Note: Descriptions are shown in the official language in which they were submitted.
CA 02336815 2001-O1-09
COMPOSITION AND PHARMACEUTICAL DOSAGE FORM FOR
COLONIC DRUG DELIVERY USING POLYSACCHARIDES
Field of the Invention
This invention relates to a composition and oral
pharmaceutical dosage form for selective delivery of
drugs to the colon. More particularly, the invention
relates to compositions and oral pharmaceutical dosage
forms for release of biologically active ingredients in
the colon while avoiding or minimizing release into the
upper gastrointestinal tract, such the stomach and
small intestine.
1~ Background of the Invention
Numerous drug entities based on oral delivery
have been successfully commercialized, but many others
are not readily available by oral administration, which
are incompatible with the physical and/or chemical
environments of the upper GI tract and/or demonstrate
poor uptake in the upper GI tract . Due to the lack of
digestive enzymes, colon is considered as suitable site
for the absorption of various drugs. However, colon
drug delivery is hardly achieved in that the oral
2~ dosage form should pass through the stomach and small
intestine, where many drugs is deactivated by their
digest materials. Colon specific drug delivery system
CA 02336815 2001-O1-09
is designed such that it remains intact in stomach and
small intestine but releases encapsulated drugs only in
colon. CSDS system is useful in administering a drug
that is irritant to the upper GI tract such as non-
steroidal anti-inflammatory agents, or drugs that are
degraded by gastric j nice or an enzyme present in the
upper GI tract such as peNtide or protein. Further,
the colonic drug delivery s_,~stem allows local, direct
treatment of colonic disease, e.g., ulcerative colitis,
Crohn's disease, or colon cancer, thus reducing the
dosage of the drugs and minimizing undesirable or
harmful side effects. Similarly, colonic drug delivery
is useful for administering drugs, e.g. non-steroidal
anti-inflammatory drugs (NSAIDS), which are irritants
to the mucosa of the upper gastrointestinal tract such
as the stomach or small intestine. Recently, it is
believed that colonic drug delivery systems maintain
the efficacy of drugs for a longer time and increase
the bioavailability of the drugs as compared to other
oral routes of administration. As the colon has a
longer retention time, drug absorption is prolonged,
and total bioavailability is increased. A. Sintov et
al., 143 Int. J. Pharma. 101-106 (1996).
Although colon is attracting interest as a site
for delivering several drugs such as unstable drugs in
upper GI tract or pocri.y-absorbed drugs, it is T~-ery
difficult to deliver the aiag unto colon effectiv<=ly.
CA 02336815 2001-O1-09
In order to deliver a drug to the colon selectively, a
composition generally should meet the following
requirements: (1) the composition is not degraded or
disintegrated in the upper GI tract; (2) the
composition does not release tl:e loaded drug in the
upper GI tract; (3) the composition releases the drug
effectively at the target site, the colon, more
particularly the ascending colon; and (9) the
composition is easy to formulate in a form suitable for
loading the drug. Further, the composition preferably
has the good processabi_lity to be manufactured.
Several approaches have been used in developing
colon-specific drug delivery systems. One is based on
the different pH of each compartment of the
gastrointestinal tract, with the pH of the proximal GI
tract being lower than that of the distal GI tract.
Thus, polymers that are insoluble at low pH and soluble
at higher pH have been used to deliver drugs to the
distal GI tract
Another approach is based on the fact that
transit time through the stomach is approximately 2
hours, whereas transit time through the small intestine
is approximately 4-6 hours. Thus, in this approach, the
delivery system is designed to withhold the release of
2~ the drugs for about 6-8 hours from the time of
administration.
Moreo~~er, it is wel lb:ic~f~r. thU- enzymes capable
CA 02336815 2001-O1-09
of reducing azo bonds or hydrolyzing glycosidic bonds,
which are not degraded in the stomach and small
intestine, are present in the colon. Thus, many
approaches to colonic drug delivery use azo bond-
s containing polymers (azo polymers) or glycosidic bond-
containing materials. The glycosidic bond-containing
polymers include disaccharides, c-~igosaccharides, and
polysaccharides.
For example, U.S. Patent No. 5,482,718; U.S.
Patent No. 4,627,851; U.S. Patent No. 4,693,895; U.S.
Patent No. 4,705,515; U.S. Patent No. 4,904,474; EP o21
032 Al; JP 34929/1991A; U.S. Patent No. 5,536,507; EP
453 001 A1; U.S. Patent No. 5, 171, 580; and EP 572 942
A2 disclose time dependent drug delivery system. They
are designed to prevent drug ~slease for a period of
time expected to be sufficient for the composition to
pass through the upper gastrointestinal tract. Further,
U.S. Patent No. 5,401,512; U.S. Patent No.
5,541,170; and WO 95/11024 describe drug compositions
for selectively releasing the drug in the colon by way
of exploiting the difference in pH between the colon
and other parts of the GI tract.
The above-mentioned compositions, however, are
not effective in delivering the drug to the colon. The
2~ pH in the terminal ileum a~.d colon is higher than other
region of the GI 'Tact and thus composition which
disintegrate at hic~ pH level have the potential for
4
CA 02336815 2001-O1-09
site specific delivery into this region. However,
because the pH is higher in the terminal ileum region
than in the colon, and the dosage forms are often
delayed at the ileo-cecal junction, the dosage forms
based on pH dependent system are often disintegrate in
the terminal ileum instead of disintegrate in colon.
Further, the colon specific delivery based o_n. GI
transit time-dependent system is hardly to be achieved.
The transit time in the upper gastrointestinal tract
tends to be highly variable among individuals.
Many approaches to colonic drug delivery use azo
bond or glycosidic bond-containing drugs, i.e. prodrug
and have been successfully come into the market. The
prodrug that is activated only in the colon requires
1~ covalent bonding between the drug molecule and carrier
molecules such that the covalent bonds are broken only
by enzymes produced by colonic bacteria. WO 84/04041,
WO 93/22334; A.D. McLeod et al., 83 J. Pharm. Sci.
1284-1288(1994); D.R. Friend et al., 27 J.Med.Chem.261-
266 (1984); B.Haeberlin et al., 10 Pharm.Res. 1553-1562
(1993); D.R. Friend et al. 28 J. Med. Chem.51-57
(1985); DR Friend, 5 S.T.P. Pharma Sci. 70-76 (1995);
J.P. Brown et al., 25 J.Med.Chem. 1300-1307(1983).
It is well knc:rn that enzymes capable of breaking
an azo, disulphide bonds and glycosidic bond are
,rresent in the color-, but not in the upper GI tract. WO
1/1605; and Fr _.Jr; 47~ A% ~,~is~lose composi-..ions
i
CA 02336815 2001-O1-09
containing an azo polymer having azo bonds as a colonic
drug delivery system. Although the composition is
relatively stable in the upper gastrointestinal tract,
the dosage form coated by azo polymer does not showed
colcn specificity effectively. Azo reductase produced
by colonic microfiora cannot easily reach the azo bond
of azo-polymers due to the hydrophobic nature of the
azo-polymer, thus resulting in slew degradation in the
colon of the composition containing the azo polymer.
P.Y. Yeh et al., 196 Macromol.Chem.Phys.2183-2202
(1965) .
A number of delivery systems based on
polysaccharides which are selectively degraded by
colonic enzyme have been reported, since
1~ polysaccharides are natural polymers with proven
minimal toxicity.
U.S. Patent No.5,505,966 discloses a
pharmaceutical composition containing calcium pectinate
as a major component and a filler such as pectin,
dextran, avicel, or mixture thereof. U.S. Patent
No.5,525,634 discloses a pharmaceutical composition
containing a synthetic or natural polymer that is
degradable by a colonic enzyme, wherein calcium
pec~inate is disclosed as an example of a natural
2J vol.~.-mer.
In U. ~. Patent No. 5, 5C!~;, X66, the calcium
sec-.mate ~cmpcsition is used ?.~ the form of a
6
CA 02336815 2001-O1-09
coacervate pellet. It is believed that calcium
pectinate, which is insoluble in water, is converted to
a water-soluble matrix by sodium ions or potassium ions
present in the digestion solution of upper GI.
Therefore, the system mainly depends on transit time of
upper GI, thus the pellets often disintegrate and
release the drug in upper GI.
U.S. Patent No. 5,525,634 suggests a compressed
tablet formulation that is prepared by pulverizing and
compressing a pharmaceutical composition containing a
drug and calcium pectinate. In the composition, the
strength of compression largely affects the system
disintegration through the GI tract. The weak
compressed tablet disintegrates easily in the upper
gastrointestinal tract by converting to a water-soluble
matrix, which caused by sodium ions or potassium ions
present in the digestion solution of upper GI. Further,
the strongly compressed tablet is hardly disintegrated
in colon. Therefore, the compositions disclosed in both
U.S. Patent No. 5,505,966 and U.S. Patent No. 5,525,634
is highly dependent on the system swelling and on the
transit time through the upper gastrointestinal tract,
and not on unique characteristics of the composition.
To solve the above problem, Adkin, D.A. et ai.
2~ s,aggests the addition of guar gurr; or pectin as a b,~:~der
~al~ium pectirlate compresseu~ tablets and cea~~v_ca
them,. v~a~ th enteric material. Adkir., D.A. et al. 14 =_~arm.
7
CA 02336815 2001-O1-09
Res. 103-107 (1997). Guar gum or pectin is used as a
binder for preventing easy disintegration in upper GI
and resulted in sustained release effect in colon.
Enteric coating is also used for preventing the rapid
swelling and disintegration in upper GI. However this
system also present slow release of drug in colon and
is highly dependent on the enteric coating thickness
and on the transit time through the upper
gastrointestinal tract, and not on unique
characteristics of 'he composition.
U.S. Patent Io.4,432,966 discloses a composition
comprising microc~ystalline cellulose and ethyl
cellulose; EP 627 173 Al describes a cellulose
composition; WO 95/35100 discloses a starch capsule and
a comp~~~ition comprising an enteric coatir:g; USP
5,422,121 discloses a composition comprising a guar gum
or locust bean gum blended with a film forming material.
The above-mentioned composition is formulated by using
polysaccharide with the film forming materials.
Generally polysaccharides have the hydrophilic moiety
and have a difficulty in fabricating the coating film
in the coated dosage form due to its physical
properties. In addition, the polysaccharide films and
matrix fabricated by compression method, they are
2~ easily disintegra=~~ in upper GI. Therefore,
pol_,~saccharides am- nixed witlu the mere hydrophobic
film:-_orming m.at=. ial in tt~e above-mentioned
CA 02336815 2004-09-16
composition. Although the blend of polysaccharide and
film forming material shows the improved film forming
properties, a hydrophobic film-forming material
generally has the lower swelling ratio than that of a.
polysaccharide. Due to the swelling ratio difference,
the coated film comprising a polysaccharide and a film
forming material is often phase-separated and bring a
crack during passage through the stomach and small
intestine. In addition, the using hydrophobic film
forming material bring the undesirable result that
disintegration of dosage form by colonic enzyme is
delayed due to the hydrophobic nature of the mixed
polymer, thus resulting in slow degradation in the
colon of the composition. It may be happened that
colonic enz~,~me from microflora cannot easily reach the
polysaccharide due to the hydrophobic nature of the
mixed polymer. Accordingly, the drug is often released
1r1 the upper l~aW .i.vla.~.c~~111d1 tract, Or It 1S :uU SlUw
drug release that the system fails to show colon
specific drug delivery.
In view of the foregoing, it will be appreciated that
providing a polysaccharide-based composition for
controlled drug delivery in the colon would be a
significant advancement in the art.
9
CA 02336815 2004-09-16
Brief Summary of the Invention
It is an object of an aspect of the present
invention to provide a composition and pharmaceutical
dosage form for delivering a drug, wherein such dosage
form is orally administered for specifically deliverer-ig
the drug to the colon of a subject in need thereof.
Another object of an aspect of the invention is to
provide a composition and pharmaceutical dosage form for
colonic drug delivery that is not degraded or
disintegrated in the upper GI tract.
Still another object of an aspect of the invention
is to provide a composition and pharmaceutical dosage
form for delivering an orally-administered drug that is
inactivated in the upper GI tract, wherein the dosage
form is a form that passes through the upper GI tract and
then releases the drug in the colon of a human subject in
need thereof.
Yet another object of an aspect of the invention :is
to provide a composition and pharmaceutical dosage form
for colonic drug delivery that releases the drug rapidly
and effectively at the target site, the colon, and
minimizes adverse systemic effects to a subject being
treated.
Another object of an aspect of the present invention
is to provide a composition and pharmaceutical dosage
form for colonic drug delivery that is easy to formulate
in a form suitable for loading the drug to be delivered.
Still another object of an aspect of the invention
is to provide a method for treating a subject through
oral administration of a pharmaceutical dosage form and
composition that achieves the foregoing objects of this
invention.
Yet another obj ect of an aspect of the invention i.s
to provide a process for preparing a pharmaceutical
dosage form and composition that achieves the foregoing
objects of this invention.
CA 02336815 2004-09-16
One aspect of this invention is a composition and
pharmaceutical dosage form designed for delivering an
orally administered drug to the colon. The composition
passes through the upper GI tract without releasing t:ze
drug, but the drug is rapidly and effectively released at
the target site in the colon, more especially in the
ascending colon, minimizing adverse systemic effects to a
human subject being treated. The composition comprises a
mixture, prepared at a pH of about 7 or above, of a
galactomannan and a polysaccharide, preferably pectin,
selected from the group consisting of pectin, derivat:ivE=_s
of pectin, and mixtures thereof. The composition forms a
strong elastic gel that is not appreciably dissolved or
disintegrated in gastric or intestinal fluids, thus
protecting drugs from being released in the upper c~I
tract. When the composition arrives in the colon, the
composition is easily degraded by synergic effect of
pectinolytic enzymes and galactomannanase, thus releasing
drugs rapidly in the colon. The ratio of the two
polysaccharides determines the rate of enzymatic
degradation of the composition and disintegration of
dosage form through GI tract, which in turn enables tree
composition to release the drug specifically in the
colon. More particularly, the ratio and coating
thickness of the two polysaccharides mixture enables the
composition to release the drug more specifically in tree
colon such as ascending, transverse, or descending colon.
Another aspect of this invention provides a colon
specific composition and a pharmaceutical dosage form
comprising the inventive colonic drug delivez~y
composition and a biologically active substance without
additional enteric coating and an independence t:o
individual variance such as pH and transit time. The
composition and dosage form of this invention can be i_n
the form of a coating material, hard capsule shel_1
material, or matrix.
11
CA 02336815 2004-09-16
In accordance with one embodiment of the present
invention, there is provided a colonic drug delivery
composition for oral delivery comprising a mixture formed
in an aqueous medium at a pH of about 7 or above of (a) a
polysaccharide selected from the group consisting of
pectin, derivatives of pectin, and mixtures thereof, and
(b) galactomannan.
In accordance with another embodiment of the present
invention, there is provided a colon selective
pharmaceutical composition for oral delivery of a drug,
nutrient, diagnostic reagent, or mixture thereof
comprising an effective amount of said drug, nutrient,
diagnostic reagent, or mixture thereof in contact wii~h a
composition comprising a mixture formed in an aqueous
medium at a pH of about 7 or above of (a) a
polysaccharide selected from the group consisting of
pectin, derivatives of pectin, and mixtures thereof, and
(b) galactomannan.
In accordance with a further embodiment, there is
provided a method for preparing a colon selective
pharmaceutical composition for oral delivery of a drug,
nutrient, diagnostic reagent, or mixture thereof
comprising forming a mixture in an aqueous medium at a pH
of about 7 or above of (a) a polysaccharide selected from
the group consisting of pectin, derivatives of pectin,
and mixtures thereof, and (b) galactomannan.
In accordance with a further embodiment is use of a
pharmaceutical composition comprising a drug, nutrient,
diagnostic reagent, or mixture thereof in contact with a
composition comprising a mixture formed in an aqueous
medium at a pH of about 7 or above of (a) a
polysaccharide selected from the group consisting of
pectin, derivatives of pectin, and mixtures thereof, and
12
CA 02336815 2004-09-16
(b) galactomannan, as a colon selective drug delivery
composition.
Brief Description of the Drawings
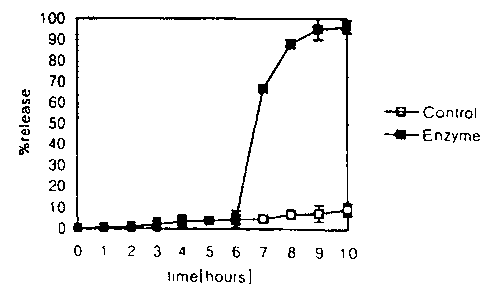
FIG. 1 shows the results of a drug release wherein
inbuprofen tablest coated with a composition according vo
the present invention comprising 15 mg/cm2 of a 4:1 weight
ratio of pectin . guar gum were incubated successive:iy
for 2 hours in simulated gastric fluid (SGF) , 4 hours :in
simulated intestinal fluid (SIF), and 4 hours in either
simulated colonic fluid (SCF; ~)or buffer (~).
12a
CA 02336815 2001-O1-09
FIG.2 shows the results of a drug release wherein
budesonide pellets coated with a 100 um film of 4:1
weight ratio of pectin , guar gum according to the
present invention were incubated successively for 2
hours in SGF, 4 hours in SIF, and 4hours in either SCF
or buffer
FIG. 3 shows the resin is of_ a drug release ~f~herein
sugar seeds coated with a 100 hm film comprising a
mixture of diclofenac sodium and a 4:1 weight ratio of
pectin . guar gum according to the present invention
were incubated successively for 2 hours in SGF, 4 hours
in SIF, and 4 hours in either SCF (~ ) or buffer
Detailed description of ~.ze invention
Before the present composition and method for
colonic drug delivery are disclosed and described, it
is to be understood that this invention is not limited
to the particular configurations, process steps, and
materials disclosed herein as such configurations,
process steps, and materials may vary somewhat. It is
also to be understood that terminology employed herein
is used for the purpose of describing particular
embodiments only and is not intended to be limiting
?> since the scope of the present in venticn will be
1_imited only by the appended claims and equip%alents
;-hereof.
13
CA 02336815 2001-O1-09
In must be noted that, as used in this
specification and the appended claims, the singular
forms "a," "an," and "the" include plural referents
unless the context clearly dictates otherwise. Thus,
for example, reference to a composition containing "a
galactomannan" includes a mixture of one or more
galactomannans, reference to " a pectin salt" includes
reference to one or more of such pectin salts, and
reference to " a coating" includes reference one or
more of such coatincs.
In describing and claiming the present invention,
the following terminology will be used in accordance
with the definitions set out herein.
As used herein, "colon-specific drug delivery
system" and similar terms mean devices and methods for
oral administration that release biologically active
ingredients in the colon without substantial release
into the upper gastrointestinal tract, e.g. stomach and
intestine.
As used herein, the term "drug" or
"pharmacologically active agent" or any other similar
term means any chemical or biological material or
compound suitable for administration by the methods
previously known in the art and/or by the methods
2~ taught in the prese:_t invention, that induces a desired
biological or pharmacological effect, which may include
but is net limited vc ;lj having a prophylactic ef=ect
14
CA 02336815 2001-O1-09
on the organism and preventing an undesired biological
effect such as preventing an infection, (2) alleviating
a condition caused by a disease, for example,
alleviating pain or inflammation caused as a result of
disease, and/or (3) either alleviating, reducing, or
completely eliminating the disease from the organism.
The effect may be local, such as providing for a local
anaesthetic effect, or it may be systemic. This
invention is not drawn to novel drugs or to ne~~ classes
of active agents. Rather it is limited to the mode of
delivery of agents of drugs that exist in the state of
the art or that may later be established as active
agents and that are suitable for delivery by the
present invention. Such substances include broad
classes of compounds normally delivered into the body.
In general, this includes but is not limited to:
antiinfectives such as antibiotics and antiviral
agents; analgesics and analgesic combinations;
anorexics; helminthics; antiarthritics; antiasthmatic
agents; anticonvulsants; antidepressants; antidiabetic
agents; antidiarrheals; antihistamines;
antiinflammatory agents; antimigraine preparations;
antinauseants ;antineoplastics; antiparkinsonism drugs;
antipruritics; antipsychotics; antipyretics;
2> anti~nasmodics; anticpolinergics; sympathomimetics;
xantr_;~ne derivatv~ves; cardiovascular preparations
i.nclv~ing potassiu-~. and calcium channel M ockers, beta-
I~
CA 02336815 2001-O1-09
M ockers, alpha-M ockers, and antiarrhythmics;
antihypertensives; diuretics and antidiuretics;
vasodilators; including general coronary,peripheral and
cerebral; central nervous system stimulants;
vasoconstrictors; cough and cold preparations including
decongestants; hormones such as estradiol and other
steroids, including corticosteroids; hypnotics;
immunosuppressives; muscle relaxants;
parasympatholytics; ps_,v~hostimulants; sedatives; and
tranquilizers; prouiotics. By the method of the present
invention, both ionized and nonionized drugs may be
delivered, as can drugs of either high or low molecular
weight.
As used herein, "nutrient" means a substance that
1~ affects the nutriti~~s or metabolic processes of the
body. Nutrients include essential nutrients, i.e. those
nutrients such as proteins, minerals, carbohydrates,
fats, and vitamins necessary for growth, normal
functioning, and maintaining life, and secondary
nutrients, i.e. substances that stimulate the
intestinal microflora to synthesize other nutrients.
As used herein, "diagnostic reagent" means a
substance used to produce a chemical reaction so as to
detect or measure anct:~.er substance.
P.s used herein, "simulated intestinal fluid" or
"SIE" means a corr;:.os_r--rn prepared by dissolving E.8
of r~aonc;basic potGss_ ~;m, phosphata in 250 ml cf wager,
16
CA 02336815 2001-O1-09
then adding 190 ml of 0.2 N NaOH, 400m1 of water and 10
g of pancreatin, and finally adding 0.2 N NaOH to
adjust the pH to 7.5, and then diluting with water to
1000 ml.
As used herein, 'simulated gastric fluid' or
'SGF' means a composition prepared by dissolving 2 g of
NaCl and 3.2 g pepsin in 7 ml of HCl, and then adding
water to 1000m1. The resulting fluid has a pH of about
1.2.
As used herein, 'derivatives of pectin' and
similar terms includes can on salts of pectin such as
sodium pectinate, potassium pectinate, and ammonium
pectinate, and the like.
As used herein, 'effective amount' means an
amount of a drug or pharmacologically active agent that
is nontoxic but sufficient to proeide the desired local
or systemic effect and performance at a reasonable
benefit/risk ratio attending any medical treatment. An
effective amount of a nutrient is an amount sufficient
to provide a selected nutritive benefit. An effective
amount of a diagnostic reagent is an amount sufficient
to be efficacious in a selected diagnostic test or
assay.
The present invention relates to a colonic drug
2> delivery ~:omposition comprising a rr.ixture of (a) a
polysacoharide selected from the group consisting of
pectin, derivatives of pectin, and mixtures thereof,
17
CA 02336815 2001-O1-09
and (b) galactomannan. The mixture is made by combining
the ingredients in an aqueous medium at a pH of about 7
or above.
In a preferred embodiment of the invention, the
_5 composition comprises (a) pectin or a derivative of
pectin or a mixture thereof and (b) galactomannan. The
composition is prepared at a pH of about 7 or above.
The ratio of ingredients is limited only by
functionality. Preferably, however, the composition has
a polysaccharide: galactomannan weight ratio of about
50:50 to about 99.9:0.01, more specifically, about 2:1
to about 5:1. A coating prepared with the instantly
claimed composition will generally have a mass to area
ratio in the range of about 1-100 mg/cm', and
preferably about 1-40 mg/ cm'. A t;ard capsule shell
will generally have a thickness of about 1-100 !~,
preferably 1-401.
A key feature of this invention is based on a
change in the properties of a mixture of these two
polysaccharides, which is degradable in the colon by
colonic bacterial enzymes, as compared to either
polysaccharide alone. When pectin or galactomannan
(such as guar gum or locust bean gumj is used alone as
a drug carrier, e.g., coating material, the coating is
easily dissolved and/or disintegra~ed in simulated
gastric fluid (SGF) and simulated intestinal fluid
;SIE') . However, a mixture of these ttr,o polysaccharides
18
CA 02336815 2001-O1-09
prepared at a pH of about 7 or above produces a strong,
elastic, and insoluble gel that is not dissolved or
disintegrated in the simulated gastric and intestinal
fluids, thus protecting drugs coated with the mixture
from being released in the upper GI tract. On the ether
hand, under conditions encountered in the colon the
coating is degraded by pectinolytic enzymes and
galactomannanase, thus disintegrating the dosage form
and rapidly releasing the drugs. When the coating is
prepared at belo~;; pH 7, such coating is easily
disintegrated and dissolved in the upper GI tract.
Pectin and galactomannan are both degradable by
colonic bacterial enzymes. When the mixture of pectin
and galactomannan arrives in the colon, it is rap-dly
degraded by the synergic action of coloni:: bacterial
enzymes. Accordingly, the composition of the present
invention can prevent the drug deactivation in upper GI
and also produces the burst-like drug release pattern
in ascending colon, which is preferred to treat local
colon disease such as ulcerative colitis, Crchn's
disease. Thus, the composition of the present invention
can be used advantageously for specific drug delivery
in the colon as compared to known drug delivery systems.
The pH at which the mixture is prepared _s a
determining factor ~f the properties of the compos--:ion.
As shown by exile=_.a;ental. data cresented herein, --1ms
of such mixtures ~._epared at a pH of about 7 or _:~ove
19
CA 02336815 2001-O1-09
were stronger, more elastic and less soluble than films
cast from mixtures prepared at a pH below 7. In
addition, all films cast with the mixtures prepared at
a ~H below 7 were easily dissolved in SIF, while films
S cast from mixtures prepared at a pH of about 7 or above
were not extensively dissolved or not dissolved at all
in SIF. Either pectin or galactomannan is soluble in
aqueous media. Below pH 7, there is no binding force
be~ween pectin and galactomannan, whereas, at about pH
7 cr above, it is likely that pectin and galactomannan
in~eract each other to form a specific complex gel.
Gaiactomannan is a non-gelling polysaccharide but it
can be gelled synergically when mixed with some
pc-!ysaccharides. Proposed binding forces are synergic
IS action of hydrogen bonding, hydrophobic force and the
formation of interjunction zone. The conformational
change of polysaccharide chains at pH 7 or above
results in the strong interaction. Synergic gellation
and interaction of galactomannan and polysaccharides
are referred to in C.M.D. Iain, et a1.,31 Adv.
Carbohydrate. Chem. Biochem. 241-312(1975).
The ratio of the two polysaccharides determines
the rate of enzymatic degradation of the composition
and dosage disintegration through GI tract, which in
2~ t~.-n enables the composition to release the drug site
srecifically in the colon. More particvalarl,~, the ratio
,,_ tl~:e t~:,o polysaccharides enables tr.e composition: tc
CA 02336815 2001-O1-09
release the drug more specifically in the colon such as
the ascending, transverse, descending colon. Between
the two polysaccharides, the more galactomannan(e.g.
guar gum) there is, the less soluble in SIF and the
less degradable by colonic enzymes. By increasing the
proportion of galactomannans, the composition releases
the drug at a site furt:ler on than the ascending colon,
e.g. the transverse or descending colon.
In addition, by changing the dimensions of the
dosage form, the site where drugs are released can be
controlled. The thickness of a coating or the diameter
of a matrix formulation which drug is incorporated can
be selected to achieve site specificity within the
colon. Thus, the ratio of polysaccharides and the
1~ dimensions of the composition are important factors for
determining the site of drug release in the colon. By
manipulation of these factors in the manufacture of
dosage forms according to the present invention, drug
delivery can be selectively targeted to the ascending
colon or more distally to the transverse or descending
colon.
Another improvement of the present invention is
that the composition can be used in many different
types of dosage forms. The composition can be used as a
coating material fir -ablets, soft capsules, gran~:les,
seeds, and the ~~ke. Further, it can be used
advantageously in hard capsules and matrix pellets.
21
CA 02336815 2001-O1-09
The composition can be used to deliver a wide
range of biologically active ingredients. For example,
topically acting drugs such as those for IBD, Crohn' s
disease, laxatives and colon cancer; systemically
acting drugs such as peptide or protein drugs, calcium
antagonists, antiasthmatics, hypoglycemic agents,
antirheumatic, and the like can be loaded and delivered
to the colon with the composition of the present
invention. Likewise, nutrients can be loaded and
delivered into the colon for a better absorption.
Further, pharmaceutically acceptable excipients may be
included in the composition.
Because both she pectin. and galactomannan
components of the composition of the present invent~_on
are specifically degraded in the colon, there is a
burst of drug release in the colon due to the synergic
effect of the colonic enzymatic activities. Compared to
the instant invention, previously known dosage forms
are much slower and gradual with respect to
disintegration, dissolution, or drug release in the
colon. The synergic effect of the enzymatic activities
is referred to in T . Ooya et al . , 25 Proc . Int' 1 Symp .
Control. Rel. Bioact. Mater. 731-732(1998).
As stated above, the composition of the present
2~ in~,~ention can be used as a coating material, hard
capsule :~hei__ material, or matrix.
I71usvrative examples of drugs that can be used
CA 02336815 2001-O1-09
in the composition and the dosage form of the invention
include: mesalamine, balsalazide, olsalazine, ibuprofen,
prednisolone, dexamethasone, budesonide, beclomethasone,
flucticasone, tioxocortal, hydrocortisone,
metronidazole, cyclosporin, methotrexate, domperidone,
5-fluorouracil, bisacodyl, senna, insulin, vassopressin,
growth hormones, colony stimulating factors, calcitonin,
immunoglobulin, glibenclimide, diltiazem, verapamil,
nifedipine, captopril, benazepril, enalapril,
theophylline, naproxen, diclofenac, acyclovir,
omeprazole, lovastatin, alendronate, desmopressin,
metformin, metoprolol, cisapride, tacrine, mixtures
thereof and probiotics. Also, the pharmaceutical
composition of the present/ invention may include
diagnostic reagents and nutrients as active substances.
The active substances that may be used in the
pharmaceutical composition of the present invention are
not limited to those mentioned above.
The composition and the dosage form of this
invention is not limited to the above mention
embodiments, and modification may be made thereto by a
person skilled in the art. The invention may be
explained by the representative examples, which are
provided only for the purpose of illustrating certain
2~ aspects of the present invention. Therefore, they are
not to be construed as limiting the scope of the
present invention In any way.
23
CA 02336815 2001-O1-09
Examples
<Example 1> Preparation of Film Made of the Composition
Pectin and guar gum (4:1 w/w) were mixed and
dissolved in distilled water to a final concentrar_ion
of 2o(w/v). The pH of aliquots of the mixture was
adjusted to pH 4, S, 6, 7, 8, and 10 with Na~CO~. F ilm
with a thi ckness of 150 ~cm were cast on a Teflon plate
with each aliquot.
Additional samples were also prepared wherein
locust bean gum was added in place of guar gum, and the
pH of these samples was adjusted to pH 4 and pH 8.
Films were cast and dried. The thickness of these dried
films was 150 ~Lni.
Further, other samples w~re prepared wherein
pectin was dissolved in distilled water, and the pH was
adjusted to pH 4 and pH 8 with Na2C03. These samples
were also cast as films and dried. The thickness of
these dried films was also 150 um.
Finally, films of guar gum and of locust bean gum
were prepared at pH 7 and pH 14 as described above.
Thickness of these dried films was also 150~m.
All prepared films were cut into pieces with a
dimension of 1 cm x 1 cm. Samples were weighed and
subjected tc a dissolution test. The medium uses fcr
testing solubility was simulated intestinal fluid ~I:~J.
After 6 yours in SIF, samples were withdrawn frc~- tue
24
CA 02336815 2001-O1-09
medium, washed, and dried. The dried samples were then
weighed and compared with pre-test weights.
The results of this experiment are presented in
Table 1(n = 3)
Table
1
Film o Remaining
after 6
hrs
Pectin/guar gum, pH 4 _ 0
Pectin/guar gum, pH 5 0
Pectin/guar gum, pH 5 15 3
Pectin/guar gum, pH 7 61 3
Pectin/guar gum, pH 8 ~0 4
Pectin/guar gum, pH 10 ~1 3
Pectin/locust bean um,pH 0
g 4
Pectin/locust bean um,pH ~,2 5
g 8
Pectin, pH 4 0
-
Pectin, pH 8 0
Guar gum, pH 7 0
Guar gum, pH 14 0
Locust bean gum, pH 7 0
Locust bean gum, pH 14 0
From Table l, it can be seen that. the films
prepared at pH 7 and above with the mixtures of pectin
and guar gum or pectin and locust bean gum were not
readily dissolved in the simulated intestinal fluid
(SIF). Films of the same mixtures prepared at below pH
7 and films of only one of the polysaccharides,
regardless of pH, were easily dissolved. Therefore, the
composition for colonic drug delivery of this invention
requires a mixture of at least two polysaccharides, e.g.
pectin and aalactomannan, prepared at a-~ envircnmental
pH of about , cr greater.
2~
CA 02336815 2001-O1-09
<Example 2> Measurement of Tensile Strength
Films were made according to the procedure of
Example 1 of a mixture of pectin and guar gum (4:1 w/w)
prepared at either pH 4 or pH 8. These films were cut
to a dimension of 1 x 7 wo. Then, the films were tested
in an Instron Tensile Strength Machine to measure the
tensile strength. The results are presented in Table 2
(n = 6)
Table 2
Film Breaking Strain at
Load ( g ) Maximum ( o )
Pectin/guar gum, 130.5 12.1 60.93 10
pH 4
Pectin/guar gum, 3,95.6 16 115.7 8.5
pH 8
These results show that the films prepared at an
environmental pH of about 7 or above have superior
tensile properties as compared to films prepared at a
pH below 7.
<Example 3> Coating of Tablets with the Composition
Tablets containing 100 mg of ibuprofen as a model
drug were coated with the composition prepared
according to the procedure of Example 1. The weight
ratio of pectin to guar gum was 4:1, and t~:~:e pr-was
adjusted t~ pH 8. The coating was processed ~::ith ~ Hi-
Coater (HCT-MINI, Freund Ind., Japan , and the comings
2~
CA 02336815 2001-O1-09
were prepared to reach amounts of 8 u~g/cvi', 151ug/cm', 261118
/ cu~2 , a n d 3 5 nag / cm2 .
Additional tablets containing 100 ttt~ of ibuprofen
as a model drug were coated with composition prepared
according to the procedure of Example 1 wherein locust
bean gum was added in place of guar gum. The weight
ratio of pectin to locust bean gum was 4:1, and the pH
was adjusted to pH 8. The coating was processed with a
Hi-Coater (HCT-MINI, Freund Ind., Japan), and the
coating were prepared to reach amount of 15 n1;/CU~'.
Control tablets containing 100 og of ibuprofen as
a model drug were coated by three other compositions.
Each of those composition was composed of pectin and
guar gum (4:1 weight ratio) at a pH of 4, pectin and
l~ locust x:ean gum (4:1 weight ratio) at a pH of 4, and
pectin of the same concentration at a pH of 8. Coating
thickness was 15 mg/cm'.
A disintegration test was performed in simulated
gastric fluid (SGF), simulated intestinal fluid (SIF)
for 24 hours with a disintegration tester (DT-400, Fine
Scientific Instrument, Korea). SGF and SIF were
prepared according to the United States Pharmacopoeia,
relevant parts of which are hereby incorporated by
reference and which is summarized herein. SCF was 50 mM
phosphate buffer, 26 pg/ml Pectinex Ultra SPL~'Ncvo
Nordisk) , and ~0 unit/ml ;alactomannanase I~lo~-o
Nordi~k).
77
CA 02336815 2001-O1-09
In addition, disintegration was tested in a fecal
solution prepared by a modification of the method of
Macfarlane et al., 60 ~.Appl.Bacteriol. 195(1986),
hereby incorporated by reference.
All disintegration test results are presented in
Table 3, expressed as disintegration time.
Tabla 3
Coating SGF SIF ' Fecal
SCF
Sol.
Pectin/guar gum ND~ aD 45 75 min
min
8 m, / oui
Pectin/guar gum ND I~TD 120 min
60 min
uig / cui'
Pectin/guar gum ND ND 150 min 190 min
2 6 mg / cm'
Pectin/gua.r gum ND ND 470 min 510 min
35 ng/cm'
Pectin/guar cum 120 60 min 30 min -b
P H 4 , 15 n1g m i
j cm~ n
Pectin/locust ND ND I 60 min 120 min
bean gum, pH 8
15 mg / cm~
Pectin/locust 120 60 min 30 min -
bean gum, pH 4 min
1_ 5 mg / cmZ
Pectin 120 30 min 21 min -
min
a Not disintegrated during 24 hours
b Not determined
<Example 4> Drug Release Test
A drug release test ~~-a~ performed with tablets
coated ~,aith the composition a.~cording to the present
in~,~erv_tion. These tablets ~~~re =.~epared by the procedure
CA 02336815 2001-O1-09
of Example 3, with a coating of 15 u1g/cui'. The tablets
were incubated successively for 2 hours in SGF, 9 hours
in SIF, and 4 hours in either SCF or 50 mM phosphate
buffer. Released ibuprofen was measured every hour by
HPLC (HP-1100, Hewlett-Packard, Column:u Bondapak C-18).
The results are illustrated in Figure 1.
The minimal drug was released in SGF and SIF. In
addition, very little drug was released in the control
buffer SCF without enzymes), whereas the entire amount
of drug was release in SCF within 1 cr 2 hours. The
data obtained in the drug release test show that the
composition can protect the laded drug from being
released in the upper GI tract, and the release of drug
is dependent only on colonic enzymes and is independent.
of pH and transit time.
<Example 5> Variation of the Ratio of the Composition
Tablets containing 100 mg ibuprofen as a model
drug were coated by the composition prepared according
to the procedure of Example 3. The ratio of pectin and
guar gum were 4:1, 4:1.5 and 4:2. The pH of all
formulations was adjusted to 8. The coating was
processed with the Hi-Coater (HCT-MINI, Freund Ind.,
Japan) to reach an amount of 15 mg/cui.
C~sintegration ~,~as performed in simulated gastric
fluid SGF), simulated intestinal flvaid ~;SIF'), and
simulated colonic fl~,~id (SCF) for 24 hours with the
~ cj
CA 02336815 2001-O1-09
disintegration tester (DT 400, Fine Scientific
Instrument, Korea). SGF and SIF were prepared as
described above, i.e. according to USP. SCF contained
50 mM phosphate buffer with 26 pg/ml Pectinex Ultra SPL
(Novo Nordisk) and 20 unit/ml galactomannanase (Novo
Nordisk) was used as SCF.
Also, instead of SCF, the fecal solution was used
as the medium for the disintegration test. The fecal
solution was prepared by a modification of the method
of Macfarlane, et al., in J. Appl. Bacteriol., 60,
p.195(1986). All disintegration test results are
presented in Table 4, expressed by disintegration time.
Table 4
Coating SGF SIF SCF Fecal
Pectin:guar gum ~'Da ND _ _
4:1 60 min 120 min
Pectin:guar gum ND ND 180 min 270 min
4:1.5
Pectin:guar gum ND ND 485 min 495 min
4:2
a Not disintegrated during 24 hours.
l~
It is clearly presented that variation in the
ratio of the composition can delay the degradation time
in the colon, thus controlling the release of drugs
selectively in the ascending, transverse or descending
colon .
<Example 6> Coating of the Soft Capsules and Hard
CA 02336815 2001-O1-09
Capsules
Soft capsules filled with mineral oil and hard
capsules filled with budesonide pellets were coated
with the composition of the present invention according
to the procedure of Example 3. The ratio of pectin to
guar gum was 4:1 and the pH was adjusted to 8. The
coating vaas applied at 15 mg; cup'.
Coated capsules were subjected to the
disintegration test under the conditions described as
above. Also, a fecal disintegration test was performed
under the conditions described above. The results ~.re
presented in Table 5 in terms of disintegration time.
Table 5
_ SGF SIF SCFFecal
Soft Capsule NDa ND 60 min pl~J min
Hard Capsule ND ND 60 min 120 min
a Not disintegrated during ?4hours
<Example 7> Coating of the pellets
Pellets containing budesonide were prepared with
a Fluid-Bed Granulator (GPCG-I, Glatt). Pellets were
then coated with the composition according to the
present invention using the Flow-Coater. Thickness of
the coated film was about 100 ~t m. The gelatin hard
capsules were filled with the coated pellets and
subjected t.o a drug release test. The test alas
performed by incubatsn:~ the dosage form in SGF _~7-
J
CA 02336815 2001-O1-09
hours, followed by incubation in SIF for 4 hours and
then SCF for 4 hours. As a control, during the SCF
incubation, half of the samples under the drug release
test were incubated in SCF without Pectinex and
galactomannanase, that is, in buffer. Released
budesonide was measured every hour by HPLC (HP-1100,
Hewlett-Packard, Column: y Bondapak C-18). The results
are similar to those of the coated tablets and are
illustrated in Figure 2.
The results show that there was minimal release
in the SGF and the SIF medium. In addition, in the SCF
buffer without enzymes (control), the drug released was
minimal also. In SCF, however, the drug was completely
released within about 1. or 2 hours. These results show
that the composition can protect the loaded drug from
being released in the upper GI tract and that its
release is dependent only on specific colonic enzymes.
Further, release is independent of the pH or transit
time, which is true for both coated tablets and pellets.
<Example 8> Manufacture of hard capsules
Hard capsules were manufactured with a
conventional pin molder. Thickness was about 100 mm.
The hard capsules t.~ere filled with pellets containing
budesonide (non dated) and were subjected to the
disintegratio:~ t~s~ according to the procedure of
Ezample 7. The results were substantially simila- to
32
CA 02336815 2001-O1-09
those of coated tablets. No disintegration was detected
in either SGF or SIF. Complete disintegration occurred
in 75 min in SCF and in 132 min in fecal solution.
These results show that the composition according to
the present invention can be applied advantageously to
the shell material of hard capsules.
<Example 9> Preparation of matrix pellets
Matrix pellets containing diclofenac sodium were
prepared using a Fluid-Bed Granulator (GPCG-I, Glatt).
Nonpareil sugar seeds were coated with a mixture of the
composit=ion according to the present invention and the
drug. The prepared pellets were subjected to the drug
release test according to the procedure of Example 4.
Results were s ~.:nilar to those obtained with ibuprofer:
and budesonide arid are illustrated in Figure 3.
As described above, the composition and the
pharmaceutical dosage form of the present invention,
which comprises a polysaccharide, preferably pectin,
and galactomannan, were prepared at an environmental pH
of about 7 or greater. The composition is not degraded
or disintegrated in the upper gastrointestinal tract,
but is degraded effec'ive~!_,~ in the colon by colonic
bacterial enzymes, ~hereNy rendering the active
subsr_an~e contained in the ~~ompcsition to be deli-~ered
iJ
CA 02336815 2001-O1-09
selectively to the colon. The drug targeting site in
the colon can be selected simply by adjusting the ratio
of galactomannan to pectin in the composition, or by
adjusting the size of the dosage form of the
composition. Since each component of the composition is
degradable only by coloni.c enzymes, a burst of drug
release in the colon can be achieved due to the
synergistic effect of the enzymatic reaction. In
addition, the degradation of the composition does nct
depend on the transit time or the pH of the upper GI
tract, thus rendering the drugs to be delivered
specifically and efficiently to the colon.
While the invention has been described with
respect to certain specific embodiments, it should be
recognized tha~ various modifications and changes may
be made by those skilled in the art, which
modifications also fall within the scope of the
invention as defined by the appended claims.
34