Note: Descriptions are shown in the official language in which they were submitted.
CA 02334215 2000-12-04
P1366PCT -1-
TRANSFORMED C~;LL LINES, WHIGH EXPRESS HETEROLOGOUS
G-PROTEIN-COUPLED RECEPTORS
Field of the Invention
The invention concerns transformed cell lines in accordance with the generic
term of the
first, independent claim,, which cell lines express heterologous G-protein-
coupled
receptors (GPC-receptors) and which cell lines are suitable for detecting
interactions
between the GPC-receptors or the signal transmission systems controlled by the
GPC-
receptors with substance, (ligands, modulators), resp., for finding
substances, which
interact with the receptors or with the signal transmission systems. The
invention further
concerns vectors for producing the cell lines and the use of these cell lines
for detecting
1 o the named interactions and for finding substances acting on the receptors
or on the signal
transmission systems (screening assays).
Prior Art
G-protein-coupled receptors (GPC-receptors) are receptors with seven
transmembrane
domains, which in conjunction with heterotrimeric guanyl-nucleotide-binding
regulatory
proteins (G-proteins) form signal transduction systems for the transmission of
many
CA 02334215 2000-12-04
P1366PCT - 2 -
extra-cellular signals [H.Ci. Dohlmann, J. Thorner, M. Carom and R. J.
Lefkowitz (1991)
Annu. Rev. Biochem., 60, 653-688]. Signal transduction systems of this kind
occur in a
broad spectrum of organisms, starting with simple fungi and extending right to
the
human being.
An example of a receptor of this kind is the somatostatin receptor, which
represents a
prototype of the GPC-receptors in mammalian cells. Somatostatin has far-
reaching
modulatory effects in the central nervous system and in the peripheral tissue
and acts on a
range of receptor subtype.
to
The signal transmission through a signal transmission system with GPC-receptor
and
G-protein has the following general characteristics: Heterotrimeric G-proteins
work as
signal transmitters between a transmembrane receptor molecule (GPC-receptor)
and an
enzyme designated as an effector, which produces a secondary messenger
substance.
Adenylate cyclase, phospholipaseC and ion channels are examples of well
investigated
effectors in mammalian systems.
G-proteins consist of a guanyl-nucleotide-binding a-subunit, a ~3-subunit and
a y-subunit
(Ga-, G(3-, Cry-subunit) [M. I. Simon, M. P. Strathmann and N. Gautam (1991)
Science,
2 0 252, 802-808] . G-proteins exist in two differing forms, depending on
whether GDP or
GTP is bound to the a-sulbunit. If GDP is bound, the G protein occurs as a
heterotrimeric
a/iy-complex. Through the binding of GTP to the G-protein, the a-subunit
dissociates
and leaves behind a [i~y-complex. Association of a Ga[i~y-complex with an
activated GPC-
receptor in the cell membrane leads to an increase of the exchange rate of GTP
for bound
GDP. In consequence, the dissociation rate of the bound Ga-subunit from the
G(i~y-
complex increases. The free a-subunit and the G(3~y-complex can transmit
signals to
cellular effectors of different signal transmission paths.
The GPC-receptors represent important target molecules for therapeutic
compounds. The
3 o human genome probably contains about 5000 different GPC-receptor genes, on
which
the new therapeutic compounds can act as ligands. For the investigation of
such
interactions between ligands and receptors and also of modulators and
corresponding
signal transmission systems, screening test systems are utilized, in which in
accordance
CA 02334215 2000-12-04
P1366PCT - 3 -
with prior art, biochemical ligand-binding studies, reporter systems in
mammalian cells
or reporter systems in yeast cells are used.
Yeast cells utilized in test systems of this kind are transformed in such a
manner, that
they express heterologous GPC-receptors. In the publication WO-92/05244 (US-
5739029), such yeast cE;lls are described. They contain a first heterologous
DNA-
sequence, which expresses a heterologous GPC-receptor, and a second
heterologous
DNA-sequence, which expresses an a-subunit of a mammalian G-protein.
1 o The endogenous GPC-receptors in yeast cells facilitate identification of
different cell
types via extra-cellular pfptides, so-called pheromones. The pheromone-
activated signal
transmission path inducE;s a development programme, which leads to the fusion
of
haploid a- and a-cells and to the formation of diploid a/a-cells [M. Whiteway
and B.
Errede (1993) in: Signal Transduction, Prokaryotic and Simple Eukaryotic
Systems, ed.
J. Kurjan and B. L. Taylor, Academic Press, pp. 189-237]. Cells of the cross-
breed type a
secrete a-factor, which in a-cells binds to the a-factor receptor (Ste2), and
cells of the
cross-breed type a secrete a-factor, which in a-cells binds to the specific a-
factor receptor
(Ste3). Both Ste2 as well as Ste3 belong to the family of the GPC-receptors.
After the
binding of the pheromone to the corresponding receptor, the pheromone receptor
2 o probably changes its conformation. This leads to the dissociation of the
Ga-subunit
(Gpal) from the G(3~y-complex (Ste4, StelB) and therefore to the activation of
the G
protein. Interestingly - and in contrast to the function of G-proteins in
mammalian
systems - the Ga-subunit has a negative influence on the signal transmission,
while the
G(3~y-sub-unit passes on the pheromone signal.
A further, fundamental difference between GPC-receptor-controlled signal
transduction
in mammals and in yeast consists of the fact, that up until now no effector
enzyme has
been discovered in yeast: cells, which generates a secondary messenger
substance as a
reaction to receptor stimulation. Probably the (3'y-complex directly passes
the pheromone
3 o signal on to the Ste20-protein kinase, which in turn activates a protein
kinase cascade,
consisting of Stell, Ste7" Fus3 and Kssl, and the "structure"-protein Ste5 [I.
Herskowitz
(1995) Cell, 80, 187-197_x.
CA 02334215 2000-12-04
P1366PCT - 4 -
Belonging to the cellular consequences of the pheromone stimulation in yeast
are the
transcriptional induction of a whole range of genes and the arrest of the cell
cycle. These
pheromone-induced genes encode for products, which are required for the
biosynthesis of
the pheromones (MFAl, :MFA2, MFal, MFa2, STE6, STE13), for the production of
the
receptors STE2 and STE;3, for the pheromone signal transmission (GPAl, FUS3),
for
cell cycle arrest (FART, CLN2, CLN3), for morphological changes and cell
fusion
(FUSl, FUS2, CHSl) and for pheromone desensitization (SST2, BARD.
Pheromone-controlled transcription is facilitated by the sequence-specific DNA-
binding
1 o protein Stel2. The transcription of the STE12 gene is not inducible
through pheromone.
It is suspected that the functionally redundant MAP-kinase -homologues Fus3
and Kssl
of the pheromone signal transduction path activate the transcription factor
Stel2 through
specific phosphorylation. Pheromone-inducible genes have cis-acting DNA-
sequences in
their promotor region, the so-called "Pheromone Response Element" (PRE). The
presence of PRE-sequences in the promotor region of a gene in yeast, however,
does not
suffice for the transcription of this gene to be pheromone-inducible. Thus,
e.g., the
STEl2-gene has several I'REs, but the expression of STEl2 is not pheromone-
inducible.
2 o Basic Features of the Invention
The invention sets itself the objective of creating transformed cell lines
(human, animal
or vegetable as well as also fungal cells), which cells express heterologous
GPC-
receptors. These transformed cell lines shall be suitable for detecting
interactions between
2 s substances (ligands, modulators) and GPC-receptors or GPC-receptor-
controlled signal
transmission systems, resp., for finding in corresponding screening assays,
substances
which interact with the receptors or the signal transmission systems. In order
to be
suitable for this purpose, the transformed cells shall manifest a high
sensitivity to
interactions of this kind.
This objective is achieved by the transformed cell lines, as they are defined
in the claims.
The cell lines in accordance with the invention (human, animal or vegetable as
well as
CA 02334215 2000-12-04
P1366PCT - 5 -
also fungal cells) have a signal transmission system with GPC-receptors, which
signal
transmission system is acaivatable through a ligand and manifests a positive
feedback.
The mechanism of the po sitive feedback consists of the fact, that
transcription of the gene
encoding for the transcription factor which is activated by the GPC-receptor,
is itself
s inducible through receptor stimulation.
The cell lines in accordance with the invention may have an endogeneous GPC-
receptor
signal transmission system with positive feedback or a positive feedback
mechanism can
be built into an existing GPC-receptor signal transmission chain by means of
1 o recombinant DNA-technology. Through the positive feedback mechanism, the
natural or
correspondingly modified signal transduction system manifests a significantly
higher
sensitivity in comparison with known, cellular systems, used for detecting the
mentioned
interactions. This means that cell lines having a positive feedback mechanism
react more
sensitively to an activation with the GPC-receptors than known cellular
systems used for
1 s such detection, irrespective of whether the receptors are the endogenous
ones or
heterologous receptors introduced through transformation.
The maize blight fungus Ustilago maydis represents an example of a cellular
test system
for GPC-receptors, in which a positive feedback mechanism exists in the
natural
2 o condition in the GPC-receptor-controlled signal transmission chain. This
positive
feedback in the pheromone-activatable signal transduction for Ustilago maydis
is
described by H. A. Hartmann, R. Kahmann and M. Bolker [EMBO J., 15, 1632-1641
(1996)]. The basidiomycet Ustilago maydis is utilized as eukaryontic model
organism. In
its pathogenic form it causes the maize blight on its host plant maize and for
this reason
2 s also serves as model system for the study of pathogenic fungi. The genetic
constitution of
U. maydis can be relatively easily modified [F. Banuette (1995) Annu. Rev.
Genet., 29,
179-208] .
It has to be assumed, that also other cell lines, in particular fugi of the
Ustilago family or
3 o the basidiomycous cells in general manifest such GPC-receptor-activatable
signal
transduction paths with positive feedback and therefore like Ustilago offer
themselves for
the mentioned test reactions in their natural condition.
_ CA 02334215 2000-12-04
P1366PCT - 6 -
According to the invention, cell lines having in a natural or genetically
modified state a
GPC-receptor-activatable signal transmission system, in which the GPC-receptor-
induced
transcription of target genes is amplified by positive feedback are used for
detecting
interactions between specific GPC-receptors or corresponding signal
transmission
s systems and test substances. The cells further have an endogenous or
heterologous
reporter gene, the expression of which is controlled by a promotor being
inducible by
activation of the receptor, whereby the expression of the reporter gene can be
detected
and quantified by measuring techniques (e.g., essential growth enzyme causing
measurable cell growth, or other enzymes, which in biochemical reactions lead
to
1 o measurable effects).
In the cells of the cell lines in accordance with the invention, the
heterologous GPC-
receptor can associate with endogenous G-protein or with heterologous G-
protein, in
particular with a heterologous a-subunit of the G-protein. In addition, the
cell can contain
is a mutation of the gene inhibiting the endogenous a-subunit responsible for
the GPC-
receptor-controlled signal. transmission and therewith facilitating
interaction between the
heterologous receptor andl the heterologous G-protein.
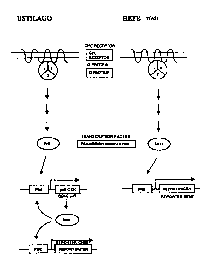
2 o Brief Description of the Figures
Figure 1 illustrates the comparison between a signal transmission system with
positive
feedback (on the left), as is present in the cell lines in accordance with the
invention (e.g., Ustilago maydis), and a signal transmission system without
2 s positive feedback (on the right), as, for example, is present in yeast
cells.
Detailed Description of the Invention
s o The term "heterologous" is used in this description in reference to the
corresponding cell
lines and therefore refers to DNA-sequences, proteins and other materials,
which are
brought into the corresponding cell line from other organisms, or to
combinations, which
do not occur in the corresponding cell lines in their natural condition.
_ CA 02334215 2000-12-04
P1366PCT - 7 -
The terms "upstream" and "downstream" are used in the following to refer to
the
direction of transcription and translation. A sequence, which is transcribed
or translated
prior to another sequence, is designated as "upstream" of the other sequence.
Methods and materials, with the help of which the cell lines in accordance
with the
invention are produced, ~~re described in detail for the example Ustilago
maydis. This,
however, shall in no manner whatsoever restrict the cell lines in accordance
with the
invention to this species. For other cell lines, the methods and materials are
to be
1 o correspondingly applied, which for one skilled in the art is possible
without any problem.
For Ustilago maydis in it~~ natural state a signal transmission system enables
identification
and fusion of compatible cell types (al and a2), in a similar way as described
for yeast
further up. This signal transmission system is controlled by pheromones and by
the
s 5 corresponding pheromone receptors [J. Kronstad and C. Staben (1997) Annu.
Rev.
Genet., 31, 245-276]. CE;lls of Ustilago maydis of the cross-breed type al
secrete the
peptide pheromone Mfal, which in a2-cells binds to the specific Mfal-receptor
(Prat).
Cells of the cross-breed type a2 secrete the Mfa2-pheromone, which only acts
in al-cells
expressing Mfa2-receptor (Pral). The pheromone receptors Pral and Prat are GPC-
2 o receptors.
Activation of the receptors Pral and Pra2 by binding the corresponding
pheromone
probably leads to the dissociation of a hetero-trimeric G-protein, of which up
until now
only the a-subunit Gpa3 is known [E. Regenfelder, T. Spellig, A. Hartmann, S.
Lauen-
25 stein, M. Bolker and R. Kahmann (1997) EMBO J., 16, 1934-1942]. Gpa3 - in
contrast
to the functional homologue in yeast (Gpal) - has a positive influence on the
pheromone
signal transmission. Interestingly, the cellular effector of Gpa3 appears to
be an
adenylcyclase (Uacl), bE;cause mutations in the gpa3-gene on the one hand
render the
pheromone signal transmission impossible and on the other hand lead to a
filament-like
3 o growth, which resembles the growth of adenylcyclase-deficient mutants.
Furthermore,
the filament-like growth of gpa3-mutants can be reversed by the addition of
cyclic AMP,
the secondary messenger substance, which is produced by the adenylcyclase [R.
Kahmann and C. Basse (1997) Trends in Plant Sci., 2, 366-368; S. Gold, G.
Duncan, K.
CA 02334215 2000-12-04
P1366PCT - 8 -
Barren and J. Kronstad (1994) Genes Dev., 8, 2805-2816]. In consequence of
this, the
pheromone signal transmission in Ustilago appears to be more similar to the
corresponding mechanisms in mammalian systems than is the case for the
pheromone-
controlled signal transmission in yeast.
In the final instance, pheromone stimulation in Ustilago results in the
transcriptional
induction of all genes, which are at the hybrid type loci a and b, i.e., mfal,
mfa2, pral,
prat, lga2, rga2, bE, bV~~ [M. Urban, R. Kahmann and M. Bolker (1996) Mol.
Gen.
Genet., 251, 31-37]. All these genes possess at least one cis-acting DNA-
sequence, the
"Pheromone Response Element" (PRE), in their associated gene-regulatory
regions. The
PRE-sequences of Ustila~;o maydis are identified by the sequence-specific DNA-
binding
protein Prfl. Pheromone stimulation leads to the activation of Prfl, which
imparts the
pheromone-inducible transcription of these genes. Since the promotor of the
prfl-gene
also has PREs, the transcription of the prfl-gene is also activated through
pheromone
stimulation (H. A. Hartmann, R. Kahmann and M. Bolker (1996) EMBO J., 15, 1632-
1641). Through the phf;romone-inducible transcription of the prfl-gene, a
positive
feedback mechanism is inherent to the pheromone signal transmission path of
Ustilago.
GPC-receptor-controlled signal transmission systems with positive feedback can
be
2 o identified by the fact, that the transcription of the gene, which codes
for the transcription
factor activated by stimulation of the GPC-receptor and as a result of this
controls the
GPC-receptor-controlled transcription of target genes, is itself induced by
stimulation of
the GPC-receptor.
2 5 The mechanism described above is schematically illustrated in Figure 1 on
the left. This
mechanism leads to a significantly higher sensitivity for detecting e.g.
binding of a ligand
to the receptor. As already mentioned above, and as illustrated in Figure 1 on
the right as
a comparison, the corresponding yeast system and other cellular systems used
for such
detection in corresponding assays, do not possess a positive feedback
mechanism. In the
3 o corresponding mechanism of yeast, expression of the GPC- receptor-
activatable
transcription factor (Stel:?) is not induced through receptor stimulation
(refer to Fig.l).
However, it is conceivable to modify yeast strains utilized for assaying
interactions
CA 02334215 2000-12-04
P1366PCT - 9 -
between heterologous receptors and ligands, in such a manner, that they have a
positive
feedback mechanism. In order to achieve this, the promotor of the STE12-gene,
which
codes for the transcription factor activated by pheromone stimulation, is
replaced by a
promotor, which is pheromone-inducible (e.g., FUSl-promotor).
Various Ustilago strains and suitable expression vectors for ustilago
transfection are
known. Expression vectors are replicatable DNA-constructs, which are utilized
to
express a heterologous DNA-sequence in a host cell. The heterologous DNA-
sequence
to be expressed has to be equipped with suitable control sequences capable of
controlling
1 o expression of the protein or protein-subunit encoded by the heterologous
DNA-sequence
in the intended host. Control sequences encompass a transcriptional promotor,
optional
cis-acting DNA-sequences in order to regulate transcription, suitable DNA-
sequences,
which impart an efficient initiation of translation, and DNA-sequences, which
control
termination of transcription and poly-adenylization of the mRNA.
Suitable vectors for the production of cell lines in accordance with the
invention
encompass plasmids, viruses and DNA-fragments being integratable into the host
genome through genetic recombination. Suitable vectors contain control
sequences,
which come from species, which are functional in the intended expression host.
Ustilago vectors can contain an autonomously replicating sequence (UARS),
which
renders the plasmid capable of replicating in the Ustilago cell to a great
number of
copies, a promotor, heterologous DNA-sequences encoding the heterologous
proteins to
be expressed, sequences for the poly-adenylization and a selectable marker
gene.
An example of a plasmid of this kind is pJW42 [J. Wang, D. W. Holden and S. A.
Leong
(1988) Proc. Natl. Acad. Sci. USA, 85, 865-869]. This plasmid contains the hph-
gene of
Escherichia coli [L. Gritz and J. Davies (1983) Genes, 25, 179-188], which
imparts
resistance against the antibiotic HygromycinB and as a result of this can be
utilized as a
3 o selectable marker. Other utilizable marker genes are, for example, the cbx-
gene of
Ustilago maydis, which. imparts resistance against the fungicide Carboxin
[P.L.E.
Broomfield and J.A. Hargreaves (1992) Curr. Genet.,22, 117-121], or the natl-
gene of
Streptomyces noursei, which imparts resistance against the antibiotic
nourseothricine [H.
CA 02334215 2000-12-04
P1366PCT - 10 -
Kruger, G. Fiedler, C. Srruth and S. Baumberg(1993) Genes, 127, 127-131].
Suitable promotor sequences comprise the promotors of the hsp70-gene [D. W.
Holden,
J. W. Kronstad and S. A. Leong (1989) EMBO J., 8, 1927-1934], of the
glyceraldehyde-
s 3-phosphate-dehydrogen~se gene [T. L. Smith and S. A. Leong (1990) Genes,
93, 111-
117] and the translation-elongation-factor gene [H. A. Hartmann, R. Kahmann
and M.
Bolker (1996) EMBO J., 15, 1632-1641]. Other promotors with the additional
advantage
of the transcriptional control through the growth conditions are the promotor
of the crgl-
gene [A. Bottin, J. Kamper and R. Kahmann (1996) Mol. Gen. Genet., 253, 342-
352],
1 o which is induced through arabinose and repressed by glucose, and the
promotor of the
sidl-gene, which is negatively regulated through the iron concentration in the
growth
medium [Z. An, B. Mei, W. M. Yuan and S. A. Leong (1997) EMBO J., 16, 1742-
1750].
In order to assure polyadenylization and mRNA-termination, it is also possible
to ligate
the termination sequences into the expression vectors associated with these
genes,
15 downstream of the heterologous sequences.
For enabling efficient expression of heterologous GPC-receptors in Ustilago,
novel
expression vectors were developed. These expression vectors contain Ustilago
maydis
hsp70-promotor and -terminator, which facilitate transcription of the cDNAs
for GPC-
2 o receptors. Between the hsp70-promotor and -terminator, additional
interfaces for
restriction enzymes are introduced, in order to simplify cloning of DNA-
sequences to be
expressed, e.g., GPCR-cI)NAs.
In order to optimize the intra-cellular localization of the heterologous GPC-
receptors to
2 s the plasma membrane in Ustilago, it is also possible to construct
expression vectors,
which contain a first sel;ment comprising Ustilago-DNA-sequences comprising at
least
one segment of the sequence encoding the amino-terminal of an Ustilago-GPC-
receptor.
DNA-sequences encoding the pheromone receptors of Ustilago (e.g., the pral-
gene,
which codes for the Mfa2-pheromone receptor, and the prat-gene, which codes
for the
3 o Mfal-pheromone receptor) are examples for Ustilago-genes, which code for
GPC-
receptors and can be utilized for constructing suitable vectors. A second
segment, which
lies downstream of the mentioned first segment and is in the correct reading
frame with
it, comprises a DNA-sequence encoding a heterologous GPC-receptor. Such
adaptations
CA 02334215 2000-12-04
P1366PCT -11-
of the translation initiation point can increase the expression of a
heterologous protein.
The first and second segments are operatively associated with a promotor, such
as, e.g.,
the constitutive hsp70-promotor or the inducible crgl-promotor, which are
operative in
Ustilago cells.
Every GPC-receptor and the corresponding DNA-sequences, which code for such
receptors, can be utilized for producing the cell lines in accordance with the
invention.
Examples of receptors of this kind are adrenergic receptors (a or ~),
adenosine- receptors,
angiotensine-receptors, bradykinine-receptors, cannabinoid-receptors,
chemokine-
1 o receptors, dopamine-receptors, glucagon-receptors, neurokinine-receptors,
neurotensine-
receptors, serotonin-recE;ptors, opiate-receptors, muscarinic-receptors,
somatostatin-
receptors and vasopressine-receptors. The term "receptor" used here also
includes sub-
types as well as their mutants and homologues and also the DNA-sequences,
which code
for them.
Every Ga-subunit and the corresponding DNA-sequences, which code for these Ga-
subunits, can be utilized for producing the cell lines in accordance with the
invention.
Examples of Ga-subunits are Gs-subunits, Go-subunits, Gq-subunits, Gi-subunits
and
Gz-subunits. The term "Ga-subunit" used here includes sub-types as well as
their mutants
2 o and homologues and also DNA-sequences, which code for these. The
functional
expression of heterologous Ga-subunits in Ustilago can easily be verified,
because a
defect in the Ustilago Ga-subunit Gpa3 leads to a characteristic, visually
observable
filament-like growth, in contrast to the yeast-like growth form of Ustilago
cells with an
intact gpa3-gene. Heterologous Ga-subunits, which take over the function of
the
endogenous Ga-subunit Gpa3 in the pheromone signal transmission chain,
complement
the filament-like growth defect of the gpa3 mutant cells to normal, yeast-like
growth and
therefore can be easily identified.
Every Gj3y-subunit and the corresponding DNA-sequences, which code for these
G(3y-
s o subunits, can be utilized for producing the cell lines in accordance with
the invention.
The term "G(3y-subunit" used here includes sub-types as well as their mutants
and
homologues and also DNA-sequences, which code for these.
CA 02334215 2000-12-04
P1366PCT - 12 -
In order to detect binding of a ligand to a heterologous GPC-receptor or in
general to
detect interaction between a modulator and the GPC-receptor-controlled signal
transmission system in the cell lines in accordance with the invention, it is
particularly
appropriate to equip the cells with a third DNA-construct, which encompasses a
s promoter and a reporter gene. The promoter is inducible through activation
of the
heterologous GPC-receptors. The reporter gene is placed downstream of the GPC-
receptor-inducible promoter and is operatively associated with it. The
expression of the
reporter gene can be recorded with measuring technology and reflects the
activation of
the heterologous GPC-receptor through suitable ligands. In the exemplary
Ustilago
1 o maydis, e.g., the promoter of the mfal-gene, the promoter of the mfa2-
gene, the
promoter of the pral-gene, the promoter of the prat-gene or the promoter of
the prfl-
gene can be utilized as GPC-receptor-inducible promoters. Various, endogenous
or
heterologous genes can be used as reporter genes. Examples for reporter genes
are the
pyr6-gene [J. W. Kronstad, J. Wang, S. F. Covert, D. W. Holden, G. L. McKnight
and S.
15 A. Leong (1989) Genes, 79, 97-106], the pyr3-gene [A. Spanos, N. Kanuga, D.
W.
Holden and G. R. Banns (1992) Genes, 117, 73-79], the lacZ-gene, the hph-gene
(hygromycine resistance) [L. Gritz and J. Davies (1983) Genes, 25, 179-188],
the ble-
gene (phleomycine resistance) [D. Drocourt, T. Calmels, J. P. Reynes, M. Baron
and G.
Tiraby (1990) Nucl. Acids Res., 18, 4009], a GFP-gene (Green Fluorescent
Protein) [T.
a o Spellig, A. Boffin and R. Kahmann (1996) Mol. Gen, Genet., 225, 503-509]
or the uidA
(GUS) gene [R. A. Jefferson, S. M. Burgess and D. Hirsh (1986) Proc. Natl.
Acad. Sci.
USA, 86, 8447-8451].
2 s Example 1:
Production of Ustilago expression vectors (pDT78 and pDT99)
For enabling U. maydis to express heterologous GPC-receptors the Ustilago
expression
vectors pDT78 and pDT99 were constructed.
For the expression vector pDT78, the 3.1 kb HindIII fragment of the
autonomously
replicating Ustilago vector pCM54 [T. Tsukuda, S. Carleton, S. Fotheringham
and W. K.
Holloman (1988) Mol. Celh Biol., 8, 3703-3709] was substituted by a 2 kb
HindIII
CA 02334215 2000-12-04
P1366PCT -13 -
fragment of the plasmid p:DWHlO [J. Wang, D.W. Holden and S. A. Leong (1988)
Proc.
Natl. Acad. Sci. USA, 85, 865-869]. This 2 kb HindIII fragment contains the
promotor
and the transcription terminator of the U. maydis hsp70 gene, separated by a
BgIII
interface. The resulting plasmid pDT48 was cut with SacI and PstI, and a 1.5
kb SacI-
s PstI fragment, isolated from the plasmid pNATl (pDT65), was introduced,
which
contains the hatl gene of Str°eptomyces nouysei, which imparts
resistance against the
antibiotic nourseothricine of the streptothricine family [H. Krugel, G.
Fiedler, C. Smith
and S. Baumberg (1993) Gene, 127, 127-131]. The expression of the natl gene in
U.
maydis is controlled through the promotor of the U. maydis glyceraldehyde-3-
phosphate
z o dehydrogenase (GAPDH) gene [T.L. Smith and S.A. Leong (1990) Genes, 93,
111-117]
and the transcription terminator of the cycl gene of Saccharomyces cerevisiae
[D. Dro-
court, T. Calmels, J.P. R.eynes, M. Baron and G. Tiraby (1990) Nucleic Acids
Res. 18,
4009]. The plasmid pD'T78 resulting from it possesses a single interface for
BgIII
between the U. maydis hsp70 promotor and the U. maydis hsp70 terminator. This
15 restriction enzyme interface can be utilized for introducing a DNA-sequence
to be
expressed in U. maydis, e.g., a cDNA, which codes for a heterologous GPC-
receptor.
The transcription of the cDNA, which specifies the heterologous GPC-receptor,
is
therefore imparted by tlhe transcription control sequences of the U. maydis
hsp70
promotor. The pDT78 expression vector and its derivatives can be introduced in
Ustilago
2 o maydis by means of known transformation methods [J. Wang, D.W. Holden and
S. A.
Leong (1988) Proc. Natl. Acad. Sci. USA, 85, 865-869] and by means of adding
the
antibiotic nourseothricine (40 ~g/ml) into the growth medium, a selection is
made for the
presence of this plasmid in U. maydis cells.
2 s In order to simplify the c;loning of DNA-sequences to be expressed in
Ustilago, further
expression plasmids werE; constructed with the hsp70 promotor, which, however,
instead
of having only one restri<;tion interface (as e.g. pDT78) between the hsp70
promotor and
terminator, have interfaces for several different restriction enzymes. This is
illustrated
here with the example of pDT99.
pDT49 is identical with the Pdt48 described above, with the exception that the
2 kb
HihdIII fragment, which contains the promotor and the transcription terminator
of the U.
maydis hsp70 gene, was introduced in the opposite orientation, i.e., the E.
coli lacZ
CA 02334215 2000-12-04
P1366PCT -14 -
promotor of the plasmid and the introduced hsp~0 promotor impart the
transcription in
the opposite direction. In order to eliminate the SmaI, BamHI, XbaI, SaII and
PstI
interfaces of pDT49, pDT49 was digested with SmaI and with PstI, the 3'-
overhang of
the PstI interface was removed with T4 DNA polymerase and the plasmid was
relegated.
s Into the BgIII interface between the hsp70 promotor and terminator of the
resulting
plasmid pDT85 a double-stranded oligonucleotide, which contains interfaces for
the
restriction enzymes KpnI, EcoRI, NotI, NcoI, MZuI, StuI, SphI, BamHI and SacII
(in this
sequence), was legated in such a manner, that the SacII interface came to lie
closer to the
hsp70 promotor than the KpnI interface. In the resulting plasmid pDT90 a 2.3
kb SacI
to fragment of the plasmid fjahCbx8 was introduced into the SacI interface,
which plasmid
contains a gene, which in U. maydis imparts resistance against the fungicide
Carboxin
[P.L.E. Broomfield and J.A. Hargreaves (1992) Curr. Genet., 22, 117-121].
pDT99 can
be introduced into U. maydis cells by means of transformation [J. Wang, D.W.
Holden
and S. A. Leong (1988) Proc. Natl. Acad. Sci. USA, 85, 865-869] and by means
of
s adding the fungicide Carboxin (2 ~g/ml) into the growth medium a selection
is made for
the presence of this plasmid in U. maydis cells.
Example 2:
2 o Expression of the human ~2-adrenergenic receptor into U ~ydis (pDT94)
In order to express the human (32-adrenergenic receptor ((32-AR) in U. maydis,
approx.
0.1 ~g DNA of the plasmid pTF3 [B.K. Kobilka, R.A.F. Dixon, T. Frielle, H.G.
Dohlman, M.A. Bolanowski, LS. Sigal, T.L. Yang-Feng, U. Francke, M.G. Carom
R.J.
2 s Lefkowitz (1987) Proc. Natl. Acad. Sci. USA, 84, 46-50], which contains a
cDNA of the
human (32-adrenergenic receptor, was amplified with the primers 5'-
CGGGATCCACAATGACCCAACCCGGCAACGGCAGCG-3' and 5'-
CGGGATCCTCAGAGCAGCGAGTCATTTGTGCTACA-3' (wherein A = adenosine;
C = cytosine; G = guanine; T = thymidine) by means of the polymerase chain
reaction
3 0 (PCR). In comparison with the human DNA sequence of the [32-adrenergenic
receptor, in
particular the environment of the translation initiation ATG codon was changed
in such a
manner, that it corresponds to the corresponding consensus sequence for
filamentous
fungi [D. J. Ballance (1991) in Molecular Industrial Mycology: Systems and
CA 02334215 2000-12-04
P1366PCT -15 -
Applications for Filamentous Fungi; S.A: Leong and R.M. Berka (eds.), Dekker,
New
York, pp 1-29]. The resulting 1.2 kb PCR product was digested with BamHI and
cloned
in the BamHI interfacf: of the BamHI linearized and dephosphorylated vector
pBLUESCRIPTII KS+ (;itratagene Inc.). The DNA sequence of the 1.2 kb BamHI b2-
AR PCR products such cloned was verified. The 1.2 kb BamHI fragment of the
resulting
plamid pDT87 was then introduced into the BgIII interface of the expression
vector
pDT78 in such a manner, that the (32-AR sequence is transcribed in the correct
orientation of the hsp70 hromotor. The resulting ~i2-AR expression plasmid
pDT94 can
now be introduced into LI, maydis cells by means of known transformation
methods [J.
Zo Wang, D.W. Holden and S. A. Leong (1988) Proc. Natl. Acad. Sci. USA, 85,
865-869].
The biochemical proof, that the human (32-AR is expressed in U. maydis, can be
supplied
by means of ligand binding studies. Such ligand binding studies can be carried
out on
membrane fractions of U. maydis cells transformed with pDT94 using, e.g., the
is radioactive marked [i2,-AR-ligand 3-[1~I]iodocyanopindolol in accordance
with
published protocols [H.)K. Dohlman, M.G. Carom A. DeBlasi, T. Frielle and R.J.
Lefkowitz (1990) Biochemistry, 29, 2335-2342].
The proof, that the human (32-AR expressed in U maydis is functional and
interacts with
2 o the pheromone signal transmission chain of U. maydis, can be provided by
introducing a
pheromone-inducible reporter gene into the U. maydis cells transformed with
pDT94.
The plasmid pMLTl contains the bacterial uidA gene, which codes for the enzyme
~i-
glucuronidase (GUS) [R. A. Jefferson, S. M. Burgess and D. Hirsh (1986) Proc.
Natl.
Acad. Sci. USA, 86, 8447-8451] and its expression is regulated through the
strongly
2 s pheromone-inducible promotor of the mfal gene. [M. Urban, R. Kahmann and
M.
Bolker (1996) Mol. Gen. Genet., 251, 31-37]. In consequence of this, the
binding of a
[32- .AR agonist to the X32-AR expressed in U maydis can be proven by
stimulating U.
maydis transform~ds, vvhich contain pDT94 and pMUl, with e.g., the (3-
adrenergenic
agonist isoproterenol. Tlhe stimulation of the receptor can be proven through
a simple
3o biochemical test for GLfS-activity, as described, e.g., in A. Gururaj Rao
and P. Flynn
(1992) in GUS Protocol;.; S.R. Gallagher (ed.), Academic Press Inc., 89-99.