Note: Descriptions are shown in the official language in which they were submitted.
CA 02336363 2000-12-29
WO 00/01840 PCT/EP99/04702
.'~r
FERMENTAT10N PROCESS TO PRODUCE CLAVULANIC ACID AT A LOW CONCENTRATION OF FREE
AMINO
ACIDS
The present invention relates to the field of the fe-mentative
production of secondary metabolites from a Streptom~ices strain on a
io suitable medium comprising carbon and nitrogen sources.
~ackaround of the invention
A large variety of different secondary metabolites is produced
~5 fermentatively from Stre tp omyces microorganisms, as for instance
lactams, polyketides and macrolides, such as clavulanic acid, pimaricine
and erythromycine. Clavulanic acid is an important inhibitor of ~i-
lactamases and is produced by various microbial strains belonging to the
genus of Streptomycetes such as S. clavuligerus ATCC 27064, S.
zo jumonjinensis (GB patent 1563103), S. katsurahamanus IFO 13716 FERM
3944 (JP patent 83009679B) and Streptomyces sp. P6621 FERM 2804
fJP patent application 55162993A).
Clavulanic acid can be produced in substantial amounts and
production conditions are being optimized continuously in order to
z5 increase the yield and the purity of the end product. The production of
clavulanic acid has been optimized with respect to continuous
fermentation (GB 1508977), feeding of carbon to a batch process (EP-
182522), maintaining ammonia at low concentrations fW0 96/18743 and
Romero J., Liras P. and Martin J.F., Anol. r i Biotechnol. (1984),
so Vol. 20, 318-325), reduction of phosphate concentration in fermentation
media during growth and production (Romero (1984), v.s., Lebrihi A.,
Germain P. and Lefebre G., y_ lied r bi I. Biotechnolog~r. (1987), Vol.
CA 02336363 2000-12-29
WO 00/01840 PCT/EP99/04702
-z-
26, 130-135 and International patent applications WO 97/19187 ate! WO-
97/39137). With respect to carbon sources it was mentioned that
triglycerides are the preferred carbon sources compared to glycerol
(Butterworth ( 1984); Biotechnology of Industrial antibiotics, E.J.
s Vandamme, 1 st edition, Marcel Dekker Inc. pages 225-235 and the
patent application WO 97/19187).
With respect to the nitrogen source a lot of research has been done
by Brana A.F., Paiva N. and Demain A.L., ,j~ Q General Microbioloav
( 1986), Vol 132, 1305-1317. It was found that growth on media
~o containing an amino acid is faster than growth on ammonia as sole
nitrogen source.
Furthermore, on a medium containing ammonia as the sole nitrogen
source, the maximum specific growth rate is < 0.05 h~', Brana ( 1986)
v.s., and Aharonowitz Y. and Demain A.L., Can J. Microbiol. (1979), Vol.
~ s 25, 61-67, while the maximum specific growth rate is > 0.05 h'' in
media containing ~ at least one amino acid like asparagine, aspartate,
glutamate, glutamine, alanine, histidine, proline, threonine or arginine.
Therefore, one would prefer the use of a medium containing one or more
amino acids. In such media, the production of biomass takes less time
zo compared to inorganic media containing only ammonia which is of course
an advantage over a slower production process.
Asparagine is described in the non-prepublished International
application WO 98/37179 as fermentation ingredient for the production of
clavulanic acid, and also for cephalosporin, e.g. cephamycin C production
zs from Str tome .tee clavuliger~s (Aharonowitz Y. and Demain A.L., v.s.).
Besides this, glutamate was shown to repress clavulanic acid formation in
a batch process (Romero (1984), v.s.), one would not be directed to use
glutamate or glutamine.
Furthermore it is even more common to use proteins as source of
so amino acids as this is much cheaper than the individual ones. The
disadvantage is obviously the heterogeneity of proteins and the
CA 02336363 2000-12-29
WO 00/01840 PCT/EP99/04702
-3-
irreproducible quality generally associated with these complex nutrients.
For clavulanic acid production typical complex nitrogen sources applied
are: fishmeal, soybean meal, peanut meal for S. ciavuligerus (EP 0 182
522 B1 and WO 97/19187), soybean meal for S. jumonjinensis (UK
s patent 1563103), soybean meal and corn steep liquor for Streptomyces
sp. P6621 FERM 2804 (WO 97/39137 + JP patent application
55162993), and soybean flour and cotton seed flour for S.
katsurahamanus (JP patent 83009679B). In an overview article it was
reported that soybean protein was the most important protein for
~o clavulanic acid production (Butterworth (1984), v.s.).
Here we describe the surprisingly advantageous application of
hydrolysed proteins preferably rich in glutamate and proline in the
fermentation broth of a secondary metabolite producing Strel toto omyces and
the particular advantage of gluten hydrolysate and casein hydrolysate in
~s this respect. Moreover we describe the unexpected advantageous
application of a source of amino acids, in particular glutamate as a feeding
nutrient in a fed batch process for the production of these compounds.
Dggcription of the Figures
Zo
Figure 1: titre of clavulanic acid during a batch fermentation at low
levels of free glutamate.
Figure 2: titre of clavulanic acid during a fed batch fermentation at
25 low levels of free glutamate.
Summary of the invention
ao The present invention provides a method for the production of
secondary metabolites by the fermentation of such a secondary
metabolite producing Stre t!a omyces on a suitable medium by keeping the
concentration of free amino acids lower than 5 g/I fermentation broth,
preferably lower than 2.5 gil and more preferably lower than 0.5 g/I,
CA 02336363 2000-12-29
WO 00/01840 PCT/EP99/04702
-4-
provided that a concentration of 4 g/I free asparagine supplied _.o a
fermentation broth of Stre tp om_~ ces clavuligerus has been excluded. This
process is especially favourable for the production of -lactams,
polyketides and macrolides, preferably clavulanic acid, pimaricine,
erythromycine, nystatine or amphotericine. According to one aspect of the
invention to maintain such low concentrations in the fermentation broth,
one or more of the amino acids, preferably glutamate or proline_ is applied
in a fed-batch or continuous mode. According to another aspect of the
invention, besides amino acids itselves, also protein hydrolysate, more
io preferably glutenhydrolysate or caseinhydrolysate and most preferably
wheat glutenhydrolysate is applied in a batch, fed-batch, semi-continuous
or continuous mode.
The present invention describes the use of media poor in free amino
acids, by the application of said amino acids in a fed batch or continuous
mode or by the application of protein hydrolysate in any mode.
For the present patent application a protein is defined as a polymer
of amino acids with a size expressed by a Molecular Weight > 20,000
Dalton which has not been processed by any means to degrade the
protein to smaller fragments. A protein hydrolysate is defined to be a
polymer of amino acids with an average size between 300 Dalton and
20,000 Dalton and a free amino acid content of less than 30% of the
is total amino acids. These protein hydrolysates can be produced either by
enzymatic or by chemical hydrolysis of the corresponding proteins.
Alternatively the advantage of using hydrolyzed proteins is achieved by
using strains improved for protease activity in a process using non-
hydrolyzed proteins as raw materials. Furthermore, glutamate stands in
3o the present application for glutam like compounds as glutamate and
glutamine. In the present application a protein extract is defined to be a
CA 02336363 2000-12-29
WO 00/01840 PCT/EP99/04702
-5-
protein hydrolysate with a molecular weight lower than 300 Daltor~,and
wherein > 30% of the amino acids are present as free amino acids.
When a protein or the hydrolysate thereof is defined as rich in
glutamate it is meant that > 15% of the amino acid content consists of
s glutamate and glutamine. Proteins described as rich in glutamate are
casein (21 %) and wheat gluten (35%).
According to one aspect of the present invention it was surprisingly
found that clavulanic acid production was especially high when a protein
hydrolysate was included in the medium, especially when it was derived
~o from wheat gluten or casein. As the production levels were reduced
dramatically when protein extracts were used of other protein sources like
yeast and corn, especially at high hydrolysis degree ( > 35%) compared to
the peptides ( < 30% free amino acids), it is shown that protein
hydrolysates should preferably contain less than 30% free amino acids,
~ s even more preferably less than 5 % and most preferably less than 1 % free
amino acids. A protein hydrolysate preferably rich in glutamate can be
used as well supplied in all forms of feeding, viz. batch, fed batch,
continuous or semi-continuous. By semi-continuous feeding is meant the
continuous addition of nutrients to the fermentation broth while
Zo intermittently a small volume of the broth is removed.
The fermentation medium may either be a defined medium
comprising (NH4)2S04, free amino acid, KH2P04, MgS04.7H20,
CaC12.2H20, 3-(N-morpholino), propanesulfonic acid, glycerol, sodium
succinate and a solution of trace elements, with a low concentration of
2s amino acid, or with a protein hydrolysate which results in a complex
medium. Also the application of a complex medium as for instance flours
from nuts, vegetables, seeds, cereals, grasses such as those useful in
fermentation industry, soybean flour, lineseed flour, peanut flour, potato
flour, sunflower, pea- or beanflour, cotton seed flour, wheat gluten, whole
so wheat, rice meal to which protein hydrolysate is added, wherein the
concentration of free amino acid is low, is part of the invention. The
CA 02336363 2000-12-29
WO 00/01840 PCT/EP99/04702
-6-
medium may also contain mixtures of mentioned flours with mixtuc~,s of
protein hydrolysates of various sources and of various peptide sizes as
desired to achieve the optimal result.
Besides the wild type strains, also microbial strains which are
s capable of being fermented in a chemically defined medium and/or
improved by subjecting a parent strain of interest to a classical mutagenic
treatment using physical means, such as UV irradiation, or a suitable
chemical mutagen, such as N-methyl-N'-nitro-N-nitrosoguanidine or
ethylmethane sulfonate may be used for the process of the present
io invention. The same does apply to a parent strain of interest to
recombinant DNA technology, whereby the parent strain is transformed
with a one or more functional genes of interest.
Any assimilable carbon source may be added to the above said
mixture, like sugars such as glucose, fructose, sucrose, maltose, lactose,
~s or polysaccarides like starch, maltodextrines and inuline or other fructose
polymers, triglycerides such as soybean oil, sunflour oil, olive oil, tri-
oleate
etc., (poly-) alcohols such as ethanol, propanol, glycerol, mannitol, or
organic acids or a salt thereof such as acetate, propionate, succinate,
adipate, malonate, citrate, lactate, gluconate etc.
Zo An inorganic nitrogen source may be added to the medium such as
ammonia and/or nitrate or any of its salts. Ureum may be used as well.
Furthermore also vitamins, and various sorts of inorganic anions such as
sulphates, phosphates, chlorides, borates, molybdate, iodate or their salts
and the cations potassium, sodium, zinc, manganese, magnesium, iron,
Zs copper, cobalt, nickel etc. may be added to the medium.
A fermentation is started by inoculating from a preculture or
inoculum fermentation at a volume of about 1 to 50% of the main
fermentation medium, particularly from 5 to 20%. The process may last
from about 24 to 400 hours and especially from 48 to 168 hours. The
so temperature will be kept between 20 and 40 °C, preferably between 25
and 35 ° C, and even more preferably between 26 and 30 ° C. The
pH can
CA 02336363 2000-12-29
WO 00/01840 PCT/EP99/04702
_7_
be maintained at pH 6 to 8 by means of titration with an alkaline
substance such as ammonia, sodium hydroxide, potassium hydroxide,
calcium hydroxide, or an organic base like lysine, arginine and histidine
and an acid substance, such as the anorganic acids like sulphuric acid and
s hydrochloric acid. Alternatively, an organic acid may be used such as
glutamate, citrate, gluconate or acetate.
The dissolved oxygen concentration may be controlled in the
optimal range for the process by varying the oxygen concentration in the
inlet gas, application of overpressure, modification of stirrerspeed and
~o airflow. The range may vary between 0 and 100% of air saturation.
The process may be carried out by controlling various non-growth
limiting nutrients in their optimal concentrations. Dependent on the
growth limiting nutrient of choice, these growth-non-limiting nutrients
may contain any relevant carbon, nitrogen, phosphor, sulphur source or
oxygen.
Carbon dioxide should be kept at non-toxic concentrations by for
instance increasing the airflow through the fermentor so that the carbon
dioxide concentration in the outlet-gas is less than 5%, preferably less
than 2.5%.
2o The fermentation can be carried out in a batch, fed batch, or (semi-)
continuous fermentation process mode.
Of course, the recovery of the impure clavulanic acid solution as
formed by the fermentative process of the present invention as well as the
subsequent conversion thereof into a pharmaceutically acceptable salt by
2s methods known in the art do form an aspect of the present invention. One
of the most advantageous procedures is the conversion of the impure
clavulanic acid into an amino salt thereof by adding the corresponding
amino salt forming compound as for instance N,N,N',N'-
tetramethylethylenediamine, 1,3-bis(di-methylamino)-2-propanol, t-
so butylamine, t-octylamine, benzhydrylamine and bis (2-(dimethyl-
aminolethyl)ether and reacting said amine clavulanate with a non-toxic
CA 02336363 2000-12-29
WO 00101840 PCT/EP99/04702
_$_
pharmaceutially acceptable salt as for instance potassium ethylhexano,~,e to
form the corresponding purified salt, for instance potassium clavulanate.
Batch fermentations of clavulanic acid comparing the use of
proteins, protein hydrolysates and protein extracts.
Streptomyces clavuligerus ATCC27064 was improved for
io clavulanic acid production by means of several rounds of classic mutation
(UV, nitroso guanidine (NTG)) and selection in shake flask cultures
whereby clavulanic acid production was tested by imidazole methods as
known in the art. The strain was conserved as vegetative mycelium
grown for 48 hours in Tryptone Soytone Broth-medium (TSB-medium) at
i5 28 °C in a shaker incubator shaken at 280 rpm and stored frozen at -
80
°C.
1 ml of the frozen mycelium was inoculated to 100 ml of a
sterilized (30 minutes, 121 °C) preculture medium containing 5-20 g/I
maltose.1 aq, 15-30 g/I bacto tryptone, 15-30 g/I bacto peptone, 1-10 g/I
zo bacto soytone, mono potassium phosphate 1-5 g/I and 0.2 g/I synthetic
antifoam.
After 72 hours of cultivation at 27 to 28 °C, 2.5% of this
preculture is transferred to a sterile production medium containing 2.5 g/1
from a complex nitrogen source such as a protein, a protein hydrolysate
z5 and/or or protein extract. The production medium further contains 50-100
g/I glycerol, 5-20 g/I soybean flour, 0.5-2 g/l mono potassium phosphate,
a suitable trace element cocktail and 0.2 - 2 g/I synthetic antifoam. After
4 days of cultivation at 28 °C and adequate shaking, the cultures were
harvested and assayed for clavulanic acid by means of standard HPLC-
ao methods.
When different nitrogen sources were compared in a batch process,
it was surprisingly found that the application of protein hydrolysates
CA 02336363 2000-12-29
WO 00/01840 PCT/EP99/04702
_g_
increased the production of clavulanic acid with at least 10% compare! to
the use of proteins. The best protein hydrolysates were those derived
from gluten, followed by casein- and soy-proteins respectively (see table
11.
Table 1. Clavulanic acid production with a mutant strain from S.
clavuligerus ATCC 27064 using different complex nitrogen sour~.es
additional to soybean flour.
Nutrient Type Supplier Class Free Yield
name
amino percentage
acid
_ csm
Potato flourAlburex Roquette protein < 1 100
Freres
Yeast extractBaker's YE Gist brocadesextract 13.0 7
(65% free
amino
acids)
Brewers YE Gist brocadesextract 7.9 1 1
(50% free
amino
acids)
Maxarome Gist brocadesextract 5.1 20
(35% free
amino
acid)
Corn Steep Phytase treatedRoquette extract 5.7 4
Liquor CSL Freres
Corn Steep Roquette extract 5.4 5
Powder Freres
Glutamate amino 25 0
acid
Caseine Casein Armor hydrolysate2.0 115
hydrolysate hydrolysee Proteines
Wheat glutenGPU Marcor Inc.hydrolysate0.1 121
hydrolysate
GPN Marcor Inc.hydrolysate0.3 136
GPA Marcor Inc.hydrolysate0.3 132
Soybean proteinMXP-90 PTI Inc. hydrolysate 115
hydrolysate
~o
~xam_I la a 2
Fermentation of clavulanic acid at low levels of free glutamate in
the medium
Batch fermentation of cfavulanic acid
CA 02336363 2000-12-29
WO 00/01840 PCT/EP99/04702
-10-
Streptomyces clavuligerus ATCC 27064 was precultivated for~26 h
at a temperature of 28°C and a start pH of 6.8 in a shaken incubator
rotated at 220 RPM on a medium containing 5 to 30 g/I glycerol, 5 to 30
g/I soy peptone, 2 to 6 g/I sodium chloride, and 0.5 to 3 g/I calcium
carbonate.
The preculture was inoculated at a volume of 10 % into 1 I of
chemically defined medium containing 10-30 g/1 glycerol, 0.5 to 3 g/I
~o KH2P04, 1 to 3 g/I (NH4)2S04, 15-25 g/I monosodium glutamate, 0.05 to
0.2 g/I FeS04~7 H20, 0.1 to 1 g/I MgS04~7 H20, 10-20 g/I 2-(N-
morpholine) propane sulfonic acid (MOPS), 0.2-1 g/I Basildon antifoam,
and a suitable trace element solution. The pH was adjusted to 6.8 with
4N NaOH. The second preculture was cultivated for 20 h at a temperature
~5 of 28°C and a start pH of 6.8 in a shaken incubator rotated at 220
RPM.
The second preculture was inoculated at a volume of 3.3 % into 29
I of a chemically defined medium containing 10 to 30 g/I glycerol, 0.5 to
1 g/I KH2P04, 0.5 to 3 g/I (NH4)2S04, 10-30 g/I monosodium glutamate,
0.05-0.15 g/I FeS04~7 H20, 0.1 to 1 g/I MgS04~7 H20, 0.1 to 1 g/I
2o Basildon antifoam, and a suitable trace elements solution.
The fermentation was carried out at 30°C and the pH was
controlled at 6.95 to 7.05 by titration with 4N NaOH and 4N H2S04. The
dissolved oxygen tension was maintained above 50 % of air saturation or
regulated at 50 % of air saturation by the stirrer speed.
Fed-batch fermentation of clavulanic acid
Streptomyces clavuligerus ATCC 27064 was precultivated for 26 h
at a temperature of 28°C and a start pH of 6.8 in a shaken incubator
rotated at 220 RPM on a medium containing 5 to 30 g/I glycerol, 5 to 30
CA 02336363 2000-12-29
WO 00/01840 PCT/EP99/04702
-11-
g/I soy peptone, 2 to 6 g/I sodium chloride, and 0.5 to 3 g/I ca4~,ium
carbonate.
The preculture was inoculated at a volume of 10 % into 1 I of
chemically defined medium containing 10-30 g/I glycerol, 0.5 to 3 g/I
KH2P04, 1 to 3 g/I (NHQ)2504, 15-25 g/I monosodium glutamate, 0.05 to
0.2 g/I FeS04~7 H20, 0.1 to 1 g/I MgS04~7 H20, 10-20 g/I 2-(N-
morpholine) propane sulfonic acid (MOPS), 0.2-1 g/I Basildon antifoam,
and a suitable trace element solution. The pH was adjusted to 6.8 with
4N NaOH. The second preculture was cultivated for 20 h at a temperature
~o of 28°C and a start pH of 6.8 in a shaken incubator rotated at 220
RPM.
The second preculture was inoculated at a volume of 4 % into 24 I
of a chemically defined medium containing 5 to 15 g/I glycerol, 0.5-2 g/I
K2HP04, 0.5 to 3 g/I (NH4)2504, 10 g/I monosodium glutamate, 0.05 to
0.15 g/I FeS04.7H20, 0.1 to 1 g/l MgS04~7H20, 0.05 to 1 g/I Basildon
i 5 antifoam, and a suitable trace elements solution.
The fermentation was carried out at 30°C and the pH was
controlled at 6.95 to 7.05 by titration with 4N NaOH and 4N H2S04. The
dissolved oxygen tension was maintained above 50 % of air saturation or
regulated at 50 % of air saturation by the stirrer speed.
2o Five hours after the phosphate was exhausted from the batch
medium a carbon feed containing 300 to 500 g glycerol/kg feed was
added at a rate of 30-60 g/h and a nitrogen-phosphate-feed containing 50
to 100 g Na-glutamate/kg feed, 5 to 10 g (NH4)2S04/ kg feed, and 5 to 10
g K2HP04/kg feed was added at a rate of 50 to 150 g/h.
Clavulanic acid and glutamate concentrations in the medium are
demonstrated in figures 1 and 2 respectively for the batch process and
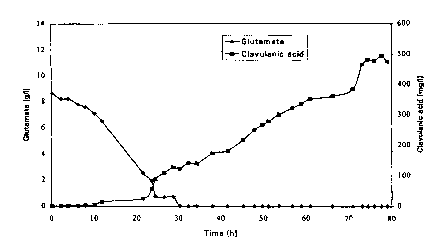
the fed batch process. From figure 1 it can seen that clavulanic acid
production starts when the glutamate concentration is dropping below 5
ao and even more preferably below 2.5 g/I and ends at 250-300 mg
cfavulanic acid /liter. Figure 2 shows that when glutamate is fed to the
CA 02336363 2000-12-29
WO 00/01840 PCT/EP99/04702
-1 z-
fermentor keeping the concentration very low ( < 1 g/L) after 30 hour".
clavulanic acid titers can increase to 500 mg/L in this experiment, giving a
doubling compared to the batch experiment.