Note: Descriptions are shown in the official language in which they were submitted.
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
RECOVERY OF HEAVY HYDROCARBONS BY IN-SITU HYDROVISBREAKING
Background of the Invention
Field of the Invention
This invention relates to a process for simultaneously upgrading and
recovering heavy
crude oils and natural bitumens from subsurface reservoirs.
Description of the Prior Art
Worldwide deposits of natural bitumens (also referred to as "tar sands") and
heavy crude
oils are estimated to total more than five times the amount of remaining
recoverable reserves of
conventional crude [References 1,5]. But these resources (herein collectively
called "heavy
hydrocarbons") frequently cannot be recovered economically with current
technology, due
principally to the high-viscosities which they exhibit in the porous
subsurface formations where
they are deposited. Since the- rate at which a fluid flows in a porous medium
is inversely
proportional to the fluid's viscosity, very viscous hydrocarbons lack the
mobility required for
economic production rates.
Steam injection has been used for over 30 years to produce heavy oil
reservoirs
economically by exploiting the strong negative relationship between viscosity
and temperature
that all liquid hydrocarbons exhibit. This relationship is illustrated in the
drawing labeled
FIGURE 6, which includes plots 601, 603, 605, and 607 of viscosity as a
function of temperature
for heavy hydrocarbons from, respectively, the Street Ranch, Saner Ranch,
Athabasca, and
Midway Sunset deposits [Reference 6].
In one method of steam-assisted production, steam is injected into a formation
through a
borehole so that a portion of the heavy oil in the formation is heated,
thereby significantly
reducing its viscosity and increasing its mobility. Steam injection is then
halted and the oil is
produced through the same borehole. In a second method, after the oil-bearing
formation is
preheated sufficiently by steam injection into all boreholes, steam is
continuously injected into
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
2
the formation through a set of injection boreholes to drive oil to a set of
production boreholes.
Referring again to FIG. 6, the plots show that heating the heavy hydrocarbons
from say
100 F, a typical temperature for the subsurface deposits in which the
hydrocarbons are found, to
400 F, a temperature that could be achieved in a subsurface deposit by
injecting steam from the
surface, reduces the viscosity of each of the four hydrocarbons by three to
four orders of
magnitude. Such viscosity reductions will not, however, necessarily result in
economic
production. The viscosity of Midway Sunset oil at 400 F approaches that of a
conventional
crude, which makes it economic to produce. But even at 400 F, the viscosities
of the bitumens
from Athabasca, Street Ranch, and Saner Ranch are 50 to 100 times greater than
the levels
required to ensure economic rates of recovery. Moreover, the high viscosities
of many heavy
hydrocarbons, when coupled with commonly encountered levels of formation
permeability, make
the injection of steam or other fluids which might be used for heating a
hydrocarbon-bearing
formation difficult or nearly impossible.
In addition to high viscosity, heavy hydrocarbons often exhibit other
deleterious
properties which cause their refining into marketable products to be a
significant challenge.
These properties are compared in Table 1 for an internationally-traded light
crude, Arabian Light,
and three heavy hydrocarbons.
Table I
Properties of Heavy Hydrocarbons Compared to a Light Crude
Light Crude Heavy Hydrocarbons
Properties Arabian Light Orinoco Cold Lake San Miguel
Gravity, API 34.5 8.2 11.4 -2 to 0
Viscosity, cp @ 100 F 10.5 7,000 10,700 >1,000,000
Sulfur,wt% 1.7 3.8 4.3 7.9to9.0
Nitrogen, wt % 0.09 0.64 0.45 0.36 to 0.40
Metals, wppm 25 559 260 109
Bottoms (975 F +), vol % 15 59.5 51 71.5
Conradson carbon residue, wt % 4 16 13.1 24.5
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
3
The high levels of undesirable components found in the heavy hydrocarbons
shown in
Table 1, including sulfur, nitrogen, metals, and Conradson carbon residue,
coupled with a very
high bottoms yield, require costly refining processing to convert the heavy
hydrocarbons into
product streams suitable for the production of transportation fuels.
Two fundamental alternatives exist for the upgrading of heavy hydrocarbon
fractions:
carbon rejection and hydrogen addition.
~ Carbon-rejection schemes break apart (or "crack") carbon bonds in a heavy
hydrocarbon
fraction and isolate the resulting asphaltenes from the lighter fractions. As
the
asphaltenes have significantly higher carbon-to-hydrogen ratios and higher
concentrations
of contaminants than the original feed, the product stream has a lower carbon-
to-
hydrogen ratio and significantly less contamination than the feed. Although
less
expensive than hydrogen-addition processes, carbon rejection has major
disadvantages-significant coke production and low yields of liquid products
which are
of inferior quality.
~ Hydrogen-addition schemes convert unsaturated hydrocarbons to saturated
products and
high-molecular-weight hydrocarbons to hydrocarbons with lower molecular
weights
while removing contaminants without creating low-value coke. Hydrogen addition
thereby provides a greater volume of total product than carbon rejection. The
liquid
product yield from hydrogen-addition processes can be 20 to 25 volume percent
greater
than the yield from processes employing carbon rejection. But these processes
are
expensive to apply and employ severe operating conditions. Catalytic
hydrogenation,
with reactor residence times of one to two hours, operate at temperatures in
the 700 to
850 F range with hydrogen partial pressures of 1,000 to 3,000 psi.
Converting heavy crude oils and natural bitumens to upgraded liquid
hydrocarbons while
still in a subsurface formation, which is the object of the present invention,
would address the
two principal shortcomings of these heavy hydrocarbon resources-the high
viscosities which
heavy hydrocarbons exhibit even at elevated temperatures and the deleterious
properties which
make it necessary to subject them to costly, extensive upgrading operations
after they have been
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
4
produced. However, the process conditions employed in refinery units to
upgrade the quality of
liquid hydrocarbons would be extremely difficult to achieve in the subsurface.
The injection of
catalysts would be exceptionally expensive, the high temperatures used would
cause unwanted
coking in the absence of precise control of hydrogen partial pressures and
reaction residence
time, and the hydrogen partial pressures required could cause random,
unintentional fracturing of
the formation with a potential loss of control over the process.
A process occasionally used in the recovery of heavy crude oil and natural
bitumen which
to some degree converts in the subsurface heavy hydrocarbons to lighter
hydrocarbons is in situ
combustion. In this process an oxidizing fluid, usually air, is injected into
the hydrocarbon-
bearing formation at a sufficient temperature to initiate combustion of the
hydrocarbon. The heat
generated by the combustion warms other portions of the heavy hydrocarbon and
converts a part
of it to lighter hydrocarbons via uncatalyzed thermal cracking, which may
induce sufficient
mobility in the hydrocarbon to allow practical rates of recovery.
While in situ combustion is a relatively inexpensive process, it has major
drawbacks. The
high temperatures in the presence of oxygen which are encountered when the
process is applied
cause coke formation and the production of olefins and oxygenated compounds
such as phenols
and ketones, which in turn cause major problems when the produced liquids are
processed in
refinery units. Commonly, the processing of products from thermal cracking is
restricted to
delayed or fluid coking because the hydrocarbon is degraded to a degree that
precludes
processing by other methods.
The present invention concems an in situ process which converts heavy
hydrocarbons to
lighter hydrocarbons that does not involve in situ combustion or the short
reaction residence
times, high temperatures, high hydrogen partial pressures, and catalysts which
are employed
when conversion reactions are conducted in refineries. Rather, conditions
which can readily be
achieved in hydrocarbon-bearing formations are utilized; viz., reaction
residence times on the
order of days to months, lower temperatures, lower hydrogen partial pressures,
and the absence
of injected catalysts. These conditions sustain what we designate as "in situ
hydrovisbreaking,"
conversion reactions within the formation which result in hydrocarbon
upgrading similar to that
achieved in refinery units through catalytic hydrogenation and hydrocracking.
The present
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
invention utilizes a unique combination of operations and associated hardware,
including the use
of a downhole combustion apparatus, to achieve hydrovisbreaking in formations
in which high-
viscosity hydrocarbons and commonly encountered levels of formation
permeability combine to
limit fluid mobility.
Following is a review of the prior art as related to the operations
incorporated into this
invention. The patents referenced teach or suggest a means for enhancing flow
of heavy
hydrocarbons within a reservoir, the use of a downhole apparatus for in situ
operations,
procedures for effecting in situ conversion of heavy crudes and bitumens, and
methods for
recovering and processing the produced hydrocarbons.
In U.S. Patent 4,265,310, CONOCO patented the application of formation
fracturing to
steam recovery of heavy hydrocarbons.
Some of the best prior art disclosing the use of downhole devices for
secondary recovery
is found in U.S. Patents 4,159,743; 5,163,511; 4,865,130; 4,691,771;
4,199,024; 4,597,441;
3,982,591; 3,982,592; 4,024,912; 4,053,015; 4,050,515; 4,077,469; and
4,078,613. Other
expired patents which also disclose downhole generators for producing hot
gases or steam are
U.S. Patents 2,506,853; 2,584,606; 3,372,754; 3,456,721; 3,254,721; 2,887,160;
2,734,578; and
3,595,316.
The concept of separating produced secondary crude oil into hydrogen, lighter
oils, etc.
and the use of hydrogen for in situ combustion and downhole steaming
operations to recover
hydrocarbons are found in U.S. Patents 3,707,189; 3,908,762; 3,986,556;
3,990,513; 4,448,251;
4,476,927; 3,051,235; 3,084,919; 3,208,514; 3,327,782; 2,857,002; 4,444,257;
4,597,441;
4,241,790; 4,127,171; 3,102,588; 4,324,291; 4,099,568; 4,501,445; 3,598,182;
4,148,358;
4,186,800; 4,233,166; 4,284,139; 4,160,479; and 3,228,467. Additionally, in
situ hydrogenation
with hydrogen or a reducing gas is taught in U.S. Patents 5,145,003;
5,105,887; 5,054,551;
4,487,264; 4,284;139; 4,183,405; 4,160,479; 4,141,417; 3,617,471; and
3,228,467.
U.S. Patents 3,598,182 to Justheim; 3,327,782 to Hujsak; 4,448,251 to Stine;
4,501,445
to Gregoli; and 4,597,441 to Ware all teach variations of in situ
hydrogenation which more
closely resemble the current invention:
~ Justheim, 3,327,782 modulates (heats or cools) hydrogen at the surface. In
order to
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
6
initiate the desired objectives of "distilling and hydrogenation" of the in
situ hydrocarbon,
hydrogen is heated on the surface for injection into the hydrocarbon-bearing
formation.
~ Hujsak, 4,448,251 teaches that hydrogen is obtained from a variety of
sources and
includes the heavy oil fractions from the produced oil which can be used as
reformer fuel.
Hujsak also includes and teaches the use of forward or reverse in situ
combustion as a
necessary step to effect the objectives of the process. Furthermore, heating
of the injected
gas or fluid is accomplished on the surface, an inefficient means of heating
compared to
using a downhole combustion unit because of heat losses incurred during
transportation
of the heated fluids to and down the borehole.
~ Stine, 4,448,251 utilizes a unique process which incorporates two adjacent,
non-
communicating reservoirs in which the heat or thermal energy used to raise the
formation
temperature is obtained from the adjacent reservoir. Stine utilizes in situ
combustion or
other methods to initiate the oil recovery process. Once reaction is achieved,
the desired
source of heat is from the adjacent zone.
~ Gregoli, 4,501,445 teaches that a crude formation is subjected to fracturing
to form "an
underground space suitable as a pressure reactor," in situ hydrogenation, and
conversion
utilizing hydrogen and/or a hydrogen donor solvent, recovery of the converted
and
produced crude, separation at the surface into various fractions, and
utilization of the
heavy residual fraction to produce hydrogen for re-injection. Heating of the
injected
fluids is accomplished on the surface which, as discussed above, is an
inefficient process.
~ Ware, 4,597,441 describes in situ "hydrogenation" (defined as the addition
of hydrogen to
the oil without cracking) and "hydrogenolysis" (defined as hydrogenation with
simultaneous cracking). Ware teaches the use of a downhole combustor.
Reference is
made to previous patents relating to a gas generator of the type disclosed in
U.S. Patents
3,982,591; 3,982,592; or 4,199,024. Ware further teaches and claims injection
from the
combustor of superheated steam and hydrogen to cause hydrogenation of
petroleum in
the formation. Ware also stipulates that after injecting superheated steam and
hydrogen,
sufficient pressure is maintained "to retain the hydrogen in the heated
formation zone in
contact with the petroleum therein for 'soaking' purposes for a period of
time." In some
CA 02335737 2006-07-07
77643-13
7
embodiments Ware includes combustion of petroleum products
in the formation- a major disadvantage, as discussed
earlier- to drive fluids from the injection to the
production wells.
None of the patents referenced above teach the
application of fracturing or related methods to the
hydrocarbon-bearing formation for the purpose of enhancing
fluid mobility. In contrast, the Gregoli and Ware patents
both teach that injected fluids must be confined with the in
situ hydrocarbons to allow time for conversion reactions to
take place. Further, none of the patents referenced include
in situ conversion exclusively without combustion of the
hydrocarbon in the formation.
Another group of U.S. Patents- including 5,145,003
and 5,054,551 to Duerksen; 4,160,479 to Richardson;
4,284,139 to Sweany; 4,487,264 to Hyne; and 4,141,417 to
Schora- all teach variations of hydrogenation with heating
of the injected fluids (hydrogen, reducing gas, steam, etc.)
accomplished at the surface. Further, Schora, 4,141,417
injects hydrogen and carbon dioxide at a temperature of less
than 300 F and claims to reduce the hydrocarbon's viscosity
and accomplish desulfurization. Viscosity reduction is
assumed primarily through the well-known mechanism involving
solution of carbon dioxide in the hydrocarbon. None of
these patents includes the use of a downhole combustion unit
for injection of hot reducing gases.
In light of the current state of the technology,
what is needed- and what has been discovered by us- is an
efficient process for converting, and thereby upgrading,
very heavy hydrocarbons in situ without combustion of the
virgin hydrocarbon and the attendant degradation of products
CA 02335737 2006-07-07
77643-13
7a
which accompany combustion operations. The process
disclosed herein permits the production and utilization of
heavy-hydrocarbon resources which are otherwise not
economically recoverable by other methods and minimizes the
amount of surface processing required to produce marketable
petroleum products.
Objectives of the Invention
The primary objective of this invention is to
provide a method for the in situ upgrading
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
8
and recovery of heavy crude oils and natural bitumens. The process includes
the heating of a
targeted portion of a formation containing heavy crude or bitumen with steam
and hot reducing
gases to effect in situ conversion reactions-including hydrogenation,
hydrocracking,
desulfurization, and other reactions-referred to collectively as
hydrovisbreaking. Fracturing of
the subsurface formation or a related procedure is employed to enhance
injection of the required
fluids and increase the recovery rate of the upgraded hydrocarbons to an
economic level.
It is another objective of this invention that no combustion of the virgin
crude or bitumen
occur in the formation so as to minimize in situ degradation of the converted
hydrocarbons. In
the instant invention, virgin hydrocarbons are only subjected to reducing
conditions after being
heated by steam injection and hot combustion gases. Formation hydrocarbons and
converted
products are therefore never subjected to the oxidation conditions encountered
in conventional in
situ combustion operations, thereby eliminating the product degradation which
results from the
formation of unstable oxygenated components.
An additional objective of this invention is the utilization of a downhole
combustion unit
to provide a thermally efficient process for the injection of superheated
steam and hot reducing
gases adjacent to the subsurface formation, thereby vastly reducing the heat
losses inherent in
conventional methods of subsurface injection of hot fluids.
A further objective of this invention is to eliminate much of the capital-
intensive
conversion and upgrading facilities, such as catalytic hydrocracking, that are
required in
conventional processing of heavy hydrocarbons by upgradingthe hydrocarbons in
situ.
Summary of the Invention
This invention discloses a process for converting heavy crude oils and natural
bitumens in
situ to lighter hydrocarbons and recovering the converted materials for
further processing on the
surface. The conversion reactions-which may include hydrogenation,
hydrocracking,
desulMzation, and other reactions-are referred to herein as hydrovisbreaking.
Continuous
recovery utilizing one or more injection boreholes and one or more production
boreholes, which
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
9
may include horizontal boreholes, may be employed. Alternatively, a cyclic
method using one or
more individual boreholes may be utilized.
The conditions necessary for sustaining the hydrovisbreaking reactions are
achieved by
injecting superheated steam and hot reducing gases, comprised principally of
hydrogen, to heat
the formation to a preferred temperature and to maintain a preferred level of
hydrogen partial
pressure. This is accomplished through the use of downhole combustion units,
which are located
in the injection boreholes at a level adjacent to the heavy hydrocarbon
formation and in which
hydrogen is combusted with an oxidizing fluid while partially saturated steam
and, optionally,
additional hydrogen are flowed from the surface to the downhole units to
control the temperature
of the injected gases.
The method of this invention also includes the creation of horizontal or
vertical fractures
to enhance the injectibility of the steam and reducing gases and the mobility
of the hydrocarbons
within the formation so that the produced fluids are recovered at economic
rates. Alternatively, a
zone of either high water saturation or high gas saturation in contact with
the zone containing the
heavy hydrocarbon or a pathway between wells created by an essentially
horizontal borehole may
be utilized to enhance inter-well communication.
Prior to its production from the subsurface formation, the heavy hydrocarbon
undergoes
significant conversion and resultant upgrading in which the viscosity of the
hydrocarbon is
reduced by many orders of magnitude and in which its API gravity may be
increased by 10 to 15
degrees or more.
Followi.ng is a summary of the process steps for a preferred embodiment to
achieve the
objectives of this invention:
a. inserting downhole combustion units within injection boreholes, which
communicate
with production boreholes by means of horizontal fractures, at or near the
level of the subsurface
formation containing a heavy hydrocarbon;
b. for a first preheat period, flowing from the surface through said injection
boreholes
stoichiometric proportions of a reducing gas mixture and an oxidizing fluid to
said downhole
combustion units and igniting same in said downhole combustion units to
produce hot
combustion gases, including superheated steam, while flowing partially
saturated steam from the
CA 02335737 2006-07-07
77643-13
surface through said injection boreholes to said downhole
combustion units to control the temperature of said heated
gases and to produce additional superheated steam;
c. injecting said superheated steam into the
5 subsurface formation to heat a region of the subsurface
formation to a preferred temperature;
d. for a second conversion period, increasing the
ratio of reducing gas to oxidant in the mixture fed to the
downhole combustion units, or injecting reducing gas in the
10 fluid stream controlling the temperature of the combustion
units, to provide an excess of reducing gas in the hot gases
exiting the combustion units;
g. continuously injecting the heated excess
reducing gas and superheated steam into the subsurface
formation to provide preferred conditions and reactants to
sustain in situ hydrovisbreaking and thereby upgrade the
heavy hydrocarbon;
h. collecting continuously at the surface, from
said production boreholes, production fluids comprised of
converted liquid hydrocarbons, unconverted virgin heavy
hydrocarbons, residual reducing gases, hydrocarbon gases,
solids, water, hydrogen sulfide, and other components for
further processing.
According to another aspect the invention provides
a process for continuously converting, upgrading, and
recovering heavy hydrocarbons from a subsurface formation,
said process being free from the injection of hot oxidizing
fluids into said subsurface formation for the purpose of
igniting a portion of said heavy hydrocarbons and being free
CA 02335737 2006-07-07
77643-13
l0a
of injection of catalysts into the subsurface formation, and
said process comprising the steps of: a. inserting a
downhole combustion unit into at least one injection
borehole which communicates with at least one production
borehole, said downhole combustion unit being placed at a
position within said injection borehole in proximity to said
subsurface formation; b. flowing from the surface to said
downhole combustion unit within said injection borehole a
set of fluids- comprised of steam, reducing gases, and
oxidizing gases- and burning at least a portion of said
reducing gases with said oxidizing gases in said downhole
combustion unit; c. injecting a gas mixture- comprised of
combustion products from the burning of said reducing gases
with said oxidizing gases, residual reducing gases, and
steam- from said downhole combustion unit into said
subsurface formation; d. recovering from said production
borehole, production fluids comprised of said heavy
hydrocarbons, which may be converted to lighter
hydrocarbons, as well as residual reducing gases, and other
components; e. continuing steps b, c, and d until the
recovery rate of said heavy hydrocarbons within said
subsurface formation in the region between said injection
borehole and said production borehole is reduced below a
level of practical operation.
According to another aspect the invention provides
a process for cyclically converting, upgrading, and
recovering heavy hydrocarbons from a subsurface formation,
said process being free from the injection of hot oxidizing
fluids into said subsurface formation for the purpose of
igniting a portion of said heavy hydrocarbons and being free
of injection of catalysts into the subsurface formation, and
CA 02335737 2006-07-07
77643-13
lOb
said process comprising the steps of: a. inserting a
downhole combustion unit into at least one injection
borehole, said downhole combustion unit being placed at a
position within said injection borehole in proximity to said
subsurface formation; b. for a first period, flowing from
the surface to said downhole combustion unit within said
injection borehole a set of fluids- comprised of steam,
reducing gases, and oxidizing gases- and burning at least a
portion of said reducing gases with said oxidizing gases in
said downhole combustion unit; c. injecting a gas mixture-
comprised of combustion products from the burning of said
reducing gases with said oxidizing gases, residual reducing
gases, and steam- from said downhole combustion unit into
said subsurface formation; d. for a second period, upon
achieving a preferred temperature within said subsurface
formation, halting injection of fluids into the subsurface
formation while maintaining pressure on said injection
borehole to allow time for a portion of said heavy
hydrocarbons in the subsurface formation to be converted
into lighter hydrocarbons; e. for a third period, reducing
the pressure on said injection borehole, in effect
converting the injection borehole into a production
borehole, and recovering at the surface production fluids,
comprised of said heavy hydrocarbons, which may be converted
to lighter hydrocarbons, as well as residual reducing gases,
and other components; f. repeating steps b through e to
expand the volume of said subsurface formation processed for
the recovery of said heavy hydrocarbons until the recovery
rate of said heavy hydrocarbons within said subsurface
formation in the vicinity of said injection borehole is
below a level of practical operation.
CA 02335737 2006-07-07
77643-13
lOc
According to another aspect the invention provides
a process for cyclically- followed by continuously-
converting, upgrading, and recovering heavy hydrocarbons
from a subsurface formation, said process being free from
the injection of hot oxidizing fluids into said subsurface
formation for the purpose of igniting a portion of said
heavy hydrocarbons and being free of injection of catalysts
into the subsurface formation, and said process comprising
the steps of: a. inserting downhole combustion units into at
least two injection boreholes, said downhole combustion
units being placed at a position within said injection
boreholes in proximity to said subsurface formation; b. for
a first period, flowing from the surface to said downhole
combustion units within said injection boreholes a set of
fluids- comprised of steam, reducing gases, and oxidizing
gases- and burning at least a portion of said reducing gases
with said oxidizing gases in said downhole combustion units;
c. injecting a gas mixture- comprised of combustion products
from the burning of said reducing gases with said oxidizing
gases, residual reducing gases, and steam- from said
downhole combustion units into said subsurface formation; d.
for a second period, upon achieving a preferred temperature
within said subsurface formation, halting injection of
fluids into the subsurface formation while maintaining
pressure on said injection boreholes to allow time for a
portion of said heavy hydrocarbons in the subsurface
formation to be converted into lighter hydrocarbons; e. for
a third period, reducing the pressure on said injection
boreholes, in effect converting the injection boreholes into
production boreholes, and recovering at the surface
production fluids, comprised of said heavy hydrocarbons,
which may be converted to lighter hydrocarbons, as well as
residual reducing gases, and other components; f. repeating
CA 02335737 2006-07-07
77643-13
lOd
steps b through e to expand the volume of said subsurface
formation processed for the recovery of said heavy
hydrocarbons until the recovery rate of said heavy
hydrocarbons within said subsurface formation in the
vicinity of said injection boreholes is below a level of
practical operation; g. from at least one injection
borehole, removing the downhole combustion unit and
permanently converting the borehole to a production
borehole; h. flowing from the surface to the remaining
downhole combustion units within the remaining injection
boreholes a set of fluids- comprised of steam, reducing
gases, and oxidizing gases- and burning at least a portion
of said reducing gases with said oxidizing gases in said
downhole combustion units; i. injecting a gas mixture-
comprised of combustion products from the burning of said
reducing gases with said oxidizing gases, residual reducing
gases, and steam- from said downhole combustion units into
said subsurface formation; j. recovering from said
production borehole, production fluids comprised of said
heavy hydrocarbons, which may be converted to lighter
hydrocarbons, as well as residual reducing gases, and other
components; k. continuing steps h, i, and j until the
recovery rate of said heavy hydrocarbons within said
subsurface formation in the region between the remaining
injection boreholes and said production borehole is reduced
below a level of practical operation.
Brief Description of the Drawings
FIG. 1 is a schematic of a preferred embodiment of
the invention in which injection boreholes and production
boreholes are utilized in a continuous fashion. Steam and
CA 02335737 2006-07-07
77643-13
10e
hot reducing gases from downhole combustion units in the
injection boreholes are flowed toward the production
boreholes where upgraded heavy hydrocarbons are collected
and produced.
FIG. 2 is a modification of FIG. 1 in which a
cyclic operating mode is illustrated whereby both the
injection and production operations occur in the same
borehole, with the recovery process operated as an injection
period followed by a production period. The cycle is then
repeated.
FIG. 3A is a plan view and FIG. 3B is a profile
view of another embodiment featuring the use of horizontal
boreholes. Injection of hot gases and steam is carried out
in vertical boreholes
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
Il
in which vertical fractures have been created. The vertical fractures are
penetrated by one or
more horizontal production boreholes to efficiently collect the upgraded heavy
hydrocarbons.
FIG. 4 is a plan view of a square production pattern showing an injection well
at the
center of the pattern and production wells at each of the corners. Contour
lines within the pattecn
show the general distribution of injectants and temperature at a time midway
through the
production period.
FIG. 5 is a graph showing the recovery of oil in three cases A, B, and C using
the process
of the invention compared with a Base Case in which only steam was injected
into the reservoir.
The production patterns of the Base Case and of Cases A and B encompass 5
acres. The
production pattern of Case C encompasses 7.2 acres. FIG. 5 shows for the four
cases the
cumulative oil recovered as a percentage of the original oil in place (OOIP)
as a function of
production time.
FIG. 6 is a graph in which the viscosities of four heavy hydrocarbons are
plotted as a
function of temperature.
Description of the Preferred Embodiments
This invention discloses a process designed to upgrade and recover heavy
hydrocarbons
from subsurface formations which may not otherwise be economically recoverable
while
eliminating many of the deleterious and expensive features of the prior art.
The invention
incorporates mtlltiple steps including: (a) use of downhole combustion units
to provide a means
for direct injection of superheated steam and hot reactants into the
hydrocarbon-bearing
formation; (b) enhancing injectibility and inter-well communication within the
formation via
formation fracturing or related methods; (c) in situ hydrovisbreaking of the
heavy hydrocarbons
in the formation by establishing suitable subsurface conditions via injection
of superheated steam
and reducing gases; (d) production of the upgraded hydrocarbons; (e)
additional processing of the
produced hydrocarbons on the surface to produce marketable products.
The process of in situ hydrovisbreaking as disclosed in this invention is
designed to
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
12
provide in situ upgrading of heavy hydrocarbons comparable to that achieved in
surface units by
modifying process conditions to those achievable within a reservoir-relatively
moderate
temperatures (625 to 750 F) and hydrogen partial pressures (500 to 1,200 psi)
combined with
longer residence times (several days to months) in the presence of naturally
occurring catalysts.
To effectively heat a heavy-hydrocarbon reservoir to the minimum desired
temperature of
625 F requires the temperature of the injected fluid be at least say 650 F,
which for saturated
steam corresponds to a saturation pressure of 2,200 psi. An injection pressure
of this magnitude
could cause a loss of control over the process as the parting pressure of
heavy-hydrocarbon
reservoirs, which are typically found at depths of about 1,500 ft, is
generally less than 1,900 psi.
Therefore, it is impractical to heat a heavy-hydrocarbon reservoir to the
desired temperature
using saturated steam alone. Use of conventionally generated superheated steam
is also
impractical because heat losses in surface piping and wellbores can cause
steam-generation costs
to be prohibitively high.
The limitation on using steam generated at the surface is overcome in this
invention by
use of a downhole combustion unit, which can provide heat to the subsurface
formation in a
more efficient manner. In its preferred-operating mode, hydrogen is combusted
with oxygen with
the temperature of the combustion gases controlled by injecting partially
saturated steam,
generated at the surface, as a cooling medium. The superheated steam resulting
from using
partially saturated steam to absorb the heat of combustion in the combustion
unit and the hot
reducing gases exiting the combustion unit are then injected into the
formation to provide the
thermal energy and reactants required for the process.
Alternatively, a reducing-gas mixture-comprised principally of hydrogen with
lesser
amounts of carbon monoxide, carbon dioxide, and hydrocarbon gases-may be
substituted for
the hydrogen sent to the downhole combustion unit. A reducing-gas mixture has
the benefit of
requiring less purification yet still provides a means of sustaining the
hydrovisbreaking reactions.
The downhole combustion unit is designed to operate in two modes. In the first
mode,
which is utilized for preheating the subsurface formation, the unit combusts
stoichiometric
amounts of reducing gas and oxidizing fluid so that the combustion products
are principally
superheated steam. Partially saturated steam injected from the surface as a
coolant is also
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
13
converted to superheated steam.
In a second operating mode, the amount of hydrogen or reducing gas is
increased beyond
its stoichiometric proportion (or the flow of oxidizing fluid is decreased) so
that an excess of
reducing gas is present in the combustion products. Alternatively, hydrogen or
reducing gas is
injected into the fluid stream controlling the temperature of the combustion
unit. This operation
results in the pressurizing of the heated subsurface region with hot reducing
gas. Steam may also
be injected in this operating mode to provide an injection mixture of steam
and reducing gas.
The downhole combustion unit may be of any design which accomplishes the
objectives
stated above. Examples of the type of downhole units which may be employed
include those
described in U.S. Patents 3,982,591; 4,050,515; 4,597,441; and 4,865,130.
The downhole combustion unit may be designed to operate in a conventional
production
well by utilizing an annular configuration so that production tubing can
extend through the unit
while it is installed downhole. With such a design, fluids can be produced
from a well
containing the unit without removing any equipment from the wellbore.
Instead of having the production tubing extending through the unit, a gas
generator of the
type disclosed in U.S. Patent Nos.3,982,591 or 4,050,515 may be used for
heating the
hydrocarbon formation and then removed from the borehole to allow a separate
production-
tubing system to be inserted into the borehole for production purposes.
Ignition of the combustible mixture formed in the downhole combustion unit may
be
accomplished by any means including the injection of a pyroFihoric fluid with
the fuel gas to
initiate combustion upon contact with the oxidant, as described in U.S. Patent
5,163,511, or the
use of an electrical spark-generating device with electrical leads extending
from the surface to the
downhole combustion unit.
The very high viscosities exhibited by heavy hydrocarbons limit their mobility
in the
subsurface formation and make it difficult to bring the injectants and the in
situ hydrocarbons
into intimate contact so that they may create the desired products. Solutions
to this problem may
take several forms: (1) horizontally fractured wells, (2) vertically fractured
wells, (3) a zone of
high water saturation in contact with the zone containing the heavy
hydrocarbon, (4) a zone of
high gas saturation in contact with the zone containing the heavy hydrocarbon,
or (5) a pathway
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
14
between wells created by an essentially horizontal hole, such as established
by Anderson, U.S.
Patents 4,037,658 and 3,994,340.
These configurations may be used in several ways. Horizontal fractures may be
used in a
continuous mode of injection and production which requires multiple wells-at
least one injector
(preferably vertical) and at least one producer (preferably vertical)-or in a
cyclic mode with at
least one well (preferably vertical). Vertical fractures may be used either in
a continuous mode
with at least one injector (preferably vertical) and at least one producer
(preferably horizontal) or
a cyclic mode with at least one injector (preferably vertical).
When a zone of high water saturation is present in contact with the zone
containing a
heavy hydrocarbon, its presence is normally due to geological processes.
Therefore, not all
formations containing heavy hydrocarbons are in contact with a zone of high
water saturation.
Doscher, U.S. Patent 3,279,538, showed how to inject steam into such a water-
saturated zone to
establish communication between multiple wells in heavy oil reservoirs. In
such a case, and also
in the case of horizontal fractures used in the continuous mode, it is
important to inject the hot
fluid rapidly enough to establish a heated zone which completely extends
between at least two
wells. Failure to establish a heated zone can allow displaced, heated, heavy
oil to migrate into
the flow path (i.e., the fracture or the water zone), lose heat, thereby
become more viscous, and
halt the recovery process. The injection into a water-saturated zone can be
used either in the
continuous or cyclic mode.
A zone of high gas saturation in contact with the zone containing a heavy
hydrocarbon
also provides a conduit for flow between wells. Sceptre Resources Ltd.
successfully used steam
injection into a gas cap.in the Tangleflags Field in Saskatchewan to recover
the heavy oil
underlying a gas zone. A similar procedure would be possible with the in situ
hydrovisbreaking
process that is the subject of the present invention. In this case, the
location of the gas zone
above the heavy hydrocarbon might lessen the efficiency of the mixing of
reactants, several of
which are in the gas phase, but its high level of communication might more
than offset this
problem. Injection into a gas zone will probably only be efficient in the
continuous mode of
operation.
Anderson, 4,037,658 and 3,994,340, patented processes for establishing
communication
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
between two wells by drilling an essentially horizontal hole connecting the
wells that is separated
from the surrounding formation by casing. One of the wells serves as a point
of injection, while
the other serves as a point of production. At the beginning of the recovery
process, steam is
injected into the injection well and flows into the horizontal casing, which
is not perforated
except at the end near the producing well. The passage of steam through the
horizontal pipe
heats the surrounding formation by conduction to the point where the viscosity
of the heavy
hydrocarbon in the formation drops low enough to permit it to flow under
typical injection
pressures. Then, hot reaction gases are injected into the formation at the
bottom of the injection
well. Since the heavy hydrocarbon is now mobile, the injectants are able to
displace heavy
hydrocarbon into the producing well through the heated annulus that surrounds
the hot,
horizontal pipe. In time the heated zone grows larger, sustaining itself from
the hot injected
fluids and the exothermic reactions that have been initiated, and no longer
requires heat from
inside the horizontal pipe.
A significant disclosure of this invention is that use of fractures within the
subsurface
formation or the other related methods just discussed are consistent with
controlling the injection
of fluids into the reaction zone. As illustrated in a following example,
creating fractures in a
reservoir can significantly enhance the rate of fluid injection and the degree
of fluid mobility
within a heavy-hydrocarbon formation resulting in greatly increased recovery
of converted
hydrocarbons.
The steps necessary to provide the conditions requirecl for the in situ
hydrovisbreaking
reactions to occur may be implemented in a continuous mode, a cyclic mode, or
a combination of
these modes. The process may include the use of conventional vertical
boreholes or horizontal
boreholes. Any method known to those skilled in the art of reservoir
engineering and
hydrocarbon production may be utilized to effect the desired process within
the required
operating parameters.
In the continuous operating mode, a number of boreholes are utilized for
injection of
steam and hot reducing gases. The injected gases flow through the subsurface
formation, contact
and react with the in situ hydrocarbons, and are recovered along with the
upgraded hydrocarbons
in a series of production boreholes. The injection and production boreholes
may be arranged in
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
16
any pattern amenable to the eflicient recovery of the upgraded hydrocarbons.
The rate of
withdrawal of fluids from the production boreholes may be adjusted to control
the pressure and
the distribution of gases within the subsurface formation.
rn the cyclic operating mode, multiple boreholes are operated independently in
a cyclic
fashion consisting of a series of injection and production periods. In the
initial injection period,
steam and hot reducing gases are injected into the region adjacent to the
wellbore. After a period
of soaking to allow conversion reactions to occur; the pressure on the
wellbore is reduced and
upgraded hydrocarbons are recovered during a production period. In subsequent
cycles, this
pattern of injection and production is repeated with an increasing extension
into the subsurface
formation.
A hybrid operating mode is also disclosed in which the subsurface formation is
first
treated using a series of boreholes employing the cyclic mode just described.
After this mode is
used to the limit of practical operation, a portion of the injection boreholes
are converted to
production boreholes and the process is operated in a continuous mode to
recover additional
hydrocarbons bypassed during the cyclic operation.
After completion of any of the procedures outlined above for recovery of
upgraded
hydrocarbons, it may be beneficial to utilize surfactants (surface active
agents such as soap)
which have been found to enhance oil recovery from steam-injection processes.
These will also
aid in oil recovery for the process of this invention. High-temperature
surfactants (surfactants
which retain their function at high temperatures) may be injected during the
period of the
operation in which the temperature of the injected fluids is less than the
limit at which they are
effective. Similarly, low-temperature surfactants-which include sodium
hydroxide, potassium
hydroxide, potassium carbonate, potassium orthosilicate, and other similar
high-pH, inorganic
compounds-may be injected. These surfactants react with the naturally
occurring carboxylic
acids in the in situ hydrocarbons to form natural surfactants, which will have
beneficial effects on
recovery of heavy hydrocarbons. These surfactants will be injected in a late
stage of the process
during the implementation of a clean-up, or scavenging phase. This phase will
take advantage of
the injection of cold or warm water to transport heat from areas depleted in
heavy hydrocarbons
to other undepleted areas, and the injected surfactants will aid in scavenging
the remaining
CA 02335737 2006-07-07
77643-13
17
hydrocarbons.
Operation of the in situ hydrovisbreaking process
will be controlled utilizing available physical
measurements. Controllable elements include the injection
pressure, injection rate, temperature, and fluid
compositions of the injected gases. In addition, the back-
pressure maintained on production boreholes may be selected
to control the distribution of production rates among
various boreholes. Measurements may be taken at the
injection boreholes, production boreholes, and observation
wells within the production patterns. All of this
information can be gathered and processed, either manually
or by computer, to obtain the optimum degree of conversion,
product quality, and recovery level of the hydrocarbon
liquids being collected.
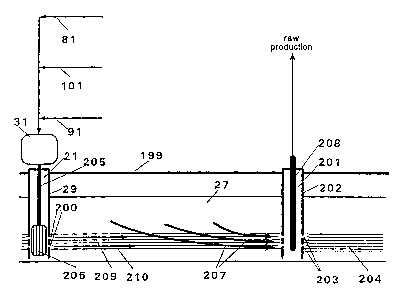
Referring to the drawing labeled FIG. 1, there is
illustrated a borehole 21 for an injection well drilled from
the surface of the earth 199 into a hydrocarbon-bearing
formation or reservoir 27. The injection-well borehole 21 is
lined with steel casing 29 and has a wellhead control system
31 atop the well to regulate the flow of reducing gas,
oxidizing fluid, and steam to a downhole combustion unit
206. The casing 29 contains perforations 200 to provide
fluid communication between the inside of the borehole 21
and the reservoir 27.
Also in FIG. 1, there is illustrated in a borehole
201 for a production well drilled from the surface of the
earth 199 into the reservoir 27 in the vicinity of the
injection-well borehole 21. The production-well borehole
201 is lined with steel casing 202. The casing 202 contains
perforations 203 to provide fluid communication between the
CA 02335737 2006-07-07
,77643-13
17a
inside of the borehole 202 and the reservoir 27. Fluid
communication within the reservoir 27 between the injection-
well borehole 21 and the production-well borehole 201 is
enhanced by hydraulically fracturing the reservoir in such a
manner as to introduce a horizontal fracture 204 between the
two boreholes.
Of interest is to inject hot gases into the
reservoir 27 by way of the injection-well borehole 21 and
continuously recover hydrocarbon products from the
production-well borehole 201. Referring again to FIG. 1,
three fluids under pressure are coupled to the wellhead
control system 31: a source of reducing gas by line 81, a
source of oxidizing-fluid by line 91, and a source of
cooling-fluid by line 101. Through injection tubing strings
205, the three fluids are coupled to the downhole combustion
unit 206. The fuel is oxidized by the oxidizing fluid in the
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
18
combustion unit 206, which is cooled by the cooling fluid. The products of
oxidation and the
cooling fluid 209 along with any un-oxidized fue1210, all of which are heated
by the exothermic
oxidizing reaction, are injected into the horizontal fracture 204 in the
reservoir 27 through the
perforations 200 in the casing 29. Heavy hydrocarbons 207 in the reservoir 27
are heated by the
hot injected fluids which, in the presence of hydrogen, initiate
hydrovisbreaking reactions. Thesc
reactions upgrade the quality of the hydrocarbons by converting their higher
molecular-weight
components into lower molecular-weight components which have less density,
lower viscosity,
and greater mobility within the reservoir than the unconverted hydrocarbons.
The hydrocarbons
subjected to the hydrovisbreaking reactions and additional virgin hydrocarbons
flow into the
perforations 203 of the casing 202 of the production-well borehole 201,
propelled by the pressure
of the injected fluids. The hydrocarbons and injected fluids arriving at the
production-well
borehole 201 are removed from the borehole using conventional oil-field
technology and flow
through production tubing strings 208 into the surface facilities. Any number
of injection wells
and production wells may be operated simultaneously while situated so as to
allow the injected
fluids to flow efficiently from the injection wells through the reservoir to
the production wells
contacting a significant portion of the heavy hydrocarbons in situ.
In the preferred embodiment, the cooling fluid is steam, the reducing gas is
hydrogen, and
the oxidizing fluid used is oxygen, whereby the product of oxidization in the
downhole
combustion unit 206 is superheated steam. This unit incorporates a combustion
chamber in
which the hydrogen and oxygen mix and react. Preferably, a'stoichiometric
mixture of hydrogen
and oxygen is initially fed to the unit during its operation. This mixture has
an adiabatic flame
temperature of approximately 5,700 F and must be cooled by the coolant steam
in order to
protect the combustion unit's materials of construction. After cooling the
downhole combustion
unit, the coolant steam is mixed with the combustion products, resulting in
superheated steam
being injected into the reservoir. Generating steam at the surface and
injecting it to cool the
downhole combustion unit reduces the amount of hydrogen and oxygen, and
thereby the cost,
required to produce a given amount of heat in the form of superheated steam.
The coolant steam
may include liquid water as the result of injection at the surface or
condensation within the
injection tubing. The ratio of the mass flow of steam passing through the
injection tubing 205 to
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
19
the mass flow of oxidized gases leaving the combustion unit 206 affects the
temperature at which
the superheated steam is injected into the reservoir 27. As the reservoir
becomes heated to the
level necessary for the occurrence of hydrovisbreaking reactions, it is
preferable that a
stoichiometric excess of hydrogen be fed to the downhole combustion unit
during its
operation-or that hydrogen be injected into the fluid stream controlling the
temperature of the
combustion unit-resulting in hot hydrogen being injected into the reservoir
along with
superheated steam. This provides a continued heating of the reservoir in the
presence of
hydrogen, which are the conditions necessary to sustain the hydrovisbreaking
reactions.
In another embodiment, a reducing-gas mixture-comprised principally of
hydrogen with
lesser amounts of carbon monoxide, carbon dioxide, and hydrocarbon gases-may
be substituted
for hydrogen. Such a mixture has the benefit of requiring less purification
yet still provides a
means of sustaining the hydrovisbreaking reactions.
FIG. 1 therefore shows a hydrocarbon-production system that continuously
converts,
upgrades, and recovers heavy hydrocarbons from a subsurface formation
traversed by one or
more injection boreholes and one or more production boreholes with inter-well
communication
established between the injection and production boreholes. The system is free
from any
combustion operations within the subsurface formation and free from the
injection of any
oxidizing materials or catalysts.
Referring to the drawing labeled FIG. 2, there is illustrated a borehole 21
for a well
drilled from the surface of the earth 199 into a hydrocarbon-bearing formation
or reservoir 27.
The borehole 21 is lined with steel casing 29 and has a wellhead control
system 31 atop the well.
The casing 29 contains perforations 200 to provide fluid communication between
the inside of
the borehole 21 and the reservoir 27. The ability of the reservoir to accept
injected fluids is
enhanced by hydraulically fracturing the reservoir to create a horizontal
fracture 204 in the
vicinity of the borehole 21.
Of interest is to cyclically inject hot gases into the reservoir 27 by way of
the borehole 21
and subsequently to recover hydrocarbon products from the same borehole.
Referring again to
FIG. 2, three fluids under pressure are coupled to the wellhead control system
31: a source of
reducing gas by line 81, a source of oxidizing-fluid by line 91, and a source
of cooling-fluid by
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
line 101. Through injection tubing strings 205, the three fluids are coupled
to a downhole
combustion unit 206. The combustion unit is of an annular configuration so
tubing strings can be
run through the unit when it is in place downhole. During the injection phase
of the process, the
fuel is oxidized by the oxidizing fluid in the combustion unit 206, which is
cooled by the cooling
fluid in order to protect the combustion unit's materials of construction. The
products of
oxidation and the cooling fluid 209 along with any un-oxidized fuel 210, all
of which are heated
by the exothermic oxidizing reaction, are injected into the horizontal
fracture 204 in the reservoir
27 through the perforations 200 in the casing 29. As in the continuous-
production process, heavy
hydrocarbons 207 in the reservoir 27 are heated by the hot injected fluids
which, in the presence
of hydrogen, initiate hydrovisbreaking reactions. These reactions upgrade the
quality of the
hydrocarbons by converting their higher molecular-weight components into lower
molecular-
weight components which have less density, lower viscosity, and greater
mobility within the
reservoir than the unconverted hydrocarbons. At the conclusion of the
injection phase of the
process, the injection of fluids is suspended. After a suitable amount of time
has elapsed, the
production phase begins with the pressure at the wellhead 31 reduced so that
the pressure in the
reservoir 27 in the vicinity of the borehole 21 is higher than the pressure at
the wellhead. The
hydrocarbons subjected to the hydrovisbreaking reactions, additional virgin
hydrocarbons, and
the injected fluids flow into the perforations 200 of the casing 29 of the
borehole 21, propelled by
the excess reservoir pressure in the vicinity of the borehole. The
hydrocarbons and injected
fluids arriving at the borehole 21 are removed from the borehole using
conventional oil-field
technology and flow through production tubing strings 208 into the surface
facilities. Any
number of wells may be operated simultaneously in a cyclic fashion while
situated so as to allow
the injected fluids to flow efficiently through the reservoir to contact a
significant portion of the
heavy hydrocarbons in situ.
As with the continuous-production process illustrated in FIG. 1, in the
preferred
embodiment the cooling fluid is steam, the fuel used is hydrogen, and the
oxidizing fluid used is
oxygen. Preferably, a stoichiometric mixture of hydrogen and oxygen is
initially fed to the
downhole combustion unit 206 so that the sole product of combustion is
superheated steam. As
the reservoir becomes heated to the level necessary for the occurrence of
hydrovisbreaking
CA 02335737 2006-07-07
77643-13
21
reactions, it is preferable that a stoichiometric excess of
hydrogen be fed to the downhole combustion unit during its
operation-or that hydrogen be injected into the fluid stream
controlling the temperature of the combustion unit-
resulting in hot hydrogen being injected into the reservoir
along with superheated steam. This provides a continued
heating of the reservoir in the presence of hydrogen, which
is the condition necessary to sustain the hydrovisbreaking
reactions.
As with the continuous-production process, in
another embodiment of the cyclic process a reducing-gas
mixture-comprised principally of hydrogen with lesser
amounts of carbon monoxide, carbon dioxide, and hydrocarbon
gases-may be substituted for hydrogen.
FIG. 2 therefore shows a hydrocarbon-production
system that cyclically converts, upgrades, and recovers
heavy hydrocarbons from a subsurface formation transversed
by one or more boreholes which have been fractured to
enhance injectivity and mobility of fluids within the
formation. The system is free from any combustion
operations within the subsurface formation and free from the
injection of any oxidizing materials or catalysts.
In yet another embodiment, horizontal well
technology is applied to the process of this invention.
This method is illustrated in FIG. 3, in which FIG. 3A shows
a plan view and FIG. 33 which shows a profile view, of one
configuration for combining vertical injection wells with
horizontal production wells. There is illustrated in
FIG. 3B a borehole 21 for an injection well drilled from the
surface of the earth 199 into a hydrocarbon-bearing
formation or reservoir 27. The borehole is lined with steel
CA 02335737 2006-07-07
77643-13
21a
casing 29 and has a wellhead control system 31 atop the
well. The casing 29 contains perforations 200 to provide
communication between the inside of the borehole 21 and the
reservoir 27. The injection well borehole 21 is
hydraulically fractured to create a vertical fracture 211.
In the plan view of FIG. 3, there are illustrated horizontal
production wells 212 with casing that is slotted to
communicate with the reservoir 27. The horizontal wells are
drilled so as to intersect the vertical fractures 211 of the
injection wells.
It is of interest to inject hot gases into the
reservoir 27 by way of one or more injection-well boreholes
and continuously recover hydrocarbon products from one or
more horizontal production wells. The wellhead control
system 31 used to regulate the flow of injected fluids on
each of the injection wells is supplied with a fuel source
by line 81, an oxidizing fluid by line 91,
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
22
and a cooling fluid by line 101. Through injection tubing strings 205, the
three fluids are coupled
to a downhole combustion unit 206. The fuel is.oxidized in the combustion unit
206, which is
cooled by the cooling fluid in order to protect the combustion unit's
materials of construction.
The products of oxidation and the cooling fluid 209 along with an un-oxidized
fuel 210, all of
which are heated by the exothermic oxidizing reaction, are injected into the
reservoir 27 through"
the perforations 200 in the casing 29. Heavy hydrocarbons 207 in the reservoir
27 are heated by
the hot injected fluids which, in the presence of hydrogen, initiate
hydrovisbreaking reactions.
These reactions upgrade the quality of the hydrocarbons by converting their
higher molecular-
weight components into lower molecular-weight components which have less
density, lower
viscosity, and greater mobility within the reservoir than the unconverted
hydrocarbons. The
hydrocarbons subjected to the hydrovisbreaking reactions and additional virgin
hydrocarbons,
propelled by the pressure of the injected fluids, flow into the vertical
fractures 211 of the
reservoir 27 and thence into the horizontal producing wells intersecting the
fractures, where they
are recovered along with the injected fluids using conventional oil-field
technology.
FIG. 3 therefore shows a hydrocarbon-recovery system that continuously
converts,
upgrades, and recovers heavy hydrocarbons from a subsurface forniation
traversed by one or
more vertical wells--used for injection-and by one or more horizontal wells-
used for
production-which are drilled within the reservoir containing the hydrocarbons.
The injection
wells may be vertically fractured and the horizontal wells drilled so as to
intersect the fractures.
Example I
Hydrovisbreaking Upgrades Many Heavy Crudes and Bitumens
Example I illustrates the upgrading of a wide range of heavy hydrocarbons that
can be
achieved through hydrovisbreaking, as confirmed by bench-scale tests.
Hydrovisbreaking tests
were conducted by World Energy Systems on four heavy crude oils and five
natural bitumens
[Reference 8]. Each sample tested was charged to a pressure vessel and allowed
to soak in a
hydrogen atmosphere at a coristant pressure and temperature. In all cases,
pressure was
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
23
maintained below the parting pressure of the reservoir from which the
hydrocarbon sample was
obtained. Temperature and hydrogen soak times were varied to obtain
satisfactory results, but no
attempt was made to optimize process conditions for the individual samples.
Table 2 lists the process conditions of the tests and the physical properties
of the heavy
hydrocarbons before and after the application of hydrovisbreaking. As shown in
Table 2,
hydrovisbreaking caused exceptional reductions in viscosity and significant
reductions in
molecular weight (as indicated by API gravity) in all samples tested.
Calculated atomic
carbon/hydrogen (C/H) ratios were also reduced in all cases.
Table 2
Conditions and Results from Hydrovisbreaking Tests on Heavy Hydrocarbons
(Example I)
Asphalt Tar Sands
Cmde/Bitumen Kem River Unknown San Miguel Slocum Rid e Trian le Athabasea Cold
Lake Primrose
l.oeation California Califomia Texas Tcxas Utah Utah Alberta Albena Albata
Test Conditions
Temperanuc, 'F 650 625 650 700 650 650 650 650 600
H= Prrssure, psi 1,000 2,000 1,000 1,000 900 1,000 1,000 1,500 1,000
Soak Time days 10 14 11 7 8 10 3 2 9
Properties Before and After H drovisbreakin Tests
Viscosity, c !00'F
Before 3,695 81,900 >8,000,000 1,379 1,070 700,000 100,000 10,700 11,472
After 31 1,000 55 6 89 77 233 233 220
Rttio 112 82 18,000 246 289 9,090 429 486 52
Gmvity, =API
Before 13 7 0 16.3 12.8 8.7 6.8 9.9 10.6
Atter 18.6 12.5 10.7 23.7 15.4 15.3 17.9 19.7 14.8
Increase 6.0 5.5 10.7 7.4 2.6 6.6 11.1 9.8 3.8
Sulfur, wt Yo
Bcfon: 1.2 1.5 7.9 0.3 0.4 3.8 3.9 4.7 3.6
0.9 .9 1.3 4.8 0.2 0.4 2.5 2.8
2.2 3.8
Y= Reduction 29 13 38 33 0 35 29 53 0
Carbon/ti ro en Ratio wt/wt
Before 7.5 7.8 9.8 8.3 7.2 8.1 7.9 7.6 8.8
ARer 7.4 7.8 8.5 7.6 7.0 8.0 7.6 N/A 7.3
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
24
In most cases the results shown in Table 2 are from single runs, except for
the San Miguel
results which are the averages of seven runs. From the multiple San Miguel
runs, data
uncertainties expressed as standard deviation of a single result were found to
be 21 cp for
viscosity, 3.3 API degrees for gravity, 0.5 wt % for sulfur content, and 0.43
for C/H ratio.
Comparing these levels of uncertainty with the magnitude of the values
measured, it is clear that
the improvements in product quality from hydrovisbreaking listed in Table 2
are statistically
significant even though the conditions under which these experiments were
conducted are at the
lower end of the range of conditions specified for this invention, especially
with regards to
temperature and reaction residence time.
Example II
Hydrovisbreaking Increases Yield of
Upgraded Hydrocarbons Compared to Conventional Thermal Cracking
Example II illustrates the advantage of hydrovisbreaking over conventional
thermal
cracking. During the thermal cracking of heavy hydrocarbons coke formation is
suppressed and
the yield of light hydrocarbons is increased in the presence of hydrogen, as
is the case in the
hydrovisbreaking process.
The National Institute of Petroleum and Energy Research conducted bench-scale
experiments on the thermal cracking of heavy hydrocarbons [Reference 7]. One
test on heavy
crude oil from the Cat Canyon reservoir incorporated approximately the
reservoir conditions and
process conditions of in situ hydrovisbreaking. A second test was conducted
under nearly
identical conditions except that nitrogen was substituted for hydrogen.
Test conditions and results are summarized in Table 3. The hydrogen partial
pressure at
the beginning of the experiment was 1,064 psi. As hydrogen was consumed
without
replenishment, the average hydrogen partial pressure during the experiment is
not known with
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
total accuracy but would have been less than the initial partial pressure. The
experiment's
residence time of 72 hours is at the low end of the range for in situ
hydrovisbreaking, which
might be applied for residence times more than 100 times longer.
Table 3
Thermal Cracking of a Heavy Crude Oil in the Presence and Absence of Hydrogen
(Example II)
Gas Atmosphere Hydrogen Nitrogen
Pressure cylinder charge, grams
Sand 500 500
Water 24 24
Heavy crude oil 501 500
Process conditions
Residence time, hours 72 72
Temperature, F 650 650
Total pressure, psi 2,003 1,990
Gas partial pressure, psi 1,064 1,092
Products, grams
Light (thermally cracked) oil 306 208
Heavy oil 148 152
Residual carbon (coke) 8 30
Gas (by difference) 39 110
Although operating conditions were not as severe in terms of residence time as
are
desired for in situ hydrovisbreaking, the yield of light oil processed in the
hydrogen atmosphere
was almost 50% greater than the light oil yield in the nitrogen atmosphere,
illustrating the benefit
of hydrovisbreaking (i.e., non-catalytic thermal cracking in the presence of
significant hydrogen
partial pressure) in generating light hydrocarbons from heavy hydrocarbons.
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
26
Example III
Commercial-Scale Application of In Situ Hydrovisbreaking
Example III indicates the viability of in situ hydrovisbreaking when applied
on a
commercial scale. The continuous recovery of commercial quantities of San
Miguel bitumen is
considered.
Bench-scale experiments and computer simulations of the application of in situ
hydrovis-
breaking to San Miguel bitumen suggest recoveries of about 80% can be
realized. The bench-
scale experiments referenced in Example II include tests on San Miguel bitumen
where an
overall liquid hydrocarbon recovery of 79% was achieved, of which 77% was
thermally cracked
oil. Computer modeling of in situ hydrovisbreaking of San Miguel bitumen
(described in
Example N following) predict recoveries after one year's operation of 88 to
90% within inverted
5-spot production patterns of 5 and 7.2 acres [Reference 3).
At a recovery level of 80%, at least 235,000 barrels (Bbl) of hydrocarbon can
be produced
from a 7.2-acre production pattern in the San Miguel bitumen formation.
Assuming the
produced hydrocarbon serves as the source of hydrogen, oxygen, and steam for
the process,
energy and material balances indicate that 103,500 Bbl of the produced
hydrocarbon would be
consumed in the production of process injectants. (The balances are based on
the fractionation of
the produced hydrocarbon into a synthetic crude oil and a residuum stream. The
residuum is
used as feed to a partial oxidation unit, which produces hydrogen for the
process as well as fuel
gas for a stearn plant and for generation of the electricity used in an oxygen
plant.) Thus, each
production pattern would provide 131,500 Bbl of net production in one year, or
about 45% of the
hydrocarbon originally in place, at an average production rate of 360 barrels
per day (BbUd).
These calculations provide a basis for the design of a commercial level of
operation in
which fifty 7.2-acre production patterns, each with the equivalent of one
injection well and one
production well, are operated simultaneously. Together, the 50 patterns would
provide gross
production averaging 32,000 BbUd, which-after surface processing-would
generate synthetic
crude oil with a gravity of approximately 25 API at the rate of 18,000 BbUd.
As the projected
life of each production pattern is one year, all injection wells and all
production wells in the
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
27
patterns would be replaced annually.
Field tests [References 2,6] and computer simulations [Reference 3] indicate a
similar
sized operation using steamflooding instead of in situ hydrovisbreaking would
produce 20,000
Bbl/d of gross production, some three-quarters of which would be consumed at
the surface in
steam generation, providing net production of 5,000 BbVd of a liquid
hydrocarbon having an AI'I
gravity of about 10 .
FIG. 4 shows the general distribution across a nominal 5 to 7-acre production
pattern of
the injectants and of the temperature within the formation at a time midway
through the
production period. The contours within the production pattern in FIG. 4 are
based on the results
of computer simulations of in situ hydrovisbreaking of the San Miguel bitumen
discussed below
in Examples IV and V.
Example IV
In Situ Hydrovisbreaking Promoted by Formation Fracturing
Example IV illustrates how formation fracturing makes possible the injection
of
superheated steam and a reducing gas into a formation containing a very
viscous hydrocarbon,
thereby promoting in situ hydrovisbreaking of the hydrocarbon. In situ
hydrovisbreaking,
conducted in the absence of fracturing, is compared through computer
simulation to in situ
hydrovisbreaking conducted with horizontal fractures introduced prior to
injecting any fluids.
A compiehensive, three-dimensional reservoir simulation model was used to
conduct the
simulations discussed in this and the following examples. The model solves
simultaneously a set
of convective mass transfer, convective and conductive heat transfer, and
chemical-reaction
equations applied to a set of grid blocks representing the reservoir. In the
course of a simulation,
the model rigorously maintains an accounting of the mass and energy entering
and leaving each
grid block. Any number of components may be included in the model, as well as
any number of
chemical reactions between the components. Each chemical reaction is described
by its
stoichiometry and reaction rates; equilibria are described by appropriate
equilibrium
CA 02335737 2000-12-20
WO 99/67505 PCTIUS99/14060
28
thermodynamic data.
Reservoir properties of the San Miguel bitumen formation, obtained from
Reference 6,
were used in the niodel. Chemical reaction data in the model were based on the
bench-scale
hydrovisbreaking experiments with San Miguel bitumen presented in Example I
and on
experience with conversion processes in commercial refineries. Two viscosity-
temperature
relationships from FIG. 6 were considered in the computer simulations without
fracturing: that
of Midway Sunset heavy crude oil and that of San Miguel bitumen. Only the
viscosity-
temperature of relationship of San Miguel bitumen was considered in the
simulation
incorporating fracturing.
Table 4
Simulation of In Situ Hydrovisbreaking in the Absence and Presence of
Formation Fracturing
(Example IV)
No Fracturing With Fracturing
Operating Mode (Cyclic) (Continuous)
Type of Hydrocarbon Heavy Crude Bitumen Bitumen
Dynamic Viscosity @ 500 F, cp~') 2 10 10
Days of Operation 70 35 79
Steam Injected, barrels (CWE)(1) 2,625 151 592,000
Hydrogen Injected, Mcf(') 3,329 185 782,000
Cumulative Production, barrels 4,940 14 175,000
Hydrocarbon Recovered, % OOIP(') 9.3 0.03 65.8
Gravity Increase, API degrees 1.2 5.8 10.0
From FIG. 6
t2~ Cold water equivalents
Thousands of standard cubic feet
~'~ Original oil in place
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
29
Simulation results are summarized in Table 4. The computer simulations show
that
without horizontal fracturing, in situ hydrovisbreaking could only be applied
with difficulty to
either a heavy crude oil having the viscosity characteristics of Midway Sunset
crude or to San
Miguel bitumen because the lack of fluid mobility within the formation caused
a very rapid
build-up of pressure when injection of steam and hydrogen was attempted. In
general, the cydes
of injection and production could be sustained for only a few minutes,
resulting in insignificant
to modest hydrocarbon production.
The final column of Table 4 lists results from the computer simulation of
continuous in
situ hydrovisbreaking in which the physical properties of a part of the
formation were altered to
simulate horizontal fracturing throughout the production unit. In this case,
significant quantities
of upgraded hydrocarbon are recovered, indicating that in situ
hydrovisbreaking can be
successfully conducted in a formation which has been fractured to enhance the
mobility of a very
viscous hydrocarbon. Recoveries greater by orders of magnitude can be
anticipated for a
fractured versus unfractured operation.
Example V
Advantages of In Situ Hydrovisbreaking Compared to Steam Drive
Example V teaches the advantages of the upgrading and increased recovery which
occur
when a heavy hydrocarbon is produced by in situ hydrovisbreaking rather than
by steam drive.
The example afso demonstrates the feasibility of applying in situ
hydrovisbreaking to recover a
very heavy hydrocarbon.
Through computer simulation, San Miguel bitumen was produced by steam drive
(FIG. 5,
"Base Case") and by in situ hydrovisbreaking (FIG. 5, "Case B") under
identical conditions. The
yield of hydrocarbons was more than 1.8 times greater from in situ
hydrovisbreaking. Moreover,
the API gravity of the hydrocarbons produced by in situ hydrovisbreaking was
increased by more
than 15 while there was no significant improvement in the gravity of the
hydrocarbons produced
by steam drive.
CA 02335737 2000-12-20
WO 99/67505 PCT/US99/14060
Table 5
ISHRE Process Compared to Steam Drive
(Example V)
Continuous Continuous
Operating Mode Steam Drive In Situ H drovisbreakin
Da s of O eration 360 360
Injection Temperature, F
Steam 600 600
H ydro en - 1,000
Cumulative Injection
Steam, barrels (CWE) 1,440,000 982,000
H dro en, Mcf 0 1,980,000
Cumulative Production
Hydrocarbon, barrels 129,000 239,000
H dro en, Mcf 0 1,639,000
Total Recovery
Hydrocarbon, %OOIP 48.6 89.9
H dro en, % injected _ 82.8
In Situ U din DAPI degrees 0 15.3
CA 02335737 2000-12-20
WO 99/67505 31 PCT/US99/14060
References
1. "Analysis of Heavy Oils: Method Development and Application to Cerro Negro
Heavy
Petroleum," Bartlesville Project Office, U.S. Department of Energy, December
1989.
2. Britton, M.W. et al.: "The Street Ranch Pilot Test of Fracture-Assisted
Steamflood
Technology," Journal of Petroleum Technology, March 1983.
3. Graue, D.J. and K. Karaoguz: "Conceptual Simulation of the In Situ
Hydrovisbreaking
Process in the San Miguel-4 Sand, Texas, for World Energy Systems," NITEC,
LLC,
October 1996.
4. Hertzberg, RH. and F. Hojabri: "The ENPEX Project - System Design and
Economic
Analysis of an Integrated Tar Sands Production and Upgrading Project."
5. Meyer, R.F. and C.J. Schenk: "An Estimate of World Resources of Heavy Crude
Oil and
Natural Bitumen," Proceedings of the Third UNTTARIUNDP International
Conference of
HC&TS, Alberta Oil Sands Technology and Research Authority.
6. Stang, H.F. and Y. Soni: "Saner Ranch Pilot Test of Fracture-Assisted
Steamflood
Technology," Journal of Petroleum Technology, June 1987.
7. Stapp, Paul R.: "In Situ Hydrogenation," Bartlesville Project Office, U. S.
Department of
Energy, December 1989.
8. Ware, C.H. and R.M. Amundson: "An Advanced Thermal EOR Technology,"
Proceedings
of the 1986 Tar Sands Symposium, Laramie, Wyoming, 1986.