Note: Descriptions are shown in the official language in which they were submitted.
CA 02336186 2000-12-18
WO 99/66104 PCT/US99/13827
METHOA FOR INHIB~G STAINS
ON ALUMIrIUNX PRODUCT SURFACES
Water stains do not generally present problems for the structural properties
and/or corrosion performance of an aluminum product. Aluminum surface
discolorations
that accompany water staining may discomfort some customers who are unfamiliar
with
the surface and corrosion properties of aluminum. Customers already aware of
the
properties of cold rolled steel may mistakenly believe that water stains on
aluminum are
the onset of "rusting", similar to that found on steel. For bright aluminum
products, such
as buffed trailer plate, rail cars, tool boxes, running boards, and tread
plate on fire trucks,
stain inhibition would preserve the buffed finish and enhance customer
satisfaction. A
simple, low-cost solution to inlu'bit water stain on aluminum could result in
a higher
degree of customer confidence in replacing stool with alu~oainum for their
products. In
addition, arsthelics of these products is important to the end customer. Waxer
star are
aesthetically unattractive and their elimination or reduction would be
valuable to the
owner whether it be an aluminum trailer, rail car, tool box or other aluminum
product.
CA 02336186 2000-12-18
WO 99/66104 PCT/US99/13827
Numerous uses for organophosphonic acids in conjunction with aluminum
arc iaaown. These include U.S. Patent Nos. 4,957,890, 5,032,237, 5,059,258,
5,103,550,
5,124,022, 5,124,289, 5,126,210, 5,132,181, 5,238,715, 5,277,788 and
5,463,804. None
of these, however, mention organophasphonic acids for the inhibition of
stains, especially
water stains, on aluminum surfaces. Most of the aforementioned patents
describe
aluminum surface pretreatments that enhance the durability of organic coatings
or
adhesively bonded joints. They do not describe the use of organophosphonics
without a
topcoat
Other methods for inhibiting coaosion with respect to aluminum and other
metals are disclosed in U.S. Patent Nos. 3,433,577, 3,672,822 and 4,427,448_
This invention addresses a low cost method for inhibiting water staining on
5000 Series, or SXXX, aluminum. alloys, most notably 5083-H321 and 5454-H32
aluminum (Aluminum Association dcsigaations). Such alloys are used to make
rail
hopper cars and buffed trailer tanks. Similar surprising and unexpected
results have been
observed when this method was practiced on 6000 Series aluminum alloys, like
the 6061-
T6 alloys used to make various products including vehicle wheels. According to
this
method, it was determined that spraying a solution consisting of about 0.25
w~/o
oct~decylphosphonic acid (or "ODPA") in an isopropanol solvent (or other
medium) onto
these aluminum alloy products, then allowing the alcohol to evaporate, is
effective for
2
CA 02336186 2000-12-18
WO 99/66104 PCT/US99/13827
inhibiting water staining. Similar effects were subsequently observed with a
carrier
composition containing octylphospbonic acid (or "OPA"). Suitable liquid
carriers
include alcohols, ketoses, ethers, aldchydcs, alkanes, and other organic
solvents witb
sufficient solubility for the organophosphonic acids. These organophosphonic
acid-
derived solutions can be applied to the metal surface by spraying, dipping,
painting, or
roll coating. It is also recognized that the stain inhibitor component can be
delivered to
the aluminum surfaces from various compositions used in the manufacture of
alumiunum
parts, including but not limited to: aqueous suspensions or solutions; metal
forming
lubricants, and metal cleaning andlor rinsing formulations; a buffing compound
or wax
that incorporates the stain inhibitor, metal heat treatment quench waters,
and/or post-
rinsing polisherslseal,ants or the like_ For certain stain inb,ibitor
compounds, it is possible
to buff a paste-like stain inhibitor directly onto the aluminum product
surface.
Farther features, objectives and advantages of the present invention will be
made clearer from the following detailed description made with reference to
the drawing
in which:
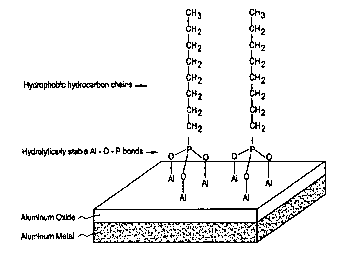
The FIGURE shows the schematic formation and orientation of
hydrolytically .stable Al-O-P bonds of the stain inhibitor, octylphosphonic
acid (OPA), as
a reaction product with an oxidized aluminum surface for effecting the stain
inbz'bition
obseivcd according to this invention.
CA 02336186 2000-12-18
WO 99/66104 PCT/US99/13827
Preliminary indications of the effectiveness of this invention, for inhibiting
stains, were observed in an accelerated corrosion test that involved
outgassing products of
cthylvinylacetate plastic pellets (obtained from Millennium Petrochemicals),
high
humidity and temperature cycling. After 12 temperature cycles, no water stains
were
observed on 5000 Series alloy samples initially etched in caustic, then
sprayed with an
ODPA containing solution. Water staining was also inlu.'bited for "mill
finish" metal
sprayed with ODPA; though some spots were interspersed with unstained surface
in the
latter case. By cont<~ast, mill-finish and etched-only samples were completely
covered
with water stains. It is believed that the difference in performance as a
result of pre-
etching were most likely due to the removal of residual rolling lubricants via
etching. In
that manner, the stain inhibiting molecules of this invention would be allowed
to
chemically bond with surface aluminum oxides. .
Chemical reaction of the inhibitor to the surface can also be achieved by
changing the means of application or using a different solvent. The surface
ODPA
inhibits access of water to the aluminum oxide and forms hydrolytically stable
bonds with
the oxide, thus inhibiting water staining. ODPA is a commercial compound
manufactured and sold by Albright & Wilson Ltd. Working solation
concentrations and
surface coverages of this invention are relatively low, which results in low
gent costs
of cents per square foot of A1 plate or sheet product. The same would be true
for other
aluminum product forms, including castings, forgings and extensions.
CA 02336186 2000-12-18
WO 99/66104 PCT/US99/13827
Another potential stain inhibitor, octylphosphonic acid (OPA), was
evaluated. It showed even better performance results than the ODPA samplings
above.
OPA has the following chemical sflructure: CH3(CH2)~P(O)(OH)z. It can be
applied with
a water and surfactant carrier as effectively as with an isopropanol carrier.
OPA is more
soluble than ODPA in isopropanol thus allowing for increased solution
concentrations.
And while OPA is not water soluble, it fo:ms a suspension of solids with
water. In either
case, no volatile organic carbons (ar VOC's) result therefrom.
Preliminary humidity test results show that OPA is highly effective for
inhibiting stains on mill finish or buffed aluminum products without cleaning,
pic~g or
pre-etching. After three hours at 50°C (125°F~ and 100% relative
humidity, the OPA
treated surface was unstained, where ~~-bnged'~, untreated surfaces were
considerably
stained.
It may also be possible to apply certain foimuIations by the methods of this
invention with no carrier solution. For example, one may directly buff a more
solid form
of OPA onto an aluminum product surface. It is also possible, actually even
more
practical depending on the aluminum surface to be treated, to incorporate the
stain
inhibiting compounds of this invention into mill lubricants for providing an
in situ type of
stain inhibition and cIinninating subsequent processing steps. it may also be
possible to
similarly add such stain inhibitors to bung, sealing and/or polishing compound
formulations.
CA 02336186 2000-12-18
WO 99/66104 PCT/US99/13827
When the aluminum to be treated is mill finish or "as buffed", a preferred
carrier/solvent is an alcohol, more preferably 2-propanol or isopropaaol.
Isopropanol is
also beneficial in that its solvent action is betievcd to displace residual
mill lubricants or
buffing compounds and wet the surface aluminum resulting in the formation of
Al-O P
bonds with the oxidized aluminum surface. Isopropanol is also non toxic. When
the
aluminum surface has been pro-cleaned or etched, the choice of solvent is not
as critical.
In many instances, water may be used to transport (or apply) such stain
inln'bitors.
In addition to forming hydrolytically stable Al-O-P bonds,
organophosphonic acids may provide yet another mechanism for stain inhibition.
For
example, when OPA or ODPA reacts on the A1 surface, the reaction end product
is
believed to orient or align so that its hydrocarbon chains extend away from
said surface.
A schematic representation of the bonding tliat is believed to take place is
shown in the
accompanying FIGURE. The latter surface takes on a "hydrophobic" or non-
wetting
quality thereby further inhibiting the conversion of oxides to hydroxides (or
effecting a
water stain thereon). Under the latter scenario, longer chained
organophosphonic acids
become the preferred stain inhibitors for this invention.
In some embodiments of this invention, a foil (and not partial or non-
uniform) haze on the aluminum product surface may form. It is preferred that
such haze
be wiped away with a dry cloth to further enhance stain inhibition. On a less
preferred
basis, this haze may removed by rinsing the aluminum product's outer surface.
CA 02336186 2000-12-18
WO 99/66104 PCT/US99/13827
Certain classes of phosphorus oxo acids, acid esters, aiid acid salts arc
effective to various degrees in preventing water stains according to this
invention.
Phosphate salts, phosphate esters, and phosphoric acids cacti impart some
stain
inhibition. In comparative tests, however, octadecylphosphonic (C-18} acid
(01~PA) and
several fluoro phosphoric acids were not as effective as OPA (C-8) in
inhibiting stains.
Poly(vinylphosphonic acid), and copoiymcrs thereof, may work even better than
OPA,
but it is currently cost prohibitive to use in commercial quantities. Some of
the
representative stain inhibitors can be grouped by the following "families":
a) acidic aluminum phosphate salts
~o
I
(NHJi ~' QO ~ P - OH
O
dibasic ammonium phosphate
b) inorganic phosphorus oxo acids
OH OH H
HO-P-OH H-P-OH H-P-OH
Ip 1I IO
O
phosphoric acid phosphorous acid hypophosphorous acid
c) organophosphonic and organophosphinic acids
H OH
CH3 - (CH~,~ - P - OH CH3 - (CH~j~ - P - OH
II 'O
O
oc~adecylphosphonic and (ODPA) octylphosphonic acid
(OPA)
7
CA 02336186 2000-12-18
WO 99/66104 PCT/US99/13827
OH
I
CFs - (CF~s - P - OH CFs - (CF~S - P - OH
II
II O
O
perfluorohexylphosphonic acid perfluorohexylphosphinic acid
(a component of the FluowetPP~ product
sold by Hoechst-Celanese)
d) phosphate acid esters_ op(o)(ot~z
oP(O)(OH)~ ~ myo-inositolhexakis(dihydrogenphosphate)
. ~ °r~~°~cvH~z "phytic acid'.
oP(o)(OH)~
OP(O)(OH)2
e) organo phosphoric acid polymers and copolymers; and
for example, polyvinyl phosphoric-co-acrylic acid)
~ phosphate ester polymers
for example, polyvinyl phosphoric acid)
This invention can be used to improve the stain inhibition of numerous
aluminum alloy surfaces, including various sheet or plate products, extrusions
and
forgings, regardless of whether such products have welded joints or other
connections. It
is best snitcd for any aluminum product that its purchaser, the end
user/consumer, would
prefer that said pmduct "look good" (i.e. brighter, less stained, etc.)
longer! This includes
a
CA 02336186 2000-12-18
WO 99/66104 PCT/US99/13827
a whole family of building/architecwral products, appliances, lighting
supplies, and other
household cosmetics like vertical blind stock. On a preferred basis, the
method of this
invention works well with 5000 and 6000 Series alloys (Aluminum Association
designation). It should also enhance the stain inhibiting performance of
products made
from other aluminum alloys,.including but not limited to 1000 and 3000 Series
alloys.
t Stud - Several sections of buffed trailer tank plate product (made
from 5454 aluminum alloy) were sprayed with two comparative stain inhibiting
compositions:
Set 1: 0.2 wt% octyiphosphonic acid (OPA) in isopropanol; and
Set 2: 0.2 wt% octadecylphosphonic acid (ODPA) in isopropanol.
Haze on both sets of sprayed plates was rinsed away with water, then gently
buffed with
dry cheesecloth. These treated plates, along with an "as-buffed" control, were
then
placed in a humidity cabinet at 50°C (I25°F) with 100% relative
humidity for 3 hours.
After exposure, the plates were removed from the cabinet, dried with a towel,
and
visually examinrcd for staining.
No noticeable loss of specularity was observed with either of the above
stain inhibition treatments. All surfaces had the same visual appearance as
the "as-
buffed" sample. After humidity exposure, however, brownish colored, water
stains were
evident over a majority of the "as-buged" and ODPA-treated surfaces. The OPA
treated
surface did not exhibit any water stains and appeared the same as unexposed
specimens.
CA 02336186 2000-12-18
WO 99/66104 PCT/US99/13827
While ODPA specimens did not fare as well as OPA in this particular study, as
compared
with its earlier positive results, different application techniques arc
believed to have
caused its reduced stain inhibiting performance here.
Second Studv - Tanker Trial results
A covered hopper trailer, made from 5454 aluminum Bulk Transportation
Sheet ('BTS") was treated with various applications according to the invention
before
being exposed to harsh, in-service conditions: From an aggressive environment
of salt air
due to seacoast proximity, and harsh winter conditions with numerous road salt
applications. Subsections of this hopper/tanker were treated as follows: (a) 1
wt.%
solution of OPA, in isopropanol, was sprayed on the first section of tanker,
dried to a
filru, water rinsed and air deed thereafter, (b) the same solution as above
was sprayed
onto another adjoining section of the same hopper/tanker, then dried to a film
and wiped
to an initial shine using cheesecloth; (c) for this section of hopper/tanker,
the treatment
material was 1 wt% OPA, suspended in water. After spraying, this water-based
solution
was allowed to sit on the product surface for about 10 minutes before being
dried and
wiped to a shine with cheesecloth. The last comparative section of
hopper/tanker was
sprayed with a S wt.% solution of OPA, in water, before being allowed to sit
for 10
minutes, then water rinsed and air dried.
After three months of service along the U.S. East Coast, this hopper/trailcr
was brought back for inspection. While OPA treatments were observed to provide
a
substantial degree of water stain inhibition over that 3 month trial period,
one of the first
io
CA 02336186 2000-12-18
WO 99/66104 PCT/US99/13827
conclusions drawn from that inspcckioa was that monthly reapplications could
ensure a
pristine, polished surface on such trailer stock.
Following a wash with non etching alkaline cleaner, various sides and
subsections of this hoppcr/tanker were photographed and closely compared by
visual
inspection. From that inspection, it was noted that the water-based sections
of treated
hopper fared better than their alcohol-based counterparts (in terms of water
staining
inhibition). In. addition, wiping to a shine after application of the OPA, as
per example
(a) above, was most effective, even more than merely applying, rinsing and air
drying, the
latter trea~m~ent resulting in a noticeable, residual haze at first
Third Stuliy - Coil Line Trial
A coil of 5182-H19 aluminum sheet was roll coated with a 5% aqueous
suspension of OPA. Phosphorus surface concentrations were measured on the
treated
surface using X-ray fluorescence spectroscopy (X~. From previous bench scale
tests,
it was observed that phosphorus surface levels of about 2 Kcps were sufficient
for
inhibiting water staining. Phosphorus surface levels on the aforementioned
sheet product
were measured at about 10 Kcps, however.
Fourth Studv - Forged Truck Wheels
Forged and polished truck wheels made from aluminum alloy 6061-T6 were treated
with
comparative solutions of 0.5 and 1 wt% OPA in isopropanol. For testing, the
treated
wheels were placed into a cabinet with condensing humidity set at
100°F. The wheels
were examined every hour for water stains. The tests were stopped after 120
hours of
m
CA 02336186 2000-12-18
WO 99/66104 PCT/US99/13827
humidity exposure. Untreated wheels (as-polished) were substantially stained
within I 1
hours of humidity exposure. The wheels that were treated with OPA, then buffed
to
shine lasted the longest without substantial water staining. On certain OPA-
treated
wheels, only a few small, widely dispersed spots were observed after 120 hours
of
exposure testing, but that level of staining was insi~ificant compared to the
gross
quantities of water sting observed on the untreated wheels after only 11 hours
of
humidity exposure.
fifth Study - Lighting Sheet
Aluminum alloy 5657-H18, used for making bright lighting sheet, was
treated with a 5 wt.% solution of OPA stain inhibitor in isopropanol. Specular
reflectance measurements showed that after buffing the resultant haze from
said sheet
surfaces, the OPA treatment did not reduce reflectivity. Furthermore, such OPA
treated
panels lasted up to 13 days in condensing humidity at 100°F without
staining, as
compared to their untreated sheet equivalents that were significantly stained
within 24
hours of such humidity exposure.
ixth Studv - Quench Water Additions
Phosphorus compounds, like those described above, were added to the
quench waters used for making extruded tubes and rolled sheet from 6061-T6
alloy. In
this comparison, the aluminum product forms were heated to about 1U00°F
before being
cold water quenched, said quenching solution containing various phosphorus
compounds.
Thereafter, those products were allowed to remain in the quench water for 24
hours. By
12
CA 02336186 2000-12-18
WO 99/66104 PCT/US99/13827
visually examining these aluminum product forms, and by further measuring the
amount
of hydroxides formed thereon using Fouricr-transform infrared spectroscopy (FT-
TR), it
was determined separately that 10 g/L solutions of dibasic ammonium phosphate -
(NH~~HP04 - and 10 g/L phytic acid best prevented the formation of water
stains on
these products. ~TUcy also prevented the formation of bayetite powders on the
interior
aluminmn surfaces of these ext<vded tubes.
13