Note: Descriptions are shown in the official language in which they were submitted.
CA 02335409 2001-02-22
P-3240P 1
Richard J. Carroll and Frank A. Augello
APPARATUS AND METHOD FOR PLASMA PREPARATION
S BACKGROUND OF THE INVENTION
1. Field of the Invention
This invention relates to a device for blood plasma preparation
for a variety of analytical assays. More particularly, the present
invention pertains to a blood collection device comprising a thixotropic
polymeric polyester gel and an anticoagulant formation. The device of
the present invention is most preferably used in nucleic acid testing,
which use amplification technologies including, but not limited to,
polymerase chain reaction (PCR), branched DNA (bDNA) and nucleic
acid sequence based amplification (NASBA).
2. Description of Related Art
New amplification technologies, such as polymerase chain
reaction (PCR), branched DNA (bDNA), and nucleic acid sequence
based amplification (NASBA), allow researchers to monitor the levels
of infectious agents in plasma. Studies have demonstrated that the
number of extracellular HIV RNA viral copies, or viral load, is a
surrogate marker for the progression of the HIV infection. Scientific
research has shown that HIV replication occurs throughout the life of
the infection. After the initial infection, the HIV viron enters
CA 02335409 2001-02-22
P-3240P 1
susceptible cells, replicates rapidly creating billions of copies of the
HIV viral RNA soon after infection. Although the HIV RNA viral load
varies across the patient population, the disease follows a specific
progressive pattern within each patient. Therefore, monitoring the
HIV RNA viral load of HIV infected patients can be used to manage
the disease. In addition, the patients' response to approved drugs, new
drugs and combination drug therapies can be evaluated by monitoring
the patient's HIV RNA viral load.
In addition to the HIV virus, there are a number of other
infectious diseases that would benefit from viral load monitoring, such
as the Hepatitis C virus.
Measurements of the viral load are determined by using
polymerase chain reaction (PCR), branched DNA (bDNA), and other
amplification techniques. The quality and consistency of the sample is
critical to obtaining optimal test results using these technologies.
There are a number of variables that influence the sample quality,
such as the collection method, centrifugation time, sample preparation
technique, transport to the test laboratory, contamination with cellular
materials, and the like.
Numerous sample types have been evaluated for nucleic acid
testing, including whole blood, serum and plasma. Studies have
shown that the HIV viral load is stable for up to 30 hours in a whole
blood sample using EDTA as the anitcoagulant. The clotting process
required to produce serum can artificially lower the viral load by
trapping viral particles in the resulting clot. Although the preferred
2
CA 02335409 2001-07-19
P-3240P 1
sample type is plasma, the preparation of a plasma sample may
adversely affect the outcome of the amplification process. For
example, if the plasma sample remains in contact with the red blood
cells, heme molecules from the hemoglobin contained within red blood
cells will interfere with PCR amplification if hemolysis occurs. In
addition, since the half-life of the neutrophils is approximately 24
hours in a blood collection tube, and as the neutrophils begin to die
they release granules which contain myeloperoxidase into the sample,
and since myeloperoxidase causes reduction in the viral load, this is
also another factor that supports the need to sequester the plasma
sample away from blood cells.
A further example of the difficulties associated with current
plasma preparation is the fact that blood collection tubes may contain
a liquid anticoagulant to prevent clotting of the sample. A liquid
anticoagulant may dilute the viral load value per volume of sample.
Therefore, the viral load value may be below the threshold of detection.
Commercially available blood collection products such as (all
sold by Becton Dickinson and Company, Franklin Lakes, NJ and all
registrations and trademarks are of Becton Dickinson and Company)
VACUTAINER*Bra.nd Hematology tubes, Catalog nos. 367650-I,
367661, 6405, 6385, 6564, 367653, 367665, 367658, 367669, 6450-8,
6535-37; 367662; VACUTAINER Brand K~EDTA tubes catalog no.
367841-2, 367856, 367861; VACUTAINER Brand PST tubes catalog
nos. 367793-4, 6698, 6595, 6672; VACUTAINER Brand CPT tubes
2~ catalog nos. 362753, 362760-1; VACUTAINER Brand SST tubes
* Trademark 3
CA 02335409 2001-02-22
P-3240P 1
catalog nos. 367782-89, 6509-17, 6590-92; and VACUTAINER Brand
ACD tubes catalog nos. 367756, 364012, 4816; may be used for nucleic
acid testing. However, these commerically available products may not
consistently provide a sample of good integrity and therefore may not
provide consistent and adequate amplification results.
Therefore, a need exists to provide a standard device designed to
collect, process, and transport plasma samples for use with
amplification technologies. Most preferably, the device should be able
to assist in standardizing specimen handling, provide a closed system,
isolate the plasma from the cellular components, produce minimal
plasma dilution, and minimize interference with the nucleic acid
testing.
4
CA 02335409 2001-02-22
P-3240P 1
SITVINIARY OF THE INVENTION
The present invention is a device for preparing a plasma
specimen suitable for diagnostic assays, such as nucleic acid testing.
The device comprises a plastic or glass tube, a means for inhibiting
blood coagulation, and a means for separating plasma from whole
blood. The device preferably further comprises a means for closing the
tube to seal a vacuum within the tube, and for providing easy access
into the tube.
Preferably, the means for inhibiting blood coagulation is an
anticoagulant formulation.
Desirably, the anticoagulant formulation comprises a mixture of
water, ethylenediaminetetraacetic acid dipotassium salt dihydrate,
also known collectively as K2EDTA or alternatively,
ethylenediaminetetraacetic acid tripotassium salt dihydrate, also
known collectively as KgEDTA. Most preferably, the anticoagulant
formulation comprises K2EDTA having a chemical composition of
2(CH2COOK)-C2-N2-H4-2(CH2COOH)-2(H20).
Most preferably, the K2EDTA formulation is spray dried over a
large surface area of the inner wall of the tube to substantially reduce
the local osmolality and concentration gradients between the
anticoagulant and cells of the blood sample, thereby substantially
minimizing the possibility of hemolysis and cell rupture within the
blood sample.
5
CA 02335409 2001-02-22
P-3240P 1
Preferably, the means for separating plasma from whole blood is
a gel formulation. The gel is desirably a thixotropic polymeric gel
formulation. The gel desirably isolates the plasma from the cells of the
blood sample in the tube by serving as a density separation medium.
As the sample is centrifuged, the gel moves to a point dividing the
heavier cellular materials and the lighter plasma fraction of the blood
sample. In other words, the plasma of the blood sample is partitioned
above the gel and separated from the remainder of the blood.
Most preferably, the tube comprises the gel positioned at the
bottom end of the tube and the anticoagulant formulation is then
spray-dried onto the interior of the tube above the gel.
The device of the present invention is useful in molecular.
diagnostic applications, including but not limited to nucleic acid
testing, RNA and DNA detection and quantification, using
amplification methods. Accordingly, the present invention provides an
improved method for handling and preparing plasma samples for
nucleic acid testing, because the separation of the plasma from the
whole blood can be accomplished at the point of collection and may
minimize any changes or degradation of the nucleic acid.
The device of the present invention provides a one-step closed
system for collecting blood, separating plasma, and transporting a
specimen for nucleic acid testing. The device substantially maximizes
the capabilities of PCR, bDNA, NASBA or other amplification
techniques, by providing a substantially consistent sample, whereby
6
CA 02335409 2001-02-22
P-3240P 1
test-to-test variability due to sample quality and variation may be
minimized and standardization of sample handling may be facilitated.
In addition, the device of the present invention provides an
isolated specimen that is protected when prompt centrifugation at the
S point of collection is employed and the stability of the specimen is
improved during transport. Additional attributes of the device of the
present invention are that a spray-dried anticoagulant formulation,
which provides a substantially stable blood-to-additive ratio over the
shelf life of the tube, whereby the device substantially isolates plasma
from cells and substantially minimizes sample degradation due to the
neutrophils and red blood cells.
Most notably is that the device of the present invention provides.
a closed system for collecting a blood specimen; means for
anticoagulating the blood without any substantial dilution; means for
facilitating separation of the plasma from the remainder of the whole
blood by a gel barrier; means for freezing the plasma within the device;
and means for transporting the specimen to an analytical site while
maintaining sample quality and integrity. Therefore the device of the
present invention provides the means to derive an undiluted plasma
within a closed-system configuration with minimal test-to-test
variations as compared to commercially available devices.
Important attributes of the device of the present invention are
that it is (i) compatible with the molecular technologies that are used
for nucleic acid testing; (ii) provides a substantially pure plasma
specimen with substantially less cellular contamination as compared
7
CA 02335409 2001-02-22
P-3240P 1
to devices that have no gel barrier and (iii) allows for an undiluted
plasma specimen which enhances the sensitivity of various molecular
technologies, especially for specimens with a low viral titer.
DESCRIPTION OF THE DRAWINGS
FIG. 1 is a perspective view of a typical blood collection tube
with a stopper.
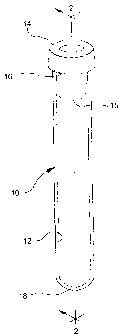
FIG. 2 is a longitudinal section view of the tube of FIGURE 1
taken along line 2-2, comprising the spray dried anticoagulant
formulation and the gel of the present invention.
CA 02335409 2001-02-22
P-3240P 1
DETAILED DESCRIPTION
The present invention may be embodied in other specific forms
and is not limited to any specific embodiments described in detail,
which are merely exemplary. Various other modifications will be
apparent to and readily made by those skilled in the art without
departing from the scope and spirit of the invention. The scope of the
invention will be measured by the appended claims and their
equivalents.
The device of the present invention preferably comprises a
spray-dried anticoagulant formulation and a gel. The device of the
present invention is most preferably a blood collection device and may
be either an evacuated blood collection device or a non-evacuated blood
collection device. The blood collection device is desirably made of
plastic, such as but not limited to polyethylene terephthalate, or
polypropylene, or glass.
Referring to the drawings in which like reference characters
refer to like parts throughout the several views thereof, FIG. 1 shows a
typical blood collection device 10, having an open end 16, a closed end
18, inner wall 12, and a stopper 14 that includes a lower annular
portion or skirt 15 which extends into and presses against the inner
wall 12 of the tube for maintaining stopper 14 in place.
FIG. 2 shows device 10 with a gel 20 and above the gel along
inner wall 12 is an anticoagulant coating 22.
9
CA 02335409 2001-02-22
P-3240P 1
A blood specimen sample of interest can be transferred into
device 10, wherein the specimen contacts the anticoagulant
formulation so that the anticoagulant formulation rapidly dissolves
into the specimen and clotting of the specimen is minimized.
After blood is collected in the device of the present invention, a
cascade reaction may occur that causes the blood to clot.
Anticoagulants are materials that are used to prevent the clotting of
blood by blocking the cascade mechanism that causes clotting. To
collect a plasma sample from whole blood, an anticoagulant must be
added immediately to preserve the integrity of the sample. There are
commercially available tubes for plasma collection that contain
numerous types of anticoagulants, such as sodium citrate, heparin,
potassium EDTA and the like. The selection of the type of
anticoagulant is important because some additives may interfere with
bDNA, PCR, or other amplification techniques used in nucleic acid
testing. For example, heparin may interfere with PCR amplification.
Preferably, the anticoagulant formulation of the present
invention comprises a mixture of water, ethylenediaminetetraacetic
acid dipotassium salt dehydrate, also know collectively as KZEDTA.
The concentration of the anticoagulant formulation is
substantially sufficient for minimizing coagulation of a blood specimen
sample. Desirably, the concentration of KZ EDTA is from about 0.2M
to about 1.0M, preferably from about 0.2M to about 0.5M and most
preferably from about 0.3M to about 0.4M.
CA 02335409 2001-02-22
P-3240P 1
The anticoagulant formulation desirably has a pH ranging from
about 5.6 to about 6.2, and preferably from about 5.8 to about 6.2.
The anticoagulant formulation of the present invention may
include, additional reagents in order to provide additional properties to
the device.
A variety of tube coatings or the addition of other compounds to
the anticoagulant formulation may be desirable. Such things include
but are not limited to silicone oils and silicone surfactants.
Preferably, the gel is a thixotropic polymeric gel. The gel
preferably has a specific gravity from about 1.040 to about 1.080 g/cm3
and most preferably from about 1.043 to about 1.050 g/cm3 so that
after centrifugation, the plasma of the blood sample is partitioned-
above the gel and separated from the remainder of the whole blood.
The thixotropic polymeric gel is substantially water insoluble
and substantially chemically inert in blood. The gel may be
formulated from dimethyl polysiloxane or polyester and a precipitated
methylated silica, wherein the methylation renders the material
partially hydrophobic.
The thixotropic polymer gel is first deposited into a tube at the
closed end, then the anticoagulant formulation of K2EDTA and water
is applied onto the inner wall of the tube above the gel in the form of
fine mist by spray coating. The applied formulation is then dried by
air jet or forced air at an elevated temperature for a period of time.
Thereafter, the tube is assembled with a closure and a vacuum is
11_
CA 02335409 2001-02-22
P-3240P 1
formed inside the tube. The device is then sterilized by gamma
irradiation or the like.
The main advantages of a tube with a spray coated
anticoagulant formulation on the inner wall are more precise, stable
and uniform anticoagulant fill and improved anticoagulant dissolution
into the specimen. Because of the fine mist of the anticoagulant
formulation, the actual surface area of anticoagulant formulation
exposed to the specimen is maximized.
The method for preparing the device of the present invention
comprises:
(a) depositing a gel into the closed end of a tube;
(b) preparing an anticoagulant formulation comprising a
mixture of water, ethylenediaminetetraacetic acid dipotassium salt
dihydrate at a concentration from about 0.2M to about 1.0M and a pH
from about 5.6 to about 6.2;
(c) applying the anticoagulant formulation to the inner wall
surface of the tube with a means that produces a fine mist of the
formulation above the gel; and
(d) drying the applied formulation by applying an air jet or
forced air to the inner wall of the coated tube at an elevated
temperature for a period of time.
It is preferable that the anticoagulant formulation is metered
and dispensed by a volumetric type device, such as a positive
displacement pump. The solution concentration (amount of
12
CA 02335409 2001-02-22
P-3240P 1
anticoagulant per unit volume of formulation) is tailored with the
dispense volume so that the desired amount of anticoagulant is
dispensed into the device. Other spraying techniques include
ultrasonic spraying.
The device of the present invention may be used to collect and
prepare a specimen for nucleic acid testing as follows:
(a) collecting a specimen such as a whole blood sample or a
pretreated cell fraction of blood into the prepared tube;
(b) mixing the specimen in the tube with the anticoagulant
solution by manual inversion;
(c) centrifuging the tube to induce separation of plasma from
the red and white blood cells and platelets so that the gel migrates to a
point intermediate to the denser white and red blood cells and
platelets and the less dense plasma fraction of the blood sample,
thereby facilitating isolation and subsequent removal of the plasma.
Various other modifications will be apparent to and may be
readily made by those skilled in the art without departing from the
scope and spirit of the invention.
13