment of
-~5 Notch that can be bound by an anti-DlE' antibody and/or bound
by an anti-Notch antibody, respectively, or bound by an
antibody specific for a D1E~:Notch complex.
Fragments and other derivatives or analogs of a
Delta:Kuz complex or of a D1E~:Notch complex can be tested for
;ZO the desired activity by procedures known in the art,
including but not limited to the assays described infra.
In specific ernbodiments, the present invention .is
directed to a Delta:Kuz complex or to a D1E~:Notch complex
comprising a fragment of one or both members of the complex.
In a preferred embodiment, these fragments consist of, but
:25
are not exclusive to fr~~gments of Kuz, identified as
interacting with Delta :in a modified yeast matrix mating
assay or genetic screen. Fragments, or proteins comprising
fragments, lacking a region of either member of the complex,
are also provided. Nuc:Leic acids encoding the foregoing are
30 provided in the present invention.
Nucleic acids encoding Delta, Notch and Kuz are
known, and in addition can be obtained by any method knawn in
_ 57 _
CA 02333893 2001-O1-11
wo ooioas9~ rc~r~rs~nssm
the art, e.g., by PCR amplification using synthetic primers
hybridizable to the 3' and 5' ends of each sequence, and/or
by cloning from a cDNA or genomic library using an
oligonucleotide specific for each nucleotide sequence.
Homologs (e. g., nucleic acids encoding Delta, Notch
and Kuz of species other than human) or other related
sequences (e. g., paralogs) can be obtained by low, moderate
or high stringency hybridization with all or a portion of the
particular human sequence as a probe, using methods well
known in the art for nucleic acid hybridization and cloning.
The encoded human Delta, Kuz and Notch proteins,
which are depicted in Figures 4A, 5A and 2, respectively (SEQ
ID NO:10, SEQ ID N0:12, and SEQ ID NOS:1 and 2, respectively)
either alone or in a complex, can be obtained by methods well
known in the art for protein purification and recombinant
protein expression. For recombinant expression of one or
more of the proteins, the nucleic acid containing all or a
portion of the nucleotide sequence encoding the protein can
be inserted into an apps.~opriate expression vector, i.e., a
vector that contains the: necessary elements for the
;Zp transcription and trans7_ation of the inserted protein coding
sequence. The necessary transcriptional and translational
signals can also be supplied by the native promoter of the
Delta, Kuz and Notch genes, and/or their flanking regions.
A variety of host-vector systems may be utilized to
express the protein coding sequence. These include but are
:>. 5
not limited to mammalian cell systems infected with virus
(e. g., vaccinia virus, adenovirus, etc.); insect cell systems
infected with virus (e.sr., baculovirus); microorganisms such
as yeast containing yea~;t vectors; or bacteria transformed
with bacteriophage, DNA, plasmid DNA, or cosmid DNA. The
'~~ expression elements of vectors vary in their strengths and
specificities. Depending on the host-vector system utilized,
- 58 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
any one of a number of suitable transcription and translation
elements may be used.
In a preferred embodiment, a Delta:Kuz complex is
obtained by expressing the entire Delta coding sequence and
the entire Kuz coding sequence in the same cell, either under
the control of the same promoter or two separate promoters.
In yet another embodiment, a derivative, fragment or homolog
of Delta and/or a derivative, fragment or homolog of Kuz are
recombinantly expressed. Preferably the derivative, fragment
or homolog of Delta and/or the Kuz protein forms a complex
with a binding partner identified by a binding assay, and
more preferably forms a complex that binds to an anti-
Deita:Kuz complex antibody. In another preferred embodiment,
a SDelta:Notch complex is obtained by expressing the entire
DlE~ coding sequence and the entire Notch coding sequence in
the same cell, either under the control of the same promoter
or two separate promoters. In yet another embodiment, a
derivative, fragment or homolog of DlE~ and/or a derivative,
fragment or homolog of Tfotch are recombinantly expressed.
Preferably the derivative, fragment or homolog of D1E~ and/'or
the Notch protein forms a complex with a binding partner
identified by a binding assay, and more preferably forms a
complex that binds to an anti-D1E~:Notch complex antibody.
Any method available in the art can be used for the
insertion of DNA fragments into a vector to construct
expression vectors containing a chimeric gene consisting of
2~5 appropriate transcriptional/translational control signals and
protein coding sequences, These methods may include in vitro
recombinant DNA and synthetic techniques and in vivo
recombinant techniques (genetic recombination?. Expressian
of nucleic acid sequence's encoding Delta, Kuz and Notch, or a
~0 derivative, fragment or homolog thereof, may be regulated by
a second nucleic acid ss~quence so that the gene or fragment
thereof is expressed in a host transformed with the
- 59 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
recombinant DNA molecule(s). For example, expression of the
proteins may be controlled by any promoter/enhancer known in
the art. In a specific embodiment, the promoter is not
native to the genes for Delta, Notch or Kuz. Promoters that
may be used include but are not limited to those described in
Section 5.1.1.
In a specific embodiment, a vector is used that
comprises a promoter ope:rably linked to nucleic acid
sequences encoding Delta, Notch and/or Kuz, or a fragment,
derivative or homolog thereof, one or more origins of
~~0 replication, and optionally, one or more selectable markers
(e. g., an antibiotic re:;istance gene). In a preferred
embodiment, a vector is used that comprises a promoter
operably linked to nucleic acid sequences encoding both Delta
and Kuz, or both D1E~ and Notch, one or more origins of
~~5 replication, and optionally, one or more selectable markers.
In another specific embodiment, an expression
vector containing the coding sequence, or a portion thereof,
of Delta and Kuz, or of D1E~ and Notch, either together or
separately, is made by :~ubcloning the gene sequences into the
:20 EcoRI restriction site of each of the three pGEX vectors
(glutathione S-transferase expression vectors; Smith and
Johnson, 1988, Gene 7:3:x-40). This allows for the expression
of products in the corresct reading frame.
Expression vecaors containing the sequences of
interest can be identified by three general approaches: (a)
.25 nucleic acid hybridization, (b) presence or absence of
"marker" gene function, and (c) expression of the inserted
sequences. In the firsi~ approach, Delta, Notch and Kuz
sequences can be detected by nucleic acid hybridization to
probes comprising sequences homologous and complementary to
30 the inserted sequences. In the second approach, the
recombinant vector/host system can be identified and selected
based upon the presence or absence of certain "marker"
- 6G -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
functions (e. g., resistance to antibiotics, occlusion body
formation in baculovirus, etc.) caused by insertion of the
sequences of interest in the vector. For example, if a Delta
or Kuz gene, or portion thereof, is inserted within the
marker gene sequence of the vector, recombinants containing
the Delta or Kuz fragment will be identified by the absence
of the marker gene function (e. g., loss of beta-galactosidase
activity). In the third approach, recombinant expression
vectors can be identified by assaying for the Delta and Kuz
expressed by the recombinant vector. Such assays can be
based, for example, on the physical or functional properties
of the interacting species in in vitro assay systems, e.g.,
formation of a Delta:Kuz complex or binding to an anti-Delta,
anti-Kuz, or anti-Delta:Kuz complex antibody.
Once recombinant Delta, Notch and Kuz molecules are
v5 identified and the complexes or individual proteins isolated,
several methods known in the art can be used to propagate
them. Using a suitable host system and growth conditions,
recombinant expression vectors can be propagated and
amplified in quantity. As previously described, the
;t0 expression vectors or dESrivatives which can be used include,
but are not limited to, human or animal viruses such as
vaccinia virus or adenovirus; insect viruses such as
baculovirus, yeast vectors; bacteriophage vectors such as
lambda phage; and plasmid and cosmid vectors.
In addition, a host cell strain may be chosen that
:35
modulates the expression of the inserted sequences, or
modifies or processes the expressed proteins in the specific
fashion desired. Expre:~sion from certain promoters can be
elevated in the presence' of certain inducers; thus expression
of the genetically-engineered Delta, Notch and/or Kuz may be
=~0 controlled. Furthermore:, different host cells have
characteristic and specific mechanisms for the translational
and post-translational processing and modification (e. g.,
- 61 -
CA 02333893 2001-O1-11
WO 00/02897 PC'T/US99/15817
glycosylation, phosphory:Lation, etc.) of proteins.
Appropriate cell lines o:r host systems can be chosen to
ensure that the desired modification and processing of the
foreign protein is achieved. For example, expression in a
bacterial system can be used to produce an unglycosylated
core protein, while expression in mammalian cells ensures
"native" glycosylation o:E a heterologous protein.
Furthermore, different vector/host expression systems may
effect processing reactions to different extents.
In other speci:Eic embodiments, the Delta, Notch
and/or Kuz protein or a :Fragment, homolog or derivative
thereof, may be expressed as fusion or chimeric protein
products comprising the protein, fragment, homolog, or
derivative joined via a peptide bond to a heterologous
protein sequence of a di:Pferent protein. Such chimeric
products can be made by :ligating the appropriate nucleic acid
sequences encoding the desired amino acids to each other by
methods known in the art, in the proper coding frame, and
expressing the chimeric products in a suitable host by
methods commonly known i:n the art. Alternatively, such a
chimeric product can be made by protein synthetic techniques,
e.g., by use of a peptide synthesizer. Chimeric genes
comprising portions of Delta, Notch and/or Kuz fused to any
heterologous protein-encoding sequences may be constructed.
A specific embodiment relates to a chimeric protein
comprising a fragment of Delta, Notch and/or Kuz of at least
six amino acids.
In a specific embodiment, fusion proteins are
provided that contain the interacting domains of the Delta
protein and Kuz, or the interacting domains of D1E~ and Notch,
and, optionally, a peptide linker between the two domains,
where such a linker promotes the interaction of the Delta and
Kuz binding domains or promotes the interaction of the DIEc
and Notch binding domains. These fusion proteins may be
- 62 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
particularly useful where the stability of the interaction is
desirable (due to the formation of the complex as an intra-
molecular reaction), f:or example, in production of antibodies
specific to the Delta:Kuz complex or specific to the
D1E~:Notch complex.
In particular, Delta, Notch and/or Kuz derivatives
can be made by altering their sequences by substitutions,
additions or deletions that provide for functionally
equivalent molecules. Due to the degeneracy of nucleotide
coding sequences, other DNA sequences that encode
substantially the same amino acid sequence as a Delta, Notch
or Kuz gene or cDNA can be used in the practice of~the
present invention. These include but are not limited to
nucleotide sequences comprising all or portions of the Delta,
Notch or Kuz genes that are altered by the substitution of
different codons that encode a functionally equivalent amino
acid residue within the sequence, thus producing a silent
change. Likewise, the Delta, Notch or Kuz derivatives of the
invention include, but are not limited to, those containing,
as a primary amino acid sequence, all or part of the amino
acid sequence of Delta" Notch or Kuz, including altered
sequences in which functionally equivalent amino acid
residues are substituted for residues within the sequence
resulting in a silent change. For example, one or more amino
acid residues within the sequence can be substituted by
another amino acid of a similar polarity that acts as a
functional equivalent, resulting in a silent alteration.
Substitutes for an amino acid within the sequence may be
selected from other members of the class to which the amino
acid belongs. For example, the nonpolar (hydrophobic) amino
acids include alanine, leucine, isoleucine, valine, proline,
phenylalanine, tryptophan and methionine. The polar neutral
amino acids include glycine, serine, threonine, cysteine,
tyrosine, asparagine, and glutamine. The positively charged
- 63 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
(basic) amino acids include arginine, lysine and histidine.
The negatively charged (acidic) amino acids include aspartic
acid and glutamic acid..
In a specific: embodiment of the invention, the
nucleic acids encoding proteins and proteins consisting of or
comprising a fragment of Delta, Notch or Kuz consisting of at
least 6 (continuous) amino acids of Delta, Notch or Kuz are
provided. In other emx>odiments, the fragment consists of at
least 10, 20, 30, 40, or 50 amino acids of Delta and Kuz or
D1E~ and Notch. In specific embodiments, such fragments are
not larger than 35, 100 or 200 amino acids. Derivatives or
analogs of Delta, Notch, and Kuz include, but are not limited,
to molecules comprising regions that are substantially
homologous to Delta, Notch or Kuz, in various embodiments, by
at least 30%, 40%, 50%, 60%, 70%, 80%, 90% or 95% identity
over an amino acid sequence of identical size or when
compared to an.aligned sequence in which the alignment is
done by a computer homology program known in the art, or
whose encoding nucleic acid is capable of hybridizing to a
sequence encoding Delta, Notch or Kuz under stringent,
moderately stringent, or nonstringent conditions.
The Delta, Notch and Kuz derivatives and analogs of
the invention can be produced by various methods known in the
art. The manipulations which result in their production can
occur at the gene or protein level. For example, the cloned
Delta, Notch and Kuz gene sequences can be modified by any of
numerous strategies known in the art (Sambrook et al., 1989,
Molecular Cloning, A Laboratory Manual, 2d Ed., Cold Spring
Harbor Laboratory Press, Cold Spring Harbor, New York). 'the
sequences can be cleaved at appropriate sites with
restriction endonuclease(s), followed by further enzymatic
:30 modification if desired, isolated, and ligated in vitro. In
the production of the gene encoding a derivative, homolog or
analog of Delta, Notch or Kuz, care should be taken to ensure
- 64 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
that the modified gene retains the original translational
reading frame, uninterrupted by translational stop signals,
in the gene region where the desired activity is encoded.
Additionally, the Delta-, Notch- and/or Kuz-
encoding nucleic acid sequence can be mutated in vitro or in
vivo, to create and/or destroy translation, initiation,
and/or termination sequences, or to create variations in
coding regions and/or form new restriction endonuclease sites
or destroy pre-existing ones, to facilitate further in vitro
modification. Any technique for mutagenesis known in the art
can be used, including but not limited to, chemical
mutagenesis and in vitro site-directed mutagenesis
(Hutchinson et al., 1978, J. Biol. Chem 253:6551-6558),
amplification with PCR primers containing a mutation, etc.
Once a recombinant cell expressing Delta, Notch
and/or Kuz, or fragment or derivative thereof, is identified,
the individual gene product or complex can be isolated and
analyzed. This is achieved by assays based on the physical
and/or functional properties of the protein or complex,
including, but not limited to, radioactive labeling of the
Product followed by analysis by gel electrophoresis,
immunoassay, cross-linking to marker-labeled product, etc.
The Delta:Kuz or DlE~:Notch complexes may be
isolated and purified by standard methods known in the art:
(either from natural sources or recombinant host cells
expressing the complexes. or proteins), including but not
i; 5
restricted to column chromatography (e. g., ion exchange,
affinity, gel exclusion, reversed-phase high pressure, fast
protein liquid, etc.), differential centrifugation,
differential solubility, or by any other standard technique
used for the purification of proteins. Functional properties
v0 may be evaluated using any suitable assay known in the art.
Alternatively, once Delta or its derivative, or Kuz
or its derivative, or Notch or its derivative, is identified,
- 65 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
the amino acid sequence. of the protein can be deduced from
the nucleic acid sequence of the chimeric gene from which it
was encoded. As a result, the protein or its derivative can
be synthesized by standard chemical methods known in the art
(e. g., Hunkapiller et al., 1984, Nature 310: 105-111).
In a specific, embodiment of the present invention,
such Delta:Kuz complexes, whether produced by recombinant DNA
techniques, chemical s~mthesis methods, or by purificatian
from native sources include, but are not limited to, those
containing, as a primaz-y amino acid sequence, all or part: of
the amino acid sequences substantially as depicted in Figures
3 and 5A-5B (SEQ ID NO~c:S, 6, 7, 8 and 9 and SEQ ID N0:12,
respectively), as well as fragments and other analogs and
derivatives thereof, including proteins homologous thereto.
In another specific embodiment of the present invention, such
D1E~:Notch complexes, whether produced by recombinant DNA
techniques, chemical synthesis methods, or by purification
from native sources include, but are not limited to, those
containing, as a primary amino acid sequence, all or part of
the amino acid sequences substantially as depicted in Figures
2 and 3 (SEQ ID NOS:S, 6, 7, 8 and 9 and SEQ ID NOS:1, 2, 3,
and 4, respectively), as well as fragments and other analogs
and derivatives thereof, including proteins homologous
thereto.
Manipulations of Delta, Notch and/or Kuz sequences
may be made at the protein level. Included within the scope
of one embodiment of the invention is a complex of a Delta
fragment or a Kuz fragment and Delta or Kuz fragments,
derivatives and analogs that are differentially modified
during or after translation, e.g., by glycosylation,
acetylation, phosphorylation, amidation, derivatization by
:30 known protecting/blocking groups, proteolytic cleavage,
linkage to an antibody molecule or other cellular ligand,
etc. Any of numerous chemical modifications may be carried
- 66 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
out by known techniques, including but not limited to
specific chemical cleava<~e by cyanogen bromide, trypsin,
chymotrypsin, papain, V8 protease, NaBH4, acetylation,
formylation, oxidation, :reduction, metabolic synthesis in the
,5 presence of tunicamycin, etc.
In specific embodiments, the Delta, Notch and/or
Kuz amino acid sequences are modified to include a
fluorescent label. In another specific embodiment, Delta,
Notch and/or Kuz are modified to have a heterofunctional
reagent; such heterofunctional reagents can be used to
crosslink the members of the complex.
In addition, complexes of analogs and derivatives
of Delta and/or Kuz, or ~D1E~ and/or Notch, can be chemically
synthesized. For example, a peptide corresponding to a
portion of Delta and/or :Kuz, which comprises the desired
domain or mediates the desired activity in vitro (e. g.,
Delta:Kuz complex formation) can be synthesized by use of a
peptide synthesizer. Furthermore, if desired, non-classical
amino acids or chemical amino acid analogs can be introduced
as a substitution or addition into the Delta and/or Kuz.
Non-classical amino acids include but are not limited to the
D-isomers of the common amino acids, a-amino isobutyric acid,
4-aminobutyric acid (4-Abu), 2-aminobutyric acid (2- Abu?,
6-amino hexanoic acid (Ahx), 2-amino isobutyric acid (2-Aib),
3-amino propionoic acid, ornithine, norleucine, norvaline,
hydroxyproline, sarcosine, citrulline, cysteic acid, t-
butylglycine, t-butylalanine, phenylglycine,
cyclohexylalanine, i3-ala.nine, fluoro-amino acids, designer_
amino acids such as fS-methyl amino acids, Ca-methyl amino
acids, Na-methyl amino acids, and amino acid analogs in
general. Furthermore, the amino acid can be D (dextrorotary)
3.0 or L ( levorotary) .
In cases where natural products are suspected of
being mutant or are isolated from new species, the amino acid
- 67 -
CA 02333893 2001-O1-11
WO 00/02897
PCT/US99/15817
sequence of Delta, Notch or Kuz isolated from the natural
source, as well as those expressed in vitro, or from
synthesized expression vectors in vivo or in vitro, can be
determined from analysis of the DNA sequence, or
alternatively, by direct sequencing of the isolated protein.
Such analysis can be performed by manual sequencing or
through use of an automated amino acid sequenator.
The Delta:Kuz or D1E~:Notch complexes can also be
analyzed by hydrophilicity analysis (Hopp and Woods, 1981,
Proc. Natl. Acad. Sci. USA 78:3824-3828). A hydrophilicity
profile can be used t« identify the hydrophobic and
hydrophilic regions of the proteins, and help predict their
orientation in designing substrates for experimental
manipulation, such as in binding experiments, antibody
synthesis, etc. Secondary structural analysis can also be
done to identify regions of Delta, Notch and/or Kuz, or their
derivatives, that assume specific structures (Chou and
Fasman, 1974, Biochemistry 13:222-23). Manipulation,
translation, secondary structure prediction, hydrophilicity
and hydrophobicity profile predictions, open reading frame
Prediction and plotting, and determination of sequence
homologies, etc., can be accomplished using computer software
programs available in the art.
Other methods of structural analysis including but
not limited to X-ray crystallography (Engstrom, 1974 Biochem.
Exp. Biol. 1i:7-13), mass spectroscopy and gas chromatography
(Methods in Protein Science, J. Wiley and Sons, New York,
1997), and computer modeling (Fletterick and Zoller, eds.,
1986, Computer Graphics and Molecular Modeling, In: Current
Communications in Molecular Biology, Cold Spring Harbor
Laboratory, Cold Spring Harbor Press, New York) can also be
employed.
- 68 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
S.c ANTIBODIES
According to one embodiment of the present
invention, a Delta cleavage peptide, its fragments or other
derivatives, or analogs thereof, may be used as an immunagen
to generate antibodies which recognize such an immunogen.
According to another embodiment of the present invention, a
soluble Delta peptide, its fragments or other derivatives, or
analogs thereof, may be: used as an immunogen to generate
antibodies which recognize such an immunogen. Such
antibodies include but are not limited to polyclonal,
monoclonal, chimeric, ~~ingle chain, Fab fragments, and an Fab
expression library. In a specific embodiment, antibodies to
human Delta cleavage peptide are produced. In another
specific embodiment, antibodies to human soluble Delta
peptide are produced.
According to another embodiment of the present
invention, the Delta:Ku.z complex or a fragment, derivative or
homolog thereof, or the DlE~:Notch complex or a fragment,
derivative or homolog thereof, may be used as an immunogen to
generate antibodies which immunospecifically bind such
i~unogen. Such antibodies include, but are not limited to,
polyclonal, monoclonal, chimeric, single chain, Fab
fragments, and an Fab expression library. In a specific
embodiment, antibodies to a complex of human Delta and human
Kuz are produced. In another specific embodiment, antibodies
to a complex of human D1E~ and human Notch are produced. In
another embodiment, a complex formed from a fragment of Delta
and a fragment of Kuz, which fragments contain the protein
domain that interacts with the other member of the complex,
are used as an immunogen for antibody production.
Various procedures known in the art may be used for
the production of polyclonal antibodies to a Delta cleavage
peptide or derivative o:r analog, or to a soluble Delta
peptide or derivative o:r analog, or to a protein complex of
- 69 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
the present invention. For the production of antibody,
various host animals can be immunized by injection with the
native Delta cleavage peptide, or D1E~ or Notch or Kuz, or a
synthetic version, or derivative (e. g., fragment) thereof,
including but not limited to rabbits, mice, rats, etc.
Various adjuvants may be used to increase the immunological
response, depending on the host species, and including but
not limited to Freund';3 (complete and incomplete), mineral
gels such as aluminum hydroxide, surface active substances
such as lysolecithin, pluronic polyols, polyanions, peptides,
oil emulsions, keyhole limpet hemocyanins, dinitrophenol, and
potentially useful human adjuvants such as BCG (bacille
Calmette-Guerin) and corynebacterium parvum.
For preparation of monoclonal antibodies directed
toward, for example, a Delta cleavage peptide sequence or
analog thereof, any technique which provides for the
production of antibody molecules by continuous cell lines in
culture may be used. F'or example, the hybridoma technique
originally developed by Kohler and Milstein (1975, Nature
256:495-497), as well a.s the trioma technique, the human B-
cell hybridoma technique (Kozbor et al., 1983, Immunology
Today 4:72), and the BBV-hybridoma technique to produce human
monoclonal antibodies (Cole et al., 1985, in Monoclonal
Antibodies and Cancer Therapy, Alan R. Liss, Inc., pp. 77-
96). In an additional embodiment of the invention,
monoclonal antibodies can be produced in germ-free animals
utilizing recent technology (PCT/US90/02545). According to
the invention, human antibodies may be used and can be
obtained by using human hybridomas (Cote et al., 1983, Proc.
Natl. Acad. Sci. U.S.A. 80:2026-2030) or by transforming
human B cells with EBV virus in vitro (Cole et al., 1985, in
Monoclonal Antibodies and Cancer Therapy, Alan R. Liss, pp.
77-96). In fact, according to the invention, techniques
developed for the production of "chimeric antibodies"
- 70 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
(Morrison et al., 1984, Proc. Natl. Acad. Sci. U.S.A.
81:6851-6855; Neuberger et al., 1984, Nature 312:604-608;
Takeda et al., 1985, Nature 314:452-454) by splicing the
genes from a mouse antibody molecule specific for, e.g.,
Delta cleavage peptide together with genes from a human
antibody molecule of appropriate biological activity can be
used; such antibodies are within the scope of this invention.
According to the invention, techniques described
for the production of single chain antibodies (U. S. Patent
4,946,778) can be adapted to produce, for example, Delta
7.0
cleavage peptide-specific single chain antibodies. An
additional embodiment of the invention utilizes the
techniques described for the construction of Fab expression
libraries (Huse et al., 1989, Science 246:1275-1281) to allow
rapid and easy identification of monoclonal Fab fragments
~-5 with the desired specificity for Delta proteins, derivatives,
or analogs.
Antibody fragments which contain the idiotype of
the molecule can be generated by known techniques. For
example, such fragments include but are not limited to: the
~~0 F(ab')Z fragment which can be produced by pepsin digestion of
the antibody molecule; t:he Fab' fragments which can be
generated by reducing the disulfide bridges of the F(ab').2
fragment, and the Fab fragments which can be generated by
treating the antibody molecule with papain and a reducing
agent.
:Z 5
In the production of antibodies, screening for the
desired antibody can be accomplished by techniques known in
the art, e.g. ELISA (en:~yme-linked immunosorbent assay). For
example, to select antibodies which recognize, for example, a
Delta cleavage peptide, one may assay generated hybridomas
30 for a product which binds to a Delta cleavage peptide. For
selection of an antibod~~r immunospecific to human Delta
cleavage peptide, one can select on the basis of positive
_ 71 _
CA 02333893 2001-O1-11
WO 00/02897 PCTNS99/15817
binding to human Delta cleavage peptide and a lack of binding
to Drosophila Delta cleavage peptide.
The foregoing antibodies can be used in methods
known in the art relating to the localization and activity of
the protein sequences of the invention, e.g., for imaging
these proteins, measuring levels thereof in appropriate
physiological samples, in diagnostic methods, etc.
In another embodiment of the invention (see infra),
anti-Delta cleavage peptide antibodies specific for the Delta
cleavage peptide and fragments thereof containing the binding
domain are Therapeutics. In yet another embodiment of the
invention, an anti-Delta:Kuz complex antibody or a fragment
thereof containing the binding domain, is a Therapeutic. In
yet another embodiment of the invention, an anti-soluble
Delta peptide antibody .or a fragment thereof containing the
:15 binding domain, is a Therapeutic.
5.3 DETECTI0~1 OF THE ACTIVE FORM OF DELTA
The present invention is directed to methods for
detecting or measuring l7elta activation by observing or
;~0 measuring Delta cleavage products that are indicative of
Delta activation. In one aspect of this embodiment of the
invention, the method for detecting or measuring Delta
activation in a cell comprises detecting or measuring the
expression of one or more Delta cleavage products selected
';5 from the group consisting of D1E~ and D1T"'. In yet another
aspect, the method comprises detecting or measuring an amino-
terminal fragment of fu:L1-length Delta beginning at amino
acid Serz3 and terminating between amino acid Cysss4 and amino
acid Alassa or Glns94 in Drosophila Delta (SEQ ID N0:9) ,
beginning at amino acid Ser22 and terminating between amino
3~ 0
acid Cyssls and amino acid Phe543 in human Delta (SEQ ID
NO:10), beginning at amino acid Ser22 and terminating between
amino acid Cyss~s and amino acid Phe543 in mouse Delta (SEQ ID
- 72 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
N0:6), beginning at amino acid Ser24 and terminating between
amino acid Cyss2, and amino acid Phessl in chick Delta (SEQ ID
N0:7), or beginning at amino acid Ser~2 and terminating
between amino acid Cyssle. and amino acid Phes49 in Xenopus
Delta (SEQ ID N0:8). In yet another aspect, the method
comprises detecting or measuring under reducing conditions, a
soluble Delta fragment of approximately 67 kilodaltons.
In another embodiment, the present invention is
also directed to methods for identifying a molecule that
modulates Delta activation by detecting or measuring a change
:l O
in the amount or pattern of Delta cleavage products. In ane
aspect of this embodiment of the invention, the method for
identifying a modulator of Notch activation comprises
providing a cell with a candidate modulator molecule and
detecting or measuring t:he expression by the cell of one or
~~5 more Notch cleavage products. selected from the group
consisting of DlE~ and D:1'''"', in which a difference in the
presence or amount of said one or more cleavage products
compared to a Delta cell not contacted with the candidate
molecule indicates that the molecule modulates Delta
a0 activity.
In an alternative aspect, the method for
identifying a modulator of Delta activation comprises
contacting a candidate modulator molecule with a full length
Delta in the presence o:E Kuz and optionally a composition
comprising cellular proteins, under conditions conducive to
.25
cleavage of the full-length Delta by Kuz and optionally one
or more components of tlae composition and detecting or
measuring the amount of Delta cleavage products DlE~ and D1T'"'
that result, in which a difference in the presence or amount
of said Notch cleavage lproducts compared to a full-length
30 Delta in presence of said composition not contacted with the
candidate molecule indicates that the molecule modulates
Delta activity.
- 73 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
Any method known in the art for detecting or
measuring the expression of Delta cleavage products
indicative of Delta activation can be used. For example, and
not by way of limitation, one such method of detection of the
active form of Delta by .detecting one or more Delta cleavage
products selected from t:he group consisting of D1E~ and D1'~',
or by detecting an amino-terminal fragment of full-length
Delta beginning at amino acid Ser23 and terminating between
amino acid Cys564 and amino acid A1a593 or Glns94 in Drosophi:la
Delta (SEQ ID N0:9), beginning at amino acid Ser2z and
terminating between amino acid Cys516 and amino acid Phe543 .in
human Delta (SEQ ID NO:10), beginning at amino acid Ser22 and
terminating between amino acid Cyss~s and amino acid Phe543 in
mouse Delta (SEQ TD N0:6), beginning at amino acid Serz4 and
terminating between amino acid Cyssz3 and amino acid PheSSI in
L5 chick Delta (SEQ ID N0:7), or beginning at amino acid Ser2:a
and terminating between amino acid Cys518 and amino acid Phe5,4
in Xenopus Delta (SEQ ID N0:8). In yet another aspect, the
method comprises detecting or measuring under reducing
conditions, a soluble Delta fragment of approximately 67
2,0 kilodaltons.
Detection of such cleavage products can be done,
e.g., by immunoprecipitating the cleavage products with an
anti-Delta antibody or binding to anti-Delta antibody on an
immunoaffinity column or' immobilized on a plate or in a well,
or visualizing the fragments by Western blotting. In a
:; 5
specific embodiment, the cleavage products can be labelled by
general cell surface labeling, or, alternatively, by pulse
labeling the cells by incubation in culture medium containing
a radioactive label, or, alternatively, it can be anti-Delta
antibody (or antibody binding partner) that is labeled rather
=s0 than the Delta cleavage products.
Another method to detect the active farm of Delta
is to use a Delta ligand or binding fragment thereof, such as
- 74 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
Notch, to bind to Delta (e. g., when the ligand is labeled),
or to recover Delta by coimmunoprecipitating with the
appropriate anti-Delta ligand antibody to co-
immunoprecipitate Delta cleavage products, etc.
Similar procedures to those described supra can be
used to make antibodies to domains of other proteins
(particularly toporythmic proteins) that bind or otherwise
interact with Delta (e. g., binding fragments of Notch).
The cell in which Delta activation is detected or
LO measured can be any cel:L, e.g., one that endogenously or
recombinantly expresses Delta. The cell can be vertebrate,
insect (e. g., Drosophil~~), C. elegans, mammalian, bovine,
murine, rat, avian, fish, primate, human, etc. The Delta
which is expressed can be vertebrate, insect, C. elegans,
mammalian, bovine, murine, rat, avian, fish, primate, human,
etc. The cell can be a cell of primary tissue, a cell line,
or of an animal containing and expressing a Delta transgene.
For example, the transgenic animal can be a Drosophila (e. g.,
melanogaster) or a C. e.Iegans. In a preferred embodiment,
the transgene encodes a human Delta. Transgenic animals can
be made by standard methods well known in the art (e.g., by
use of P element transposons as a vector in Drosophila).
5.4 METHODS OF IDENTIFYING MODULATORS OF DELTA
ACTIVATION
In one embodiment of the invention, methods are
provided for the identification of modulators, e.g.,
inhibitors, antagonists, or agonists, of Delta activation by
detecting the ability of the modulators to effect cleavage of
full length Delta. In one aspect of this embodiment of the
invention, the method for identifying a modulator of Delta
activation comprises providing a cell with a candidate
modulator molecule and detecting or measuring the expression
by the cell of one or more Delta cleavage products selected
_ 75 _
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
from the group consisting of D1E~ and D1T"', in which a
difference in the presence or amount of said one or more
cleavage products compared to a Delta cell not contacted with
the candidate molecule indicates that the molecule modulates
Delta activity. In yet another aspect, the method comprises
providing a cell with a candidate modulator molecule and
detecting or measuring t;he amount of the expression by the
cell of an amino-terminal fragment of full-length Delta
beginning at amino acid Serz3 and terminating between amino
acid Cyss64 and amino acid Ala59a or G1n594 in Drosophila Delta
7~ 0
(SEQ ID N0:9), beginning at amino acid Serzz and terminating
between amino acid CysslE; and amino acid Phe54a in human Delta
(SEQ ID NO:10), beginning at amino acid Serzz and terminating
between amino acid Cyssl~; and amino acid Phesaa in mouse Delta
(SEQ ID N0:6), beginning at amino acid Serz4 and terminating
~5 between amino acid Cyssza and amino acid Phe5s1 in chick Delta
(SEQ ID N0:7), or beginning at amino acid Serzz and
terminating between amino acid Cyssla and amino acid Phe544 in
Xenopus Delta (SEQ ID N0:8); in which a difference in the
presence or amount of said fragment compared to a Delta cell
a0 not contacted with the candidate molecule indicates that the
molecule modulates Delta activity.
In yet another aspect, the method comprises
providing a cell with a candidate modulator molecule and
detecting or measuring the expression by the cell of a
soluble Delta fragment of approximately 67 kilodaltons, in
:Z 5
which a difference in the presence or amount of said soluble
fragment compared to a Delta cell not contacted with the
candidate molecule indicates that the molecule modulates
Delta activity.
In yet another aspect of this embodiment of the
30 invention, the method for identifying a modulator of Delta
activation comprises contacting a candidate modulator
molecule with a full length Delta in the presence of Kuz and
- 76 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99115817
optionally a composition comprising cellular proteins, under
conditions conducive to cleavage of the full-length Delta by
Kuz and optionally one o:r more components of the composition,
and detecting or measuring the amount of Delta cleavage
,5 products D1E~ and/or DlTh' that result, in which a difference in
the presence or amount of said Delta cleavage products)
compared to a full-length Delta in presence of said
composition not contacted with the candidate molecule
indicates that the molecule modulates Delta activity. In
another aspect, the method for identifying a modulator of
Delta activation comprises contacting a candidate modulator
molecule with a full length Delta in the presence of Kuz and
optionally a composition comprising cellular proteins, under
conditions conducive to cleavage of the full-length Delta by
Kuz and optionally one or more components of the composition,
Z5 and detecting or measuring an amino-terminal fragment of
full-length Delta beginning at amino acid SerZ, and
terminating between amino acid Cyss64 and amino acid Alas93 or
Glns94 in Drosophila Delta (SEQ ID N0:9), beginning at amino
acid Ser22 and terminating between amino acid Cyssl6 and amino
acid Phes4a in human Delta (SEQ ID NO:10) , beginning at amino
acid Ser22 and terminating between amino acid Cyssls and amino
acid Phes,3 in mouse Delta (SEQ ID N0:6), beginning at amino
acid Ser29 and terminating between amino acid Cysszs and amino
acid Phessl in chick Delta (SEQ ID N0:7), or beginning at
amino acid Ser22 and terminating between amino acid CysslB and
amino acid Phes44 in Xenopus Delta (SEQ ID N0:8), in which a
difference in the presence or amount of said fragment
compared to a full-length Delta in presence of said
composition not contacted with the candidate molecule
indicates that the molecule modulates Delta activity.
In yet another aspect, the method for identifying a
modulator of Delta activation comprises contacting a
candidate modulator molecule with a full length Delta in the
_ 77 _
CA 02333893 2001-O1-11
WO 00!02897 PCT/US99/15817
presence of Kuz and optionally a composition comprising
cellular proteins, under conditions conducive to cleavage of
the full-length Delta by Kuz and optionally one or more
components of the composition and detecting or measuring the
amount of a soluble Delta fragment of approximately 67
kilodaltons, in which a difference in the presence or amount
of said soluble fragment: compared to a full-length Delta in
presence of said composition not contacted with the candidate
molecule indicates that the molecule modulates Delta
activity.
:l O
In a specific aspect of the embodiment using a
composition comprising cellular proteins, the composition
comprising cellular proteins is a cell lysate made from cells
which recombinantly express Delta. In another specific
aspect of this embodiment, the composition comprising
cellular proteins is a cell lysate made from cells which
endogenously express Delta.
Detection or measurement of Delta cleavage products
can be carried out by methods well known in the art and/or
those methods disclosed in Section 5.1, supra.
The cells used in the methods of this embodiment
can either endogenously or recombinantly express Delta.
Examples of the cell types and Delta protein that can be
expressed are described in Section 5.1. Recombinant Delta
expression is carried out by introducing Delta encoding
nucleic acids into expression vectors and subsequently
introducing the vectors into a cell to express Delta or
simply introducing Delta encoding nucleic acids into a cell
for expression. Nucleic acids encoding vertebrate and nan-
vertebrate Delta have been cloned and sequenced and their
expression is well known in the art. See, for example,
International Publication WO 97/01571, which is incorporated
by reference in their entirety herein. Expression can be:
from expression vectors'. or intrachromosomal.
_ 78 _
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
Any method knovan to those of skill in the art for
the insertion of Delta-DIVA into a vector may be used to
construct expression vectors containing a chimeric gene
consisting of appropriate transcriptional/translational
,~ control signals and the protein coding sequences. These
. methods may include in vitro recombinant DNA and synthetic
techniques and in vivo recombinants (genetic recombination).
Expression of nucleic acid sequence encoding a Delta protein
may be regulated by a second nucleic acid sequence so that
the Delta protein is expressed in a host transformed with the
ly
recombinant DNA molecule. For example, expression of a De:Lta
protein may be controlled by any promoter/enhancer element
known in the art. Promoters which may be used to control
Delta gene expression include, but are not limited to, those
described in Section 5.1.
15 In the methods of the invention in which full-
length Delta is incubated with compositions comprising
cellular proteins (e.g., cell lysates or cell fractions) in
the presence of candidate cleavage (and thus Delta
activation) modulators the expression of Delta should be such
20 that full length Delta is expressed and proteolytic cleavage
of Delta is kept to a minimum such that Delta cleavage
products are easily detected over any background proteolysis.
There are several methods known in the art to keep
proteolysis to a minimum. For example, one manner to keep
25 Delta cleavage to a minimum is to express Delta in cells
concurrently with Hrefel.din A treatment. Another manner is
to express Delta in cells which do not contain Kuz or to
express Delta in an in vitro transcription-translation system
in the presence of a protease inhibitor such as
phenylmethylsulfonylfluoride (PMSF).
3. 0
_ 79 _
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
5.5 METHODS GF IDENTIFYING MODULATORS OF KUZ
ACTIVATION
In one embodiment of the invention, methods are
provided for the identification of modulators, e.g.,
inhibitors, antagonists, or agonists, of Kuz function by
detecting the ability of: the modulators to effect cleavage of
full length Delta. In one aspect of this embodiment of the
invention, the method for identifying a modulator of Kuz
function comprises providing a Delta expressing cell with a
candidate modulator molecule and detecting or measuring the
v0 expression by the cell of one or more Delta cleavage products
selected from the group consisting of D1E~ and D1T'"', in which a
difference in the presence or amount of said one or more
cleavage products compai:ed to a Delta cell not contacted with
the candidate molecule indicates that the molecule modulates
;15 Kuz function. In yet another aspect, the method comprises
providing a Delta expressing cell with a candidate modulator
molecule and detecting or measuring the amount of the
expression by the cell of an amino-terminal fragment of full-
length Delta beginning at amino acid Serz3 and terminating
20 between amino acid Cys$6,4 and amino acid A1a593 or G1n594 in
Drosophila Delta (SEQ I17 N0:9), beginning at amino acid Serzz
and terminating between amino acid CysS~s and amino acid Phe5a3
in human Delta (SEQ ID 110:10) , beginning at amino acid Serzz
and terminating between amino acid CysS,s and amino acid Phe5,3
in mouse Delta (SEQ ID 1V0:6), beginning at amino acid Serz4
25 and terminating between amino acid Cyssza and amino acid Phessl
in chick Delta (SEQ ID N0:7), or beginning at amino acid Serzz
and terminating between amino acid CysslB and amino acid Phe544
in Xenopus Delta (SEQ I:D N0:8); in which a difference in the
presence or amount of said fragment compared to a Delta cell
30 not contacted with the candidate molecule indicates that the
molecule modulates Kuz function.
- 80 -
CA 02333893 2001-O1-11
WO 00/02897 PGT/IJS99J15817
In yet another. aspect, the method comprises
providing a Delta expressing cell with a candidate modulator
molecule and detecting or measuring the expression by the
cell of a soluble Delta fragment of approximately 67
kilodaltons, in which a difference in the presence or amount
of said soluble fragment: compared to a Delta cell not
contacted with the candidate molecule indicates that the
molecule modulates Kuz function.
In yet another aspect of this embodiment of the
invention, the method for identifying a modulator of Kuz
:l0
function comprises contacting a candidate modulator molecule
with a full length Delta. in the presence of Kuz and
optionally a composition comprising cellular proteins, under
conditions conducive to cleavage of the full-length Delta by
Kuz and optionally one o~r more components of the composition,
~~5 and detecting or measuring the amount of Delta cleavage
products D1E~ and/or D1'I'"' that result, in which a difference in
the presence or amount of said Delta cleavage products)
compared to a full-length Delta in presence of said
composition not contacted with the candidate molecule
20 indicates that the molecule modulates Kuz activity. In
another aspect, the method for identifying a modulator of Kuz
function comprises contacting a candidate modulator molecule
with a full length Delta in the presence of Kuz and
optionally a composition comprising cellular proteins, under
conditions conducive to cleavage of the full-length Delta by
Kuz and optionally one or more components of the composition,
and detecting or measuring an amino-terminal fragment of
full-length Delta beginning at amino acid Ser23 and
terminating between amino acid Cysse4 and amino acid A1a593 or
G1n594 in Drosophila Delta (SEQ ID N0:9), beginning at amino
acid Ser22 and terminating between amino acid Cyssls and amino
acid Phe54a in human Delt~~ {SEQ ID NO:10), beginning at amino
acid Ser22 and terminating between amino acid Cyssls and amino
_ 81 _
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
acid Phesa3 in mouse Dell~a (SEQ ID N0:6), beginning at amino
acid Ser24 and terminating between amino acid Cys523 and amino
acid Phe55, in chick Delta (SEQ ID N0:7) , or beginning at
amino acid Ser2z and terminating between amino acid Cyssle and
amino acid Phe544 in Xen«pus Delta (SEQ ID N0:8), in which a
difference in the presence or amount of said fragment
compared to a full-length Delta in presence of said
composition not contacted with the candidate molecule
indicates that the molecule modulates Kuz function.
In yet another aspect, the method for identifying a
modulator of Kuz function comprises contacting a candidate
modulator molecule with a full length Delta in the presence
of Kuz and optionally a composition comprising cellular
proteins, under conditions conducive to cleavage of the full-
length Delta by Kuz and optionally one or more components of
the composition and detecting or measuring the amount of a
soluble Delta fragment of approximately 67 kilodaltons, i.n
which a difference in the presence or amount of said soluble
fragment compared to a full-length Delta in presence of said
composition not contacted with the candidate molecule
indicates that the molecule modulates Kuz function.
In a specific aspect of the embodiment using a
composition comprising cellular proteins, the composition
comprising cellular proteins is a cell lysate made from cells
which recombinantly express Kuz. In another specific aspect
of this embodiment, the composition comprising cellular
proteins is a cell lysa.te made from cells which endogenously
express Kuz.
Detection or measurement of Delta cleavage products
can be carried out by methods well known in the art and/ar
those methods disclosed in Section 5.1, supra.
The cells used in the methods of this embodiment
can either endogenously or recombinantly express Kuz.
Examples of the cell types and Kuz protein that can be
- 82 -
CA 02333893 2001-O1-11
WO 00/02897
PCTlUS99/15817
expressed are described in Section 5.1. Recombinant Kuz
expression is carried out by introducing Kuz encoding nucleic
acids into expression vectors and subsequently introducing
the vectors into a cell to express Kuz or simply introducing
Kuz encoding nucleic acids into a cell for expression.
Nucleic acids encoding vertebrate and non-vertebrate Kuz have
been cloned and sequenced and their expression is well known
in the art. See, for example, International Publication WO
98/08933, which is incorporated by reference in its entirety
herein. Expression can be from expression vectors or
intrachromosomal.
Any method known to those of skill in the art for
the insertion of Kuz-DIVA into a vector may be used to
construct expression vectors containing a chimeric gene
consisting of appropriate transcriptional/translational
control signals and thE~ protein coding sequences. These
methods may include in vitro recombinant DNA and synthetic
techniques and in vivo recombinants (genetic recombination).
Expression of nucleic acid sequence encoding a Delta or Kuz
protein may be regulated by a second nucleic acid sequence so
that the Kuz protein i:~ expressed in a host transformed with
the recombinant DNA molecule. For example, expression of a
Kuz protein may be controlled by any promoter/enhancer
element known in the art. Promoters which may be used to
control Delta gene expression include, but are not limited
to, those described in Section 5.1.
In the methods of the invention in which full-
length Delta and Kuz is incubated with compositions
comprising cellular proteins (e. g., cell lysates or cell
fractions) in the presence of candidate cleavage (and thus
Delta activation) modulators the expression of Delta should
:30 be such that full length Delta is expressed and proteolyt:ic
cleavage of Delta is kept to a minimum such that Delta
cleavage products are easily detected over any background
- 83 -
CA 02333893 2001-O1-11
WO 00/OZ897 PCT/US99/15817
proteolysis. There are several methods known in the art to
keep proteolysis to a minimum. For example, one manner to
keep Delta cleavage to a. minimum is to express Delta in cells
concurrently with Brefeldin A treatment. Another manner i.s
to express Kuz in cells which do not contain Delta or to
express Kuz in an in vitro transcription-translation system
in the presence of a protease inhibitor such as
phenylmethylsulfonylfluaride (PMSF).
5.6 METHODS OF IDENTIFYING MODULATORS OF DELTA:KUZ
COMPLEX OR D1=~:NOTCH COMPLEX ACTIVITY
Delta:Kuz or DlE~:Notch complexes, and derivatives,
fragments and analogs thereof, nucleic acids encoding Delta,
Notch and Kuz as well a:> derivatives, fragments and analogs
of the nucleic acids, can be used to screen for compounds
~.5 that bind to, or modulate the function of a Delta:Kuz complex
or a D1E~:Notch complex, complex member encoding nucleic
acids, complex member proteins, and derivatives of the
foregoing, and thus, have potential use as agonists or
antagonists of Delta:Kuz or D1E~:Notch complex activity or
formation. The present invention is thus directed to assays
~~~ for detecting molecules that specifically bind to, or
modulate the function of, Delta, Notch and Kuz nucleic acids,
proteins or derivatives of the nucleic acids and proteins.
For example, recombinants cells expressing both Delta and Kuz
nucleic acids can be usESd to recombinantly produce the
a5 complexes or proteins in these assays, to screen for
molecules that bind to, or interfere with, or promote
Delta:Kuz complex formai:ion or activity. In preferred
embodiments, polypeptide analogs that have superior
stabilities but retain the ability to form a Delta:Kuz or
:30 DlE~:Notch complex (e. g., Delta and Kuz or D1E~ and Notch
modified to be resistant to proteolytic degradation in the
binding assay buffers, or to be resistant to oxidative
- 84 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
degradation), are used t~o screen for modulators of Delta
activity or Kuz activity or Delta:Kuz complex activity or
formation, or are used to screen for modulators of DlEc
activity or Notch activity or DlEC:Notch complex activity or
formation. Such resistant molecules can be generated, e.g.,
by substitution of amino acids at proteolytic cleavage sites,
the use of chemically derivatized amino acids at proteolytic
susceptible sites, and the replacement of amino acid residues
subject to oxidation, i.e. methionine and cysteine.
A molecule (e.g., a putative binding partner or
modulator of Delta:Kuz or DlEC:Notch complex activity or
formation} is contacted with the Delta:Kuz or DlEC:Notch
complex, or fragment thereof, respectively, under conditions
conducive to binding or modulation, and then a molecule that
specifically bind to or modulate Delta:Kuz or DlEC:Notch
complex activity or formation is identified. Similar methods
can be used to screen for molecules that bind to or modulate
the function of Delta:Kuz or DlEC:Notch complex encoding
nucleic acids or derivatives thereof.
A particular aspect of the present invention
relates to identifying molecules that inhibit or promote
formation or degradation of a Delta:Kuz or DlEC:Notch complex,
e.g., using the method described for screening inhibitors
using the modified yeast matrix mating test described in
International Patent Publication WO 97/47763 entitled
"Identification and Comparison of Protein-Protein
Interactions that Occur in Populations and Identification of
Inhibitors of These Interactions", which is incorporated by
reference herein in its entirety.
In one embodiment of the invention, a molecule that
modulates activity of Delta or Kuz, or a complex of Delta and
Kuz, is identified by contacting one or more candidate
molecules with Delta in the presence of Kuz; and measuring
the amount of complex that forms between Delta and Kuz;
- 85 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
wherein an increase or decrease in the amount of complex that
forms relative to the <~mount that forms in the absence of_ the
candidate molecules) :indicates that the molecules)
modulates the activity of Delta or Kuz or said complex of
Delta and Kuz. In preferred embodiments, a modulator is
identified by administering a candidate molecule to a
transgenic non-human animal expressing both Delta and Kuz
from promoters that are not the native Delta or the native
Kuz promoters, more preferably where the candidate molecule
is also recombinantly expressed in the transgenic non-human
animal. Alternatively, the method for identifying such a
modulator car. be carried out in vitro, preferably with
purified Delta, purified Kuz, and a purified candidate
molecule.
In another embodiment of the invention, a molecule
that modulates activity of DlEC or Notch, or a complex of DlEc
and Notch, is identified by contacting one or more candidate
molecules with DlEC in t:he presence of Notch; and measuring
the amount of complex that forms between DlEC and Notch;
wherein an increase or decrease in the amount of complex that
forms relative to the amount that forms in the absence of the
candidate molecules) indicates that the molecules)
modulates the activity of DlEC or Notch or said complex of DlEc
and Notch. In preferred embodiments, a modulator is
identified by administering a candidate molecule to a
transgenic non-human animal expressing both DlEC and Notch
from promoters that axe not the native DlEC or the native
Notch promoters, more preferably where the candidate molecule
is also recombinantly expressed in the transgenic non-human
animal. Alternatively, the method for identifying such a
modulator can be carried out in vitro, preferably with
purified D1E', purified Notch, and a purified candidate
molecule.
- 86 -
CA 02333893 2001-O1-11
WO 00/02897 PC'T/US99/15817
Methods that can be used to carry out the foregoing
are commonly known in the art. Agents/molecules to be
screened can be provided as mixtures of a limited number of
specified compounds, or ;as compound libraries, peptide
libraries and the like ass described in Section 5.7, infra.
Agents/molecules to be screened may also include all forms of
antisera, antisense nucleic acids, etc., that can modulate
complex activity or formation.
5.7 CANDIDATE MOLECULES
Any molecule known in the art can be tested for its
ability to modulate Delta activation or Kuz function as
measured by the expression of one or more of the Delta
cleavage products disclosed herein. Furthermore, any
molecule known in the art can be tested for its ability to
modulate Delta:Kuz complex function, or for its ability to
modulate D1E~:Notch complex function. For identifying a
molecule that modulates lDelta activation or Kuz function,
candidate molecules can be directly provided to a cell
expressing Delta or Kuz or, in the case of candidate
Proteins, can be provided by providing their encoding nucleic
acids under conditions i:n which the nucleic acids are
recombinantly expressed to produce the candidate proteins
within the Delta or Kuz expressing cell. In an embodiment of
the invention directed t~o the assay using full-length Delta
and a composition comprising cellular proteins, candidate
molecules can also be added to a composition comprising
cellular proteins (whole cell lysates, membrane fraction,
etc.), preferably derived from cells endogenously or
recombinantly expressing Delta.
This embodiment of the invention is well suited to
screen chemical libraries for molecules which modulate, e.g.,
inhibit, antagonize, or agonize, Delta activation or Kuz
function or complex function. The chemical libraries can be
_ 87 _
CA 02333893 2001-O1-11
WO 00/02897 PCTNS99/15817
peptide libraries, pepti~domimetic libraries, other non-
peptide synthetic organic libraries, etc.
Exemplary libraries are commercially available from
several sources (ArQule, Tripos/PanLabs, ChemDesign,
Pharmacopoeia). In some cases, these chemical libraries are
generated using combinatorial strategies that encode the
identity of each member ~of the library on a substrate to
which the member compound is attached, thus allowing direct
and immediate identification of a molecule that is an
effective modulator. Thus, in many combinatorial approaches,
l~ the position on a plate ~of a compound specifies that
compound's composition. Also, in one example, a single plate
position may have from 1-20 chemicals that can be screened by
administration to a well containing the interactions of
interest. Thus, if modulation is detected, smaller and
1:5 smaller pools of interacting pairs can be assayed for the
modulation activity. By such methods, many candidate
molecules can be screened.
Many diversity libraries suitable for use are known
in the art and can be used to provide compounds to be tested
20 according to the present invention. Alternatively, libraries
can be constructed using standard methods. Chemical
(synthetic) libraries, recombinant expression libraries, or
polysome-based libraries are exemplary types of libraries
that can be used.
The libraries can be constrained or semirigid
(having some degree of structural rigidity), or linear or
nonconstrained. The library can be a c~NA or genomic
expression library, random peptide expression library or a
chemically synthesized random peptide library, or non-peptide
library. Expression libraries are introduced into the cells
in which the assay occurs, where the nucleic acids of the
library are expressed to produce their encoded proteins.
_ 88 _
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
In one embodiment, peptide libraries that can be
used in the present invention may be libraries that are
chemically synthesized in vitro. Examples of such libraries
are given in Houghten et al., 1991, Nature 354:84-86, which
,~ describes mixtures of free hexapeptides in which the first
and second residues in each peptide were individually and
specifically defined; Lam et al., 1991, Nature 354:82-84,
which describes a "one bead, one peptide" approach in which a
solid phase split synthe:~is scheme produced a library of
peptides in which each bead in the collection had immobilized
l~~ thereon a single, random sequence of amino acid residues;
Medynski, 1994, Bio/Technology 12:709-710, which describes
split synthesis and T-back synthesis methods; and Gallop et
al., 1994, J. Medicinal Chemistry 37(9):1233-1251. Simply by
way of other examples, a combinatorial library may be
1!~ prepared for use, according to the methods of Ohlmeyer et
al., 1993, Proc. Natl. Ac;ad. Sci. USA 90:10922-10926; Erb et
al.', 1994, Proc. Natl. Ac;ad. Sci. USA 91:11422-11426;
Houghten et al., 1992, Biotechniques 13:412; Jayawickreme et
al., 1994, Proc. Natl. Ac:ad. Sci. USA 91:1614-1618; or Salmon
2~~ et al., 1993, Proc. Natl. Acad. Sci. USA 90:11708-11712. PCT
Publication No. WO 93/20242 and Brenner and Lerner, 1992,
Proc. Natl. Acad. Sci. USA 89:5381-5383 describe "encoded
combinatorial chemical libraries," that contain
oligonucleotide identifiers for each chemical polymer library
member.
2 !~
Further, more general, structurally constrained,
organic diversity (e.g., nonpeptide) libraries, can also be
used. By way of example, a benzodiazepine library (see e.g.,
Bunin et al., 1994, Proc. Natl. Acad. Sci. USA 91:4708-4712)
may be used.
Conformational7_y constrained libraries that can be
used include but are not limited to those containing
invariant cysteine residues which, in an oxidizing
_ 89 _
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99115817
environment, cross-link by disulfide bonds to form cystines,
modified peptides (e. g., incorporating fluorine, metals,
isotopic labels, are phosphorylated, etc.), peptides
containing one or more non-naturally occurring amino acids,
non-peptide structures, and peptides containing a significant
fraction of Y-carboxyglutamic acid.
Libraries of non-peptides, e.g., peptide
derivatives (for example, that contain one or more non-
naturally occurring amino acids) can also be used. One
example of these are peptoid libraries (Simon et al., 1992,
1.0
Proc. Natl. Acad. Sci. USA 89:9367-9371). Peptoids are
polymers of non-natural amino acids that have naturally
occurring side chains attached not to the alpha carbon but to
the backbone amino nitrogen. Since peptoids are not easily
degraded by human digestive enzymes, they are advantageously
1.5 more easily adaptable to drug use. Another example of a
library that can be used, in which the amide functionalities
in peptides have been permethylated to generate a chemically
transformed combinatorial library, is described by Ostresh et
al., 1994, Proc. Natl. A.cad. Sci. USA 91:11138-11142).
20 The members of the peptide libraries that can be
screened according to the invention are not limited to
containing the 20 naturally occurring amino acids. In
particular, chemically synthesized libraries and polysome
based libraries allow th.e use of amino acids in addition to
the 20 naturally occurring amino acids (by their inclusion in
~; 5
the precursor pool of amino acids used in library
production). In specific embodiments, the library members
contain one or more non-natural or non-classical amino acids
or cyclic peptides. Non.-classical amino acids include but:
are not limited to the ~~-isomers of the common amino acids,
?.0 a_amino isobutyric acid, 4-aminobutyric acid, Abu, 2-amino
butyric acid; Y-Abu, e-Ahx, 6-amino hexanoic acid; Aib, 2-
amino isobutyric acid; 3-amino propionic acid; ornithine;
- 90 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
norleucine; norvaline, h,ydroxyproline, sarcosine, citrulline,
cysteic acid, t-butylglycine, t-butylalanine, phenylglycine,
cyclohexylalanine, i3-ala.nine, designer amino acids such as f~-
methyl amino acids, Ca-methyl amino acids, Na-methyl amino
acids, fluoro-amino acids and amino acid analogs in general.
Furthermore, the amino acid can be D (dextrorotary) or L
(levorotary) .
Further, toporythmic proteins, derivatives and
fragments thereof, can b~e tested for the ability to modulate
Delta activation. Toporythmic proteins, and more generally,
members of the "Notch cascade" or the "Notch group" of genes,
include Notch, Delta, Serrate, Kuz, and other members of the
Delta/Serrate family, which are identified by genetic (as
detected phenotypically, e.g., in Drosophila) or molecular
interaction (e. g., binding in vitro). See, International
publications W0 92/19734, WO 97/18822, WO 96/27610, and WO
97/01571 and references therein, for examples of vertebrate
and non-vertebrate members of the Notch family of genes.
Screening the libraries can be accomplished by any
of a variety of commonly known methods. See, e.g., the
following references, which disclose screening of peptide
libraries: Parmley and Smith, 1989, Adv. Exp. Med. Biol.
251:215-218; Scott and Smith, 1990, Science 249:386-390;
Fowlkes et al., 1992, BioTechniques 13:422-427; Oldenburg et
al., 1992, Proc. Natl. Acad. Sci. USA 89:5393-5397; Yu et
al., 1994, Cell 76:933-945; Staudt et al., 1988, Science
241:577-580; Bock et al., 1992, Nature 355:564-566; Tuerk et
al., 1992, Proc. Natl. A.cad. Sci. USA 89:6988-6992; Ellington
et al., 1992, Nature 355:850-852; U.S. Patent No. 5,096,815,
U.S. Patent No. 5,223,409, and U.S. Patent No. 5,198,346, all
to Ladner et al.; Rebar and Pabo, 1993, Science 263:671-673;
and International Patent Publication No. WO 94/18318.
In a specific embodiment, screening can be carried
out by contacting the library members with Delta or Kuz or' a
- 91 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
protein complex or the present invention (or encoding nucleic
acid or derivative) immobilized on a solid phase, and
harvesting those library members that bind to the protein or
complex (or encoding nucleic acid or derivative). Examples
of such screening methods, termed "panning" techniques, are
described by way of example in Parmley and Smith, 1988, Gene
73:305-318; Fowlkes et al., 1992, BioTechniques 13:422-427;
International Patent Publication No. WO 94/18318; and in
references cited hereinabove.
In a specific embodiment, fragments and/or analogs
of Delta or Kuz, especially peptidomimetics, are screened for
activity as competitive or non-competitive inhibitors of
Delta:Kuz complex formation, which thereby inhibit Delta:Kuz
complex activity or formation.
Methods for screening may involve labeling the
proteins or complex proteins of the present invention with
radioligands (e.g. , 1251 or 'H) , magnetic ligands (e.g. ,
paramagnetic beads covalently attached to photobiotin
acetate), fluorescent ligands (e.g., fluorescein or
rhodamine), or enzyme ligands (e. g., luciferase or beta-
galactosidase). The reactants that bind in solution can then
be isolated by one of many techniques known in the art,
including but not restricted to, co-immunoprecipitation of
the labeled protein or complex moiety using antisera against
the unlabeled binding partner (or labeled binding partner
with a distinguishable marker from that used on the second
labeled protein or complex moiety), immunoaffinity
chromatography, size exclusion chromatography, and gradient
density centrifugation. In a preferred embodiment, the
labeled binding partner is a small fragment or peptidomimetic
that is not retained by a commercially available filter.
Upon binding, the labeled species is then unable to pass
through the filter, providing for a simple assay of complex
formation.
- 92 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
Methods commonly known in the art are used to label
proteins. Suitable labeling methods include, but are not:
limited to, radiolabeling by incorporation of radiolabeled
amino acids, e.g. , 3H-lE~ucine or 35S-methionine, radiolabeling
by post-translational iodination with lzsl or 1311 using the
chloramine T method, Bolton-Hunter reagents, etc., or
labeling with '2P using phosphorylase and inorganic
radiolabeled phosphorous, biotin labeling with photobiotin-
acetate and sunlamp exposure, etc.
5.8 THERAPEUTIC USES
The invention provides for treatment of disorders
of cell fate or differentiation by administration of a
therapeutic compound of the invention. Such therapeutic
compounds (termed herein "Therapeutics") include: Delta
cleavage peptides, Delt~a:Kuz and D1E~:Notch protein complexes
and analogs and derivatives (including fragments) thereof
(e.g., as described hereinabove); antibodies thereto (as
described hereinabove); nucleic acids encoding the Delta
cleavage peptides, analogs, or derivatives (e.g., as
:20 described hereinabove) as well as the protein complexes of
the present invention; and Delta, Notch and Kuz antisense
nucleic acids. In addition, such Therapeutics include
soluble Delta peptides and derivatives and analogs thereof,
antibodies thereto, nucleic acids encoding the soluble Delta
:25 peptides, derivatives, or analogs, and soluble Delta peptide
antisense nucleic acids.. In a particular embodiment, the
Therapeutic is a peptide, comprising a fragment of a Delta
protein of about amino acid Cys516 to about amino acid Phe543
in human Delta (SEQ ID N0:10), of about amino acid CysSls to
about amino acid Phe543 i.n mouse Delta (SEQ ID N0:6), of about
:30 amino acid Cyssz3 to about amino acid Phe551 in chick Delta
(SEQ ID N0:7), of about amino acid Cys518 to about amino acid
Phes44 in Xenopus Delta (SEQ ID N0:8), and the sequence of
- 93 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
about amino acid Cysss4 to about amino acid A1a593 or G1n594 in
Drosophila Delta (SEQ ID N0:9). In specific embodiments, the
peptide comprises 25, 30, 35, 40, 50, 100, 150, 200 or 250
contiguous amino acids of a Delta protein. Antagonist
Therapeutics of the invention are those Therapeutics which
antagonize, or inhibit, Delta function and/or Notch function
(since Delta is a Notch ligand) and/or Kuz function (since
Kuz binds to and proteolytically processes Delta). Such
Antagonist Therapeutics are most preferably identified by use
of known convenient in vitro assays, e.g., based on their
:1 O
ability to inhibit binding of Delta to another protein (e. g.,
a Notch protein or a Kuz protein), or inhibit any known Notch
or Delta or Kuz function as preferably assayed in vitro o:r in
cell culture, although genetic assays (e. g., in Drosophila)
may also be employed. In a preferred embodiment, the
'~5 Antagonist Therapeutic :is a Delta cleavage peptide which
mediates binding to Kuz, or an antibody thereto. In other
specific embodiments, such an Antagonist Therapeutic is a
nucleic acid capable of expressing a molecule comprising a
Delta cleavage peptide which binds to Kuz, or a Delta
20 antisense nucleic acid (see Section 5.11 herein). It should
be noted that preferably, suitable in vitro or in vivo
assays, as described infra, should be utilized to determine
the effect of a specific Therapeutic and whether its
administration is indicated for treatment of the affected
~:5 tissue, since the developmental history of the tissue may
determine whether an Antagonist or Agonist Therapeutic is
desired.
In addition, t:he mode of administration, e.g.,
whether administered in soluble farm or administered via its
encoding nucleic acid for intracellular recombinant
-~0 expression, of the Delta cleavage peptide or derivative or
protein complex or derivative can affect whether it acts as
an agonist or antagonist:.
- 94 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
The Agonist The:rapeutics of the invention, as
described supra, promote Delta function or Notch function or
Kuz function. Such Agoni.st Therapeutics include but are not
limited to proteins and derivatives comprising the portions
of Delta that mediate binding to Kuz, and nucleic acids
encoding the foregoing (urhich can be administered to express
their encoded products in vivo).
Further descriptions and sources of Therapeutics of
the inventions are found in Sections 5.1 through 5.7 herein.
Molecules which retain, or alternatively inhibit, a
1 Ci
desired Delta property, e~.g., binding to Kuz, binding to an
intracellular ligand, can be used therapeutically as
inducers, or inhibitors, respectively, of such property and
its physiological correlates. In a specific embodiment, a
peptide (e. g., in the range of 6-50 or 100-200 amino acids;
-'' and particularly of about 25, 30, 35, 50, 100 or 150 amino
acids) containing the sequence of a portion of Delta which
binds to Kuz is used to antagonize Delta or Notch function.
In a specific embodiment, such an Antagonist Therapeutic is
used to treat or prevent human or other malignancies
2p associated with increased Notch expression (e. g., cervical
cancer, colon cancer, breast cancer, squamous adenocarcimas
(see infra)). Derivative s or analogs of Delta can be tested
for the desired activity by procedures known in the art,
including but not limited to the assays described in the
2~~ examples infra. In one specific embodiment, peptide
libraries can be screened to select a peptide with the
desired activity; such screening can be carried out by
assaying, e.g., for binding to Kuz.
Other Therapeutics include molecules that bind to a
Kuz. Thus, the invention also provides a method for
30 identifying such molecules. Such molecules can be identified
by a method comprising contacting a plurality of molecules
(e. g., in a peptide library, or combinatorial chemical
_ 95 _
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
library) with the Kuz protein under conditions conducive to
binding, and recovering any molecules that bind to the Kuz
protein.
The Agonist and Antagonist Therapeutics of the
invention have therapeutic utility for disorders of cell
fate. The Agonist Therapeutics are administered
therapeutically (including prophylactically): (1) in diseases
or disorders involving an absence or decreased (relative to
normal, or desired) levels of Notch or Delta or Kuz function,
for example, in patients where Delta protein is lacking,
genetically defective, biologically inactive or underactive,
or underexpressed; and (2) in diseases or disorders wherein
in vitro (or in vivo) assays (see infra) indicate the utility
of Delta agonist administration. The absence or decreased
levels in Notch or Delta or Kuz function can be readily
detected, e.g., by obtaining a patient tissue sample (e. g.,
from biopsy tissue) and assaying it in vitro for protein
levels, structure and/or activity of the expressed Notch or
Delta or Kuz protein. Many methods standard in the art can
be thus employed, including but not limited to immunoassays
to detect and/or visualize Notch or Delta or Kuz protein
(e. g., Western blot, immunoprecipitation followed by sodium
dodecyl sulfate polyacrylamide gel electrophoresis,
immunocytochemistry, etc.) and/or hybridization assays to
detect Notch or Delta or Kuz expression by detecting and/or
visualizing respectively Notch or Delta or Kuz mRNA (e. g.,
Northern assays, dot blots, in situ hybridization, etc.)
In vitro assays which can be used to determine
whether administration of a specific Agonist Therapeutic or
Antagonist Therapeutic is indicated, include in vitro cell
culture assays in which a patient tissue sample is grown in
culture, and exposed to or otherwise administered a
Therapeutic, and the effect of such Therapeutic upon the
tissue sample is observed. In one embodiment, where the
- 96 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
patient has a malignancy, a sample of cells from such
malignancy is plated out or grown in culture, and the cells
are then exposed to a Therapeutic. A Therapeutic which
inhibits survival or growth of the malignant cells (e.g., by
promoting terminal differentiation) is selected for
therapeutic use in vivo. Many assays standard in the art can
be used to assess such survival and/or growth; for example,
cell proliferation can be assayed by measuring 3H-thymidine
incorporation, by direct cell count, by detecting changes in
transcriptional activity of known genes such as proto-
oncogenes (e. g., fos, myc) or cell cycle markers; cell
viability can be assessed by trypan blue staining,
differentiation can be assessed visually based on changes in
morphology, etc. In a specific aspect, the malignant cell
cultures are separately exposed to (1) an Agonist
Therapeutic, and (2) an .Antagonist Therapeutic; the result of
the assay can indicate which type of Therapeutic has
therapeutic efficacy.
In another embodiment, a Therapeutic is indicated
for use which exhibits the desired effect, inhibition or
Promotion of cell growth, upon a patient cell sample from
tissue having or suspected of having a hyper- or
hypaproliferative disorder, respectively. Such hyper- or
hypoproliferative disorders include but are not limited to
those described in Sections 5.8.1 through 5.8.3 infra.
In another specific embodiment, a Therapeutic is
indicated for use in treating nerve injury or a nervous
system degenerative disorder (see Section 5.8.2) which
exhibits in vitro promotion of nerve regeneration/neurite
extension from nerve cells of the affected patient type.
In addition, administration of an Antagonist
Therapeutic of the invention is also indicated in diseases or
disorders determined or known to involve a Notch or Delta or
Kuz dominant activated phenotype ("gain of function"
_ 97 _
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
mutations.) Administration of an Agonist Therapeutic is
indicated in diseases or disorders determined or known to
involve a Notch or Delta or Kuz dominant negative phenotype
("loss of function" mut:ations). The functions of various
structural domains of the Notch protein have been
investigated in vivo, by ectopically expressing a series of
Drosophila Notch deletion mutants under the hsp70 heat-shock
promoter, as well as eye-specific promoters (see Rebay et
al., 1993, Cell 74:319-329). Two classes of dominant
phenotypes were observed, one suggestive of Notch loss-of
function mutations and the other of Notch gain-of-function
mutations. Dominant "activated" phenotypes resulted from
overexpression of a protein lacking most extracellular
sequences, while dominant "negative" phenotypes resulted .from
overexpression of a protein lacking most intracellular
:15 sequences. The results indicated that Notch functions as a
receptor whose extracellular domain mediates ligand-binding,
resulting in the transmission of developmental signals by the
cytoplasmic domain,
In various specific embodiments, in vitro assays
can be carried out with representative cells of cell types
involved in a patient's disorder, to determine if a
Therapeutic has a desired effect upon such cell types.
In another embodiment, cells of a patient tissue
sample suspected of being pre-neoplastic are similarly plated
';5 out or grown in vi tro, and exposed to a Therapeutic . The
Therapeutic which results in a cell phenotype that is more
normal (i.e., less representative of a pre-neoplastic state,
neoplastic state, malignant state, or transformed phenotype)
is selected for therapeutic use. Many assays standard in the
art can be used to assess whether a pre-neoplastic state,
neoplastic state, or a t:ransformed or malignant phenotype, is
present. For example, characteristics associated with a
transformed phenotype (a set of in vitro characteristics
98 _
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
associated with a tumoric~enic ability in vivo) include a more
rounded cell morphology, looser substratum attachment, loss
of contact inhibition, loss of anchorage dependence, release
of proteases such as pla:aminogen activator, increased sugar
,5 transport, decreased serum requirement, expression of fetal
antigens, disappearance of the 250,000 dalton surface
protein, etc. (see Luria et al., 1978, General Virology, 3d
Ed., John Wiley & Sons, New York pp. 436-446).
In other specific embodiments, the in vitro assays
l~~ described supra can be carried out using a cell line, rather
than a cell sample derived from the specific patient to be
treated, in which the ce7_1 line is derived from or displays
characteristics) associated with the malignant, neoplastic
or pre-neoplastic disorder desired to be treated or
prevented, or is derived from the neural or other cell type
1'S upon which an effect is desired, according to the present
invention.
The Antagonist Therapeutics are administered
therapeutically (including prophylactically): (1) in diseases
or disorders involving increased (relative to normal, or
Zy desired) levels of Notch or Delta or Kuz function, for
example, where the Notch or Delta or Kuz protein is
overexpressed or overact»ve; and (2) in diseases or disorders
wherein in vitro (or in vivo) assays indicate the utility of
Delta antagonist administration. The increased levels of
Notch or Delta or Kuz function can be readily detected by
2 5
methods such as those described above, by quantifying protein
and/or RNA. In vitro assays with cells of patient tissue
sample or the appropriate: cell line or cell type, to
determine therapeutic utulity, can be carried out as
described above.
3D
- 99 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/IJS99/15817
5.8.1 MALIGNANCIES
Malignant and pre-neoplastic conditions which can
be tested as described supra for efficacy of intervention
with Antagonist or Agoni;st Therapeutics, and which can be
~~ treated upon thus observing an indication of therapeutic
utility, include but are not limited to those described below
in Sections 5.8.1 and 5.9.1.
Malignancies and related disorders, cells of which
type can be tested in vitro (and/or in vivo), and upon
1~~ observing the appropriate assay result, treated according to
the present invention, include but are not limited to those
listed in Table 1 (for a review of such disorders, see
Fishman et al., 1985, Me~alicine, 2d Ed., J.B. Lippincott Co.,
Philadelphia):
1!i
TABLE 1
MALIGNANCIF3S AND RELATED DISORDERS
Leukemia
acute leukemia
acute lymophocytic leukemia
acute myelocytic leukemia
myeloblastic
promyelocyt is
myelomonocytic
monocytic
erythroleukemia
chronic leukemia
chronic myelocytic (granulocytic) leukemia
chronic lvymphocytic leukemia
Polycythemia vera
Lymphoma
Hodgkin's disease
non-Hodgkin's disease
Multiple myeloma
Waldenstrom's macroglobulinemia
30 Heavy chain disease
Solid tumors
sarcomas and carcinomas
fibrosarcoma
myxosarcoma
- 100 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/1581?
liposarcoma
chondrosarcoma
osteogeni~~ sarcoma
chordoma
angiosarcoma
endotheliosarcoma
lymphangiosarcoma
lymphangioendotheliosarcoma
synovioma
mesothel ioma
Ewing' s tumor
1 a i omyosa:rcoma
rhabdomyo;sarcoma
colon carcinoma
pancreatic cancer
breast cancer
ovarian cancer
prostate cancer
squamous cell carcinoma
basal cell carcinoma
adenocarc:inoma
sweat gland carcinoma
sebaceous gland carcinoma
papillary carcinoma
papillary adenocarcinomas
cystadenocarcinoma
medullary carcinoma
bronchogenic carcinoma
renal cell carcinoma
hepatoma
bile duct carcinoma
choriocarcinoma
seminoma
embryonal carcinoma
Wilms' tumor
cervical cancer
testicular tumor
lung carcinoma
small cell lung carcinoma
bladder carcinoma
epithelial carcinoma
glioma
astrocytoma
medulloblastoma
craniopha:ryngioma
ependymoma
pinealoma
hemangiob:lastoma
acoustic neuroma
of igodend:rogl ioma
menangioma
- 101 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
melanoma
neurobl a s t oma
retinoblastoma
In specific embodiments, malignancy or
dysproliferative changes (such as metaplasias and dysplasias)
are treated or prevented in epithelial tissues such as those
in the cervix, esophagus, and lung.
Malignancies o:E the colon and cervix exhibit
1~~ increased expression of human Notch relative to such non-
malignant tissue (see PCT Publication no. WO 94/07474
published April 14, 1994, incorporated by reference herein in
its entirety). Thus, in specific embodiments, malignancies
or premalignant changes of the colon or cervix are treated or
prevented by administering an effective amount of an
1!i
Antagonist Therapeutic, ~e.g., a Delta cleavage peptide, that
antagonizes Notch function. The presence of increased Notch
expression in colon, and cervical cancer suggests that many
more cancerous and hyperproliferative conditions exhibit
upregulated Notch. Thus, in specific embodiments, various
2~~ cancers, e.g., breast cancer, squamous adenocarcinoma,
seminoma, melanoma, and :lung cancer, and premalignant changes
therein, as well as other hyperproliferative disorders, can
be treated or prevented lby administration of an Antagonist
Therapeutic that antagonizes Notch function.
2 !5
5.8.2 NERVOUS SYSTEM DISORDERS
Nervous system disorders, involving cell types
which can be tested as described supra for efficacy of
intervention with Antagonist or Agonist Therapeutics, and
3~0 which can be treated upon thus observing an indication of
therapeutic utility, include but are not limited to nervous
system injuries, and diseases or disorders which result in
either a disconnection of axons, a diminution or degeneration
- 102 -
CA 02333893 2001-O1-11
WO 00!02897 PCT/US99l15817
of neurons, or demyeiination. Nervous system lesions which
may be treated in a patient (including human and non-human
mammalian patients) according to the invention include but:
are not limited to the following lesions of either the
central (including spinal cord, brain) or peripheral nervaus
systems:
(i) traumatic lesions, including lesions caused by
physical injury or associated with surgery,
for example, lesions which sever a portion of
the nervous system, or compression injuries;
~.0
(ii) ischemic lesions, in which a lack of oxygen in
a portion of the nervous system results in
neuronal injury or death, including cerebral
infarction or ischemia, or spinal cord
infarction or ischemia;
1.5 (iii) malignant lesions, in which a portion of the
nervous system is destroyed or injured by
malignant tissue which is either a nervous
system associated malignancy or a malignancy
derived from non-nervous system tissue;
2.0 (iv) infectious lesions, in which a portion of the
nervous system is destroyed or injured as a
result of infection, for example, by an
abscess o~r associated with infection by human
immunodeficiency virus, herpes zoster, or
herpes simplex virus or with Lyme disease,
~; 5
tuberculosis, syphilis;
(v) degenerative lesions, in which a portion of:
the nervous system is destroyed or injured as
a result of a degenerative process including
but not limited to degeneration associated
''0 with Park:inson's disease, Alzheimer's disease,
Huntington's chorea, or amyotrophic lateral
sclerosis.;
- 103 -
CA 02333893 2001-O1-11
wo ooiozs9~ rcT~s~nssm
(vi) lesions associated with nutritional diseases
or disorders, in which a portion of the
nervous system is destroyed or injured by a
nutritional disorder or disorder of metabolism
including but not limited to, vitamin B12
deficiency, folic acid deficiency, Wernicke
disease, tobacco-alcohol amblyopia,
Marchiafava-Bignami disease (primary
degeneration of the corpus callosum), and
alcoholic cerebellar degeneration;
(vii) neurological lesions associated with systemic
diseases :including but not limited to diabetes
(diabetic neuropathy, Bell's palsy), systemic
lupus erythematosus, carcinoma, or
sarcoidos:is;
1!5 (viii) lesions caused by toxic substances including
alcohol, lead, or particular neurotoxins; and
{ix) demyelina'ted lesions in which a portion of 'the
nervous system is destroyed or injured by a
demyelinating disease including but not
limited to multiple sclerosis, human
immunodeficiency virus-associated myelopathy,
transverse myelopathy or various etiologies,
progressive multifocal leukoencephalopathy,
and central pontine myelinolysis.
Therapeutics which are useful according to the
25 invention for treatment ~of a nervous system disorder may be
selected by testing for :biological activity in promoting the
survival or differentiation of neurons {see also Section
5.8). For example, and not by way of limitation,
Therapeutics which elicit any of the following effects may be
30 useful according to the invention:
(i) increased survival time of neurons in culture;
- 104 -
CA 02333893 2001-O1-11
WO 00/02897
PCT/US99/15817
(ii) increased sprouting of neurons in culture or
in vi vo;
(iii) increased production of a neuron-associated
molecule in culture or in vi vo, e.g., choline
acetylt:ransferase or acetylcholinesterase with
respect to motor neurons; or
(iv) decreasESd symptoms of neuron dysfunction .zn
vi vo .
Such effects may be measured by any method known in the art.
In Preferred, non-limiting embodiments, increased survival of
neurons may be measured by the method set forth in Arakawa et
al. (1990, J. Neurosci. 10:3507-3515); increased sprouting of
neurons may be detected by methods set forth in Pestronk et
al. (1980, Exp. Neurol. 70:65-82) or Brown et al. (1981, Ann.
Rev. Neurosci. 4:17-42); increased production of neuron--
associated molecules may be measured by bioassay, enzymatic
assay, antibody binding, Northern blot assay, etc., depending
on the molecule to be measured; and motor neuron dysfunction
may be measured by assessing the physical manifestation o.f
motor neuron disorder, e.g., weakness, motor neuron
conduction velocity, or functional disability.
In a specific embodiments, motor neuron disorders
that may be treated according to the invention include but
are not limited to disorders such as infarction, infection,
exposure to toxin, trauma, surgical damage, degenerative
a5 disease or malignancy that may affect motor neurons as well
as other components of t:he nervous system, as well as
disorders that selectively affect neurons such as amyotrophic
lateral sclerosis, and including but not limited to
progressive spinal muscular atrophy, progressive bulbar
palsy, primary lateral ~~clerosis, infantile and juvenile
~~0 muscular atrophy, progressive bulbar paralysis of childhood
(Fazio-Londe syndrome), poliomyelitis and the post polio
- 105 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
syndrome, and Hereditary Motorsensory Neuropathy (Charcot-
Marie-Tooth Disease).
5.8.3 TISSUE REPAIR AND REGENERATION
In another embodiment of the invention, a
Therapeutic of the invention is used for promotion of tissue
regeneration and repair, including but not limited to
treatment of benign dysproliferative disorders. Specific
embodiments are directedl to treatment of cirrhosis of the
liver (a condition in which scarring has overtaken normal
7.0
liver regeneration proceases), treatment of keloid
(hypertrophic scar) formation (disfiguring of the skin in
which the scarring proceas interferes with normal renewal),
psoriasis (a common skin condition characterized by excessive
proliferation of the skin and delay in proper cell fate
~~5 determination), and baldness (a condition in which terminally
differentiated hair follicles (a tissue rich in Notch) fail
to function properly). In another embodiment, a Therapeutic
of the invention is usedl to treat degenerative or traumatic
disorders of the sensory epithelium of the inner ear.
:: 0
5.9 PROPHYLACTIC USES
5.9.1 MALIGNANCIES
The Therapeutics of the invention can be
administered to prevent progression to a neoplastic or
s;5 malignant state, including but not limited to those disorders
listed in Table 1. Such administration is indicated where
the Therapeutic is shown in assays, as described supra, to
have utility for treatment or prevention of such disorder..
Such prophylactic use i~c indicated in conditions known or
suspected of preceding progression to neoplasia or cancer,. in
-~0 particular, where non-neoplastic cell growth consisting of
hyperplasia, metaplasia, or most particularly, dysplasia has
occurred (for review of such abnormal growth conditions, see
- 106 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
Bobbins and Angell, 1976,. Basic Pathology, 2d Ed., W.H.
Saunders Co., Philadelphia, pp. 68-79.) Hyperplasia is a
form of controlled cell proliferation involving an increase
in cell number in a tissue or organ, without significant
,~ alteration in structure or function. As but one example,
endometrial hyperplasia often precedes endometrial cancer.
Metaplasia is a form of controlled cell growth in which one
type of adult or fully differentiated cell substitutes for
another type of adult cell. Metaplasia can occur in
epithelial or connective tissue cells. Atypical metaplasia
1 iD
involves a somewhat disorderly metaplastic epithelium.
Dysplasia is frequently a forerunner of cancer, and is found
mainly in the epithelia; it is the most disorderly form of
non-neoplastic cell growth, involving a loss in individual
cell uniformity and in the architectural orientation of
15 cells. Dyspiastic cells often have abnormally large, deeply
stained nuclei, and exhibit pleomorphism. Dysplasia
characteristically occurs where there exists chronic
irritation or inflammation, and is often found in the cervix,
respiratory passages, oral cavity, and gall bladder.
20 Alternatively or in addition to the presence of
abnormal cell growth characterized as hyperplasia,
metaplasia, or dysplasia., the presence of one or more
characteristics of a transformed phenotype, or of a malignant
phenotype, displayed in vivo or displayed in vitro by a cell
sample from a patient, c:an indicate the desirability of
prophylactic/therapeutic: administration of a Therapeutic of
the invention. As mentioned supra, such characteristics of a
transformed phenotype include morphology changes, looser
substratum attachment, 7_oss of contact inhibition, loss of
anchorage dependence, protease release, increased sugar
~~0 transport, decreased serum requirement, expression of fetal
antigens, disappearance of the 250,000 dalton cell surface
- 107 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
protein, etc. (see also id., at pp. 84-90 for characteristics
associated with a trans:Eormed or malignant phenotype).
In a specific embodiment, leukoplakia, a benign-
appearing hyperplastic or dysplastic lesion of the
epithelium, or Bowen's disease, a carcinoma in situ, are pre-
neoplastic lesions indicative of the desirability of
prophylactic intervention.
In another embodiment, fibrocystic disease (cystic
hyperplasia, mammary dysplasia, particularly adenosis (benign
epithelial hyperplasia)) is indicative of the desirability of
7! 0
prophylactic intervention.
In other embodiments, a patient which exhibits one
or more of the following predisposing factors for malignancy
is treated by administration of an effective amount of a
Therapeutic: a chromosomal translocation associated with a
~~5 malignancy (e. g., the Philadelphia chromosome for chronic
myelogenous leukemia, t(14;18) for follicular lymphoma,
etc.), familial polypos~.s or Gardner's syndrome (possible
forerunners of colon cancer), benign monoclonal gammopathy (a
possible forerunner of multiple myeloma), and a first degxee
~;0 kinship with persons hatring a cancer or precancerous disease
showing a Mendelian (genetic) inheritance pattern (e. g.,
familial polyposis of the colon, Gardner's syndrome,
hereditary exostosis, polyendocrine adenomatosis, medullary
thyroid carcinoma with amyloid production and
~,5 pheochromocytoma, Peutz--Jeghers syndrome, neurofibromatosis
of Von Recklinghausen, retinoblastoma, carotid body tumor,
cutaneous melanocarcinoma, intraocular melanocarcinoma,
xeroderma pigmentosum, ataxia telangiectasia, Chediak-Higashi
syndrome, albinism, Fanc;oni's aplastic anemia, and Bloom's
syndrome; see Bobbins and Angell, 1976, Basic Pathology, 2d
30 Ed., W.B. Saunders Co., Philadelphia, pp. 112-113) etc.)
In another specific embodiment, an Antagonist
Therapeutic of the invention is administered to a human
- 108 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
patient to prevent progression to breast, colon, or cervical
cancer.
5.9.2 OTHER DISORDERS
In other embodiments, a Therapeutic of the
invention can be administered to prevent a nervous system
disorder described in e~ection 5.8.2, or other disorder (e. g.,
liver cirrhosis, psoriasis, keloids, baldness) described in
Section 5.8.3.
5.10 DEMONSTRATION OF THERAPEUTIC OR PROPHYLACTIC
UTILITY
The Therapeutics of the invention can be tested in
vivo for the desired therapeutic or prophylactic activity.
For example, such compounds can be tested in suitable animal
model systems prior to testing in humans, including but not
limited to rats, mice, chicken, cows, monkeys, rabbits, etc.
For in vivo testing, prior to administration to humans, any
animal model system known in the art may be used.
5.11 USE OF ANTISENSE OLIGONUCLEOTIDES FOR
SUPPRESSION OF DELTA ACTIVATION OR DELTA:KUZ
OR D1=~:NOTCH COMPLEX ACTIVITY OR FORMATION
In a specific embodiment of the present invention,
Delta cleavage peptide, Delta, Kuz, Notch, and Delta:Kuz or
D1E~:Notch complex activity and/or formation, is inhibited by
use of antisense nucleic acids for Delta, Notch and/or Kuz.
The present invention provides the therapeutic or
prophylactic use of nuc:Leic acids of at least six nucleotides
that are antisense to a gene or cDNA encoding Delta, Notch
and/or Kuz, or a portion thereof. An "antisense" nucleic
acid as used herein refers to a nucleic acid capable of
hybridizing to a portion of a Delta, Notch or Kuz RNA
(preferably mRNA) by virtue of some sequence complementarily.
The antisense nucleic acid may be complementary to a coding
- 109 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
and/or noncoding region of a Delta, Notch or Kuz mRNA. Such
antisense nucleic acids that inhibit Delta cleavage peptide
activity or Delta:Kuz complex formation or activity or
D1E~:Notch complex formation or activity have utility as
~~ Therapeutics, and can be used in the treatment or prevention
of disorders as described, supra.
The antisense nucleic acids of the invention can be
oligonucleotides that aces double-stranded or single-stranded,
RNA or DNA, or a modification or derivative thereof, which
can be directly administered to a cell, or which can be
l l)
produced intracellularly by transcription of exogenous,
introduced sequences.
In another embodiment, the present invention is
directed to a method for inhibiting the expression of Delta
cleavage peptide nucleic acid sequences, in a prokaryotic or
1'~ eukaryotic cell, comprising providing the cell with an
effective amount of a composition comprising an antisense
nucleic acid of Delta cleavage peptide, or a derivative
thereof, of the invention.
The antisense nucleic acids are of at least six
20 nucleotides and are preferably oligonucleotides, ranging from
6 to about 200 nucleotides. In specific aspects, the
oligonucleotide is at least 10 nucleotides, at least 15
nucleotides, at least 100 nucleotides, or at least 200
nucleotides. The oliganucleotides can be DNA or RNA or
chimeric mixtures, or derivatives or modified versions
thereof, and either single-stranded or double-stranded. The
oligonucleotide can be modified at the base moiety, sugar
moiety, or phosphate backbone. The oligonucleotide may
include other appending groups such as peptides, agents
facilitating transport across the cell membrane (see, e.g.,
Letsinger et al., 1989, Proc. Natl. Acad. Sci. U.S.A.
86:6553-6556; Lemaitre et al., 1987, Proc. Natl. Acad. Sci.
84:648-652; International Patent Publication No. WO 88/09810)
- 110 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99I15817
or blood-brain barrier (see, e.g., International Patent
Publication No. WO 89/101:34), hybridization-triggered
cleavage agents (see, e.g., Krol et al., 1988, BioTechniques
6:958-976), or intercalating agents (see, e.g., Zon, 1988,
Pharm. Res. 5:539-549).
In a preferred .aspect of the invention, a Delta
cleavage peptide antisense oligonucleotide is provided,
preferably as single-stranded DNA. The oligonucleotide may
be modified at any position in its structure with
constituents generally known in the art.
The antisense oligonucleotides may comprise at
least one modified base moiety which is selected from the
group including but not limited to 5-fluorouracil,
5-bromouracil, 5-chlorouracil, 5-iodouracil, hypoxanthine,
xanthine, 4-acetylcytosine, 5-(carboxyhydroxylmethyl)uracil,
5-carboxymethylaminomethyl-2-thio-uridine,
5-carboxymethylaminomethyluracil, dihydrouracil,
beta-D-galactosylqueosine, inosine, N6-isopentenyladenine,
1-methylguanine, 1-methylinosine, 2,2-dimethylguanine,
2-methyladenine, 2-methylguanine, 3-methylcytosine,
2p. 5-methylcytosine, N6-adenine, 7-methylguanine,
5-methylaminomethyluracil., 5-methoxyaminomethyl-2-thiouracil,
beta-D-mannosylqueosine, 5N-methoxycarboxymethyluracil,
5-methoxyuracil, 2-methyl.-thio-N6-isopentenyladenine,
uracil-5-oxyacetic acid I;v), wybutoxosine, pseudouracil,
queosine, 2-thiocytosine, 5-methyl-2-thiouracil,
2 ~i
2-thiouracil, 4-thiouracil, 5-methyluracil,
uracil-5-oxyacetic acid naethylester, uracil-5-oxyacetic acid
(v), 5-methyl-2-thiouracil, 3-(3-amino-3-N-2-carboxypropyl)
uracil, (acp3)w, and 2,6--diaminopurine.
In another embodiment, the oligonucleotide
3« comprises at least one modified sugar moiety selected from
the group including, but not limited to, arabinose,
2-fluoroarabinose, xylulose, and hexose.
- 111 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
In yet another embodiment, the oligonucleotide
comprises at least one modified phosphate backbone selected
from the group consisting of a phosphorothioate, a
phosphorodithioate, a plzosphoramidothioate, a
phosphoramidate, a phoshhordiamidate, a methylphosphonate, an
alkyl phosphotriester, and a formacetal, or an analog of the
foregoing.
In yet another embodiment, the oligonucleotide is a
2-a-anomeric oligonucleotide. An a-anomeric oligonucleotide
forms specific double-stranded hybrids with complementary RNA
:L 0
in which, contrary to the usual f3-units, the strands run
parallel to each other (Gautier et al., 1987, Nucl. Acids
Res. 15:6625-6641).
The oligonucle:otide may be conjugated to another
molecule, e.g., a peptide, hybridization-triggered cross-
-~5 linking agent, transport. agent, hybridization-triggered
cleavage agent, etc.
Oligonucleotides of the invention may be
synthesized by standard methods known in the art, e.g., by
use of an automated DNA synthesizer (such as are commercially
~;0 avail-able from Biosearch, Applied Biosystems, etc.). As
examples, phosphorothioate oligo-nucleotides may be
synthesized by the method of Stein et al. (1988, Nucl. Acids
Res. 16:3209), methylphosphonate oligonucleotides can be
prepared by use of controlled pore glass polymer supports
{Sarin et al., 1988, Proc. Natl. Acad. Sci. U.S.A. 85:7448-
7451), etc.
In a specific embodiment, the antisense
oligonucleotides comprise catalytic RNAs, or ribozymes {see,
e.g., International Patent Publication No. WO 90/11364;
Sarver et al., 1990, Science 247:1222-1225). In another
e~odiment, the oligonucleotide is a 2'-0-
methylribonucleotide (moue et al., 1987, Nucl. Acids Res.
- 112 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/1.5817
15:6131-6148), or a chime:ric RNA-DNA analog (Inoue et al.,
1987, FEBS Lett. 215:327--330).
In an alternative embodiment, the antisense nucleic
acids of the invention are produced intracellularly by
~~ transcription.from an exogenous sequence. For example, a
' vector can be introduced in vivo such that it is taken up by
a cell, within which cell the vector or a portion thereof :is
transcribed, producing an antisense nucleic acid (RNA) of the
invention. Such a vector can remain episomal or become
chromosomally integrated, as long as it can be transcribed to
produce the desired anti;sense RNA. Such vectors can be
constructed by recombinant DNA technology methods standard in
the art. Vectors can be plasmid, viral, or others known in
the art to be capable of replication and expression in
mammalian cells. Expression of the sequences encoding the
antisense RNAs can be by any promoter known in the art to act
in mammalian, preferably human, cells. Such promoters can be
inducible or constitutive. Such promoters include, but are
not limited to, the SV40 early promoter region (Bernoist and
Chambon, 1981, Nature 290:304-310), the promoter contained in
the 3' long terminal repeat of Rous sarcoma virus (Yamamoto
et al., 1980, Cell 22:787-797), the herpes thymidine kinase
promoter (Wagner et al., 1981, Proc. Natl. Acad. Sci. U.S.A.
78:1441-1445), the regulatory sequences of the
metallothionein gene (Brinster et al., 1982, Nature 296:39-
42), etc.
The antisense nucleic acids of the invention
comprise a sequence complementary to at least a portion of an
RNA transcript of a Delta, Notch or Kuz gene, preferably a
human Delta, Notch or Kuz gene. However, absolute
complementarily, although preferred, is not required. A
?.0 sequence "complementary to at least a portion of an RNA," as
referred to herein, means a sequence having sufficient
complementarily to be able to hybridize with the RNA, forming
- 113 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
a stable duplex; in the case of double-stranded antisense
nucleic acids, a single strand of the duplex DNA may thus be
tested, or triplex form<~tion may be assayed. The ability to
hybridize will depend on both the degree of complementarily
and the length of the antisense nucleic acid. Generally, the
longer the hybridizing nucleic acid, the more base mismatohes
with a RNA it may contain and still form a stable duplex (or
triplex, as the case may be). One skilled in the art can
ascertain a tolerable degree of mismatch by use of standard
DLO procedures to determine the melting point of the hybridized
complex.
The antisense nucleic acid can be used to treat (or
prevent) disorders of a cell type that expresses, or
preferably overexpresse.>, the Delta cleavage peptide or the
Delta:Kuz complex or the DlE~:Notch complex. In a preferred
~~5 embodiment, a single-stranded Delta, Notch or Kuz DNA
antisense oligonucleotidle, both single-stranded Delta, Notch
and Kuz antisense oligonucleotides, or a single-stranded
Delta:Kuz DNA antisense fusion sequence, is used.
Cell types that express or overexpress Delta, Natch
20 and/or Kuz RNA can be if.entified by various methods known in
the art. Such methods include, but are not limited to,
hybridization with Delta-, Notch- and Kuz-specific nucleic
acids (e. g., by Northern. blot hybridization, dot blot
hybridization, or in situ hybridization), or by observing the
25 ability of RNA from the cell type to be translated in vitz-o
into Delta or Kuz by immunohistochemistry, Western blot
analysis, ELISA, etc. In a preferred aspect, primary tissue
from a patient can be assayed for Delta, Notch and/or Kuz
expression prior to treatment, e.g., by immunocytochemistry,
in situ hybridization, or any number of methods to detect
30 protein or mRNA expression.
Pharmaceutical compositions of the invention (see
Section 5.7, infra), comprising an effective amount of an
- 114 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
antisense nucleic acid in a pharmaceutically acceptable
carrier can be administered to a patient having a disease or
disorder that is of a type that expresses or overexpresses,
for example a Delta:Kuz complex.
The amount of an antisense nucleic acid that will
be effective in the treatment of a particular disorder or
condition will depend on the nature of the disorder or
condition, and can be determined by standard clinical
techniques. Where possible, it is desirable to determine the
antisense cytotoxicity in vitro, and then in useful animal
model systems, prior to '.testing and use in humans.
In a specific embodiment, pharmaceutical
compositions comprising Delta and Kuz antisense nucleic acids
are administered via liposomes, microparticles, or
microcapsules. In various embodiments of the invention, it
may be useful to use such compositions to achieve sustained
release of the Delta and/or Kuz antisense nucleic acids. In
a specific embodiment, it may be desirable to utilize
liposomes targeted via antibodies to specific identifiable.
central nervous system cell types (Leonetti et al., 1990,
Proc. Natl. Acad. Sci. U.S.A. 87:2448-2451; Renneisen et al.,
1990, J. Biol. Chem. 265:16337-16342).
5.12 THERAPEUTIC/PROPHYLACTIC ADMINISTRATION AND
COMPOSITIONS
The invention provides methods of treatment (and
prophylaxis) by administration to a subject of an effective
amount of a Therapeutic of the invention. In a preferred
aspect, the Therapeutic is substantially purified. The
subject is preferably an. animal, including but not limited to
animals such as cows, pigs, chickens, etc., and is preferably
3,0 a mammal, and most preferably human.
Various delivery systems are known and can be used
to administer a Therapeutic of the invention, e.g.,
- 115 -
CA 02333893 2001-O1-11
WO 00!02897 PCT/US99/15817
encapsulation in liposomes, microparticles, microcapsules,
expression by recombinant cells, receptor-mediated
endocytosis (see, e.g., Wu and Wu, 1987, J. Biol. Chem.
262:4429-4432), construction of a Therapeutic nucleic acid as
part of a retroviral or other vector, etc. Methods of
introduction include but are not limited to intradermal,
intramuscular, intraperitoneal, intravenous, subcutaneous,
intranasal, epidural, a:nd oral routes. The compounds may be
administered by any convenient route, for example by infusion
or bolus injection, by .absorption through epithelial or
:1 O
mucocutaneous linings (~e.g., oral mucosa, rectal and
intestinal mucosa, etc.) and may be administered together
with other biologically active agents. Administration can be
systemic or local. In addition, it may be desirable to
introduce the pharmaceutical compositions of the invention
~~5 into the central nervoua system by any suitable route,
including intraventricular and intrathecal injection;
intraventricular injection may be facilitated by an
intraventricular cathetE:r, for example, attached to a
reservoir, such as an Ornmaya reservoir. Pulmonary
;~0 administration can also be employed, e.g., by use of an
inhaler or nebulizer, and formulation with an aerosolizing
agent.
In a specific embodiment, it may be desirable to
administer the pharmaceutical compositions of the invention
locally to the area in need of treatment; this may be
s". 5
achieved by, for example, and not by way of limitation, local
infusion during surgery, topical application, e.g., in
conjunction with a wound dressing after surgery, by
injection, by means of a, catheter, by means of a suppository,
or by means of an implant, said implant being of a porous,
30 non-porous, or gelatinous material, including membranes, such
as sialastic membranes, or fibers. In one embodiment,
administration can be by direct injection at the site (or
- lls -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
former site) of a malignant tumor or neoplastic or pre-
neoplastic tissue.
In another embodiment, the Therapeutic can be
delivered in a vesicle, in particular a liposome (see Langer,
Science 249:1527-1533 (1990); Treat et al., in Liposomes in
the Therapy of Infectious Disease and Cancer, Lopez-Berestein
and Fidler (eds.), Liss,, New York, pp. 353-365 (1989);
Lopez-Berestein, ibid., pp. 317-327; see generally ibid.)
In yet another embodiment, the Therapeutic can be
delivered in a controlled release system. In one embodiment,
a pump may be used (see Langer, supra; Sefton, CRC Crit. Ref.
Biomed. Eng. 14:201 (1987); Buchwald et al., Surgery 88:507
(1980); Saudek et al., N. Engl. J. Med. 321:574 (1989)). In
another embodiment, polymeric materials can be used (see
Medical Applications of Controlled Release, Langer and Wise
(eds.), CRC Pres., Boca Raton, Florida (1974); Controlled
Drug Bioavailability, Drug Product Design and Performance,
Smolen and Ball (eds.), Wiley, New York (1984); Ranger and
Peppas, J. Macromol. Sci. Rev. Macromol. Chem. 23:61 (1983);
see also Levy et al., Science 228:190 (1985); During et al.,
Win. Neurol. 25:351 (1989); Howard et al., J. Neurosurg.
71:105 (1989)). In yet another embodiment, a controlled
release system can be placed in proximity of the therapeutic
target, i.e., the brain, thus requiring only a fraction of
the systemic dose (see, e.g., Goodson, in Medical
Applications of Controlled Release, supra, vol. 2, pp.
115-138 (1984)).
Other control:Led release systems are discussed in
the review by Langer (Science 249:1527-1533 (1990)).
In a specific embodiment where the Therapeutic is a
nucleic acid encoding a protein Therapeutic, the nucleic acid
can be administered in ,vivo to promote expression of its
encoded protein, by con:atructing it as part of an appropriate
nucleic acid expression vector and administering it so that
- 117 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
it becomes intracellular, e.g., by use of a retroviral vector
(see U.S. Patent No. 4,980,286), or by direct injection, or
by use of microparticle bombardment (e. g., a gene gun;
Biolistic, Dupont), or coating with lipids or cell-surface
receptors or transfecting agents, or by administering it in
linkage to a homeobox-like peptide which is known to enter
the nucleus (see e.g., J~oliot et al., 1991, Proc. Natl. Acad.
Sci. USA 88:1864-1868), etc. Alternatively, a nucleic acid
Therapeutic can be introduced intracellularly and
incorporated within host: cell DNA for expression, by
1.0
homologous recombinatiors.
In specific embodiments directed to treatment or
prevention of particular disorders, preferably the following
forms of administration are used:
Preferred Forms of
1.5 Disorder Administration
Cervical cancer Topical
Gastrointestinal cancer Oral; intravenous
Lung cancer Inhaled; intravenous
Leukemia Intravenous; extracorporeal
Metastatic carcinomas Intravenous; oral
s'. 0
Brain cancer Targeted; intravenous;intrathecal
Liver cirrhosis Oral; intravenous
Psoriasis Topical
Keloids Topical
Baldness Topical
''S Spinal cord injury Targeted; intravenous; intrathecal
Parkinson's disease Targeted; intravenous; intrathecal
Motor neuron disease Targeted; intravenous; intrathecal
Alzheimer's disease Targeted; intravenous; intrathecal
:30 The present invention also provides pharmaceutical
compositions. Such compositions comprise a therapeutically
effective amount of a Therapeutic, and a pharmaceutically
- 118 -
CA 02333893 2001-O1-11
WO 00/02897
PCT/US99/15817
acceptable carrier. In a specific embodiment, the term
"pharmaceutically acceptable" means approved by a regulatory
agency of the Federal or a state government or listed in the
U.S. Pharmacopeia or other generally recognized pharmacopeia
for use in animals, and more particularly in humans. The
term "carrier" refers to a diluent, adjuvant, excipient, or
vehicle with which the therapeutic is administered. Such
pharmaceutical carriers can be sterile liquids, such as water
and oils, including those of petroleum, animal, vegetable or
synthetic origin, such as peanut oil, soybean oil, mineral
oil, sesame oil and the. like. Water is a preferred carrier
when the pharmaceutical composition is administered
intravenously. Saline solutions and aqueous dextrose and
glycerol solutions can also be employed as liquid carriers,
particularly for inject~able solutions. Suitable
pharmaceutical excipients include starch, glucose, lactose,
sucrose, gelatin, malt,. rice, flour, chalk, silica gel,
sodium stearate, glycerol monostearate, talc, sodium
chloride, dried skim milk, glycerol, propylene, glycol,
water, ethanol and the like. The composition, if desired,
can also contain minor amounts of wetting or emulsifying
agents, or pH buffering agents. These compositions can take
the form of solutions, suspensions, emulsion, tablets, pills,
capsules, powders, sustained-release formulations and the
like. The composition can be formulated as a suppository,
with traditional binders and carriers such as triglycerides.
Oral formulation can include standard carriers such as
pharmaceutical grades of mannitol, lactose, starch, magnesium
stearate, sodium saccharine, cellulose, magnesium carbonate,
etc. Examples of suitable pharmaceutical carriers are
described in "Remington.'s Pharmaceutical Sciences" by E.W.
Martin. Such compositions will contain a therapeutically
effective amount of the Therapeutic, preferably in purified
form, together with a suitable amount of carrier so as to
- 119 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
provide the form for proper administration to the patient.
The formulation should suit the mode of administration.
In a preferred embodiment, the composition is
formulated in accordance with routine procedures as a
pharmaceutical composition adapted for intravenous
administration to human beings. Typically, compositions for
intravenous administration are solutions in sterile isotonic
aqueous buffer. Where necessary, the composition may also
include a solubilizing agent and a local anesthetic such as
lignocaine to ease pain at the site of the injection.
Generally, the ingredients are supplied either separately or
mixed together in unit dosage form, for example, as a dry
lyophilized powder or water free concentrate in a
hermetically sealed container such as an ampoule or sachette
indicating the quantity of active agent. Where the
composition is to be administered by infusion, it can be
dispensed with an infusion bottle containing sterile
pharmaceutical grade water or saline. Where the composition
is administered by injection, an ampoule of sterile water for
injection or saline can be provided so that the ingredients
may be mixed prior to administration.
The Therapeutics of the invention can be formulated
as neutral or salt forms. Pharmaceutically acceptable salts
include those formed with free amino groups such as those
derived from hydrochloric, phosphoric, acetic, oxalic,
tartaric acids, etc., and those formed with free carboxyl
groups such as those derived from sodium, potassium,
ammonium, calcium, ferric hydroxides, isopropylamine,
triethylamine, 2-ethylamino ethanol, histidine, procaine,
etc.
The amount of the Therapeutic of the invention
which will be effective in the treatment of a particular
disorder or condition will depend on the nature of the
disorder or condition, and can be determined by standard
- 120 -
CA 02333893 2001-O1-11
WO 00/02897 PCTNS99/15817
clinical techniques. In addition, in vitro assays may
optionally be employed to help identify optimal dosage
ranges. The precise dose to be employed in the -formulation
will also depend on the route of administration, and the
.5 seriousness of the disease or disorder, and should be decided
according to the judgment of the practitioner and each
patient's circumstances. However, suitable dosage ranges for
intravenous administration are generally about 20-500
micrograms of active compound per kilogram body weight.
Suitable dosage ranges for intranasal administration are
1 i7
generally'about 0.01 pg/:kg body weight to 1 mg/kg body
weight. Effective doses may be extrapolated from dose-
response curves derived :from in vitro or animal model test
systems.
Suppositories generally contain active ingredient
1!' in the range of 0.5% to :l0% by weight; oral formulations
preferably contain 10% to 95% active ingredient.
The invention also provides a pharmaceutical pack
or kit comprising one or more containers filled with one or.
more of the ingredients of the pharmaceutical compositions of
2~~ the invention. Optional:Ly associated with such containers)
can be a notice in the form prescribed by a governmental
agency regulating the manufacture, use or sale of
pharmaceuticals or biological products, which notice reflects
approval by the agency o:f manufacture, use or sale for human
administration.
2 !i
5.13 DIAGNOSTI1~ UTILITY
Delta cleavage peptides, soluble Delta peptides,
analogs, derivatives, and subsequences thereof, Delta
cleavage peptide encodin<~ nucleic acids (and sequences
3~~ complementary thereto), soluble Delta peptide encoding
nucleic acids (and sequences complementary thereto), anti-
Delta cleavage peptide antibodies, anti-soluble Delta peptide
- 121 -
CA 02333893 2001-O1-11
WO 00/02897
PCT/US99/15817
antibodies, and anti-Delta:Kuz and anti-D1E~~Notch complex
antibodies have uses in. diagnostics. Such molecules can be
used in assays, such as immunoassays, to detect, prognose,
diagnose, or monitor various conditions, diseases, and
disorders affecting Delta cleavage peptide expression, or
monitor the treatment thereof. In a particular example, such
an immunoassay is carried out by a method comprising
contacting a sample derived from a patient with an anti-Delta
cleavage peptide antibody under conditions such that
immunospecific binding can occur, and detecting or measuring
the amount of any immunospecific binding by the antibody. In
a specific aspect, such binding of antibody, in tissue
sections, preferably in conjunction with binding of anti-Kuz
or anti-Notch antibody can be used to detect aberrant Delta,
Notch and/or Kuz localization or aberrant levels of D1E~:Notch
:15 or Delta-Kuz colocalizat:ion in a disease state. In a
specific embodiment, antibody to Delta cleavage peptide can
be used to assay in a patient tissue or serum sample for 'the
presence of Delta cleavage peptide where an aberrant level of
Delta cleavage peptide is an indication of a diseased
~~0 condition. Aberrant levels of Delta binding ability in an
endogenous Notch or Kuz protein, or aberrant levels of
binding ability to Kuz (or other Delta ligand, e.g., Notch)
in an endogenous Delta cleavage peptide may be indicative of
a disorder of cell fate Ce.g., cancer, etc.) By "aberrant
levels," is meant increased or decreased levels relative t:o
s. 5
that present, or a standard level representing that present,
in an analogous sample from a portion of the body or from a
subject not having the disorder.
The immunoassays which can be used include but are
not limited to competitive and non-competitive assay systems
30 using techniques such as western blots, radioimmunoassays,
ELISA (enzyme linked immunosorbent assay), "sandwich"
immunoassays, immunoprecipitation assays, precipitin
- 122 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99I15817
reactions, gel diffusion precipitin reactions,
immunodiffusion assays, agglutination assays, complement-
fixation assays, immunoradiometric assays, fluorescent
immunoassays, protein A immunoassays, to name but a few.
Delta, Notch and Kuz genes and related nucleic acid
sequences and subsequences, including complementary
sequences, and other toporythmic gene sequences, can also be
used in hybridization assays. Delta, Notch and Kuz nucleic
acid sequences, or subsequences thereof comprising about a.t
least 8 nucleotides, can be used as hybridization probes.
Hybridization assays can be used to detect, prognose,
diagnose, or monitor conditions, disorders, or disease states
associated with aberrant changes in Delta expression and/or
activity as described supra. In particular, such a
hybridization assay is carried out by a method comprising
1.5 contacting a sample containing nucleic acid with a nucleic
acid probe capable of hybridizing to Delta, Notch or Kuz DNA
or RNA, under conditions. such that hybridization can occur-,
and detecting or measuring any resulting hybridization.
Additionally, since Delta binds to Notch and Kuz,
;;0 Delta or a binding portion thereof can be used to assay for
the presence and/or amounts of Notch or Kuz in a sample,
e.g., in screening for malignancies which exhibit increased
Notch expression such a~~ colon and cervical cancers.
5.14 ANIMAL MODELS
a5
The present invention also provides animal models.
In one embodiment, animal models for diseases and disorders
involving Delta cleavages peptide, soluble Delta peptide, and
Delta:Kuz and D1E~:Notch complexes are provided. These
include, but are not limited to, disorders of cell fate and
:30 differentiation such as cancer. Such animals can be
initially produced by promoting homologous recombination or
insertional mutagenesis between Delta, Notch and Kuz genes in
- 123 -
CA 02333893 2001-O1-11
WO 00/02897 PCTNS99/15817
the chromosome, and exogenous Delta, Notch and Kuz genes that
have been rendered bio7.ogically inactive or deleted
(preferably by insertion of a heterologous sequence, e.g., an
antibiotic resistance gene). In a preferred aspect,
homologous recombination is carried out by transforming
embryo-derived stem (ES.) cells with a vector containing,
e.g., the insertionally inactivated Delta and Kuz gene, such
that homologous recombination occurs, followed by injecting
the transformed ES cells into a blastocyst, and implanting
the blastocyst into a foster mother, followed by the birth of
the chimeric animal ("knockout animal~~) in which a Delta
and/or Kuz gene has been inactivated or deleted (Capecchi,
1989, Science 244;1288-1292). The chimeric animal can be
bred to produce additional knockout animals. Such animals
can be mice, hamsters, cheep, pigs, cattle, etc., and are
:15 preferably non-human mammals. In a specific embodiment, a
knockout mouse is produced.
Such knockout animals are expected to develop, or
be predisposed to developing, diseases or disorders
involving, but not restricted to, disorders of cell fate and
:;p differentiation, etc., and thus, can have use as animal
models of such diseases and disorders, e.g., to screen fox- or
test molecules (e. g., potential Therapeutics) for disorders
of cell fate and differentiation, and ather diseases.
In a different: embodiment of the invention,
~;5 transgenic animals that have incorporated and express (or
overexpress or mis-express) a functional Delta and/or Kuz
gene, e.g. by introducing the Delta and Kuz genes under the
control of a heterologous promoter (i.e., a promoter that is
not the native Delta or Kuz promoter) that either
overexpresses the protein or proteins, or expresses them in
30 tissues not normally expressing the complexes or proteins,
can have use as animal models of diseases and disorders
characterized by elevated levels of Delta:Kuz complexes.
- 124 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99J15817
Such animals can be used to screen or test molecules for the
ability to treat or prevent the diseases and disorders cited
supra.
In one embodiment, the present invention provides a
recombinant non-human animal in which both an endogenous
Delta gene and an endogenous Kuz have been deleted or
inactivated by homologous recombination or insertional
mutagenesis of said animal or an ancestor thereof. In
another embodiment, the invention provides a recombinant non-
human animal containing both a Delta gene and a Kuz gene in
:l O
which the Delta gene is under the control of a promoter that
is not the native Kuz gene promoter and the Kuz gene is under
the control of a promotE;r that is not the native Kuz gene
promoter. In a specific; embodiment, the invention provides a
recombinant non-human animal containing a transgene
'~5 comprising a nucleic acid sequence encoding a chimeric
protein comprising a Delta cleavage peptide of at least 6
amino acids fused via a covalent bond to a fragment of Kuz
protein of at least 6 amino acids.
:ZO 6. THE NOTCH LIGAND DELTA IS CLEAVED BY THE
DISINTEGRIN METALLOPROTEASE KUZBANIAN
The Notch signaling pathway defines an evolutionary
conserved cell interaction mechanism which throughout
development controls the' fate of cells by modulating their
response to developmental signals (Artavanis-Tsakonas et al.,
a5 1995, Science 268:225-2.12; Fleming et al., 1998, Trends in
Cell Biology 7:437-441). The Notch receptor is cleaved in
the trans-Golgi network as it traffics towards the plasma
membrane eventually forming a ligand competent, heterodimeric
molecule (Blaumueller et: al., 1997, Cell 90:281-291). Both
:3~ known ligands, Delta and Serrate are thought to act as
transmembrane proteins interacting via their extracellular
domains with the receptor expressed on adjacent cells
- 225 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
(Fleming et al., 1998, Trends in Cell Biology 7:437-441;
Muskavitch, 1994, Developmental Biology 166:415-430). Given
the similar phenotypes between loss of Notch signaling and
loss of function mutat:tons in the Kuzbanian (Kuz) gene, a
gene encoding a putative member of the ADAM family of
metalloproteases (RookE~ et al., 1996, Nature 273:1227-1231),
it has been suggested that Kuz may be involved in the
cleavage of the Notch x-eceptor (Pan and Rubin, 1997, Cell
90:271-280. This hypothesis is not corroborated by recent
biochemical studies which indicate that the functionally
crucial cleavage of Notch in the traps Golgi network is
catalyzed by a furin-like convertase (Logeat, et al., 1998,
Proc. Nat. Acad. Sci. L;fSA 95:8108-8112). Consistent with
this, furin is known to act in this sub-cellular compartment,
as opposed to ADAM proteases, such as Kuz, which are thought
to act on the cell surface (Wolfsberg et al., 1995, Journal
of Cell Biology 131:275-278).
A genetic screen aimed in identifying modifiers of
the phenotypes associated with the constitutive expression of
a dominant negative transgene of Kuz (KuzDN) in developing
imaginal discs, has uncovered Delta as an interacting gene
(Wu et al., unpublished observation). Flies expressing this
dominant negative construct, even though they also carry a
wild type complement of Kuz become semi-lethal when
heterozygous for a loss of function Delta mutation (Xu et
al., unpublished observation). In contrast, Delta
duplications rescue the phenotypes associated with KuzDN
(Figures 6A-6F). KuzDN flies display extra vein material,
especially deltas, at tine ends of the longitudinal veins,
wing notching (observed with a low penetrance), extra
bristles on the notum, and have small, rough eyes (Figures 6A
~30 and 6E). When KuzDN flies carry three, as opposed to the
normal two, copies of wild type Notch, the bristle and eye
phenotype are not affected (Xu et al., unpublished
- 126 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/158t7
observation), nor are the vein deltas altered (Figure 6D).
On the other hand, the R:uzDN phenotypes are effectively
suppressed by Delta duplications (Figures 6B and 6F).
Indicating that a higher copy number of Delta molecules is
capable of overriding the effect of the KuzDN construct.
The interactic>n between Delta and Kuz was further
explored by examining the relationship between the protein
products of their respecaive genes. A monoclonal antibody
was raised against an e~sa racellular Delta epitope generated
by using a fusion protein generated by using a PCR product. of
~~~ the the entire extracell.ular domain of Drosophila Delta using
the primers 5' GAGTTGCGC'CTGAAGTACTT 3' (SEQ ID N0:14) and 5'
GGTCGCTCCATATTGGTGGG 3' (SEQ ID N0:15) and subsequent cloning
into the SmaI site of pC~EX3 and StuI site of pMAL. A
monoclonal cell line (C594.9B, designated "9B") was created
~~5 by standard protocols and screening of hybridoma supernatants
was done by immunostaining of Delta expressing S2 cells.
Ascites fluid was made and used at 1/3000-1/10,000 dilutian
for western blotting followed by detection with peroxidase
labelled anti-mouse antibody and chemiluminescent development
~,~ with a luminol substrate (see Rand et al., 1997, Protein
Science 6:2059-2071). Using this antibody, the Delta
antigen in S2 cells, which stably express full length Delta,
was examined (Figure 7A). S2 cells are known to express wild
type Kuz endogenously (F?an and Rubin, 1997, Cell 90:271-2f30).
The presence of an immunoreactive fragment in the culture
~~5 media that migrated faster than full length Delta was
observed exclusively in the media. It is noted that this
fragment, as with full .length Delta, was fully 40-fold more
immunoreactive with 9B under non-reducing conditions. Full
length Delta is associated with the cell pellet whereas, this
~3~ fragment is almost exclusively in the media suggesting it is
a soluble, proteolytic :Fragment derived from full length
Delta (herein referred to as "DlE~"). The size of this
- 127 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
fragment under reducing conditions is approximately 67,000
Daltons, consistent the extracellular domain of Delta, which
is estimated to be 65,000 Daltons (Figure 7D). DlEC was
subsequently affinity purified from the culture medium and
subjected to amino acid sequence analysis to determine the N-
terminal amino acid sequence. Briefly, Drosophila 52 cells
expressing Delta were induced with 0.7 mM CuS04 in serum free
media for two days and the media was collected and
precipitated with 70~ ammonium sulfate. The precipitate was
collected by centrifugation and subsequently resuspended and
dialyzed against 20 mM HEPES, 150 mM NaCl, 2 mM CaCl2, pH
7.4. This sample was passed over Sepharose beads coupled
with monoclonal antibody 9B, washed with 1.0 M NaCl and
eluted with 25 mM glycine, pH 2.8 and immediately neutralized
with 1.0 M Tris-HC1. N-terminal amino acid analysis was
performed with an Applied Biosystems gas phase amino acid
sequencer.
The amino acid sequence of DlEC is consistent with
the predicted polypeptide processing site and is conserved
among the Drosophi3a, Xe.nopus and human Delta homologs
(Figure 7E).
It is concluded that full length Delta in S2 cells
is cleaved at the surface to release a fragment containing
most or all of the extracellular domain of Delta (DlEC).
Western blot analysis of Drosophila embryos reveals the
existence of both full length Delta (D1FL) and a fragment with
the same mobility as DlEC implicating this same Delta derived
product is present in vivo (Figure 7B). It is noted that
between D1FL and DlEC additional potentially transient
proteolytic products are detectable with the 9B antibody
(Figure 7B, lane "10 embryos" and Figure 8D, lane "kuz +/-").
The possibility that the generation of DlEC can be
influenced by Kuz was examined by cotransfection experiments
in S2 cells which, as mentioned earlier, are known to express
- 128 -
CA 02333893 2001-O1-11
WO 00/02897
PCT/US99/15817
wild type Kuz endogenously (Pan and Rubin, 1997, Cell 90:271-
280). In transient transfections, cotransfection of Delta
with Kuz showed a remai:kable increase in the D1E~ fragment in
the culture medium compared to Delta transfection alone
(Figure 8A). This increase in D1E~ corresponds to a decrease
in D1FL consistent with the notion that D1FL is the precursor
of the D1E~ product. In addition, these data indicate that
transfection of Kuz acts additively to the endogenous Kuz in
the S2 cells. Supporting this hypothesis, cotransfection
with KuzDN has a dramatic inhibitory effect on D1E~ production
(Figure 8A). Under identical experimental conditions
cotransfection of Kuz or KuzDN has no effect on the
proteolytic processing of Notch (Figure 8B). These
observations demonstrate that Kuz plays a prominent role in
the processing of Delta, one that is not as clear in the
7L5 processing of Notch. In agreement with this conclusion, it
has been found D1E~ production was markedly inhibited by the
metalloprotease inhibitors EDTA and 1,10-phenanthroline
(Figure 8C), while no effect was observed with serine
protease inhibitors (PMSF and aprotinin), cysteine protease
inhibitor (leupeptin) or aspartyl protease inhibitor
(pepstatin) .
With the D1E~ product showing to be present in
embryos (Figure 7B), we sought to examine the role of Kuz in
generating this product in vivo. kuz maternal null embryos
with either one (kuz +/-) or no (kuz -/-) zygotic copies of
kuz were created by crossing female flies carrying kuz
germline clones with kuz +/- male flies (Rooke et al., 199&,
Nature 273:1227-1231). kuz -/- embryos were clearly
distinguished from kuz +,/- embryos by the absence of
malpighian tubules and lack of movement. Extracts prepared
~~ from a collection of nine of each type of embryo show the
distinct absence of the DIES and higher levels of D1FL in the
Kuz -/- embryos as compared to Kuz +/- (Figure 8D). Re-
- 129 -
CA 02333893 2001-O1-11
WO 00/02897
PCT/US99/15817
probing the same membrane with anti-Notch antibody showed no
difference in processing of Notch in the Kuz +/- and Kuz, -/-
embryos. Furthermore, analysis of 14 randomly selected
individual embryos showed eight embryos having significantly
higher levels of D1FL, analogous to the kuz -/- embryos
(Figure 8D) and consistent with the predicted outcome of the
cross. These observations indicate that Kuz mediated the
proteolytic processing of Delta in vivo.
Although kuz mutations have multiple defects
indicating an involvement in different processes (Rooke et
al., 1996, Nature 273:1227-1231), its phenotype partially
overlaps with that of Delta. Inactivation of kuz during
embryogenesis causes a more extensive neurogenic phenotype
than Delta mutations, nevertheless, it is clear that in the
ventrolateral region the neural hypertrophy in the two
mutations is identical.. In adult mosaic clones, a small
percentage of kuz mutant cells, on the clone border develop
into multiple bristles (Rooke et al., 1996, Nature 273:1227-
1231). Delta mosaic clones present a more complicated
situation. While cells on the border of the clones mutant
;20 for weak delta alleles commit to epidermal fate, it is
evident that cells mutant for strong delta alleles will
develop multiple bristlE~s at a low frequency (Figure 3 in
Heitzler and Simpson, 1991, Cell, 1083-1092), the phenotype
observed in kuz mutants. It is clear, however, that with
~,5 strong kuz and delta alleles, all extra neurons derive from
genotypically mutant cells.
The above observations are distinct from a second
function of kuz which has been termed neural promotion
function (Rooke et al., 1996, Science 273:1227-1231; Rooke
and Xu, 1998, Bioassays 20:209-214). This function prevents
cells in the center of kuz clones to develop bristles in
contrast to the multiple bristle phenotype of del to clones.
The genetic data, including the mosaic analyses, are
- 130 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
compatible with the hypothesis that the processing of Delta
protein is mediated by B;uz. These findings are also
compatible with earlier genetic studies linking kuz with
Notch activity (Pan and Rubin, 1997, Cell 90:271-280;
Sotillos et al., 1997, Development 124:4769-4779; Wen et al.,
1997, Development 124:4759-4767).
Adhesion assays have demonstrated that Notch-Delta
interactions are physically mediated by the extracellular
domains of the respective proteins (Fehon, et al., 1990, Cell
61:523-534). Furthermore deletion analyses have defined
specific sequences that are responsible for this interaction
(Rebay, et al., 1991, Cell 67:687-699; deletion mutants of
Delta lacking the DSL domain fail to bind Notch (M.
Muskavitch, personal communication); Fleming et al., 1997,
Development 124:2973-2981). These assays were done under
conditions where the Delta and the Notch proteins are
overexpressed in S2 cells and full length Delta is clearly
detected on the cell surface (data not shown). Interest was
expressed to examine if D1E~ is capable of binding to Notch.
Addition of D1E~ to Notch expressing S2 cells
followed by sedimentation through a sucrose cushion resulted
in specific binding of D1E~ to the Notch cells as compared to
S2 cells alone (Figure 9A) suggesting a D1E~:Notch complex
forms on these cells. These results were extended by
analyzing the ability of D1E~ to compete for full length Delta
binding to Notch in a cell aggregation assay. In order to
quantify the Notch/Delta interactions we have developed a
turbidimetric assay which allows us to measure aggregation in
a reproducible manner. Expression of Notch and Delta in S2
cells are induced for 16 hours with 0.085 mM and 0.022 mM
CuS04, respectively. The cells are then centrifuged and
raised in serum free media to an equivalent density yielding
between 20-30% T3zoam (-2x.106 cells/mL) in a Benchtop
spectrophotometer. Blank values are set with M3 media alone.
- 131 -
CA 02333893 2001-O1-11
WO 00/02897 PC'T/US99/15817
400 ~L of Notch and 400 ~L of Delta cells are then pipeted
into a 1.4 mL black sided, stoppered quartz cuvette and
quickly inverted three times. The T3zonm is read immediately
to determine the time "2;ero" value. The cuvette is then
rocked horizontally on a~ Thermolyne vari-mixer at 20
oscillations per minute and subsequent T32onm readings are
taken at one minute intervals. Change in T3aonm (relative to
time zero) is then plotted versus time. The effect of Dl~c
was compared to a concentrate of media from DECN-S2 cells
(closed squares) (Rebay et al., 1993, Cell 74:318-329)
7.0
prepared in parallel as these cells were transfected in the
same manner with an irrelevant construct.
Pre-incubation. of the Notch cells with DlEc
concentrate resulted in a dramatic reduction in the initial
rate of aggregation with. Delta cells (Figure 9B). The
~~5 competitive effect of DISC was sensitive to the concentration
added and the time of preincubation with the Notch cells.
Furthermore, pre-incubation of the Delta cells with DlEC had
no effect on subsequent aggregation with Notch cells
indicating DlEc specifically binds to Notch in a competitive
2;0 manner with respect to full length Delta.
The biological activity of DlEC was examined in a
cell culture assay which was carried out as follows. Low
density primary cultures of cortical neurons were prepared
from embryonic day 15.5 to 16.5 mouse embryos. Single cell
suspensions in Dulbecco's modified Eagle medium high
2. 5
glucose/F12 (1:1), N2 Supplement, 2.5 mM L-glutamine and 5-
10% fetal bovine serum were seeded on 5 mm diameter glass
coverslips precoated with 15 ~g/ml poly-ornithine and 2
~g/cm2 laminin. After 10 days in culture, neurons (<1000/em2)
were growing on a monolayer of glial cells. To examine the
f0 activity of DlEC, cultures were treated for 14-17 hours with a
1:10 dilution of either 5X ~ECN, 5X DlEC, purified DlEc
(approximately 0.04 AZeonm/ml in 25 mM glycine, 30 mM Tris;HCl,
- 132 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99115817
pH 7.0) made in culture medium. At least three independent
culture wells were examined for each condition during one
experimental trial. Cells were fixed in 4~ paraformaldehyde,
stained overnight with a mouse monoclonal antibody against
neuron-specific class III (3-tubulin (TuJl, 1:500; BabCo,
Berkeley, CA) and visualized with Cy3 conjugated anti-mouse
secondary antibody (Jackson Immunoresearch Laboratories).
Immunolabeled neurons were imaged with a Spot2 camera
(Diagnostic Instruments) using a 40X objective on a Zeiss
Axioplan 2 microscope and imported into Adobe Photoshop 4.0
(Adobe Systems, San Jose, CA). Neurite length was measured
in five to ten randomly selected images from each coverslip
using NIH Image 1.61 software and the data were analyzed with
Sigma Plot 4.0 statistical software (SPSS).
Primary cultures of mouse cortical neurons
expressing Notch endogenously develop dendritic processes
(Figure 9C). It has been demonstrated that ligand-dependent
Notch activation in vitro in cortical neurons expressing
endogenous Notch receptors causes morphological changes and
retraction of neurites. The same effects were observed when
the neurons were cultured in the presence of enriched DlEc
containing media or purified DlEC (Figure 9C). These data
show that DlEC has biological activity consistent with the
notion that it acts as an agonist. Similar effects of
neurite outgrowth have also been observed with a soluble form
of vertebrate Jagged (un.published observation).
Amino acid sequence analysis was performed on the
soluble Delta peptide. As described above, the molecular
weight of DlEC estimated from SDS-PAGE analysis is consistent
with DlEC being comprised of most if not all of the
extracellular portion of the Delta protein. In addition, the
N-terminal sequence of D~lEC is consistent with the predicted
N-terminus of full length Delta (DlEC is not proteolytically
clipped at the N-terminu.s). Further, as described above, DlEc
- 133 -
CA 02333893 2001-O1-11
WO 00/02897 PCTNS99/15817
likely arises due to proteolytic processing at a cleavage
sites) between the ninth EGF repeat and the transmembrane
domain in a region designated the juxtamembrane domain. The
sequence analysis was carried out by C-terminal sequencing
and by tryptic digest/liquid chromatography/mass spectrometry
(LC/MS) of purified DlEr derived from Drosophila Delta
expressed in S2 cells. This analysis was carried out at the
Harvard Microchemistry Facility, Cambridge MA.
The data generated by the C-terminal sequencing
showed that the terminal residue was alanine. The amino acid
residue preceding the terminal residue showed heterogeneity
with glycine being the most prevalent followed by asparagine,
leucine, and arginine. 'These data indicate that DlEc
terminates at more than .one position which indicates that
more than one proteolytic processing site exists. However,
C-terminal sequencing is very difficult to perform and the
confidence of residues beyond the terminal residue drops off
significantly. However, analysis of the Drosophila Delta
juxtamembrane domain (residues 564-594 of Drosophila Delta)
shows four of six possible alanine residues that would give a
terminal sequence consistent with the C-terminal sequencing
data, i . e. , DAs,6, GAsel, LAs9,, and NAs9a, (Figure 11) . Our data
indicated an alanine at ;position 591, in contrast to the
sequence data of Vassin, et a1.,1987, EMBO J. 6:3431-3440,
which disclosed a threonine at that position.
The tryptic digest peptide analysis was consistent
with the C-terminal sequencing data. 24 tryptic digest
peptides derived from Drosophila D1~~ were positively
identified by LC/MS and their sequences determined. Five
peptides were identified that terminated in the juxtamembrane
domain. Two of the peptides terminated at residue Alas9a and
two other peptides terminated at residue Alasel. These data
demonstrate that two prevalent forms of DlE~ terminate at
amino acid residues 581 .and 593. The fifth peptide
- 134 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
terminated at amino acid position GlnS~e, which was not
detected in the C-terminal analysis. The resolution of Alase~
and A1a593 by both analytical methods together indicates tl~iat
the primary forma of D1E~ are generated by cleavage at these
sites, although additional cleavage sites remain a
possibility. The nature' of these analyses do not permit a
quantitative assessment of the relative proportion of the
various species, thus it: cannot be concluded which of the
cleavage sites are preferred.
In conclusion, genetic and biochemical data show
~~0 that Delta is cleaved to produce an active, functionally
important extracellular fragment that is biologically active
with an apparent agonistic function in the Notch pathway.
Previous studies involving the in vivo expression of
artificially truncated notch ligands in Drosophila and other
1~' systems have demonstrated both antagonistic and agonistic
activities (Sun et al., 1997, Development 124:3439-3448;
Fitzgerald et al., 1995, Development 121:4275-4282; Li et
al., 1998, Immunity 8:43.-55; Wang et al., 1998, Neuron 21:63-
75) . It is clear that ~~oluble forms of Delta (D1S) can aca
20 as antagonists in the developing Drosophila eye (Sun et al.,
1997, Development 124:39:39-3448). However, D1E~ is not
identical to D1S and therefore it is plausible that the two
molecules may be functic>nally different. Figure 10 is a
schematic comparing DlE~ and D1S.
Although Kuz does not appear to be responsible for
the constitutive cleavage of Notch, the possibility that Kuz
can cleave Notch at alternative sites remains. In this
regard, it has been claimed that KuzDN is able to inhibit
transactivation of a target gene of the Notch pathway induced
by ligand binding to thE: receptor (Logeat, et al., 1998,
proc. Natl. Acad. Sci. I:fSA 95:8108-8112). However it is
possible that this effect does not reflect Notch cleavage but
rather the cleavage of Delta to produce an active ligand.
- 135 -
CA 02333893 2001-O1-11
WO 00/02897 PCT/US99/15817
Klueg et al., 1998, Mol. Cell Biol. 9:1709-1723 ("Klueg")
have recently reported the processing of Delta during normal
embryogenesis demonstrating the existence of Delta fragments,
one of which is consistent with DIES. We note the
intermediate forms detected in the 16-20 hour embryos
(Figures 7B, 8D, kuz +/~-) are not present in Kuz mutants
(Figure 8D, kuz -/-), r<~ising the possibility that the
generation of these products may also be mediated by Kuz.
The present invention is not to be limited in scope
by the specific embodiments described herein. Indeed,
:L 0
various modifications of the invention in addition to those
described herein will become apparent to those skilled in the
art from the foregoing description and accompanying figures.
Such modifications are .intended to fall within the scope of
the appended claims.
Various publications are cited herein, the
disclosures of which arES incorporated by reference in their
entireties.
a0
a5
:30
- 136 -